Abstract
The current work proposes a strategy for the valorization of compost obtained from municipal solid waste, through its activation with H2SO4 and thermal treatment at 400–800 °C to produce low-cost catalysts for wet peroxide oxidation. All the developed materials were catalytically active for the aqueous solution oxidation of 2-nitrophenol (C2-NP,0 = 0.5 g L−1) and 4-nitrophenol (C4-NP,0 = 5.0 g L−1). In particular, using the catalysts prepared by thermal activation of the compost (with and without subsequent treatment with H2SO4), complete removal of both model pollutants was achieved after 24 h (at 50 °C, initial pH 3, Ccat = 2.5 g L−1, and employing a stoichiometric quantity of H2O2 needed for the total mineralization of the pollutants). In a cyclohexane–water mixture (simulating biphasic oily wastewater), 4-nitrophenol is also completely removed by the catalyst not treated with H2SO4. In contrast, the removal of 2-nitrophenol decreased to 93% due to its higher lipophilic character. Thus, this work demonstrates that active catalysts for wet peroxide oxidation can be obtained by using as a precursor a matured compost derived from municipal solid waste.
1. Introduction
Municipal solid waste comprises the waste produced in households and similar establishments, with a diverse composition depending on communities, but with significant organic matter (accounting for around 30–50%). In 2018, 492 kg of municipal solid waste was generated per capita in the European Union, EU-27 [1], usually collected and transported to management centers equipped with mechanical and biological treatment units. This waste is mechanically and manually separated (sorting) into discarded, recyclable, and organic waste streams. The organic fraction is then treated by anaerobic digestion and composting, allowing the production of biogas and a maturated compost. The amount of municipal solid waste used for composting in the EU-27 has been increasing gradually every year, reaching 37 million tonnes (83 kg per capita) in 2018, up from 14 million tonnes (33 kg per capita) in 1995 [1]. In the northeast of Portugal, using a mechanical and biological treatment, 2,700,000 KW and 15,000 tons of matured compost per 50,000 tons of undifferentiated waste are produced each year [2]. The compost can be used as fertilizer, but it is not economically attractive (price around 0.015 €/kg), and the high quantity of compost generated exceeds the demand required for agriculture applications, resulting in the accumulation at the landfill sites. In addition, the expected coming directives regarding applying the “end-of-waste” criteria are leading to barriers to the use of fertilizers with an origin in waste [3]. This fact supports the need to adopt new strategies to decrease the disposal of the non-demanded matured compost under the principles of the circular economy.
In the last few decades, the scientific community has devoted special efforts to producing low-cost materials from different waste sources, such as activated carbon materials from raw biomasses [4,5,6]. Activated carbons are prepared by physical (partial oxidation under steam, CO2, and air) or by chemical (carbonaceous precursor soaked in a dehydrating agent, such as inorganic acids, hydroxides, or chloride salts) activation methods. Chemical activation can be done before calcination of the carbonaceous precursor or conducted in one-step (simultaneous activation and calcination) [4,5]. Some inorganic materials, such as activated clays, are also prepared by similar methods, in which activation is typically done with an inorganic acid, followed by a thermal treatment [7,8]. Most studies dealing with the development of activated clays or carbon materials have been focused on improving the design of these materials for environmental applications, mainly for wastewater treatment [5,9,10]. As activation methods improved the textural properties of the resultant activated materials, most of them found application in adsorption processes [4,10] and catalysis [9,11].
Catalytic wet peroxide oxidation (CWPO) is a promising advanced oxidation technology, able to operate at moderate conditions (T = 25−130 °C, P = 1−5 atm), using H2O2 as the oxidant to degrade organic pollutants contained in wastewater, when a suitable catalytic system is considered [12], such as catalysts based on activated clays [9], activated carbons [11], among others. We recently demonstrated that CWPO could be applied in the treatment of oily wastewater, in which lipophilic organic pollutants are mostly present in the oily phase, whereas H2O2 is present in the aqueous phase [13]. In this situation, the oxidation of the lipophilic organic pollutants may be controlled by the mass transfer of the pollutants from the oil phase to the aqueous phase and the properties of the pollutant partition coefficient between phases. In this sense, the pollutants’ lipophilic character plays an important role, since high hydrophilic pollutants will be preferably present in the aqueous phase (low coefficient partition and more concentrated in the aqueous phase), allowing their contact with hydrogen peroxide. Nitrophenols (NPs), whether mono-, di- or tri-nitrophenols, are well-known to be lipophilic, highly toxic, inhibitory, and bio-refractory organic compounds [14,15], being commonly present in wastewaters generated in plastic, pharmaceutical, pesticide, synthetic dyes, and explosive industries, reaching high concentrations in these effluents [16,17]. In particular, 2-nitrophenol (2-NP) and 4-nitrophenol (4-NP) are isomers with great different lipophilic character due to the position of the nitro group with respect to the hydroxyl group. The logarithmic cyclohexane-water partition coefficient of 2-NP is log(P) = 1.5, while for 4-NP it takes the value of −1.9 [18], meaning that 2-NP has a more lipophilic character, in a cyclohexane-water mixture being preferably found in the oily phase than in the aqueous phase. In contrast, 4-NP is more concentrated in the aqueous phase than in the oily phase in the cyclohexane-water biphasic system, at the same conditions. The removal efficiency of lipophilic organic pollutants in oily wastewater by CWPO is then strongly affected by the pollutants’ properties and those of the developed catalyst.
This work aims to assess an alternative strategy for the valorization of accumulated matured compost obtained from municipal solid waste, by producing catalysts for the CWPO of 2-NP and 4-NP in the aqueous phase and in a cyclohexane–water mixture simulating oily wastewater. The valorization is carried out by sulfuric acid activation and thermal treatment of the matured compost.
2. Results and Discussion
2.1. Characterization of Catalysts
The elemental analysis of the compost (C) and of the materials prepared by acid activation and thermal treatments is summarized in Table 1. As observed, the C, H, S and N content of the samples ranged within the following values: 17.6–22.0%, 0.4–2.3%, 0.4–8.2% and 0.0–1.7%, respectively. The C/H ratio increases due to the carbonization of the organic content during the thermal treatment (from 9.3 to 17.2 when the thermal treatment is carried out at 400 °C, and to 44.3 when the thermal treatment is carried out at 800 °C). This increase was similar to that found in the thermal treatment of the sample activated with H2SO4 (C-S) to produce C-S-800, as evidenced by the increase in the C/H value of 11.8 for the precursor to 44.3 for the calcined sample. This increment of C/H was also observed in previous work on biochar and activated carbon preparation from raw biomasses, and was attributed to the dehydration and aromatization reactions during the pyrolysis processes [19].

Table 1.
Elemental analysis, ashes and remaining residua of the materials determined by thermogravimetric analysis.
The content of sulfur has a higher value in the samples treated with H2SO4 without further thermal treatment (6.8% and 8.2% for C-S and C-800-S, respectively), likely due to the formation of surface sulfur groups, as observed in carbonaceous materials [20]. However, these functional groups are desorbed due to subsequent thermal treatment, as evidenced by the S values determined for C-S-800 and C-800-S-800 (0.8% and 0.6%, respectively).
The sum of C, H, S, and N does not reach more than 33.4% in all the catalysts prepared, since all of them contain inorganic compounds and other heteroatoms in its composition. The inorganic content was determined from the incineration of the samples during the TGA runs (Figure 1). As observed, the content in ashes increases as a consequence of the thermal treatment, from 55.5% in the dried homogenized compost (sample C) to 64.9% and 81.5% in the samples calcined at 400 and 800 °C, respectively, likely due to the carbon released in the form of volatile organic compounds during the thermal treatment. The treatment with H2SO4 leads to a decrease in the inorganic content (from 55.5% to 34.3% and from 81.5% to 69.2% in the samples C, C-S, C-800, and C-800-S, respectively), which is also increased by the subsequent thermal treatment at 800 °C (65.9% and 76.7% for C-S-800 and C-800-S-800, respectively).
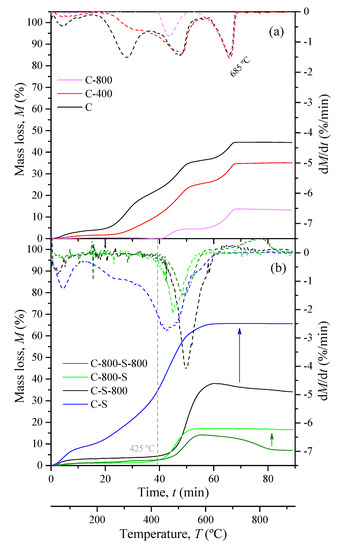
Figure 1.
Mass loss obtained by TGA and corresponding TGA derivatives of the samples prepared by thermal treatment (a) and both thermal and H2SO4 treatment (b).
The non-identified content (species different from C, H, N, S, and ashes) in the catalysts (up to 36.1% in all samples prepared) is usually ascribed to other heteroatoms, mainly oxygen [21]. As observed, this value significantly increases because of the acid treatment (from 18.6% in the C sample to 36.1% in the C-S sample). This can be ascribed to the functionalization with sulfur-containing and oxygen-containing groups, as a consequence of the acid treatment. However, the non-identified content decreases considerably for the catalysts prepared by thermal treatment at 800 °C, reaching values close to zero.
The TGA curve profile during the oxidation of the samples in the thermogravimetric analysis (Figure 1) shows an increment of the mass loss, more evident at temperatures higher than 400 °C. As observed, at the end of the analysis (>800 °C), the mass loss ranges from ca. 15% to 65%, so a remaining of 35–85% corresponds to the inorganic content (ashes). It is also possible to observe that the samples prepared only by thermal treatment (C-400 and C-800) show similar profiles characterized by a peak (in the derivative curve) found at ca. 685 °C (Figure 1a), which is absent in all samples prepared by activation with H2SO4 (Figure 1b). In fact, these samples show the highest mass losses centered between ca. 350 and ca. 625 °C.
The TGA curve profile during the oxidation of the samples in the thermogravimetric analysis (Figure 1) shows an increment of the mass loss, more evident at temperatures higher than 400 °C. As observed, at the end of the analysis (>800 °C), the mass loss ranges from ca. 15% to 65%, so a remaining of 35–85% corresponds to the inorganic content (ashes). It is also possible to observe that the samples prepared only by thermal treatment (C-400 and C-800) show similar profiles characterized by a peak (in the derivative curve) found at ca. 685 °C (Figure 1a), which is absent in all samples prepared by activation with H2SO4 (Figure 1b). In fact, these samples show the highest mass losses centered between ca. 350 and ca. 625 °C.
The morphology of selected samples (C-800 and C-800-S) was investigated by SEM (Figure 2). The SEM micrographs show size particles ranging from ca. 10 to 100 µm for both samples. As expected, heterogeneous morphology and composition is also observed due to the origin of the precursor (Figure S1). Some agglomerates of iron oxide were found only for sample C-800 (Figure S1a). In sample C-800-S, sulfur is identified (Figure S1b) as a consequence of the sulfuric acid treatment and, as observed, some metals remain after the treatment with acid.
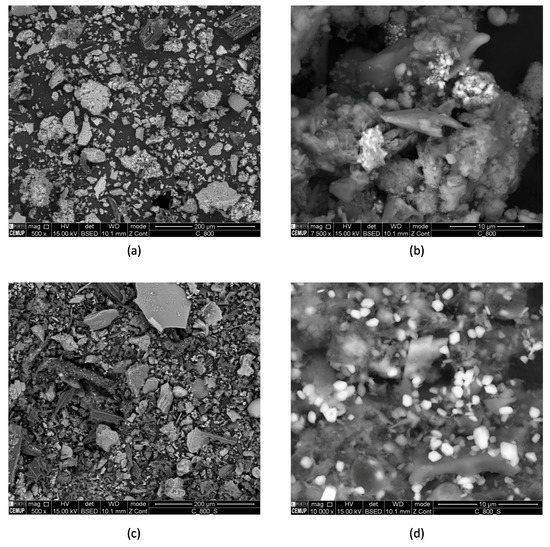
Figure 2.
Scanning electronic microphotographs of the C-800 (a,b) and C-800-S (c,d).
The acidity and basicity of the catalysts are summarized in Table 2. As observed, most samples show a higher concentration of basic sites than acid sites, mainly due to the content of alkaline earth metals present in the compost [22]. Only the sample treated with sulfuric acid after its thermal treatment (C-800-S) shows higher acidity (1.66 mmol g−1) than basicity (1.45 mmol g−1), likely due to an extensive functionalization with surface sulfur-containing groups [20]. The decrease in the basicity of the samples with the treatment with H2SO4 can be ascribed to the leaching of the alkaline earth metals from the precursor during the acid attack and also for the formation of thiol and sulfonic acid groups, as reported elsewhere [20].

Table 2.
Acid-base characterization of the catalysts.
The N2 adsorption and desorption isotherm curves of the materials are depicted in Figure 3. As observed, C, C-400, and C-S show isotherms of type III, according to the IUPAC classification, with no identification of monolayer formation (no adsorption at low relative pressures). This means that adsorbent–adsorbate interactions are relatively weak for these materials (≤400 °C), and that the adsorbed molecules are clustered around the most favorable sites on the surface, characteristic of nonporous or macroporous solids. In fact, the BET specific surface area is low (SBET =11−22 m2 g−1), and no micropores are found (null Smic and Vmic) in these materials. In contrast, the catalysts treated at 800 °C (C-800, C-S-800, C-800-S-800, and C-800-S) show adsorption at low relative pressures (Figure 3) and present improved textural properties. The highest porosity development was achieved by the sequential treatment with H2SO4 and thermal treatment at 800 °C (C-S-800), leading to a BET specific surface area of 279 m2 g−1 and a total pore volume of 172 mm3 g−1. The BET specific surface area was found to decrease in the following order: C-S-800 (279 m2 g−1) > C-800-S-800 (107 m2 g−1) > C-800-S (91 m2 g−1) > C-800 (77 m2 g−1) > C-400, C-S, C (< 25 m2 g−1). Thus, the materials prepared by both activation with H2SO4 and thermal treatment at 800 °C, regardless of the order (C-S-800, C-800-S-800 and C-800-S), show a higher BET specific surface area than the sample prepared only by thermal treatment at 800 °C (C-800).
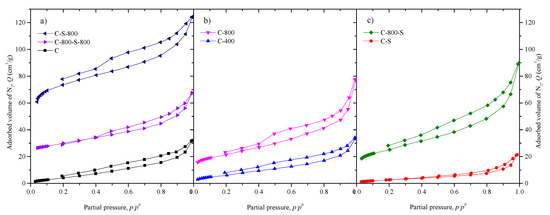
Figure 3.
N2 adsorption-desorption isotherms at 77 K of the activated (a), thermal-treated (b) and functionalized materials (c).
Only the materials calcined at 800 °C show a significant quantity of pores in the range of micropores (Smic = 12−92 m2 g−1 and 14%−53% of the pore volume corresponds to micropores, determined as Vmic/VTotal). The total pore volume increases in the following order C-S (21 mm3 g−1) < C (36 mm3 g−1) < C-400 (38 mm3 g−1) < C-800 (85 mm3 g−1) < C-800-S-800 (87 mm3 g−1) < C-800-S (103 mm3 g−1) < C-S-800 (172 mm3 g−1).
2.2. CWPO of 2-NP and 4-NP in the Aqueous Phase
The concentration decay curves of 2-NP and H2O2 during the oxidation runs carried out with the thermally treated materials are depicted in Figure 4a,b, respectively. As observed, all the tested materials show high catalytic activity in the CWPO of 2-NP, the conversions of 2-NP and H2O2 being higher in the presence of the catalysts (X2-NP > 81% and XH2O2 > 77% after 24 h) than in the non-catalytic run (X2-NP = 16% and XH2O2 = 16% after 24 h). The complete removal of 2-NP after 24 h was achieved with all materials subjected to thermal treatment at 800 °C, which can be explained by its significant micropore surface area. Among these materials, the higher removal rates were obtained with the materials also subjected to treatment with sulfuric acid, removing 99.9% of 2-NP in just 2 h, in opposition to the non-functionalized material (C-800) that allow the removal of 29% of 2-NP at the same time. Interestingly, only this material was able to completely consume the H2O2 fed to the reactor (100% of conversion after 1 h), in opposition to the S-treated catalysts (C-S-800, C-800-S-800, and C-800-S) that led to conversions of H2O2 from 77% to 90% after 24 h. This may be explained by the non-efficient decomposition of H2O2 with the catalyst C-800, resulting in the formation of non-reactive species (O2 and H2O) due to parasite reactions.
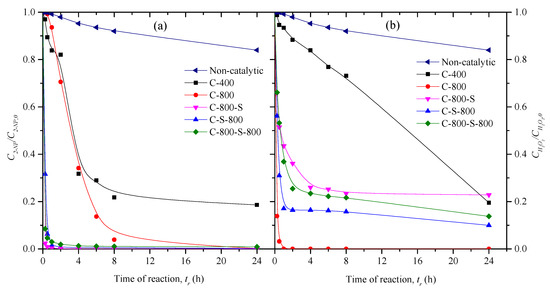
Figure 4.
Concentration decay curves of 2-NP (a) and H2O2 (b), in the CWPO of 2-NP carried out with the thermal treated materials. Operating conditions: 50 °C, pH0 = 3, Ccat = 2.5 g L−1, C2-NP,0 = 0.5 g L−1 and the stoichiometric quantity of H2O2 needed for the total mineralization of 2-NP.
The contribution of adsorption in removing 2-NP with all materials tested was not higher than 8% after 24 h of contact time, as determined by pure adsorption runs at the same operating conditions, but without H2O2. The higher contribution of adsorption was found with sample C-S-800 (8%), ascribed to its higher porosity (SBET = 279 m2 g−1). Other materials thermally treated at 800 °C show an adsorption contribution from 3% to 5% after 24 h of contact time. Adsorption was negligible when using sample C-400. Taking into account the negligible contribution of adsorption, the conversions of 2-NP achieved in the catalytic oxidation runs can only be ascribed to the catalytic activity of the materials.
In a previous work, the complete removal of 2-NP was achieved only after 24 h using carbon nanotubes as catalyst under similar operating conditions (50 °C, pH0 = 3, Ccat = 0.25 g L−1, C2-NP,0 = 0.5 g L−1 and the stoichiometric amount of H2O2 needed for the total mineralization of 2-NP) [13]. In the present study, the complete removal of 2-NP is achieved at a lower time (<2 h). Although using a higher quantity of catalysts, it should be emphasized that they were prepared from a low-cost precursor (mature compost obtained from municipal solid waste).
The concentration decay curves of 4-NP and H2O2 during the CWPO of 4-NP are depicted in Figure 5. As can be observed, the catalysts prepared in this study from solid waste also show high catalytic activity in the peroxide oxidation of 4-NP, evidencing their versatility in the degradation of two nitrophenols with different lipophilic properties [18]. However, the performances of the catalysts in the oxidation of 4-NP (Figure 5) are different to those found for the CWPO of 2-NP (Figure 4), since the S-untreated materials (C-400 and C-800) led to complete removal of 4-NP after 24 h, whereas C-S-800 and C-800-S-800 allow the degrading of 76% of 4-NP under the same operating conditions (Figure 5). Additionally, C-400 and C-800 show an induction period close to 2 h, in which the conversions of 4-NP are negligible. In a previous work dealing with the CWPO of 4-NP with carbon black as the catalyst, an induction period close to 2 h was also observed, which was mitigated decreasing the initial pH from 3 to 2 or by increasing the temperature of the reaction from 50 to 80 °C [23].
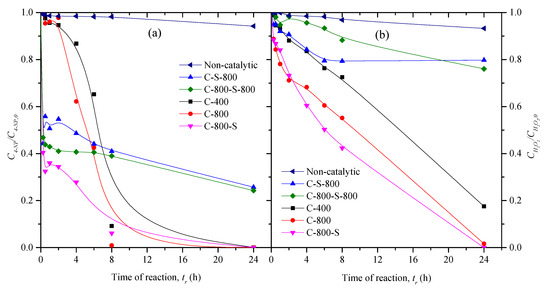
Figure 5.
Concentration decay curves of 4-NP (a) and H2O2 (b) in the CWPO of 4-NP carried out with the thermally treated materials. Operating conditions: 50 °C, pH0 = 3, Ccat = 2.5 g L−1, C4-NP,0 = 5.0 g L−1, and the stoichiometric quantity of H2O2 needed for the total mineralization of 4-NP.
Regarding the catalysts treated with sulfuric acid (C-800-S, C-S-800, and C-800-S-800), these materials show higher 4-NP degradation rates at the beginning of the reaction (X4-NP = 44−67% after 30 min), likely due to its acidic character (Table 2). However, the conversions of 4-NP obtained with C-S-800 and C-800-S-800 after 24 h are lower (76%) when compared to the other catalysts. This can be ascribed to the low conversions of H2O2 during the CWPO runs with these materials (XH2O2 = 20%−24% after 24 h). The consumption of H2O2 was found to be higher (XH2O2 > 82% under the same operational conditions) than with the other catalysts (C-400, C-800, and C-800-S), meaning that the treatments conducted to prepare the materials led to the development of different catalytic activities in a competitive process that requires both the catalytic decomposition of H2O2 and the catalytic degradation of the organic pollutant [24,25]. In this sense, the higher basicity of the character of C-400 and C-800 plays a significant role in the global decomposition of H2O2 and the strong acidity of C-800-S in its efficiency [25].
Taking into consideration the complex matrix of the catalysts and their high concentration in ashes, the iron content was determined for the liquid samples taken from the medium of the reaction after 24 h in the CWPO of 2-NP and 4-NP runs. In all samples, the iron content was lower than the limit of detection, so no leaching of iron was observed from the catalyst, and no contribution of the Fenton reaction can occur during the CWPO runs.
2.3. Oxidation Runs in Biphasic Systems
The conversion of 2-NP and 4-NP (calculated from the concentration of 2-NP and 4-NP, respectively, in both oily and aqueous phase) obtained during catalytic wet peroxide oxidation carried out in a cyclohexane-water biphasic mixture with all catalysts after 24 h (Figure 6) shows the complete conversion of 4-NP and over 90% conversion of 2-NP. As observed, the materials prepared from the solid waste show noticeable catalytic activity for both model pollutants. Removals of 2-NP higher than 89% were obtained with all catalysts after 24 h, and conversions of 4-NP were found to range from 27% to 99% in the same operating conditions. The higher conversion of the most lipophilic pollutant (2-NP) can be ascribed to its lower initial concentration in these experiments (0.5 g L−1) when compared to the 4-NP concentration (5.0 g L−1). These different initial concentrations were chosen to be equivalent to those employed in the aqueous system (i.e., to allow comparison between each other). As expected, the presence of oil led to lower removals of 2-NP (89%−93% after 24 h) than those obtained by CWPO in the aqueous phase (up to 99.9% after 8 h), likely due to the mass transfer limitation of 2-NP from the oil phase to the aqueous phase. In previous work, lower conversions of 2-NP were obtained by CWPO in cyclohexane-water medium with carbon nanotubes as the catalyst, although using a higher concentration of 2-NP (5.0 g L−1) [13].
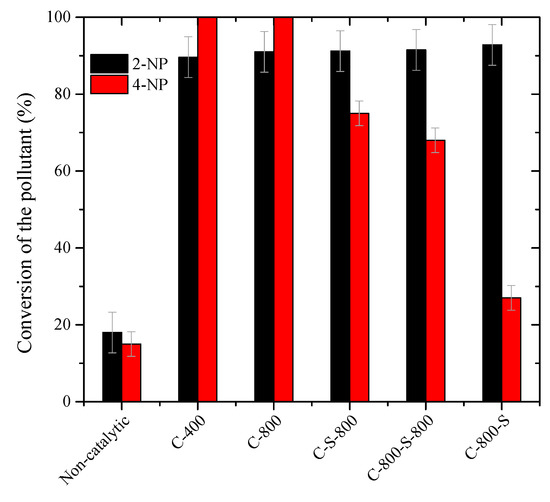
Figure 6.
Conversion after 24 h of 2-nitrophenol and 4-nitrophenol by CWPO using as catalysts the materials prepared by thermal treatment. Operating conditions: 50 °C, pH0 of aqueous phase 3, Ccat = 2.5 g L−1 and the stoichiometric amount of H2O2 needed for the total mineralization of the targeted pollutant (0.5 g L−1 for 2-NP and 5.0 g L−1 for 4-NP).
The effect of the catalyst is more notorious in the CWPO of 4-NP, likely due to the higher hydrophilic character of 4-NP, thus being found preferentially in the aqueous phase. S-untreated catalysts (C-400 and C-800) show higher catalytic activities than the other catalyst, allowing us to remove the model pollutant 4-NP completely after 24 h. There are no reported studies regarding the catalytic wet peroxide oxidation of 4-NP in an immiscible oil–water medium to the best of our knowledge.
Neither cyclohexanol nor cyclohexanone was detected in any oxidation experiment performed. Thus, the oxidation of cyclohexane does not occur under the studied operating conditions, and only oxidation of 2-NP and 4-NP occurred in the CWPO runs. Furthermore, the adsorption contribution in the biphasic oxidation runs was negligible for both 2-NP and 4-NP.
3. Materials and Methods
3.1. Materials and Reagents
The matured compost was supplied by the waste management company Resíduos do Nordeste, EIM. As obtained from the anaerobic digestion and composting, the compost had an organic matter and moisture content of 48.8% and 29.6%, respectively (more details were summarized in a previous work [22]). The activation method used to produce the catalytic materials considered the use of sulfuric acid (H2SO4, 96–98 wt.%) obtained from Riedel-de-Haën. The reactants involved in the CWPO runs were 2-NP (C6H4NO2OH, 98 wt.%), 4-NP (C6H4NO2OH, 98 wt.%), and hydrogen peroxide (H2O2, 30%, w/v), provided by Sigma-Aldrich (St. Louis, MO, USA), Acros Organics, and, respectively. Titanium (IV) oxysulfate (TiOSO4, 15 wt.% in dilute sulfuric acid, H2SO4 99.99%), hydrochloric acid (HCl, 37 wt.%), and sodium sulfite (Na2SO3, 98 wt.%), used in the analytical methods, were purchased from Sigma-Aldrich. Sodium hydroxide (NaOH, 98 wt.%) was obtained from Panreac. The mobile phase used in the HPLC analyses was composed of methanol (CH3OH, HPLC grade), glacial acetic acid (CH3COOH, analytical reagent grade), and acetonitrile (C2H3N, 99.99%), available from Fisher Chemical (Waltham, MA, USA). All chemicals were used as received without further purification. Distilled water was used throughout the work. Pure N2 and synthetic air for thermal treatment in materials preparation and thermogravimetric analysis were supplied by Air Liquid.
3.2. Preparation of Materials
The catalysts were prepared from the matured compost by activation with H2SO4, followed by thermal treatment (400−800 °C), according to procedures described elsewhere [21]. Prior to the production of the materials, the compost was washed with water (100 g L−1) under vigorous stirring in order to homogenize the precursor and to remove suspended solids. The suspension was later filtered and the homogenized solid dried overnight at 60 °C. Afterward, the mature compost was sieved to obtain particle sizes of 53–106 µm. Then, two samples were prepared by thermal treatment. For the first sample, 5 g of homogenized compost was heated under an N2 flow (100 N cm3 min−1) at 120 °C for 1 h and 400 °C for 4 h, resulting in sample C-400. A second sample was prepared by heat treatment at 120, 400, and 600 °C, for 1 h at each temperature, and then heating at 800 °C for 4 h, resulting in sample C-800. In addition, three more materials were produced: treated with H2SO4 (i) before (C-S-800) and (ii) after (C-800-S) the thermal treatment described at 800 °C, and (iii) without thermal treatment (C-S). To obtain these three samples, 2.5 g of the precursor was immersed in 50 mL of an 18 M H2SO4 solution for 3 h at 150 °C, as previously studied in the modification of carbon materials [20,21]. Posteriorly, all samples were thoroughly washed with distilled water until the neutrality of the rinsing waters was reached and further dried overnight in an oven at 110 °C. Finally, a sample was prepared by the thermally treating the sample C-800-S, as described at 800 °C, resulting in the catalyst C-800-S-800. All samples are shown in Table 1.
3.3. Techniques of Characterization
The prepared materials were analyzed by elemental, thermogravimetric, porosity, and surface area analysis and by an adaptation of Boehm’s method to determine its acidity and basicity, following methodologies described in a previous work [25]. Briefly, the composition of the materials was determined by elemental analysis (Carlo Erba Instrument EA 1108) to quantify C, H, N, and S contents. Thermogravimetric analyses (TGA) were performed in triplicate on a Netzsch STA 409 PC equipment, heating the samples from 30 to 920 °C at 10 °C min−1 under a continuous flow of synthetic air at 40 mL min−1. N2 adsorption was conducted at −196 °C using a Quantachrome NOVA TOUCH LX4 apparatus. Prior to the N2 adsorption runs, the degasification of the catalysts was conducted at 300 °C during 16 h. BET and external specific surface areas (SBET and Sext, respectively) and total pore volume were determined as described elsewhere by the BET method and t-method. The microporous surface area (Smic) was determined subtracting Sext from SBET, and the total pore volume (VTotal) was calculated at p/p0 = 0.98.
The acidity and basicity of the materials were determined by acid-base titration using NaOH and HCl solutions, and phenolphthalein as an indicator, according to a method previously described [25]. Briefly, for the determination of acidity, 0.2 g of the sample was mixed with 25 mL of a 0.02 M NaOH solution. The resultant suspension was stirred for 48 h at room temperature. Subsequently, the suspension was filtered, and 20 mL of the resulting liquid was titrated with a 0.02 M HCl solution to determine unreacted OH– (phenolphthalein was used as the indicator). The acidity was calculated by the difference between the initial NaOH and the determined amount of NaOH by titration. The determination of the basicity was done in the same way, using 0.02 M HCl as the initial solution and titrating with 0.02 M NaOH.
The scanning electron microscopy characterization of the materials was performed with a FEI Quanta 400 FEG ESEM/EDAX Genesis X4M instrument, coupled to an energy dispersive X-ray spectroscopy (Waltham, MA, USA) detector to obtain information on the chemical composition.
3.4. CWPO Runs
The CWPO runs with 2-NP and 4-NP were carried out in a batch system using a 500 mL magnetically stirred (600 rpm) round-bottom flask as reactor, equipped with a condenser and a heating bath [23]. Briefly, 100 mL of the target pollutant solution was first loaded to the reactor and then heated to 50 °C. Upon stabilizing the temperature, the solution pH was adjusted to the initial pH (pH0) of 3 using a 1 M H2SO4 solution, and the selected quantity of catalyst (2.5 g L−1) was then loaded to the aqueous solution. The volume of a 30% w/v H2O2 solution was calculated to use the stoichiometric dosage of H2O2 needed for the complete mineralization of nitrophenol (C2-NP,0 = 0.5 g L−1 and C4-NP,0 = 5.0 g L−1) and added to the solution. That moment was considered the initial time, t0 = 0 min. Adsorption runs were carried out under the same operational conditions, but in the absence of H2O2, to evaluate the adsorption contribution on the removal of the pollutants by the catalyst surface.
To simulate the treatment of oily wastewater by CWPO, a mixture of 50 mL of cyclohexane containing the most lipophilic pollutant (0.5 g L−1 of 2-NP) with 50 mL of water at pH 3 containing the hydrophilic pollutant (5.0 g L−1 of 4 NP) was used for the biphasic oxidation of 2-NP and 4-NP. The concentration of the model pollutant in each phase was followed, and CWPO started when the equilibrium concentration of the pollutant between the oily phase and the aqueous phase was reached. The CWPO runs in the biphasic system were carried out at the same operating conditions as described for the aqueous phase CWPO runs (pH = 3, 50 °C and the stoichiometric dosage of H2O2 needed for the complete mineralization of the target pollutant). Adsorption runs under biphasic conditions were also performed.
Selected experiments were performed in triplicate to assess the reproducibility of the experimental results. All the experiments (oxidation runs performed in both aqueous and L-L biphasic systems) were conducted for 24 h and monitored by taking several samples from the reactor at previously selected times. The aliquots were immediately centrifuged to separate the solid catalyst from the liquid phase and analyzed.
3.5. Analysis of Aqueous and Oily Samples
The aqueous samples were analyzed by UV-Vis spectrophotometry (T70 spectrometer, PG Instruments Ltd., Leicestershire, UK), and HPLC (Jasco, Tokyo, Japan), to determine the concentrations of H2O2 and model pollutants, respectively, as previously described [26]. Samples taken to monitor the concentration of H2O2 during the CWPO experiments were analyzed immediately. For that, 1 mL of filtered sample was added to 1 mL of H2SO4 solution (0.5 mol L−1) with 0.1 mL of TiOSO4 in a 20 mL volumetric flask, and the total volume filled up with distilled water. The resultant mixture was diluted with distilled water and further analyzed by UV–Vis spectrophotometry at a wavelength of 405 nm. The samples taken to determine the concentration of 2-NP and 4-NP were first mixed with the stoichiometry quantity of Na2SO3 needed to consume all H2O2 present in the samples. Then, both 2-NP and 4-NP were monitored using a Jasco HPLC system at a wavelength of 277 and 318 nm (UV-2075 Plus detector). A Kromasil 100-5-C18 column was used as a stationary phase feeding 1 mL min−1 (PU-2089 Plus) of the eluent composed by an A:B (40:60) mixture containing (A) 3% acetic acid and 1% acetonitrile in methanol and (B) 3% acetic acid in ultrapure water.
The samples of the organic phase were analyzed by GC-FID (Bruker Scion 436-GC) to determine the concentration of the target nitrophenol compound, as well as to evaluate the oxidation of cyclohexane, following the evolution of cyclohexanol and cyclohexanone. With this purpose, aliquots were prepared without further derivation, but adding a small quantity of Na2SO3 to remove sample moisture. The injector and detector temperatures were set at 260 and 270, respectively. Complete separation of the compounds was achieved on a 50 m × 0.25 mm CP-Sil 88 chromatography column using the following temperature program in the oven: a first isotherm step at 160 °C for 5 min, followed by a heating ramp of 5 °C min−1 that takes 2 min to reach 170 °C, then a heating ramp of 10 °C min−1 that takes 5 min to reach 220 °C and a final isothermal step at this temperature for 5 min.
The aliquots’ iron content at the end of the CWPO runs was determined by atomic absorption spectroscopy in a Varian SpectrAA 220 equipment (Varian, Steinhausen, Switzerland) as described in previous works [27].
4. Conclusions
The possibility to prepare active catalysts for peroxide oxidation from a seemingly useless precursor, such as matured compost obtained in the units of mechanical and biological treatment of municipal solid waste, has been demonstrated. This possibility allows for reducing the accumulation of the non-demanded compost at waste management centers, being transformed into a product with higher added value.
Catalysts can be prepared by grinding and sieving the compost, followed by its thermal treatment and activation, as is done for activated clays or carbon solid materials, leading to obtaining low-cost heterogeneous catalysts with high activity for peroxide oxidation of organic pollutants.
The prepared catalysts not only show interesting catalytic activity but also versatility, as they were able to oxidize 2-NP and 4-NP in both aqueous solutions and oily systems (simulated with a cyclohexane-water biphasic mixture).
The catalysts are particularly useful in the treatment of lipophilic organic pollutants present in oily wastewater. It is worth pointing out that the catalyst prepared by pyrolysis of the compost at 800 °C, without further treatment (C-800), and the catalyst prepared by thermal treatment at 800 °C and subsequent treatment with H2SO4 (C-800-S) are able to entirely remove both 2-NP and 4-NP in aqueous solution at the tested operating conditions by CWPO (50 °C, initial pH 3, Ccat = 2.5 g L−1 and a stoichiometric amount of H2O2 needed for the complete mineralization of C2-NP,0 = 0.5 g L−1 and C4-NP,0= 5.0 g L−1). In biphasic oxidation, 4-NP is also completely degraded with C-800, whereas the removal of 2-nitrophenol is decreased due to its higher lipophilic character.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/11/1243/s1, Figure S1: SEM micrographs and corresponding EDS spectra of C-800 (a) and C-800-S (b).
Author Contributions
Conceptualization: J.L.D.d.T. and H.T.G.; application of methodology: J.L.D.d.T. and G.F.P.; writing—original draft preparation: J.L.D.d.T.; writing—review: A.M.T.S., J.L.F. and H.T.G.; supervision: H.T.G.; compost supply: P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by project “VALORCOMP − Valorización de compost y otros desechos procedentes de la fracción orgánica de los residuos municipales”, 0119_VALORCOMP_2_P, and project “AIProcMat@N2020 − Advanced Industrial Processes and Materials for a Sustainable Northern Region of Portugal 2020”, reference NORTE-01-0145-FEDER-000006, supported by NORTE 2020, under the Portugal 2020 Partnership Agreement, through FEDER; CIMO (UIDB/00690/2020) through FEDER under Program PT2020 and Base Funding − UIDB/50020/2020 of the Associate Laboratory LSRE-LCM − funded by national funds through FCT/MCTES (PIDDAC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eurostat. Municipal Waste Statistics-Statistics Explained (Data Extracted in October 2020). Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics#Municipal_waste_treatment (accessed on 23 October 2020).
- Resíduos do Nordeste, E. Serviços-Unidade de Tratamento Mecânico e Biológico. Available online: https://www.residuosdonordeste.pt/tratamentoMecanicoBiologico (accessed on 23 October 2020).
- Saveyn, H.; Eder, P. End-of-waste criteria for biodegradable waste subjected to biological treatment (compost & digestate): Technical proposals. JRC Sci. Policy Rep. 2014, 1–308. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrilla, J.; Sanchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; Xu, M.; Ning, C.; Sze Ki Lin, C.; McKay, G. A critical review on preparation, characterization and utilization of sludge-derived activated carbons for wastewater treatment. Chem. Eng. J. 2015, 260, 895–906. [Google Scholar] [CrossRef]
- Ahmed, M.J. Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption: Review. J. Environ. Chem. Eng. 2016, 4, 89–99. [Google Scholar] [CrossRef]
- Rezende, M.J.C.; Pinto, A.C. Esterification of fatty acids using acid-activated Brazilian smectite natural clay as a catalyst. Renew. Energy 2016, 92, 171–177. [Google Scholar] [CrossRef]
- Sihvonen, S.K.; Schill, G.P.; Lyktey, N.A.; Veghte, D.P.; Tolbert, M.A.; Freedman, M.A. Chemical and physical transformations of aluminosilicate clay minerals due to acid treatment and consequences for heterogeneous ice nucleation. J. Phys. Chem. A 2014, 118, 8787–8796. [Google Scholar] [CrossRef]
- Santos Silva, A.; Seitovna Kalmakhanova, M.; Kabykenovna Massalimova, B.; Sgorlon, G.J.; Jose Luis, D.d.T.; T Gomes, H. Wet Peroxide Oxidation of Paracetamol Using Acid Activated and Fe/Co-Pillared Clay Catalysts Prepared from Natural Clays. Catalysts 2019, 9, 705. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Martin-Martinez, M.; Gomes, H.T.; Ovejero, G.; García, J. Enhancement of p-nitrophenol adsorption capacity through N 2-thermal-based treatment of activated carbons. Appl. Surf. Sci. 2017, 414, 424–434. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Barreiro, M.F.F.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Lignin-based activated carbons as metal-free catalysts for the oxidative degradation of 4-nitrophenol in aqueous solution. Appl. Catal. B 2017, 219, 372–378. [Google Scholar] [CrossRef]
- Rueda Márquez, J.; Levchuk, I.; Sillanpää, M. Application of Catalytic Wet Peroxide Oxidation for Industrial and Urban Wastewater Treatment: A Review. Catalysts 2018, 8, 673. [Google Scholar] [CrossRef]
- de Diaz Tuesta, J.L.; F Machado, B.; Serp, P.; T Silva, A.M.; Faria, J.L.; TGomes, H. Janus amphiphilic carbon nanotubes as Pickering interfacial catalysts for the treatment of oily wastewater by selective oxidation with hydrogen peroxide. Catal. Today 2020, 356, 205–215. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. Degradation of nitrophenols by Fenton and photo-Fenton processes. J. Photochem. Photobiol. A Chem. 2005, 170, 83–95. [Google Scholar] [CrossRef]
- Trapido, M.; Veressinina, Y.; Kallas, J. Degradation of aqueous nitrophenols by ozone combined with UV-radiation and hydrogen peroxide. Ozone Sci. Eng. 2001, 23, 333–342. [Google Scholar] [CrossRef]
- Orshansky, F.; Narkis, N. Characteristics of organics removal by PACT simultaneous adsorption and biodegradation. Water Res. 1997, 31, 391–398. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Q.; Zhang, G.; Chen, J.; Fei, Z.; Liu, F. Adsorption of phenolic compounds from aqueous solutions by a water-compatible hypercrosslinked polymeric adsorbent. Chemosphere 2002, 47, 981–989. [Google Scholar] [CrossRef]
- Abraham, M.H.; Du, C.M.; Platts, J.A. Lipophilicity of the nitrophenols. J. Org. Chem. 2000, 65, 7114–7118. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef]
- Gomes, H.T.; Miranda, S.M.; Sampaio, M.J.; Figueiredo, J.L.; Silva, A.M.T.; Faria, J.L. The role of activated carbons functionalized with thiol and sulfonic acid groups in catalytic wet peroxide oxidation. Appl. Catal. B 2011, 106, 390–397. [Google Scholar] [CrossRef]
- de Diaz Tuesta, J.L.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Removal of Sudan IV from a simulated biphasic oily wastewater by using lipophilic carbon adsorbents. Chem. Eng. J. 2018, 347, 963–971. [Google Scholar] [CrossRef]
- Mohsen, K.; de Diaz Tuesta, J.L.; Gonçalves, C.N.d.P.; Gomes, H.T.; Rodrigues, A.E.; José, A.C.S. Integrated Management of Environment by Employing Derived Compost from Municipal Solid Wastes as a Source of Biochar for CO2 Capture. Chem. Eng. Technol. 2020, 43, 1336–1349. [Google Scholar]
- Diaz de Tuesta, J.L.; Quintanilla, A.; Casas, J.A.; Morales-Torres, S.; Faria, J.L.; Silva, A.M.T.; Gomes, H.T. The pH effect on the kinetics of 4-nitrophenol removal by CWPO with doped carbon black catalysts. Catal. Today 2020, 356, 216–225. [Google Scholar] [CrossRef]
- Diaz de Tuesta, J.L.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Kinetic modeling of wet peroxide oxidation with a carbon black catalyst. Appl. Catal. B 2017, 209, 701–710. [Google Scholar] [CrossRef]
- Diaz de Tuesta, J.L.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. P-,B-and N-doped carbon black for the catalytic wet peroxide oxidation of phenol: Activity, stability and kinetic studies. Catal. Commun. 2017, 102, 131–135. [Google Scholar] [CrossRef]
- Martin-Martínez, M.; Ribeiro, R.S.; Machado, B.F.; Serp, P.; Morales-Torres, S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Role of nitrogen doping on the performance of carbon nanotube catalysts: A catalytic wet peroxide oxidation application. ChemCatChem 2016, 8, 2068–2078. [Google Scholar] [CrossRef]
- Cardoso, J.; Gomes, H.; Brito, P. Viability of the Use of Leachates from a Mechanical Biological Municipal Solid Waste Treatment Plant as Fertilizers. Recycling 2019, 4, 8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).