Thermodynamics of Chemical Processes in the System of Nanocrystalline Iron–Ammonia–Hydrogen at 350 °C
Abstract
1. Introduction
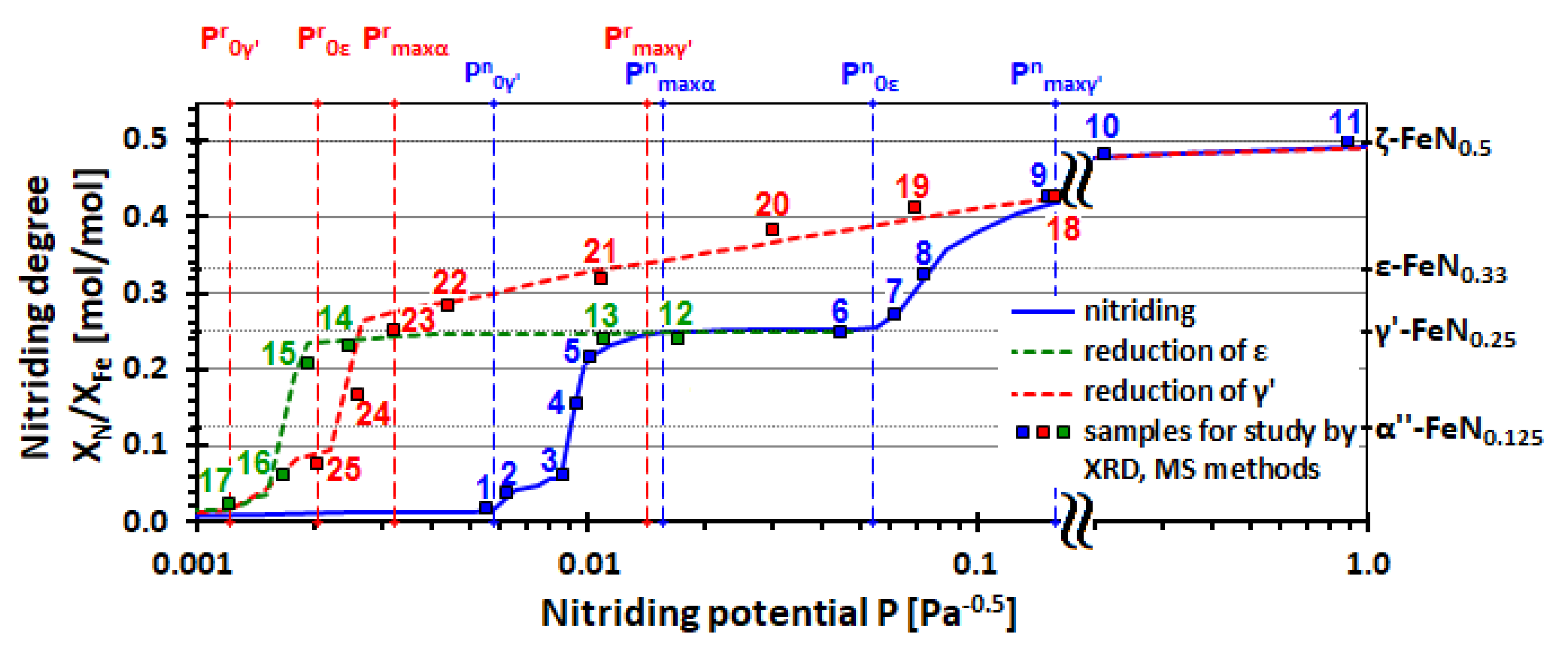
2. Results and Discussion
3. Experimental
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pye, D. Practical Nitriding and Ferritic Nitrocarburizing, 1st ed.; ASM International: Cleveland, OH, USA, 2003. [Google Scholar]
- Grosch, J. Heat Treatment with Gaseous Atmospheres. In Steel Heat Treatment Handbook, 2nd ed.; Totten, G.E., Ed.; Taylor & Francis: Portland, OR, USA, 1997; pp. 415–474. [Google Scholar]
- Davis, J.R. Metals Handbook Desk Edition, 2nd ed.; ASM International: Cleveland, OH, USA, 1998. [Google Scholar]
- Alpaslan, Ö.; Atar, E.; Çimenoğlu, H. Tribological behaviour of duplex treated AISI 4140 steel. JESTECH 2012, 15, 39–43. [Google Scholar]
- Chen, H.T.; Yan, M.F.; You, Y. Effect of N distribution on elastic and electronic properties of hexagonal ε-Fe6Nx by first-principles calculations. J. Magn. Magn. Mater. 2014, 354, 200–204. [Google Scholar] [CrossRef]
- Mittemeijer, E.J.; Sommers, M.A.J. Thermochemical Surface Engineering of Steels, 1st ed.; Woodhead Publishing: London, UK, 2015. [Google Scholar]
- Bell, T.; Akamatsu, K. Stainless Steel 2000: Thermochemical Surface Engineering of Stainless Steel, 1st ed.; Maney Publishing: London, UK, 2001. [Google Scholar]
- Jack, K.H. The iron-nitrogen system: The preparation and the crystal structures of nitrogen-austenite (γ) and nitrogen-martensite (a’). Proc. Royal Soc. 1951, 208, 200–215. [Google Scholar]
- Jack, K.H. The iron-nitrogen system: The crystal structures of ε-phase iron nitrides. Acta Crystallogr. 1952, 5, 404–411. [Google Scholar] [CrossRef]
- Jack, K.H. The synthesis, structure, and characterization of α″-Fe16N2 (invited). J. Appl. Phys. 1994, 76, 6620–6625. [Google Scholar] [CrossRef]
- Wriedt, H.A.; Gokcen, N.A.; Nafziger, R.H. The Fe-N (Iron-Nitrogen) System. Bull. Alloy Phase Diagr. 1987, 8, 355–377. [Google Scholar] [CrossRef]
- Göhring, H.; Fabrichnaya, O.; Leineweber, A.; Mittemeijer, E.J. Thermodynamics of the Fe-N and Fe-N-C Systems: The Fe-N and Fe-N-C Phase Diagrams Revisited. Metall. Mater. Trans. A 2016, 47, 6173–6186. [Google Scholar] [CrossRef]
- Du Marchie van Voorthuysen, E.H.; Chechenin, N.C.; Boerma, D.O. Low-Temperature extension of the Lehrer diagram and the iron-nitrogen phase diagram. Metall. Mater. Trans. A 2002, 33, 2593–2598. [Google Scholar] [CrossRef]
- Tong, W.P.; Tao, N.R.; Wang, Z.B.; Zhang, H.W.; Lu, J.; Lu, K. The formation of ε-Fe3–2N phase in a nanocrystalline Fe. Scr. Mater. 2004, 50, 647–650. [Google Scholar] [CrossRef]
- Tong, W.P.; He, C.S.; He, J.C.; Zuo, L.; Tao, N.R.; Wang, Z.B. Strongly enhanced nitriding kinetics by means of grain refinement. Appl. Phys. Lett. 2006, 89, 021918/1–021918/3. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Sun, J.; Bao, Y.; Zhuang, L.; Ning, H. Low temperature nitriding of iron by alternating current pretreatment. Surf. Coat. Technol. 2006, 200, 6666–6670. [Google Scholar] [CrossRef]
- Tong, W.P.; Liu, C.Z.; Wang, W.; Tao, N.R.; Wang, Z.B.; Zuo, L.; He, J.C. Gaseous nitriding of iron with a nanostructured surface layer. Scr. Mater. 2007, 57, 533–536. [Google Scholar] [CrossRef]
- Wohlfarth, E.P. Recent developments in solid state magnetism with special reference to transition metal compounds. Pure Appl. Chem. 1985, 57, 1403–1406. [Google Scholar] [CrossRef]
- Nakajima, H.; Ohashi, Y.; Shiiki, K. Effect of N atom and lattice constant on electronic structures andmagnetic properties of Fe16N2 calculated by band structure calculation based on local-density approximation. J. Magn. Magn. Mater. 1997, 167, 259–263. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Shivaprasad, S.M.; Gajbhiye, N.S. Variation of magnetic ordering in ε-Fe3N nanoparticles. Chem. Phys. Lett. 2010, 496, 122–127. [Google Scholar] [CrossRef]
- Schnepp, Z.; Thomas, M.; Glatzel, S.; Schlichte, K.; Palkovits, R.; Giordano, C. One pot route to sponge-like Fe3N nanostructures. J. Mater. Chem. 2011, 21, 17760–17764. [Google Scholar] [CrossRef]
- Tagawa, K.; Kita, E.; Tasaki, A. Synthesis of fine Fe4N powder and its magnetic characteristics. Jpn. J. Appl. Phys. 1982, 21, 1596–1598. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Smith, P.A.I. Magnetic nitrides. J. Magnet. Magnet. Mater. 1999, 200, 405–424. [Google Scholar] [CrossRef]
- Bezdicka, P.; Kláriková, A.; Paseka, I.; Závêta, K. Magnetic properties of α’-FeNx and α”-Fe16N2 nitrides. J. Alloys Comp. 1998, 274, 10–17. [Google Scholar] [CrossRef]
- Utsushikawa, Y.; Niizuma, K. The saturation magnetization of Fe-N films prepared by nitriding treatment in N2 plasma. J. Alloys Comp. 1995, 222, 188–192. [Google Scholar] [CrossRef]
- Wilk, B.; Arabczyk, W. Badanie procesów azotowania i redukcji w układzie nanokrystaliczne żelazo-amoniak-wodór. Przem. Chem. 2014, 93, 1036–1040. [Google Scholar]
- Wilk, B.; Arabczyk, W. Investigation of nitriding and reduction processes in a nanocrystalline iron–ammonia–hydrogen system at 350 °C. Phys. Chem. Chem. Phys. 2015, 17, 20185–20193. [Google Scholar]
- Arabczyk, W.; Ekiert, E.; Pelka, R. Size-dependent transformation of α–Fe into γ’-Fe4N in nanocrystalline the Fe−NH3−H2 system. J. Phys. Chem. C 2016, 120, 17989–17995. [Google Scholar] [CrossRef]
- Wilk, B.; Kiełbasa, K.; Arabczyk, W. Azotowanie nanokrystalicznego żelaza mieszaniną amoniaku i wodoru w temperaturze 300 °C. Przem. Chem. 2015, 94, 1816–1820. [Google Scholar]
- Barański, A.; Bielański, A.; Pattek, A. Kinetics of reduction of iron catalysts for ammonia synthesis. J. Catal. 1972, 26, 286–294. [Google Scholar] [CrossRef]
- Liu, H. Amonia Synthesis Catalyst: Inovation and Practice; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2013. [Google Scholar]
- Arabczyk, W.; Narkiewicz, U.; Moszyński, D. Double-layer model of the fused iron catalyst for ammonia synthesis. Langmuir 1999, 15, 5785–5789. [Google Scholar] [CrossRef]
- Pelka, R.; Arabczyk, W. Studies of the Kinetics of Reaction between Iron Catalysts and Ammonia—Nitriding of Nanocrystalline Iron with Parallel Catalytic Ammonia Decomposition. Top. Catal. 2009, 52, 1506–1516. [Google Scholar] [CrossRef]
- Moszyńska, I.; Moszyński, D.; Arabczyk, W. Zjawisko histerezy procesów azotowania i redukcji w układzie nanokrystaliczne żelazo–amoniak–wodór. Przem. Chem. 2009, 88, 526–529. [Google Scholar]
- Kiełbasa, K.; Pelka, R.; Arabczyk, W. Studies of the kinetics of ammonia decomposition on promoted nanocrystalline iron using gas phases of different nitriding degree. J. Phys. Chem. A 2010, 114, 4531–4534. [Google Scholar] [CrossRef]
- Yu, M.; Xu, Y.; Mao, Q.; Li, F.; Wang, C. Electromagnetic and absorption properties of nano-sized and micro-sized Fe4N particles. J. Alloy. Compd. 2016, 656, 362–367. [Google Scholar] [CrossRef]
- Nishimaki, K.; Ohmae, S.; Yamamoto, T.A.; Katsura, M. Formation of iron-nitrides by the reaction of iron nanoparticles with a stream of ammonia. Nanostruct. Mater. 1999, 12, 527–530. [Google Scholar] [CrossRef]
- Wohlschlögel, M.; Welzel, U.; Mittemeijer, E.J. Unexpected formation of ε iron nitride by gas nitriding of nanocrystalline α-Fe films. Appl. Phys. Lett. 2007, 91, 141901/1–141901/3. [Google Scholar] [CrossRef]
- Moszyński, D.; Moszyńska, I. Przemiany fazowe podczas procesów azotowania nanokrystalicznego żelaza. Przem. Chem. 2013, 92, 1332–1335. [Google Scholar]
- Moszyński, D. Nitriding of nanocrystalline iron in the atmospheres with variable nitriding potential. J. Phys. Chem. C 2014, 118, 15440–15447. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Arabczyk, W. Studies of the ammonia decomposition over a mixture of α-Fe(N) and γ’-Fe4N. Pol. J. Chem. Technol. 2013, 15, 97–101. [Google Scholar]
- Moszyński, D.; Moszyńska, I.; Arabczyk, W. The transformation of α-Fe into γ’-Fe4N in nanocrystalline Fe-N system: Influence of Gibbs-Thomson effect. Appl. Phys. Lett. 2013, 103, 253108/1–253108/4. [Google Scholar] [CrossRef]
- Moszyński, D.; Kiełbasa, K.; Arabczyk, W. Influence of crystallites’ size on iron nitriding and reduction of iron nitrides in nanocrystalline Fe-N system. Mater. Chem. Phys. 2013, 141, 674–679. [Google Scholar] [CrossRef]
- Wilk, B.; Pelka, R.; Arabczyk, W. Study of the iron catalyst for ammonia synthesis by CPPR method. J. Phys. Chem. C 2017, 121, 8548–8556. [Google Scholar] [CrossRef]
- Arabczyk, W.; Ekiert, E.A.; Pelka, R. Hysteresis phenomenon in a reaction system of nanocrystalline iron and a mixture of ammonia and hydrogen. Phys. Chem. Chem. Phys. 2016, 18, 25796–25800. [Google Scholar] [CrossRef]
- Pelka, R. A method of determining nanoparticle size distribution in iron ammonia synthesis catalyst by measuring mass changes during the nitriding process. Catal. Today 2017, 286, 118–123. [Google Scholar] [CrossRef]
- Lehrer, E. Über das Eisen-Wasserstoff-Ammoniak-Gleichgewicht. Z Electrochem. 1930, 36, 383–392. [Google Scholar]
- Kunze, J. Nitrogen and Carbon in Iron and Steel Thermodynamics, 1st ed.; Akademie-Verlag: Berlin, Germany, 1990. [Google Scholar]
- Du Marchie van Voorthuysen, E.H.; Feddes, B.; Chechenin, N.G.; Inia, D.K.; Vredenberg, A.M.; Boerma, D.O. Low-temperature nitridation of iron layers in NH3–H2 Mixtures. Phys. Stat. Sol. A 2000, 177, 127–133. [Google Scholar] [CrossRef]
- Sokołowska, A.; Rudnicki, J.; Beer, P.; Małdziński, L.; Tacikowski, J.; Baszkiewicz, J. Nitrogen transport mechanisms in low temperature ion nitriding. Surf. Coat. Technol. 2001, 142–144, 1040–1045. [Google Scholar] [CrossRef]
- Arabczyk, W.; Wróbel, R. Study of the kinetics of nitriding of nanocrystalline iron using TG and XRD methods. Solid State Phenom. 2003, 94, 185–188. [Google Scholar] [CrossRef]
- Pelka, R.; Arabczyk, W. Modelling of nanocrystalline iron nitriding process—Influence of specific surface area. Chem. Pap. 2011, 65, 198–202. [Google Scholar] [CrossRef]
- Li, J.; Yuan, W.; Peng, X.; Yang, Y.; Xu, J.; Wang, X.; Hong, B.; Jin, H.; Jin, D.; Ge, H. Synthesis of fine α”-Fe16N2 powders by low-temperature nitridation of α-Fe from magnetite nanoparticles. AIP Adv. 2016, 6, 125104/1–125104/5. [Google Scholar] [CrossRef]
- Dirba, I.; Schwöbel, C.A.; Diop, L.V.B.; Duerrschnabel, M.; Molina-Luna, L.; Hofmann, K.; Komissinskiy, P.; Kleebe, H.-J.; Gutfleisch, O. Synthesis, morphology, thermal stability and magnetic properties of α”-Fe16N2 nanoparticles obtained by hydrogen reduction of γ-Fe2O3 and subsequent nitrogenation. Acta Mater. 2017, 123, 214–222. [Google Scholar] [CrossRef]
- Borsa, D.M.; Boerma, D.O. Phase identification of iron nitrides and iron oxy-nitrides with Mössbauer spectroscopy. Hyperfine Interact. 2003, 151, 31–48. [Google Scholar] [CrossRef]
- Ksenofontov, V.; Reiman, S.; Waldeck, M.; Niewa, R.; Kniep, R.; Gütlich, P. In situ—High temperature Mössbauer spectroscopy of iron nitrides and nitridoferrates. Z. Anorg. Allg. Chem. 2003, 629, 1787–1794. [Google Scholar] [CrossRef]
- Qi, Q.; O’Donnell, K.; Touchais, E.; Coey, J.M.D. Mössbauer spectra and magnetic properties of iron nitrides. Hyperfine Interact. 1994, 94, 2067–2073. [Google Scholar] [CrossRef]
- Hinomura, T.; Nasu, S. 57Fe Mössbauer study of Fe nitrides. Il Nuovo Cimento 1996, 18, 253–257. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Wilk, B. Studies of phase transitions occurring in the system of nanocrystalline Fe/NH3/H2. Mater. Chem. Phys. 2019, 237, 121853/1–121853/10. [Google Scholar] [CrossRef]
- Arabczyk, W.; Jasińska, I. Obecny stan wiedzy o katalizatorze żelazowym do syntezy amoniaku. Przem. Chem. 2006, 85, 130–137. [Google Scholar]
- Lendzion-Bieluń, Z. A comparison of the distribution of promoters in reduced and oxidized form of iron catalyst for ammonia synthesis. Pol. J. Chem. 2007, 81, 433–440. [Google Scholar]
- Arabczyk, W.; Jasińska, I.; Lubkowski, K. The surface properties of iron catalyst for ammonia synthesis. React. Kinet. Catal. Lett. 2004, 83, 385–392. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Wilk, B.; Hełminiak, A. Sposób Badania Nanomateriałów Poprzez Określenie ich Właściwości Fizykochemicznych. PL 229686 B1, 31 August 2018. [Google Scholar]
- Jasińska, I.; Arabczyk, W. Kinetic studies of the recrystallization process of iron catalyst for ammonia synthesis. Chem. Pap. 2005, 59, 496–499. [Google Scholar]
- Mittemeijer, E.J.; Slycke, J.T. Chemical potentials and activities of nitrogen and carbon imposed by gaseous nitriding and carburizing atmospheres. Surf. Eng. 1996, 12, 152–162. [Google Scholar] [CrossRef]
- Ekiert, E.A.; Pelka, R.; Lubkowski, K.; Arabczyk, W. The possibility of implementation of spent iron catalyst for ammonia synthesis. Pol. J. Chem. Technol. 2009, 11, 28–33. [Google Scholar] [CrossRef]

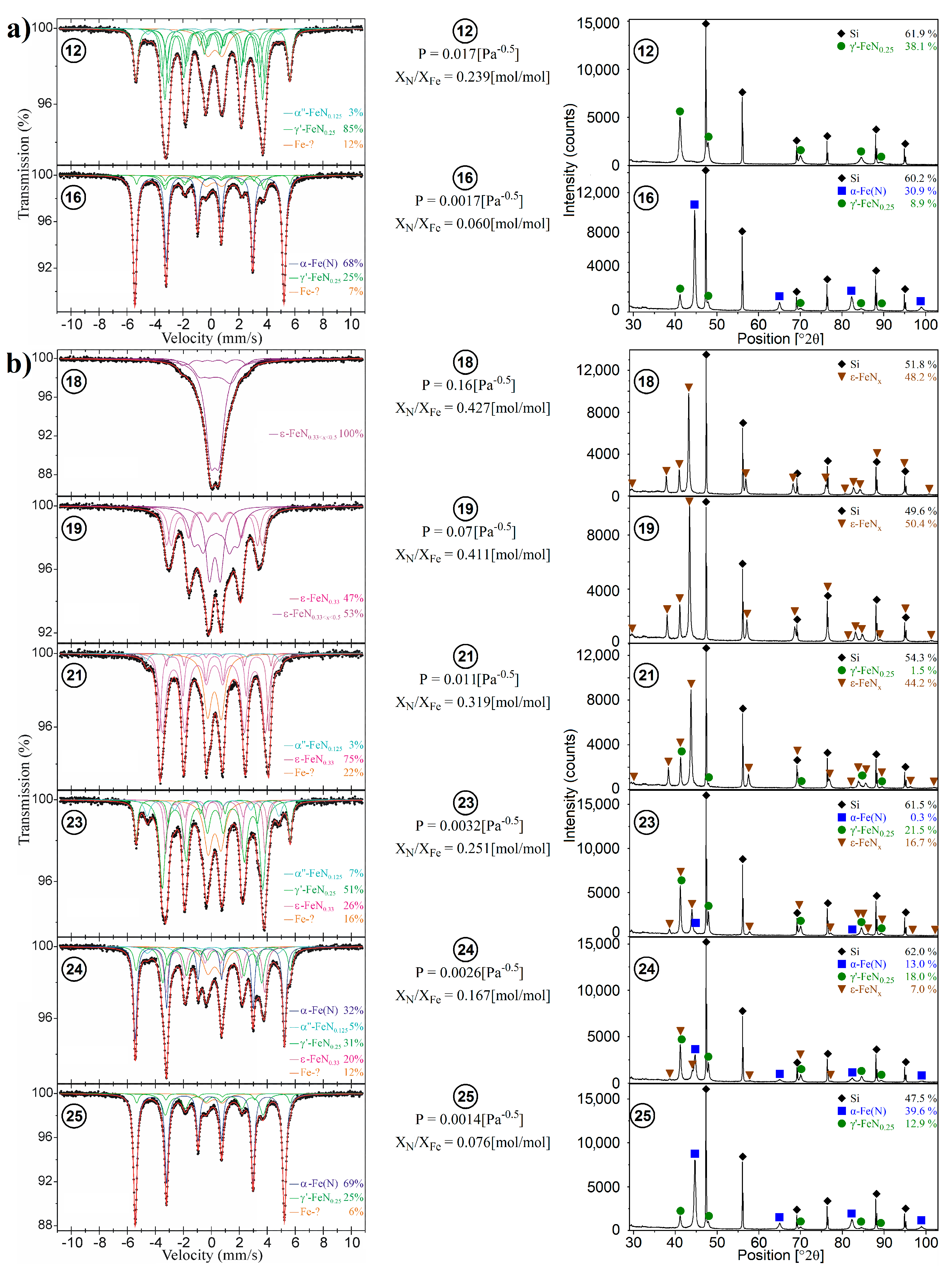
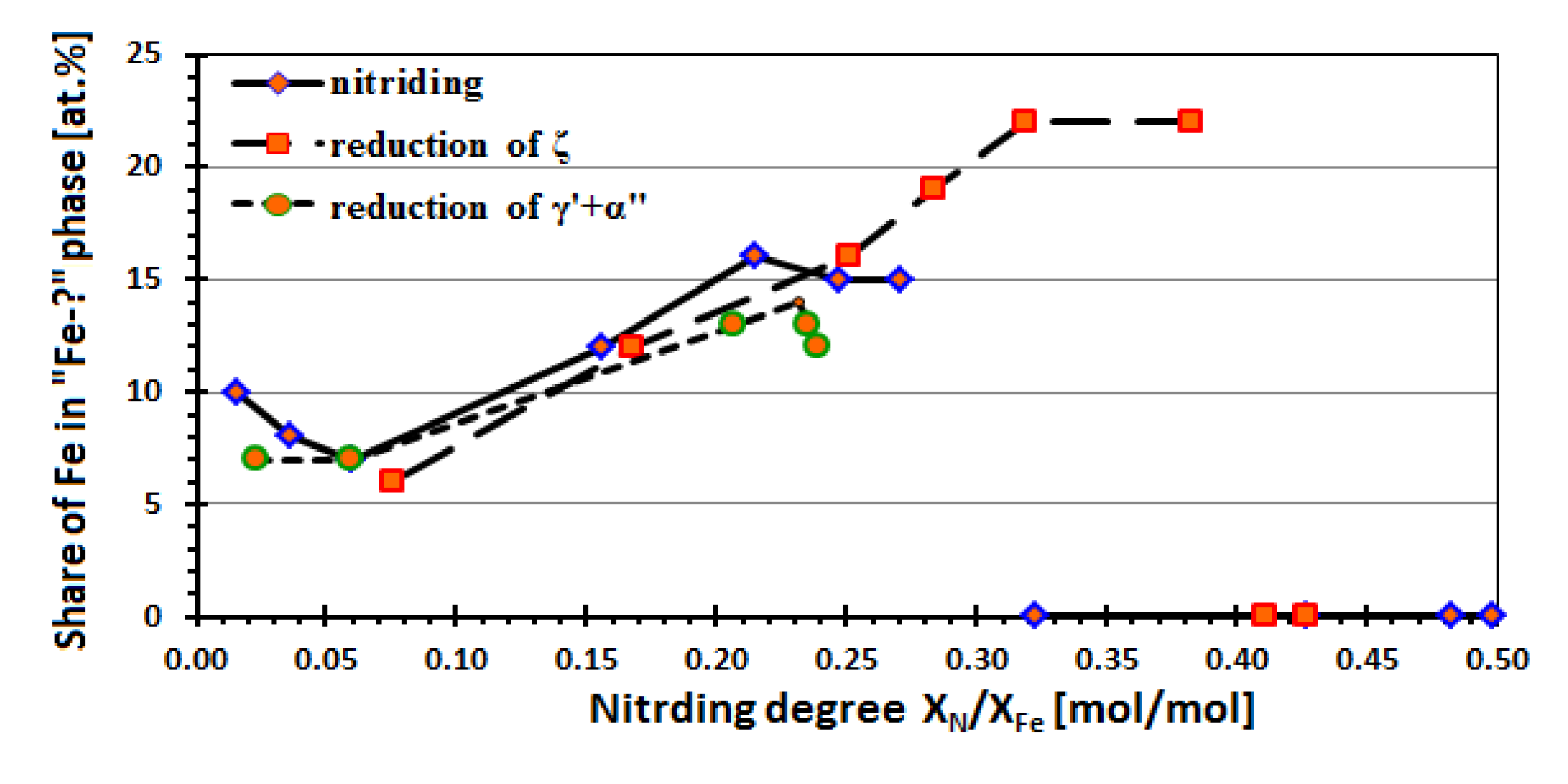
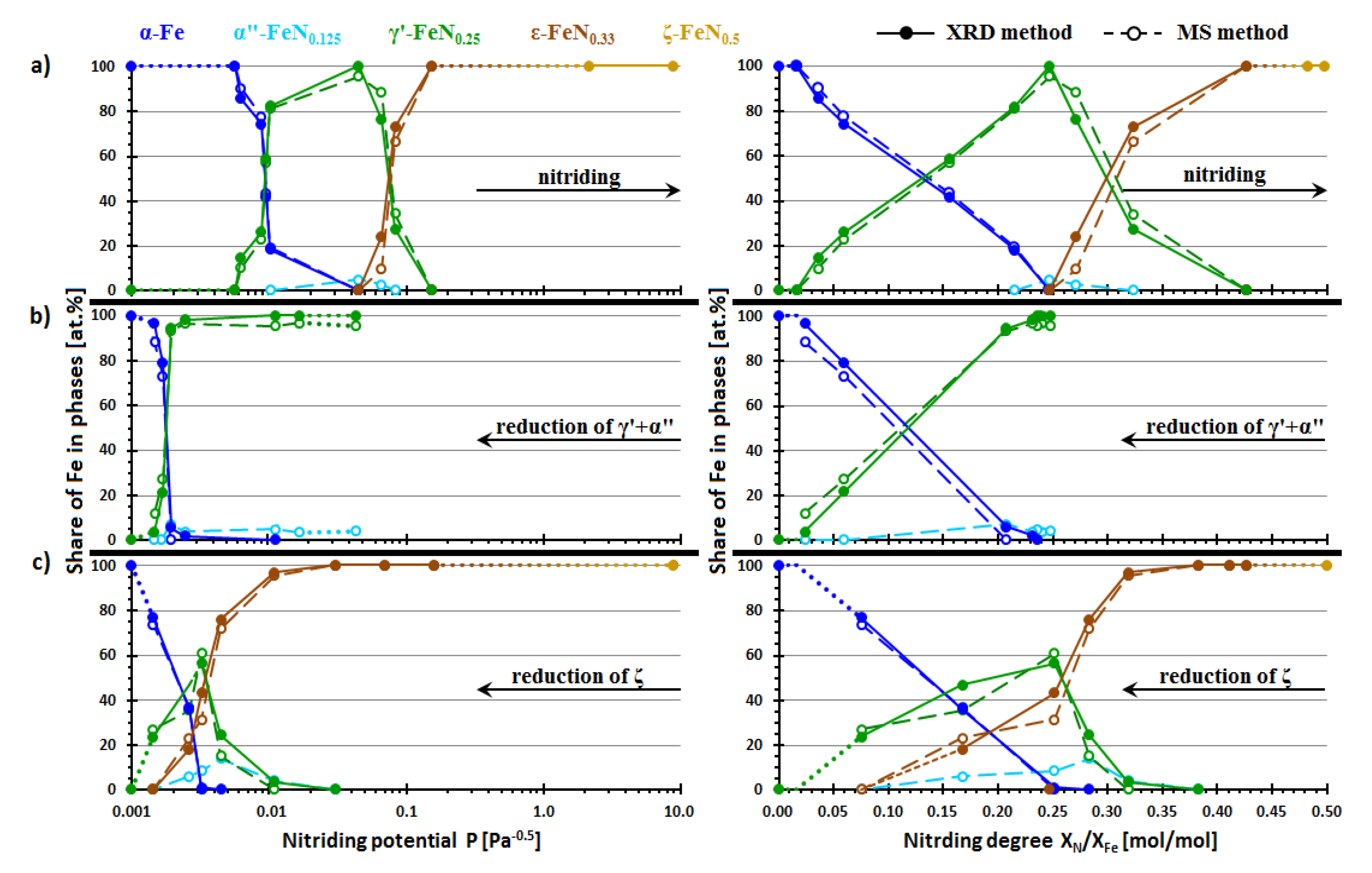

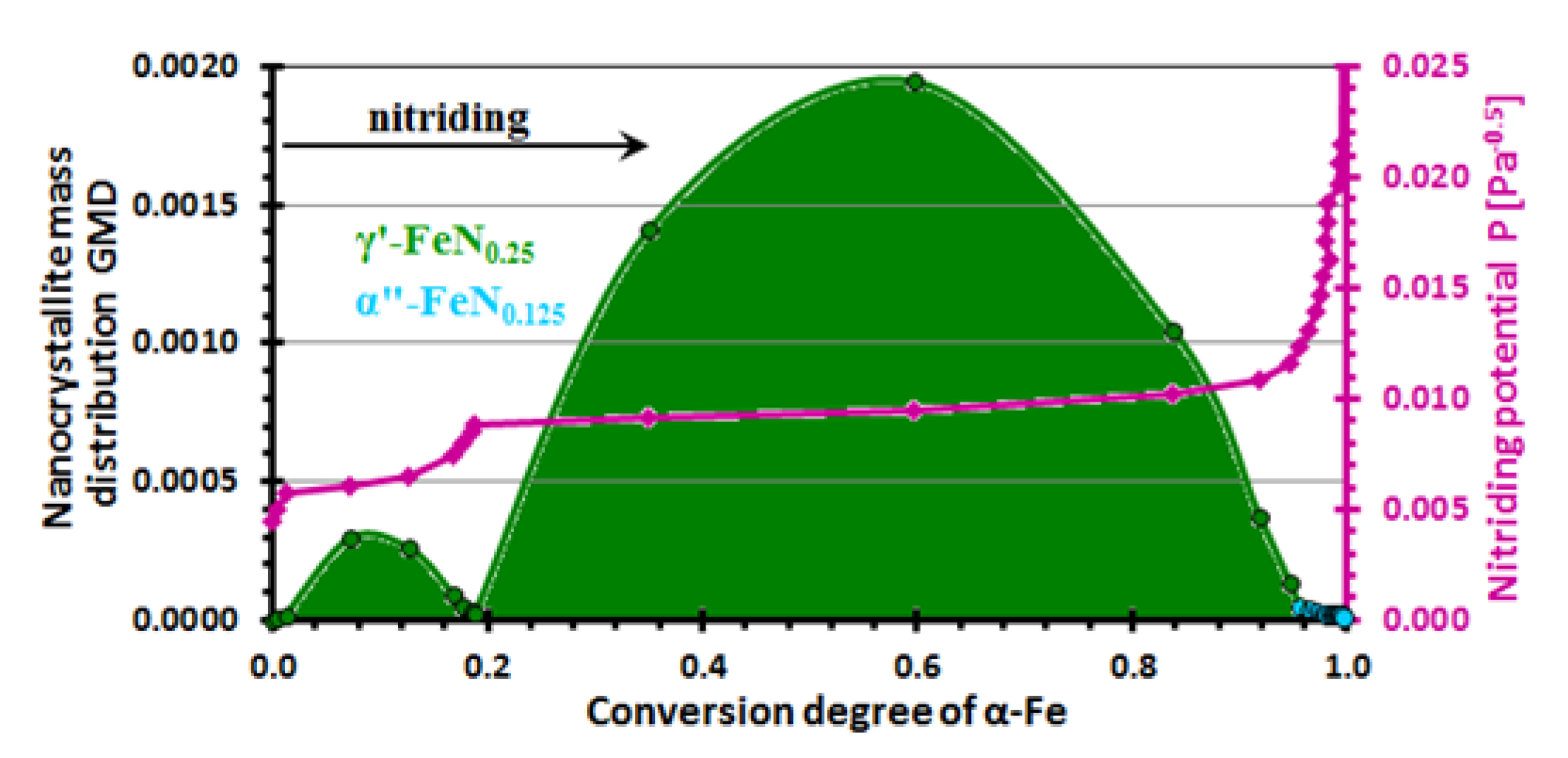
| Phase | A (%) | C (%) | IS (mm/s) | QS (mm/s) | B (T) | Γ (mm/s) | Sample |
|---|---|---|---|---|---|---|---|
| α-Fe | 90 | 90 | 0.01 | 0.00 | 32.98 | 0.18 | 1 |
| Fe-? | 10 | 10 | 0.28 | 1.25 | - | 1.5 | |
| α”-FeN0.125 | 7 | 7 | 0.22 | 0.02 | 29.19 | 0.37 | 23 |
| γ’-FeN0.25 | 51 | 11 | 0.23 | 0.02 | 34.12 | 0.19 | |
| 40 | 0.30 | -0.20 | 22.25 | 0.36 | |||
| ε-FeN0.33 | 26 | 9 | 0.44 | 0.27 | 22.30 | 0.22 | |
| 17 | 0.30 | 0.20 | 22.02 | 0.18 | |||
| Fe-? | 16 | 16 | 0.34 | 0.92 | - | 0.58 | |
| γ’-FeN0.25 | 85 | 19 | 0.21 | 0.07 | 34.11 | 0.27 | 12 |
| 18 | 0.28 | -0.37 | 21.62 | 0.26 | |||
| 21 | 0.42 | 0.20 | 21.40 | 0.27 | |||
| 26 | 0.25 | 0.11 | 21.65 | 0.25 | |||
| α”-FeN0.125 | 3 | 3 | 0.24 | 0.06 | 29.0 | 0.42 | |
| Fe-? | 12 | 12 | 0.40 | 1.05 | - | 0.9 | |
| ε-FeN0.33 | 75 | 22 | 0.33 | -0.03 | 24.19 | 0.19 | 21 |
| 50 | 0.32 | -0.04 | 22.79 | 0.43 | |||
| 3 | 0.49 | 0.32 | 23.13 | 0.10 | |||
| α”-FeN0.125 | 3 | 3 | 0.08 | 0.30 | 29.56 | 0.28 | |
| Fe-? | 22 | 22 | 0.33 | 0.97 | - | 0.56 | |
| ε-FeN0.33<x<0.5 | 100 | 18 | 0.30 | 0.08 | 15.00 | 0.90 | 9 |
| 51 | 0.37 | 0.69 | - | 0.67 | |||
| 31 | 0.49 | -0.05 | 7.01 | 0.88 | |||
| ζ-FeN0.5 | 100 | 92 | 0.42 | 0.25 | - | 0.27 | 11 |
| 8 | 0.34 | 0.66 | - | 0.22 |
| Sample No. (Figure 1) | P [Pa−0.5] | XN/XFe [mol/mol] | The Relative Content of Iron for Given Phases Found in Studied Samples [at. %] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XRD Results | 57Fe-MS Results | ||||||||||||
| α-Fe(N) | γ’-FeN0.25 | ε-FeNx | ζ-FeN0.5 | α-Fe(N) | α”-FeN0.125 | γ’-FeN0.25 | ε-FeN0.33 | ε-FeN0.33<x<0.5 | ζ-FeN0.5 | Fe-? | |||
| The nitriding process of nanocrystalline iron | |||||||||||||
| 1. | 0.0056 | 0.016 | 100.0 | - | - | - | 90 | - | - | - | - | - | 10 |
| 2. | 0.0062 | 0.036 | 85.4 | 14.6 | - | - | 83 | - | 9 | - | - | - | 8 |
| 3. | 0.0088 | 0.060 | 74.1 | 25.9 | - | - | 72 | - | 21 | - | - | - | 7 |
| 4. | 0.0095 | 0.155 | 41.6 | 58.4 | - | - | 38 | - | 50 | - | - | - | 12 |
| 5. | 0.010 | 0.215 | 18.1 | 81.9 | - | - | 16 | - | 68 | - | - | - | 16 |
| 6. | 0.045 | 0.247 | - | 100.0 | - | - | - | 4 | 81 | - | - | - | 15 |
| 7. | 0.066 | 0.271 | - | 76.1 | 23.9 | - | - | 2 | 75 | - | 8 | - | 15 |
| 8. | 0.083 | 0.323 | - | 27.4 | 72.6 | - | - | - | 34 | 24 | 42 | - | - |
| 9. | 0.15 | 0.427 | - | - | 100.0 | - | - | - | - | - | 100 | - | - |
| 10. | 2.14 | 0.482 | - | - | - | 100.0 | - | - | - | - | - | 100 | - |
| 11. | pure NH3 | 0.498 | - | - | - | 100.0 | - | - | - | - | - | 100 | - |
| The reduction process of a mixture of nanocrystalline iron nitrides α” and γ’ | |||||||||||||
| 12. | 0.017 | 0.239 | - | 100.0 | - | - | - | 3 | 85 | - | - | - | 12 |
| 13. | 0.011 | 0.235 | - | 100.0 | - | - | - | 4 | 83 | - | - | - | 13 |
| 14. | 0.0025 | 0.231 | 1.8 | 98.2 | - | - | - | 3 | 83 | - | - | - | 14 |
| 15. | 0.0019 | 0.207 | 5.7 | 94.3 | - | - | - | 6 | 81 | - | - | - | 13 |
| 16. | 0.0017 | 0.060 | 78.7 | 21.3 | - | - | 68 | - | 25 | - | - | - | 7 |
| 17. | 0.0015 | 0.024 | 96.5 | 3.5 | - | - | 82 | - | 11 | - | - | - | 7 |
| The reduction process of nanocrystalline iron nitride ζ | |||||||||||||
| 18. | 0.16 | 0.427 | - | - | 100.0 | - | - | - | - | - | 100 | - | - |
| 19. | 0.07 | 0.411 | - | - | 100.0 | - | - | - | - | 47 | 53 | - | - |
| 20. | 0.03 | 0.383 | - | - | 100.0 | - | - | - | - | - | 78 | - | 22 |
| 21. | 0.011 | 0.319 | - | 3.3 | 96.7 | - | - | 3 | - | 75 | - | - | 22 |
| 22. | 0.0044 | 0.283 | - | 24.2 | 75.8 | - | - | 11 | 12 | 58 | - | - | 19 |
| 23. | 0.0032 | 0.251 | 0.8 | 56.3 | 42.9 | - | - | 7 | 51 | 26 | - | - | 16 |
| 24. | 0.0026 | 0.167 | 35.7 | 46.5 | 17.8 | - | 32 | 5 | 31 | 20 | - | - | 12 |
| 25. | 0.0014 | 0.076 | 76.5 | 23.5 | - | - | 69 | - | 25 | - | - | - | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilk, B.; Błachowski, A.; Lendzion-Bieluń, Z.; Arabczyk, W. Thermodynamics of Chemical Processes in the System of Nanocrystalline Iron–Ammonia–Hydrogen at 350 °C. Catalysts 2020, 10, 1242. https://doi.org/10.3390/catal10111242
Wilk B, Błachowski A, Lendzion-Bieluń Z, Arabczyk W. Thermodynamics of Chemical Processes in the System of Nanocrystalline Iron–Ammonia–Hydrogen at 350 °C. Catalysts. 2020; 10(11):1242. https://doi.org/10.3390/catal10111242
Chicago/Turabian StyleWilk, Bartłomiej, Artur Błachowski, Zofia Lendzion-Bieluń, and Walerian Arabczyk. 2020. "Thermodynamics of Chemical Processes in the System of Nanocrystalline Iron–Ammonia–Hydrogen at 350 °C" Catalysts 10, no. 11: 1242. https://doi.org/10.3390/catal10111242
APA StyleWilk, B., Błachowski, A., Lendzion-Bieluń, Z., & Arabczyk, W. (2020). Thermodynamics of Chemical Processes in the System of Nanocrystalline Iron–Ammonia–Hydrogen at 350 °C. Catalysts, 10(11), 1242. https://doi.org/10.3390/catal10111242






