Tel-Cu-NPs Catalyst: Synthesis of Naphtho[2,3-g]phthalazine Derivatives as Potential Inhibiters of Tyrosinase Enzymes and Their Investigation in Kinetic, Molecular Docking, and Cytotoxicity Studies
Abstract
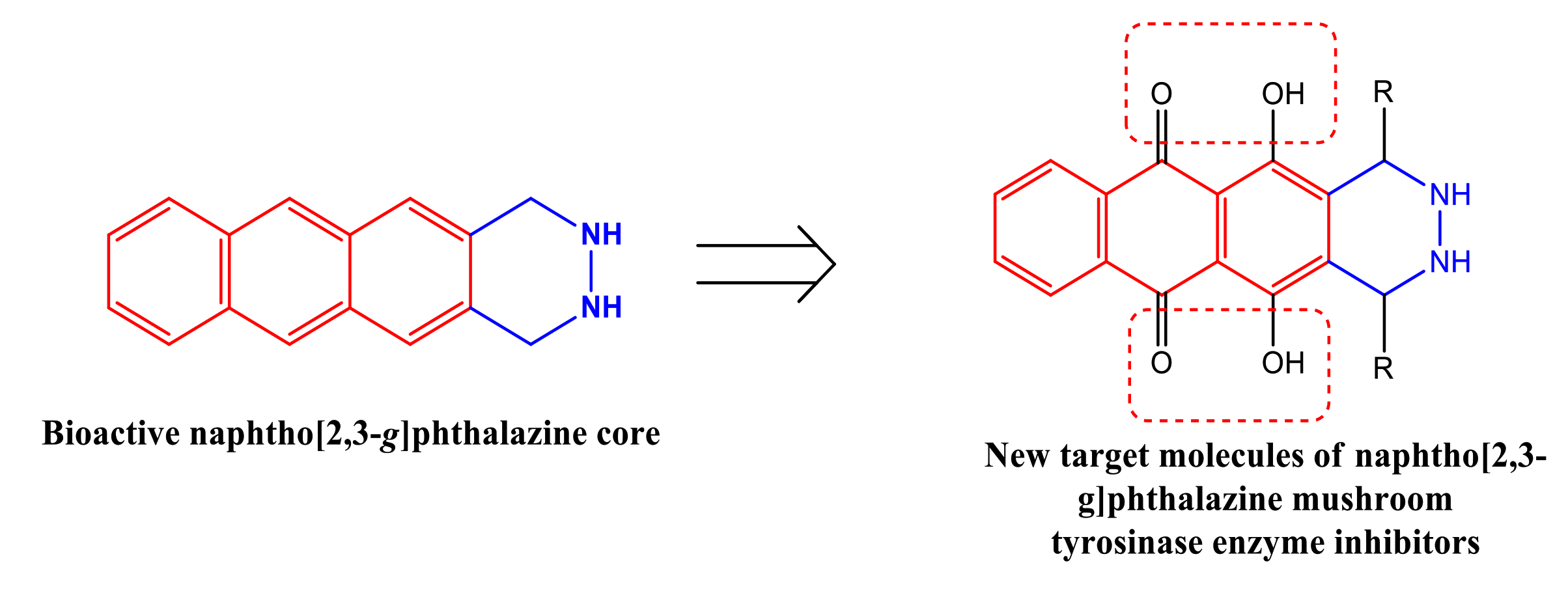
1. Introduction
2. Results and Discussion
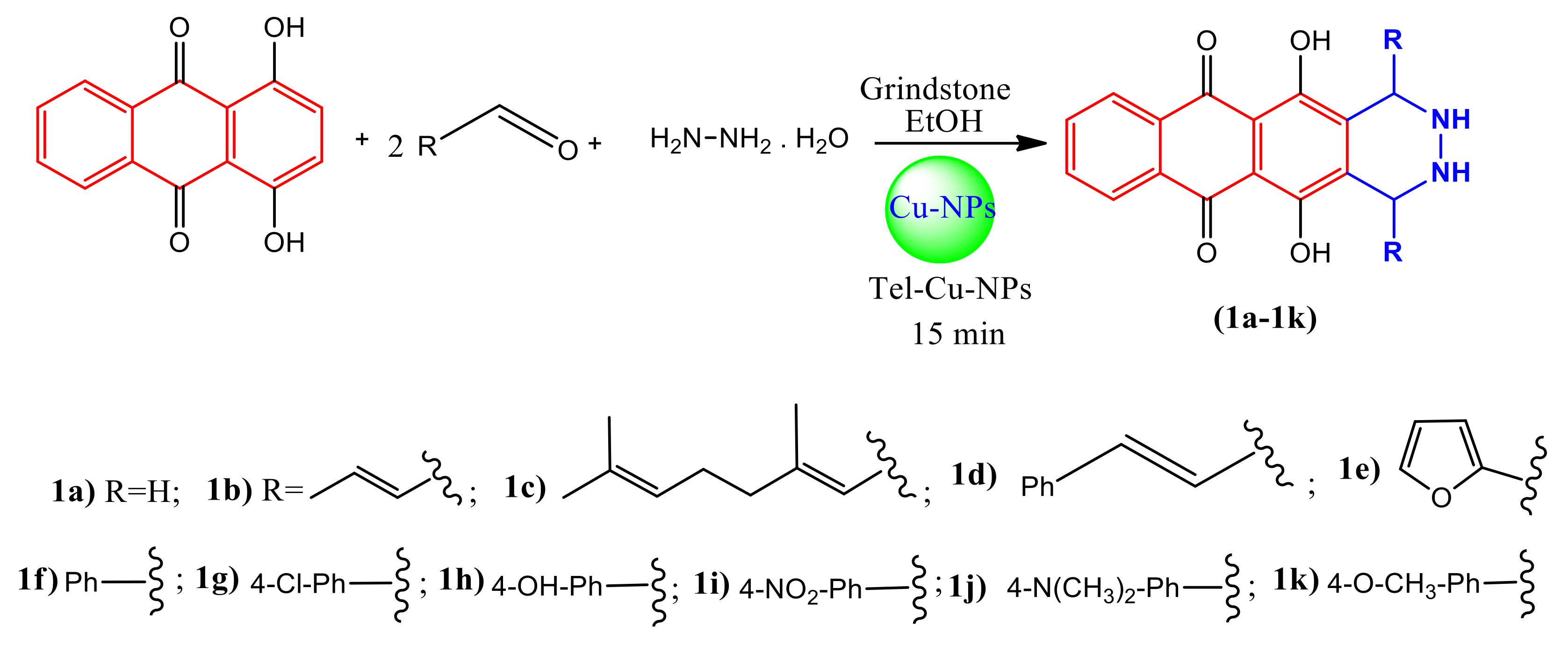
2.1. Chemistry
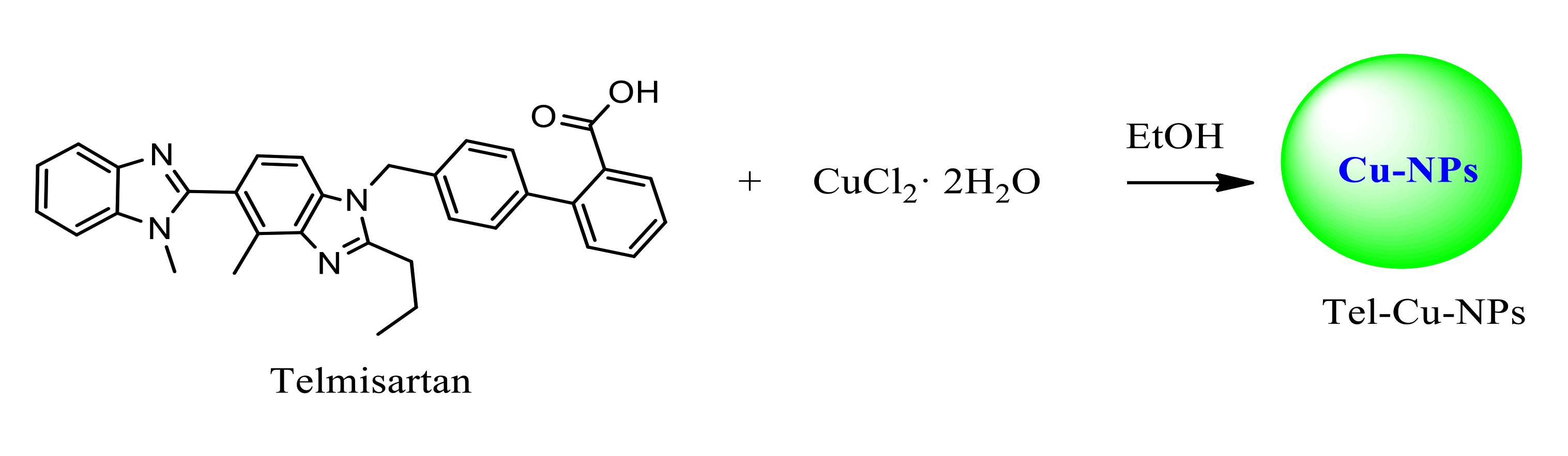
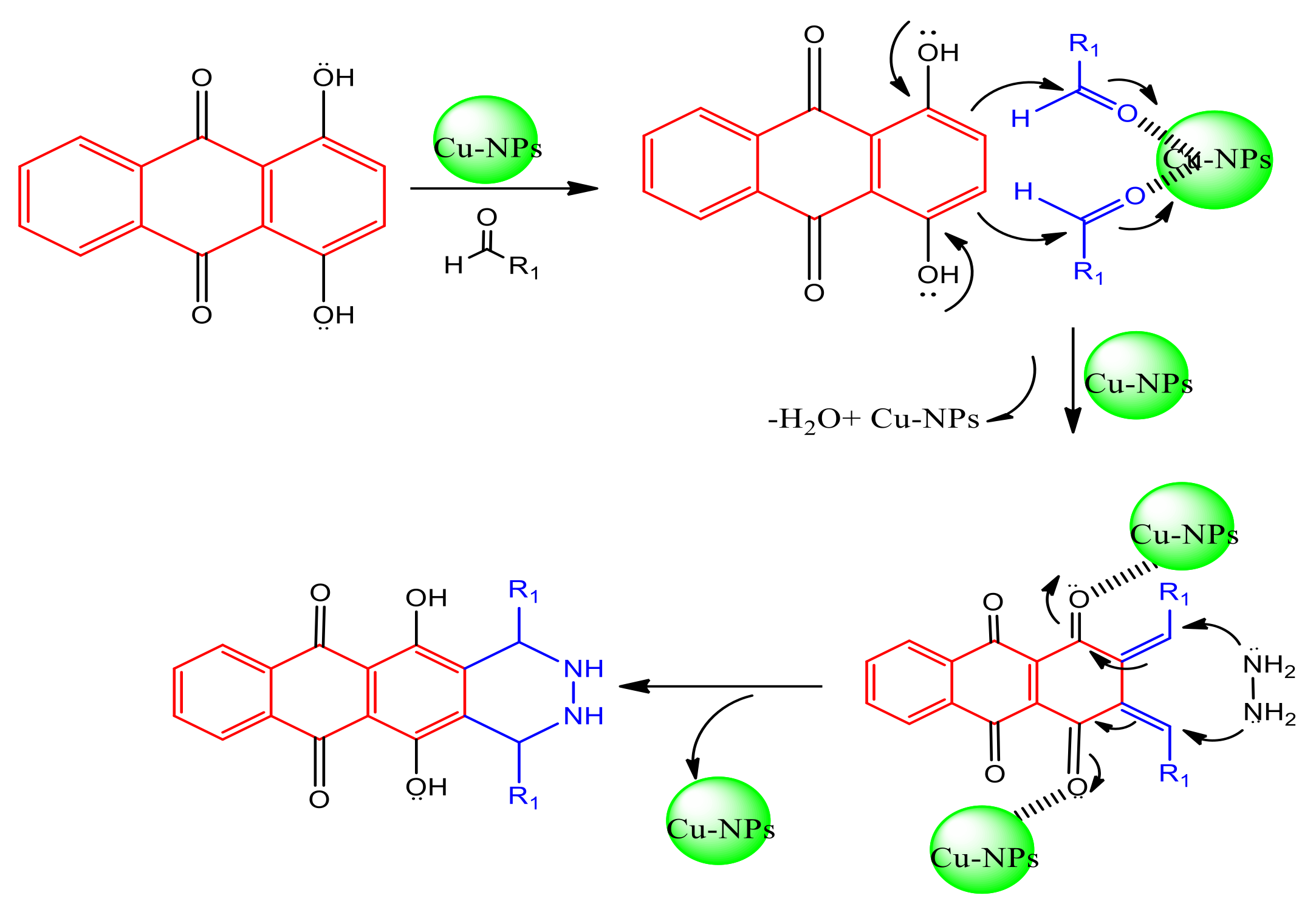
2.1.1. Synthesis of Catalysis
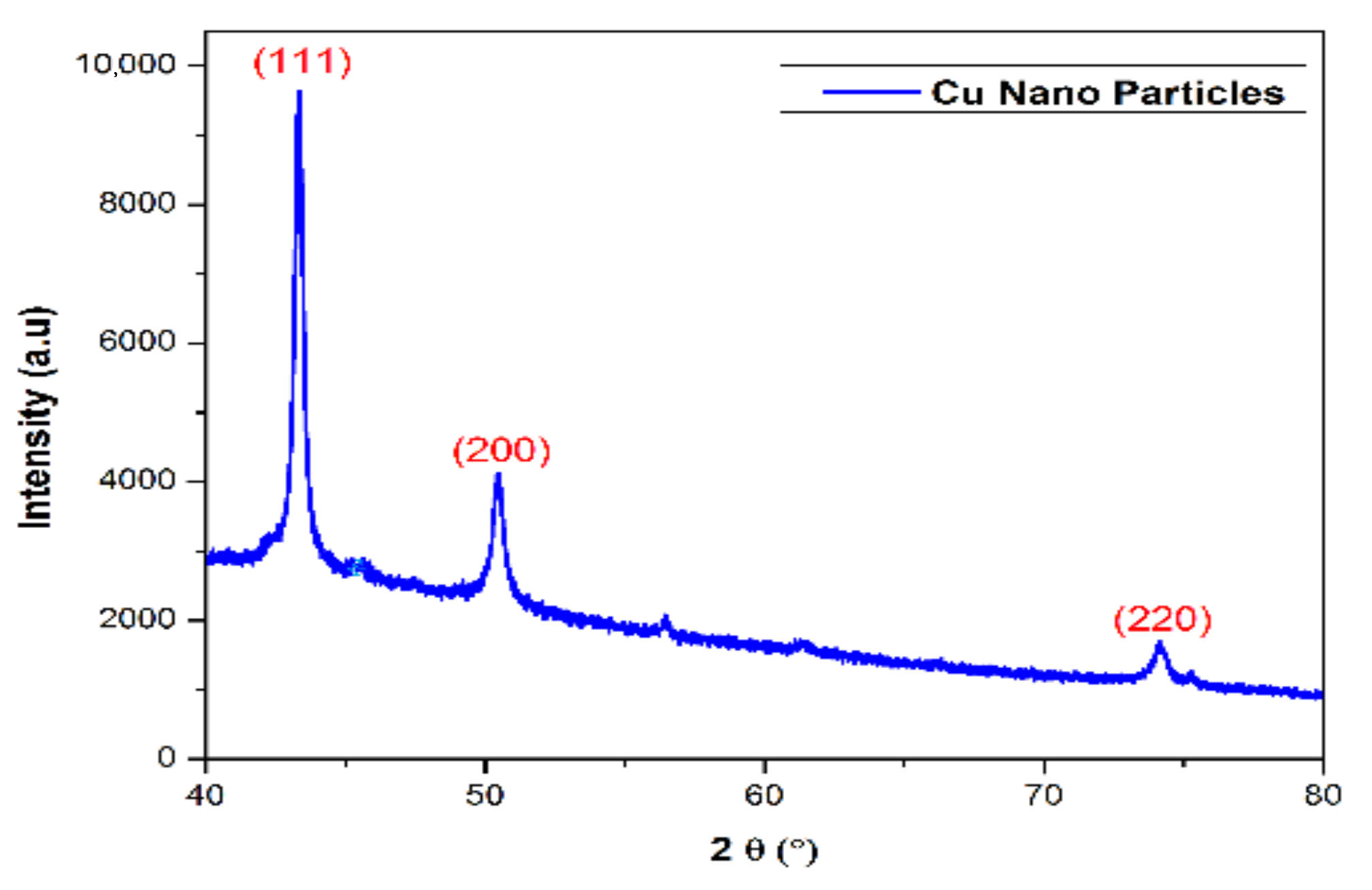
2.1.2. Powder X-Ray Diffraction Studies
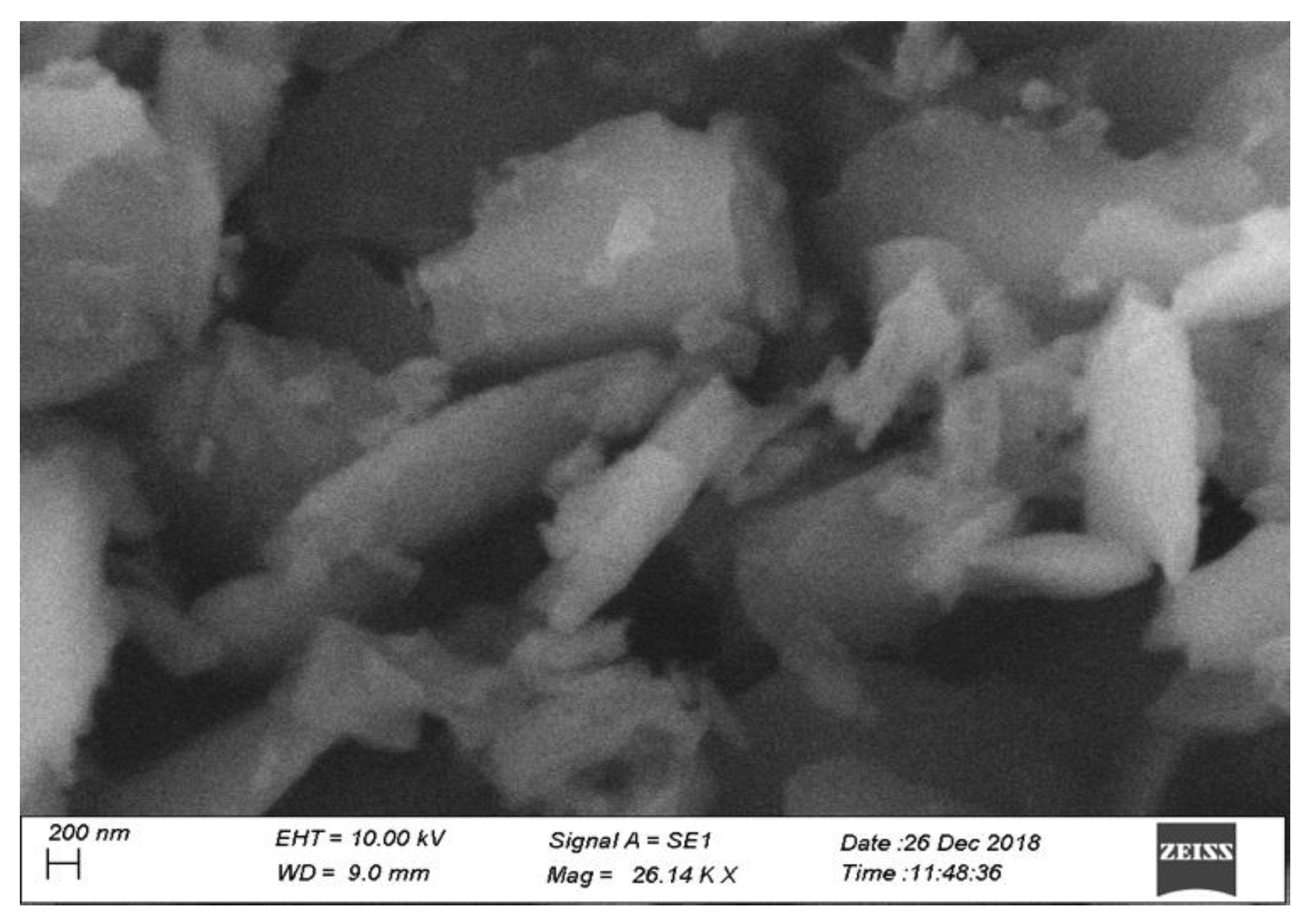
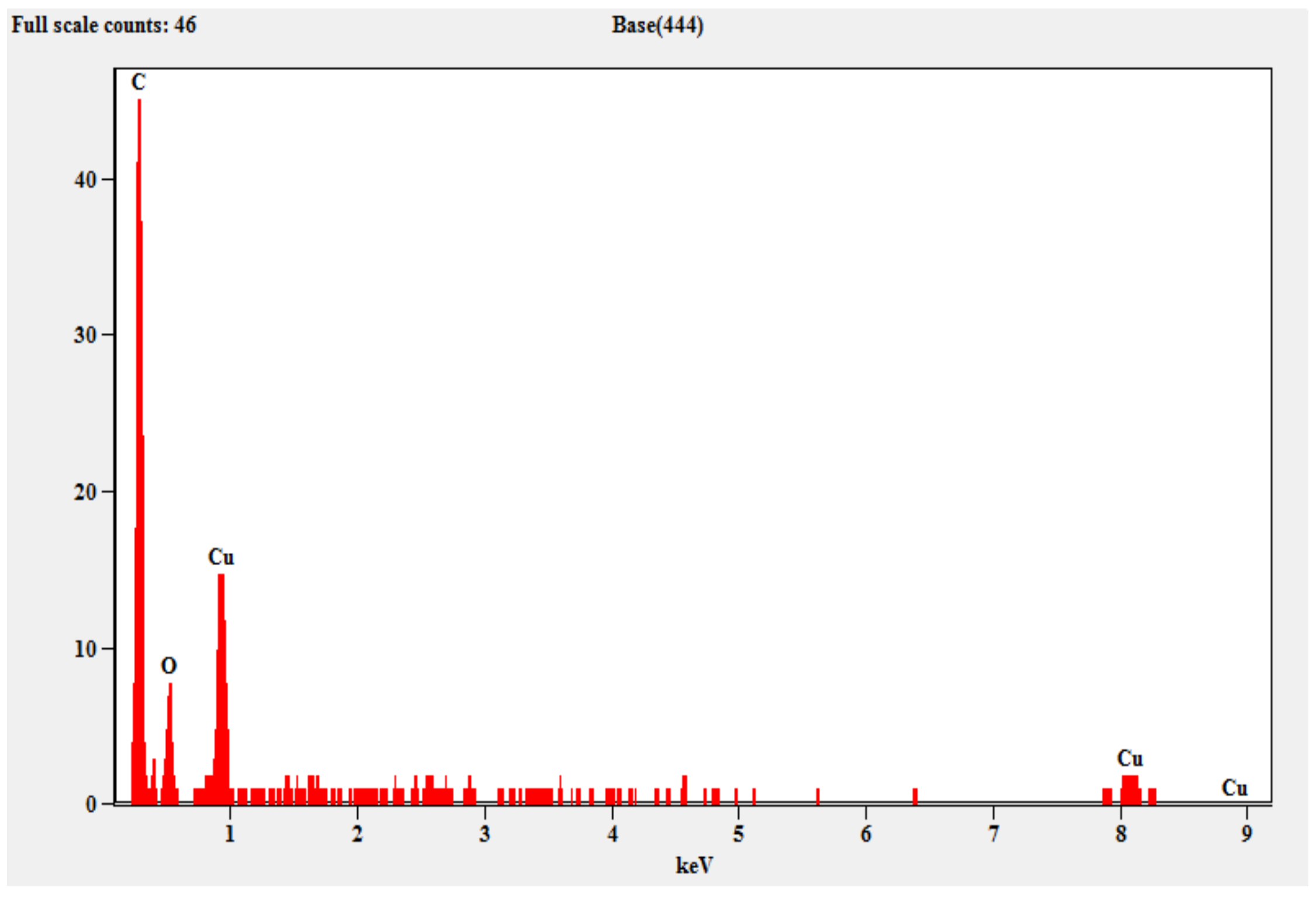
2.1.3. SEM and EDX Analysis
2.1.4. Catalyst Recovery Studies
2.2. Biological Activity
2.2.1. Antityrosinase Activity
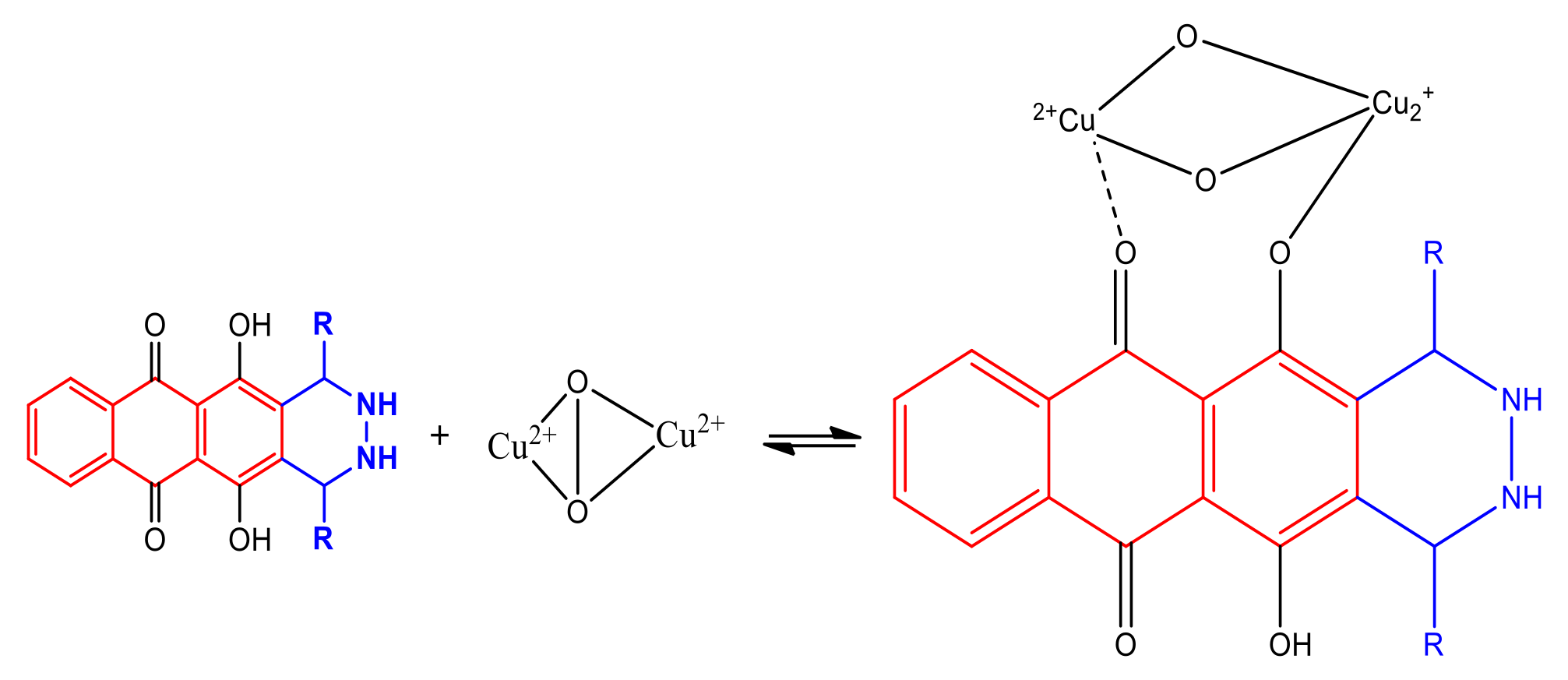
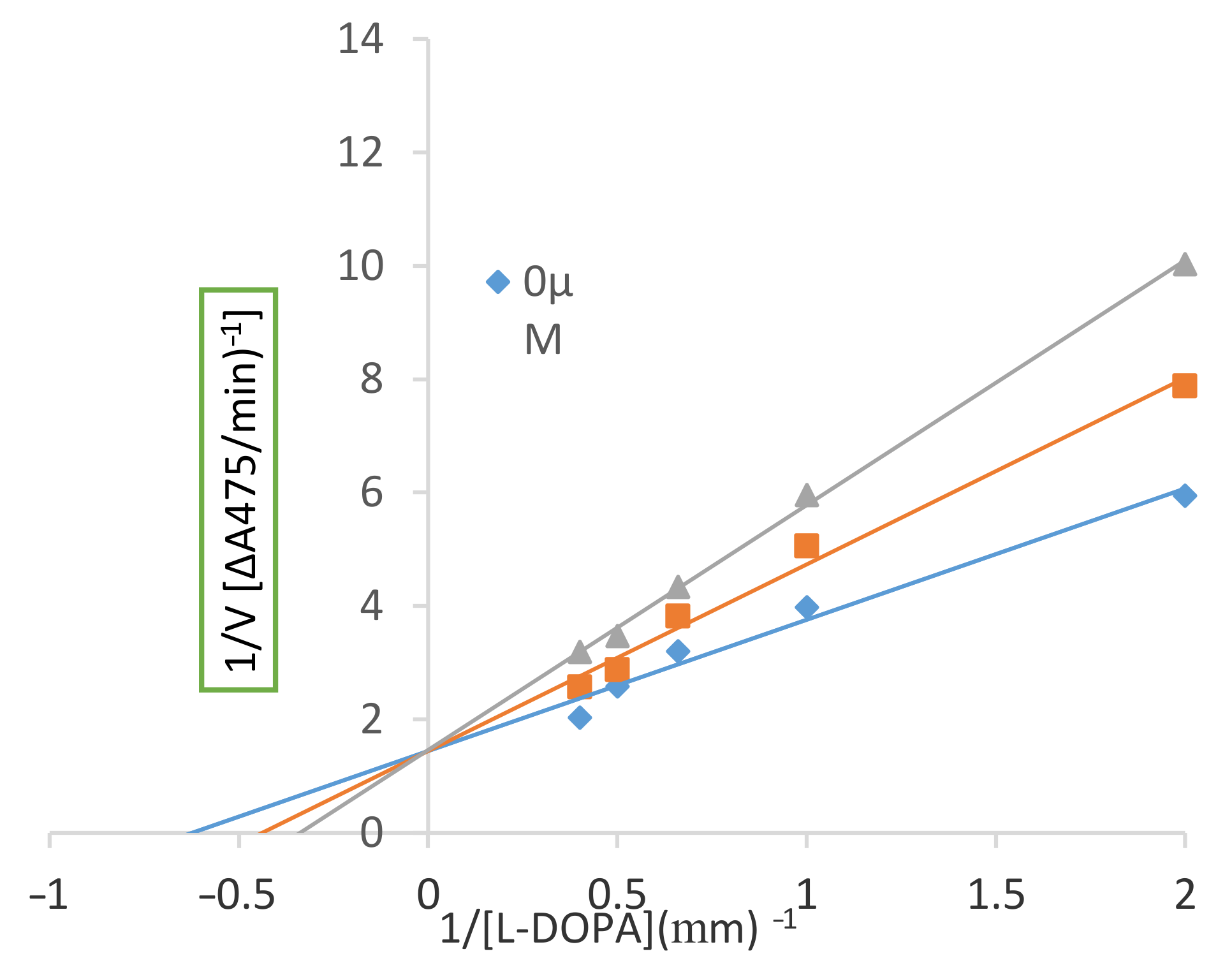
2.2.2. Inhibitory Mechanism
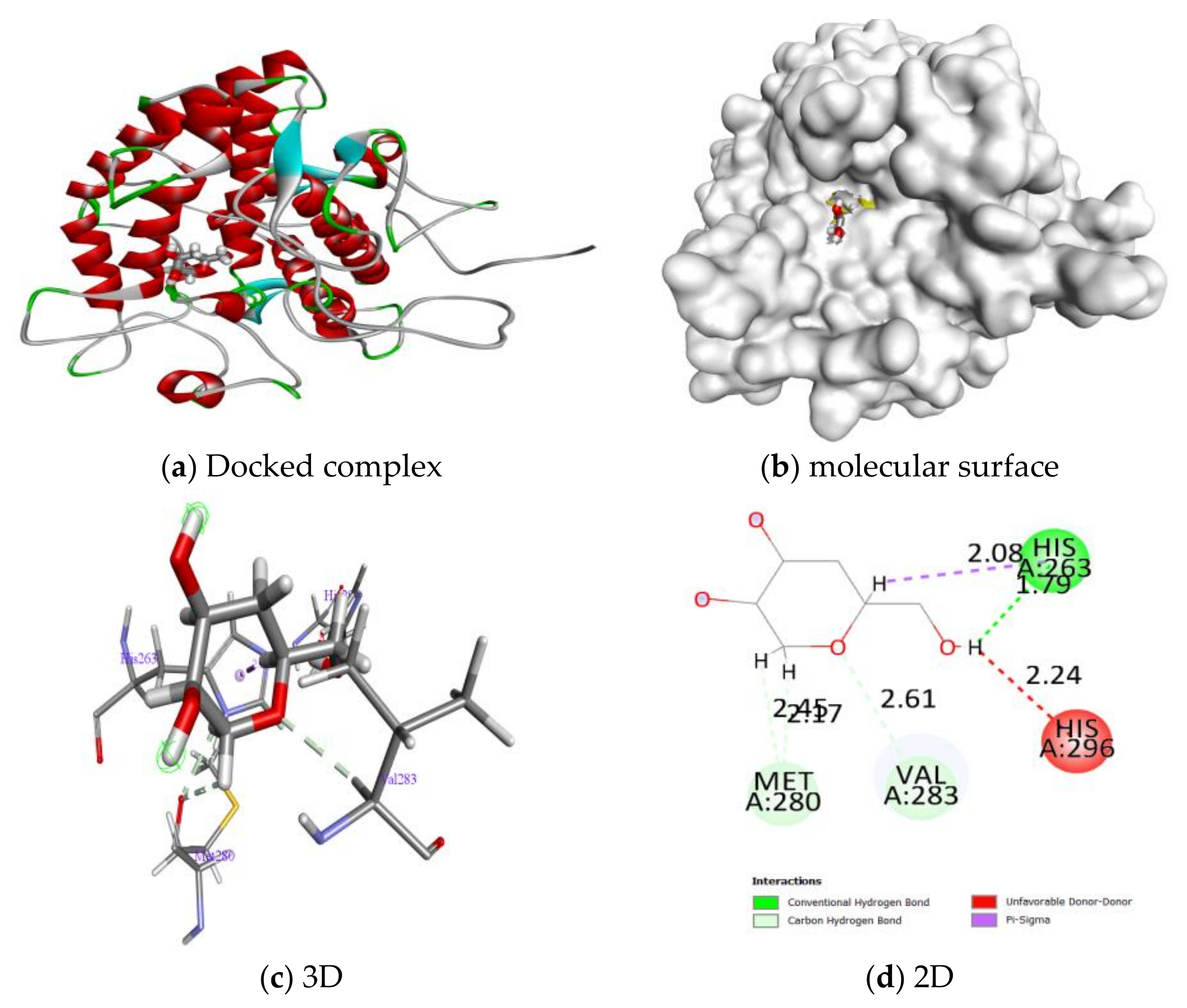
2.2.3. Molecular Docking
2.2.4. Cytotoxicity Activity
3. Experimental Section
3.1. Synthesis
3.2. Biological Activity
3.2.1. Anti-Tyrosinase Activity
3.2.2. Molecular Docking
Grid Generation and Molecular Docking
3.2.3. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toda, F.; Tanaka, K.; Sekikawa, A. Host–guest complex formation by a solid–solid reaction. J. Chem. Soc. Chem. Commun. 1987, 4, 279. [Google Scholar] [CrossRef]
- Mannich, C. Eine Synthese von β-ketonbasen. Arch. Pharm. 1917, 255, 261–276. [Google Scholar] [CrossRef]
- Manabe, K.; Kobayashi, S. Mannich-Type Reactions of Aldehydes, Amines, and Ketones in a Colloidal Dispersion System Created by a Brønsted Acid−Surfactant-Combined Catalyst in Water. Org. Lett. 1999, 1, 1965–1967. [Google Scholar] [CrossRef]
- Jing-Bo, Y.; Gang, P.; Zhi-Jiang, J.; Zi-Kun, H.; Wei-Ke, S. Mechanochemical Oxidative Mannich Reaction: Evaluation of Chemical and Mechanical Parameters for the Mild and Chemoselective Coupling of N-tert-butoxycarbonyltetrahydroquinolines and Ketones. Eur. J. Org. Chem. 2016, 32, 5340–5344. [Google Scholar]
- Khanna, G.; Aggarwal, K.; Khuran, J.L. An efficient and confluent approach for the synthesis of novel 3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione derivatives by a three component reaction in ionic liquid. RSC Adv. 2015, 5, 46448–46454. [Google Scholar] [CrossRef]
- Ghomi, J.S.; Zahedi, S. Novel ionic liquid supported on Fe3O4 nanoparticles and its application as a catalyst in Mannich reaction under ultrasonic irradiation. Ultrason Sonochem 2017, 34, 916–923. [Google Scholar] [CrossRef]
- Ling-Ling, W.; Yang, X.; Da-Cheng, Y.; Zhi, G.; Yan-Hong, H. Biocatalytic asymmetric Mannich reaction of ketimines using wheat germ lipase, Catal. Sci. Technol. 2016, 6, 3963–3970. [Google Scholar]
- Mostafa, A.A.F.; SathishKumar, C.; Al-Askar, A.M.; Sayed, S.R.; SurendraKumar, R.; Idhayadhulla, A. Synthesis of novel benzopyran-connected pyrimidine and pyrazole derivatives via a green method using Cu(II)-tyrosinase enzyme catalyst as potential larvicidal, antifeedant activities. RSC Adv. 2019, 9, 25533–25543. [Google Scholar] [CrossRef]
- Whitaker, J.R. Polyphenol Oxidase. In Food Enzymes; Springer: Boston, MA, USA, 1995. [Google Scholar] [CrossRef]
- Fenoll, L.G.; Penalver, M.J.; RodríguezLopez, J.N.; Varon, R.; GarcíaCanovas, F.; Tudela, J. Tyrosinase kinetics: Discrimination between two models to explain the oxidation mechanism of monophenol and diphenol substrates. Int. J. Biochem. Cell. Biol. 2004, 36, 235–246. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Vashi, N.A.; Kundu, R.V. Facial hyperpigmentation: Causes and treatment. Br. J. Dermatol. 2013, 169, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Tyrosinase-Expressing Neuronal Cell Line as in vitro Model of Parkinson’s disease. Int. J. Mol. Sci. 2010, 11, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Van Gorkom, B.A.; De Vries, E.G.; Karrenbeld, A.; Leibeuker, J.H. Review article: Anthranoid laxatives and their potential carcinogenic effects, Aliment. Pharmacol. Ther. 1999, 13, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, J.P.; Fosso, M.Y.; Bearss, J.; Chang, C.W.T. Synthesis and anticancer structure activity relationship investigation of cationic anthraquinone analogs. Eur. J. Med. Chem. 2014, 77, 96–102. [Google Scholar] [CrossRef]
- Khan, N.; Karodi, R.; Siddiqui, A.; Thube, S.; Rub, R. Development of anti-acne gel formulation of anthraquinones rich fraction from Rubia cordifolia (Rubiaceae). Int. J. Appl. Res. Nat. Prod. 2011, 4, 28–36. [Google Scholar]
- Davis, R.; Agnew, P.; Shapiro, E. Antiarthritic activity of anthraquinones found in aloe for podiatric medicine. J. Am. Podiatr. Med. Assoc. 1986, 76, 61–66. [Google Scholar] [CrossRef]
- Wuthi-udomlert, M.; Kupittayanant, P.; Gritsanapan, W. In vitro evaluation of antifungal activity of anthraquinone derivatives of Senna alata. J. Health Res. 2010, 24, 117–122. [Google Scholar]
- Fosso, M.Y.; Chan, K.Y.; Gregory, R.; Chang, C.W.T. Library Synthesis and Antibacterial Investigation of Cationic Anthraquinone Analogs. ACS Comb. Sci. 2012, 14, 231–235. [Google Scholar] [CrossRef]
- Barnard, D.L.; Fairbairn, D.W.; O’Neill, K.L.; Gage, T.L.; Sidwell, R.W. Anti-human cytomegalovirus activity and toxicity of sulfonated anthraquinones and anthraquinone derivatives. Antiviral. Res. 1995, 28, 317–329. [Google Scholar] [CrossRef]
- Seo, E.J.; Ngoc, T.M.; Lee, S.M.; Kim, Y.S.; Jung, Y.S. Chrysophanol-8-O-glucoside, an Anthraquinone Derivative in Rhubarb, Has Antiplatelet and Anticoagulant Activities. J. Pharmacol. Sci. 2012, 118, 245–254. [Google Scholar] [CrossRef]
- Jackson, T.C.; Verrier, J.D.; Kochanek, P.M. Anthraquinone-2-sulfonic acid (AQ2S) is a novel neurotherapeutic agent. Cell Death Dis. 2013, 4, 451. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, A.K.; Chauhan, P.; Chaudhary, S. A novel sulfur-incorporated naphthoquinone as a selective “turn-on” fluorescence chemical sensor for rapid detection of Ba2+ ion in semi-aqueous medium. Sens. Actuators B Chemical. 2019, 294, 116–122. [Google Scholar] [CrossRef]
- Kumar, P.; Ghosh, A.; Jose, D.A. A simple colorimetric sensor for the detection of moisture in organic solvents and building materials: Applications in rewritable paper and fingerprint imaging. Analyst 2019, 144, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ciria, M.; Sanz, A.M.; Yunta, M.J.R.; Gómez-Contreras, F.; Navarro, P.; Sánchez-Moreno, M.; Boutaleb-Charki, S.; Osuna, A.; Castiñeiras, A.; Pardo, M.; et al. 1,4-Bis (alkyla mino) benzo[g]phthalazines able to form dinuclear complexes of Cu(II) which as free ligands behave as SOD inhibitors and show efficient in vitro activity against Trypanosoma cruzi. Bioorg. Med. Chem. 2007, 15, 2081–2091. [Google Scholar]
- Battaini, G.; Monzani, E.; Casella, L.; Santagostini, L.; Pagliarin, R. Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid. J. Biol. Inorg. Chem. 2000, 5, 262–268. [Google Scholar] [CrossRef]
- Naeimi, H.; Brojerdi, S.S. Facile and Efficient One-Pot Synthesis of Anthraquinones from Benzene Derivatives Catalyzed by Silica Sulfuric Acid. Polycycl. Aromat. Compd. 2014, 34, 504–517. [Google Scholar] [CrossRef]
- Xia, L.L.; Idhayadhulla, A.; Lee, Y.R.; Wee, Y.J.; Kim, S.H. Anti-tyrosinase, antioxidant, and antibacterial activities of novel 5-hydroxy-4-acetyl-2,3-dihydronaphtho[1,2-b]furans. Eur. J. Med. Chem. 2014, 86, 605–612. [Google Scholar]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Farid, R.; Day, T.; Friesner, R.A.; Pearlstein, R.A. New insights about HERG blockade obtained from protein modeling, potential energy mapping, and docking studies. Bioorg. Med. Chem. 2006, 14, 3160–3173. [Google Scholar] [CrossRef]
- Saeed, A.; Mahesar, P.A.; Channar, P.A.; Abbas, Q.; Larik, F.A.; Hassan, M.; Raza, H.; Seo, S.Y. Synthesis, molecular docking studies of coumarinyl-pyrazolinyl substituted thiazoles as non-competitive inhibitors of mushroom tyrosinase. Bioorg. Chem. 2017, 74, 187–196. [Google Scholar]
- Larik, F.A.; Saeed, A.; Channar, P.A.; Muqadar, U.; Abbas, Q.; Hassan, M.; Seo, S.Y.; Bolte, M. Design, synthesis, kinetic mechanism and molecular docking studies of novel 1-pentanoyl-3-arylthioureas as inhibitors of mushroom tyrosinase and free radical scavengers. Eur. J. Med. Chem. 2017, 141, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Ashraf, Z.; Abbas, Q.; Raza, H.; Seo, S.Y. Exploration of Novel Human Tyrosinase Inhibitors by Molecular Modeling, Docking and Simulation Studies. Interdiscip. Sci. 2018, 10, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Moydeen, M.; Al-Deyab, S.S.; Aseer, M.; Idhayadhulla, A. Synthesis of new morpholine-connected pyrazolidine derivatives and their antimicrobial, antioxidant, and cytotoxic activities. Bioorg. Med. Chem. Lett. 2017, 27, 66–71. [Google Scholar] [CrossRef] [PubMed]
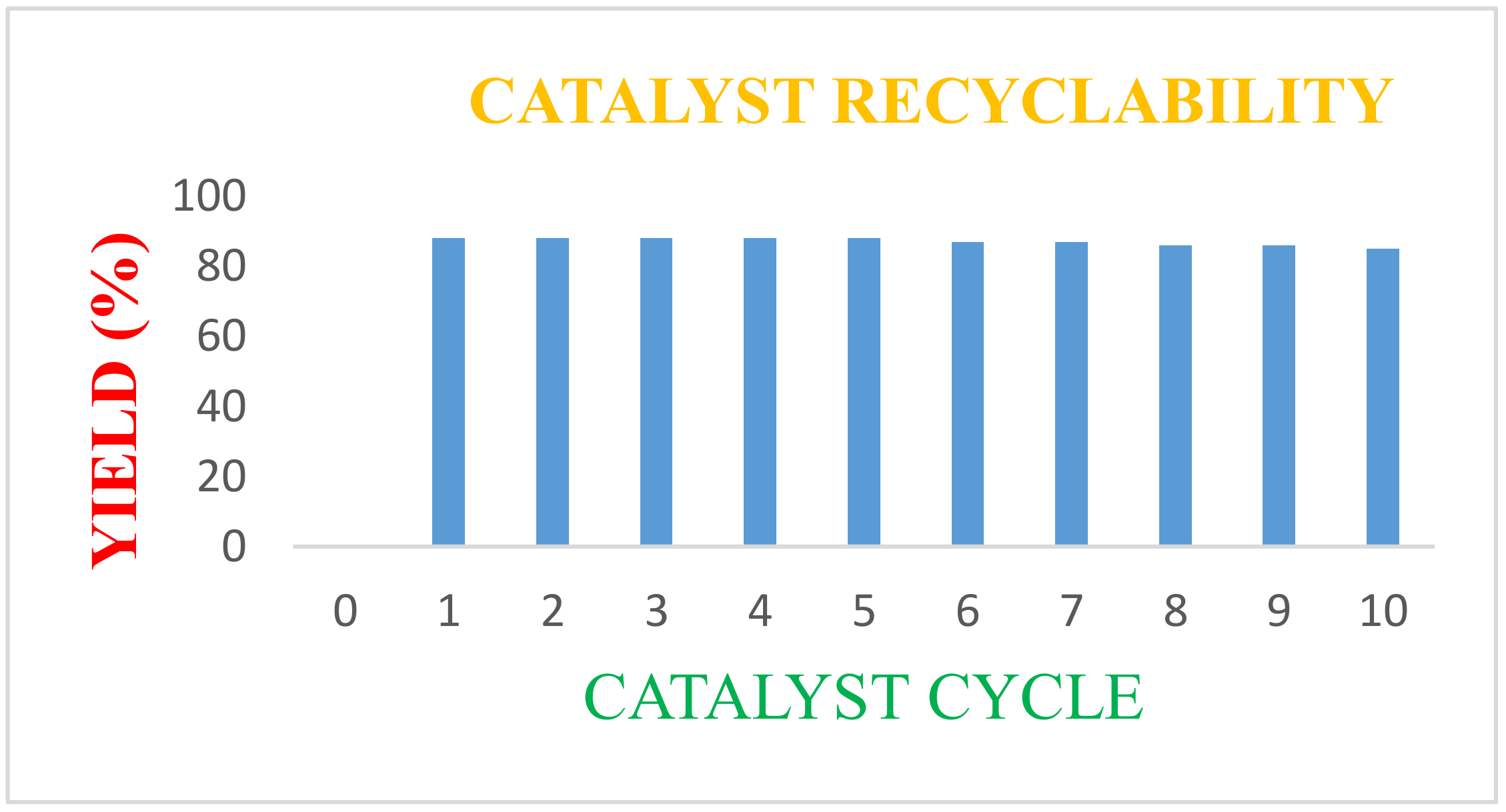

| Entry | Catalyst Use | Yield (%) |
|---|---|---|
| 1 | 1st | 86 |
| 2 | 2nd | 85 |
| 3 | 3rd | 84 |
| 4 | 4th | 84 |
| 5 | 5th | 83 |
| 6 | 6th | 81 |
| 7 | 7th | 84 |
| 8 | 8th | 82 |
| 9 | 9th | 81 |
| 10 | 10th | 81 |
| Compound | Concentration (µg/mL) a | IC50 µg/mL (µM) | ||
|---|---|---|---|---|
| 25 | 50 | 100 | ||
| 1a | 11.02 ± 0.15 | 22.81 ± 0.19 | 46.74 ± 0.98 | >100 |
| 1b | 03.05 ± 0.28 | 17.84 ± 0. 12 | 36.52 ± 0.98 | >100 |
| 1c | 58.75 ± 0.34 | 72.84 ± 0. 65 | 88.52 ± 0.57 | 06.26 (11.5) |
| 1d | 18.75 ± 0.22 | 37.84 ± 0.43 | 68.52 ± 0.18 | 71.46 (142.7) |
| 1e | 13.75 ± 0.56 | 24.84 ± 0.23 | 55.52 ± 0.06 | 91.51 (213.6) |
| 1f | 20.75 ± 0.66 | 53.84 ± 0.19 | 78.52 ± 0.63 | 56.24 (125.4) |
| 1g | 14.75 ± 0.54 | 31.84 ± 0.13 | 62.52 ± 0.17 | 80.68 (155.9) |
| 1h | 19.75 ± 0.47 | 23.84 ± 0.24 | 43.52 ± 0.05 | >100 |
| 1i | 16.75 ± 0.38 | 21.84 ± 0.29 | 40.52 ± 0.29 | >100 |
| 1j | 19.75 ± 0.23 | 34.84 ± 0.34 | 54.52 ± 0.88 | 88.59 (165.7) |
| 1k | 21.75 ± 0.17 | 41.84 ± 0.10 | 67.52 ± 0.15 | 69.18 (136.0) |
| Kojic acid | 55.61 ± 0.87 | 68.10 ± 0.11 | 84.12 ± 0.18 | 11.09 (78.0) |
| Ligand | Dock Score | H. Bond | Glide E. Model | No of Interactions | Interacting Residues | Bond Length |
|---|---|---|---|---|---|---|
| 1c | −5.6 | −2.4 | −36.865 | 3 | Arg268, Ser282, and Val283 | 5.85, 4.25, and 3.62 |
| Kojic acid | −5.2 | −2.2 | −17.030 | 1 | His263 | 1.79 |
| Comp. No. | HepG2 | MCF-7 | HeLa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GI50 (µM) | TGI (µM) | LC50 (µM) | GI50 (µM) | TGI (µM) | LC50 (µM) | GI50 (µM) | TGI (µM) | LC50 (µM) | |
| 1a | 3.60 ± 0.18 | 8.90 ± 0.74 | 15.40 ± 0.12 | 0.71 ± 0.08 | 14.60 ± 0.70 | 22.60 ± 0.32 | 3.50 ± 0.19 | 07.80 ± 0.16 | 14.80 ± 0.10 |
| 1b | 0.05 ± 0.02 | 0.19 ± 0.05 | 0.93 ± 0.02 | 0.15 ± 0.02 | 0.38 ± 0.34 | 0.89 ± 0.08 | 0.21 ± 0.17 | 0.59 ± 0.10 | 01.58 ± 0.18 |
| 1c | 0.01 ± 0.02 | 0.15 ± 0.31 | 0.61 ± 0.11 | 0.03 ± 0.00 | 0.29 ± 0.41 | 0.98 ± 0.09 | 0.04 ± 0.06 | 0.58 ± 0.19 | 0.98 ± 0.09 |
| 1d | 0.16 ± 0.07 | 0.49 ± 0.28 | 1.20 ± 0.01 | 9.80 ± 0.95 | 18.20 ± 0.74 | 36.20 ± 0.63 | 4.30 ± 0.20 | 09.40 ± 0.12 | 15.10 ± 0.10 |
| 1e | 4.70 ± 0.10 | 10.30 ± 0.24 | 28.20 ± 0.40 | 10.0 ± 0.34 | 20.10 ± 0.64 | 42.00 ± 0.41 | 10.10 ± 0.24 | 21.30 ± 0.14 | 36.70 ± 1.23 |
| 1f | 6.20 ± 0.94 | 13.20 ± 0.10 | 22.30 ± 0.52 | 09.5 ± 0.15 | 15.60 ± 0.24 | 32.90 ± 0.35 | 5.70 ± 0.03 | 19.20 ± 0.19 | 26.50 ± 0.95 |
| 1g | 0.13 ± 0.05 | 0.28 ± 0.12 | 0.56 ± 0.02 | 0.48 ± 0.08 | 0.95 ± 0.04 | 1.59 ± 0.74 | 0.06 ± 0.01 | 0.16 ± 0.06 | 0.30 ± 0.05 |
| 1h | 6.10 ± 0.12 | 12.80 ± 0.13 | 26.90 ± 0.22 | 11.60 ± 0.91 | 26.70 ± 0.28 | 49.50 ± 0.74 | 13.30 ± 0.14 | 29.40 ± 0.18 | 52.00 ± 0.04 |
| 1i | 12.30 ± 0.18 | 26.80 ± 0.25 | 45.30 ± 0.19 | 1.28 ± 0.10 | 2.91 ± 0.32 | 6.49 ± 0.74 | 4.10 ± 0.18 | 09.20 ± 0.06 | 15.80 ± 0.62 |
| 1j | 6.30 ± 0.42 | 13.10 ± 0.65 | 26.90 ± 0.72 | 2.80 ± 0.65 | 4.90 ± 0.34 | 1.20 ± 0.08 | 6.10 ± 0.07 | 13.90 ± 0.10 | 30.10 ± 0.18 |
| 1k | 15.50 ± 0.02 | 30.10 ± 0.31 | 87.20 ± 0.11 | 17.10 ± 0.74 | 26.10 ± 0.41 | 49.30 ± 0.09 | 19.60 ± 0.06 | 31.90 ± 0.19 | 87.60 ± 0.19 |
| Std. | 0.01 ± 0.00 | 0.13 ± 0.01 | 0.58 ± 0.02 | 0.02 ± 0.00 | 0.21 ± 0.06 | 0.74 ± 0.09 | 0.05 ± 0.01 | 0.41 ± 0.10 | 0.88 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvaraj, K.; Daoud, A.; Alarifi, S.; Idhayadhulla, A. Tel-Cu-NPs Catalyst: Synthesis of Naphtho[2,3-g]phthalazine Derivatives as Potential Inhibiters of Tyrosinase Enzymes and Their Investigation in Kinetic, Molecular Docking, and Cytotoxicity Studies. Catalysts 2020, 10, 1442. https://doi.org/10.3390/catal10121442
Selvaraj K, Daoud A, Alarifi S, Idhayadhulla A. Tel-Cu-NPs Catalyst: Synthesis of Naphtho[2,3-g]phthalazine Derivatives as Potential Inhibiters of Tyrosinase Enzymes and Their Investigation in Kinetic, Molecular Docking, and Cytotoxicity Studies. Catalysts. 2020; 10(12):1442. https://doi.org/10.3390/catal10121442
Chicago/Turabian StyleSelvaraj, Keerthana, Ali Daoud, Saud Alarifi, and Akbar Idhayadhulla. 2020. "Tel-Cu-NPs Catalyst: Synthesis of Naphtho[2,3-g]phthalazine Derivatives as Potential Inhibiters of Tyrosinase Enzymes and Their Investigation in Kinetic, Molecular Docking, and Cytotoxicity Studies" Catalysts 10, no. 12: 1442. https://doi.org/10.3390/catal10121442
APA StyleSelvaraj, K., Daoud, A., Alarifi, S., & Idhayadhulla, A. (2020). Tel-Cu-NPs Catalyst: Synthesis of Naphtho[2,3-g]phthalazine Derivatives as Potential Inhibiters of Tyrosinase Enzymes and Their Investigation in Kinetic, Molecular Docking, and Cytotoxicity Studies. Catalysts, 10(12), 1442. https://doi.org/10.3390/catal10121442






