Optimization of a Catalytic Chemoenzymatic Tandem Reaction for the Synthesis of Natural Stilbenes in Continuous Flow
Abstract
1. Introduction
2. Results and Discussion
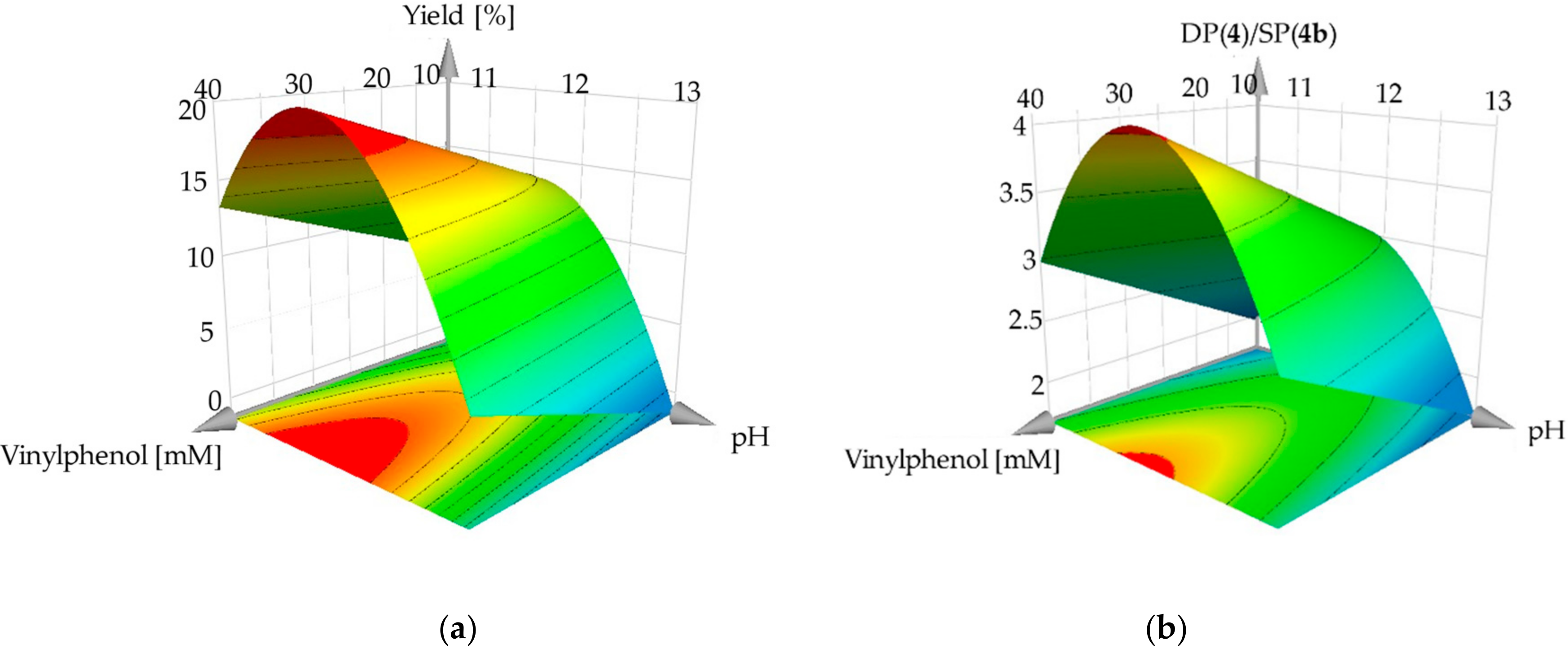
2.1. Optimization of Process Conditions in Batch
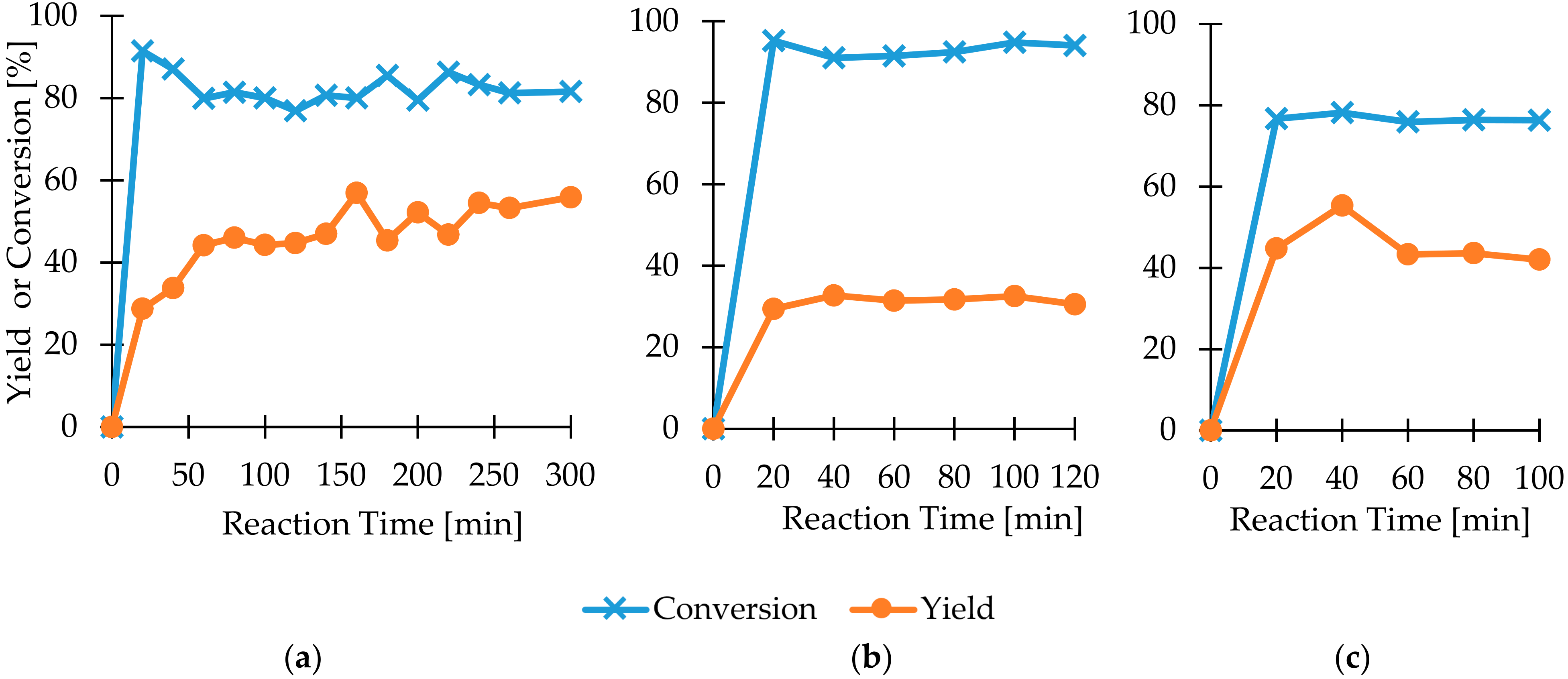
2.2. Heck Coupling in Continuous Flow
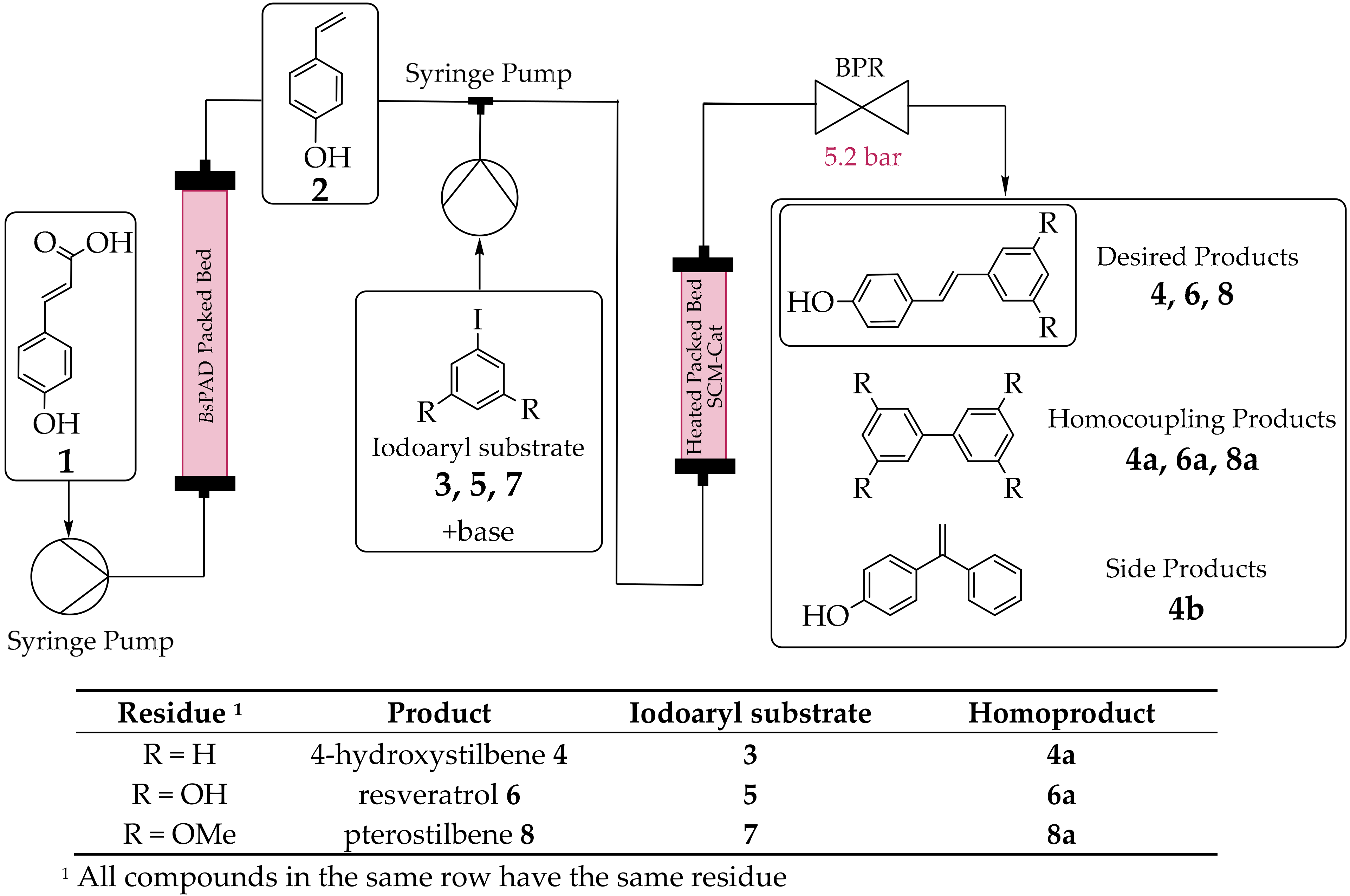
2.3. Chemoenzymatic Cascade in Flow
2.4. Palladium Reverse Flow Reactor
3. Materials and Methods
3.1. General Information
3.2. Flow Equipment
3.3. Analytical Methods
3.4. Synthesis of Palladium Catalyst Ce0.2Sn0.79Pd0.01O2−δ Using the Solution Combustion Method (SCM)
3.5. Enzyme Immobilization for the Preparation of Alginate Beads
3.6. Heck Coupling Reaction in Batch
3.7. Single Step Heck Coupling Flow Experiments
3.8. Chemoenzymatic Tandem Flow Experiments
3.9. Leaching Experiment
3.10. Synthesis of Iodoaryl Substrates 5, 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Groger, H.; Hummel, W. Combining the ‘two worlds’ of chemocatalysis and biocatalysis towards multi-step one-pot processes in aqueous media. Curr. Opin. Chem. Biol. 2014, 19, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Vennestrøm, P.N.R.; Christensen, C.H.; Pedersen, S.; Grunwaldt, J.-D.; Woodley, J.M. Next-generation catalysis for renewables: Combining rnzymatic with inorganic heterogeneous catalysis for bulk chemical production. ChemCatChem 2010, 2, 249–258. [Google Scholar] [CrossRef]
- Chiusoli, G.P.; Maitlis, P.M. (Eds.) Metal-Catalysis in Industrial Organic Processes; Royal Society of Chemistry: Cambridge, UK, 2006. [Google Scholar]
- Faber, K. Biotransformations in Organic Chemistry: A Textbook, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Yang, Y.; Zhang, J.; Wu, D.; Xing, Z.; Zhou, Y.; Shi, W.; Li, Q. Chemoenzymatic synthesis of polymeric materials using lipases as catalysts: A review. Biotechnol. Adv. 2014, 32, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, M.J.; Hall, M.; Faber, K.; van Spanning, R.J.M.; Ruijter, E.; Orru, R.V.A. Stereoselective chemoenzymatic cascade synthesis of the bis-THF core of acetogenins. Eur. J. Org. Chem. 2019, 2019, 1092–1101. [Google Scholar] [CrossRef]
- Grunwald, P. (Ed.) Pharmaceutical Biocatalysis: Chemoenzymatic Synthesis of Active Pharmaceutical Ingredients; Pan Stanford Publishing Pte Ltd.: Singapore, 2019. [Google Scholar]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The hitchhiker’s guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous process technology: A tool for sustainable production. Green Chem. 2014, 16, 55–62. [Google Scholar] [CrossRef]
- Hessel, V. Process windows—Gate to maximizing process intensification via flow chemistry. Chem. Eng. Technol. 2009, 32, 1655–1681. [Google Scholar] [CrossRef]
- Newman, S.G.; Jensen, K.F. The role of flow in green chemistry and engineering. Green Chem. 2013, 15, 1456–1472. [Google Scholar] [CrossRef]
- Ley, S.V. On being green: Can flow chemistry help? Chem. Rec. 2012, 12, 378–390. [Google Scholar] [CrossRef]
- King, A.O.; Larsen, R.D.; Negishi, E.-I. Palladium-Catalyzed Heterogeneous Hydrogenation. In Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E.-I., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Li, M.B.; Posevins, D.; Geoffroy, A.; Zhu, C.; Backvall, J.E. Efficient heterogeneous palladium-catalyzed oxidative cascade reactions of enallenols to furan and oxaborole derivatives. Angew. Chem. Int. Ed. Engl. 2020, 59, 1992–1996. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon−Carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Alonso, F.; Tyurin, V. The Suzuki-Miyaura reaction after the Nobel prize. Coord. Chem. Rev. 2019, 385, 137–173. [Google Scholar] [CrossRef]
- Torborg, C.; Beller, M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Neves, A.R.; Lucio, M.; Lima, J.L.C.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Ho, C.T.; Chen, Y.K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Savio, M.; Ferraro, D.; Maccario, C.; Vaccarone, R.; Jensen, L.D.; Corana, F.; Mannucci, B.; Bianchi, L.; Cao, Y.; Stivala, L.A. Resveratrol analogue 4,4′-dihydroxy-trans-stilbene potently inhibits cancer invasion and metastasis. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Gomez Baraibar, A.; Reichert, D.; Mugge, C.; Seger, S.; Groger, H.; Kourist, R. A one-pot cascade reaction combining an encapsulated decarboxylase with a metathesis catalyst for the synthesis of bio-based antioxidants. Angew. Chem. Int. Ed. Engl. 2016, 55, 14823–14827. [Google Scholar] [CrossRef]
- Grabner, B.; Schweiger, A.K.; Gavric, K.; Kourist, R.; Gruber-Woelfler, H. A chemo-enzymatic tandem reaction in a mixture of deep eutectic solvent and water in continuous flow. React. Chem. Eng. 2020, 5, 263–269. [Google Scholar] [CrossRef]
- Baidya, T.; Gupta, A.; Deshpandey, P.A.; Madras, G.; Hegde, M.S. High oxygen storage capacity and high rates of CO oxidation and NO reduction: Catalytic properties of Ce1-xSnxO2 and Ce0.78Sn0.2Pd0.02O2-δ. J. Phys. Chem. C 2009, 113, 4059–4068. [Google Scholar] [CrossRef]
- Lichtenegger, G.J.; Maier, M.; Hackl, M.; Khinast, J.G.; Gössler, W.; Griesser, T.; Kumar, V.S.P.; Gruber-Woelfler, H.; Deshpande, P.A. Suzuki-Miyaura coupling reactions using novel metal oxide supported ionic palladium catalysts. J. Mol. Catal. A Chem. 2017, 426, 39–51. [Google Scholar] [CrossRef]
- Gavric, K. Development of a Continuous Chemo-Enzymatic Two-Step Synthesis of Resveratrol Derivatives. Master’s Thesis, Graz University of Technology, Graz, Austria, 20 January 2020. [Google Scholar]
- Kashin, A.S.; Ananikov, V.P. Catalytic C-C and C-heteroatom bond formation reactions: In situ generated or preformed catalysts? Complicated mechanistic picture behind well-known experimental procedures. J. Org. Chem. 2013, 78, 11117–11125. [Google Scholar] [CrossRef] [PubMed]
- Ananikov, V.P.; Beletskaya, I.P. Toward the ideal catalyst: From atomic centers to a “cocktail” of catalysts. Organometallics 2012, 31, 1595–1604. [Google Scholar] [CrossRef]
- Cantillo, D.; Kappe, C.O. Immobilized transition metals as catalysts for cross-couplings in continuous flow-a critical assessment of the reaction mechanism and metal leaching. ChemCatChem 2014, 6, 3286–3305. [Google Scholar] [CrossRef]
- Bourouina, A.; Meille, V.; de Bellefon, C. A flow split test to discriminating between heterogeneous and homogeneous contributions in Suzuki coupling. J. Flow Chem. 2018, 8, 117–121. [Google Scholar] [CrossRef]
- Barreiro, E.M.; Hao, Z.; Adrio, L.A.; van Ommen, J.R.; Hellgardt, K.; Hii, K.K. Spatial, temporal and quantitative assessment of catalyst leaching in continuous flow. Catal. Today 2018, 308, 64–70. [Google Scholar] [CrossRef]
- Lichtenegger, G.J.; Maier, M.; Khinast, J.G.; Gruber-Wölfler, H. Continuous Suzuki-Miyaura reactions with novel Ce-Sn-Pd oxides and integrated crystallization as continuous downstream protocol. J. Flow Chem. 2016, 6, 244–251. [Google Scholar] [CrossRef]
- Fan, X.; Manchon, M.G.; Wilson, K.; Tennison, S.; Kozynchenko, A.; Lapkin, A.A.; Plucinski, P.K. Coupling of Heck and hydrogenation reactions in a continuous compact reactor. J. Catal. 2009, 267, 114–120. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Wikström, C.; Wold, S. Design of Experiments: Principles and Applications, 3rd ed.; Umetrics AB: Umea, Sweden, 2008. [Google Scholar]
- Muller, J.I.; Kusserow, K.; Hertrampf, G.; Pavic, A.; Nikodinovic-Runic, J.; Gulder, T.A.M. Synthesis and initial biological evaluation of myxocoumarin B. Org. Biomol. Chem. 2019, 17, 1966–1969. [Google Scholar] [CrossRef]
- Csuk, R.; Albert, S. A short synthesis of rhaponticin and its 3″-fluoroanalog via a Wittig/Heck-Mizoroki Route. Z. Naturforsch. 2011, 66b, 311–316. [Google Scholar]
- Frank, A.; Eborall, W.; Hyde, R.; Hart, S.; Turkenburg, J.P.; Grogan, G. Mutational analysis of phenolic acid decarboxylase from Bacillus subtilis (BsPAD), which converts bio-derived phenolic acids to styrene derivatives. Catal. Sci. Technol. 2012, 2, 1568. [Google Scholar] [CrossRef]
- Brazier, J.B.; Nguyen, B.N.; Adrio, L.A.; Barreiro, E.M.; Leong, W.P.; Newton, M.A.; Figueroa, S.J.A.; Hellgardt, K.; Hii, K.K.M. Catalysis in flow: Operando study of Pd catalyst speciation and leaching. Catal. Today 2014, 229, 95–103. [Google Scholar] [CrossRef]
- Civicos, J.F.; Alonso, D.A.; Najera, C. Oxime palladacycle-catalyzed Suzuki–Miyaura alkenylation of aryl, heteroaryl, benzyl, and allyl chlorides under microwave irradiation conditions. Adv. Synth. Catal. 2011, 353, 1683–1687. [Google Scholar] [CrossRef]
- Amalfitano, C.; Evidente, A.; Surico, G.; Tegli, S.; Bertelli, E.; Mugnai, L. Phenols and stilbene polyphenols in the wood of esca-diseased grapevines. Phytopathol. Mediterr. 2000, 39, 178–183. [Google Scholar]
- Orgovan, G.; Gonda, I.; Noszal, B. Biorelevant physicochemical profiling of (E)- and (Z)-resveratrol determined from isomeric mixtures. J. Pharm. Biomed. Anal. 2017, 138, 322–329. [Google Scholar] [CrossRef]


| Heck Flow Experiments | Tandem Flow Experiments | ||||||
|---|---|---|---|---|---|---|---|
| 4 1 | 6 1 | 8 1 | 4 2 | 6 2 | 8 2 | ||
| Maximum Yield [%] 3 | 71 | 25 | 50 | 57 | 33 | 62 | |
| Time Average Yield [%] 4 | 50 | 22 | 48 | 54 | 32 | 50 | |
| Time Average Conversion of 2 [%] 4 | 99 | 96 | 78 | 85 | 93 | 77 | |
| Max. Prod. Concentration [mM] 3 | 7.1 | 2.5 | 5.0 | 5.4 | 3.1 | 5.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lackner, F.; Hiebler, K.; Grabner, B.; Gruber-Woelfler, H. Optimization of a Catalytic Chemoenzymatic Tandem Reaction for the Synthesis of Natural Stilbenes in Continuous Flow. Catalysts 2020, 10, 1404. https://doi.org/10.3390/catal10121404
Lackner F, Hiebler K, Grabner B, Gruber-Woelfler H. Optimization of a Catalytic Chemoenzymatic Tandem Reaction for the Synthesis of Natural Stilbenes in Continuous Flow. Catalysts. 2020; 10(12):1404. https://doi.org/10.3390/catal10121404
Chicago/Turabian StyleLackner, Florian, Katharina Hiebler, Bianca Grabner, and Heidrun Gruber-Woelfler. 2020. "Optimization of a Catalytic Chemoenzymatic Tandem Reaction for the Synthesis of Natural Stilbenes in Continuous Flow" Catalysts 10, no. 12: 1404. https://doi.org/10.3390/catal10121404
APA StyleLackner, F., Hiebler, K., Grabner, B., & Gruber-Woelfler, H. (2020). Optimization of a Catalytic Chemoenzymatic Tandem Reaction for the Synthesis of Natural Stilbenes in Continuous Flow. Catalysts, 10(12), 1404. https://doi.org/10.3390/catal10121404





