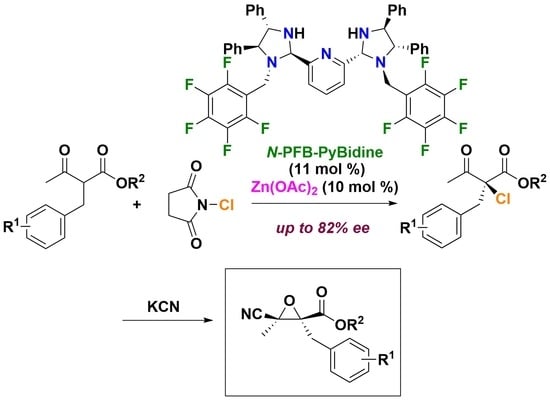
Catalytic Asymmetric Chlorination of β-Ketoesters Using N-PFB-PyBidine-Zn(OAc)2
Abstract
:1. Introduction
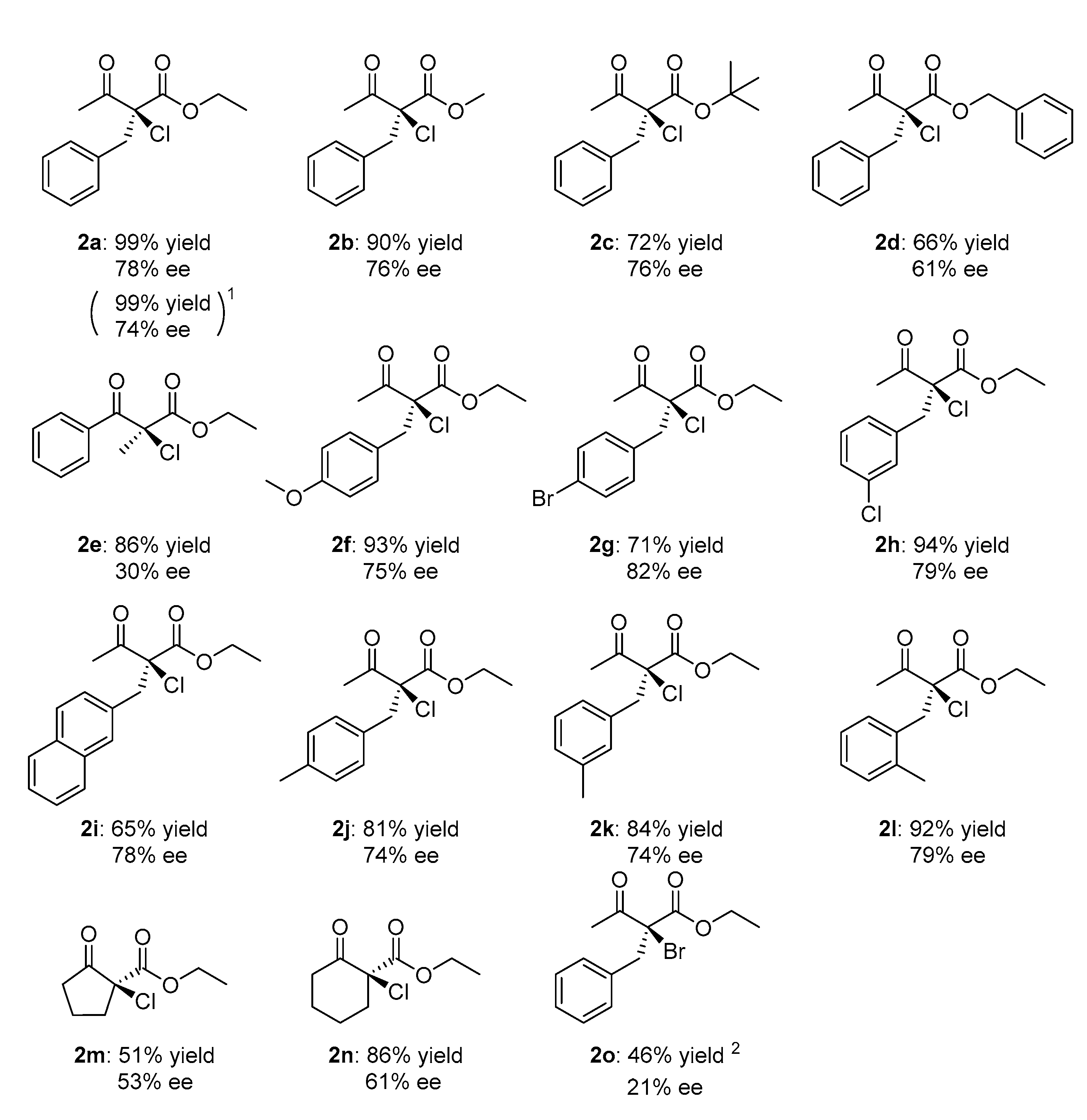
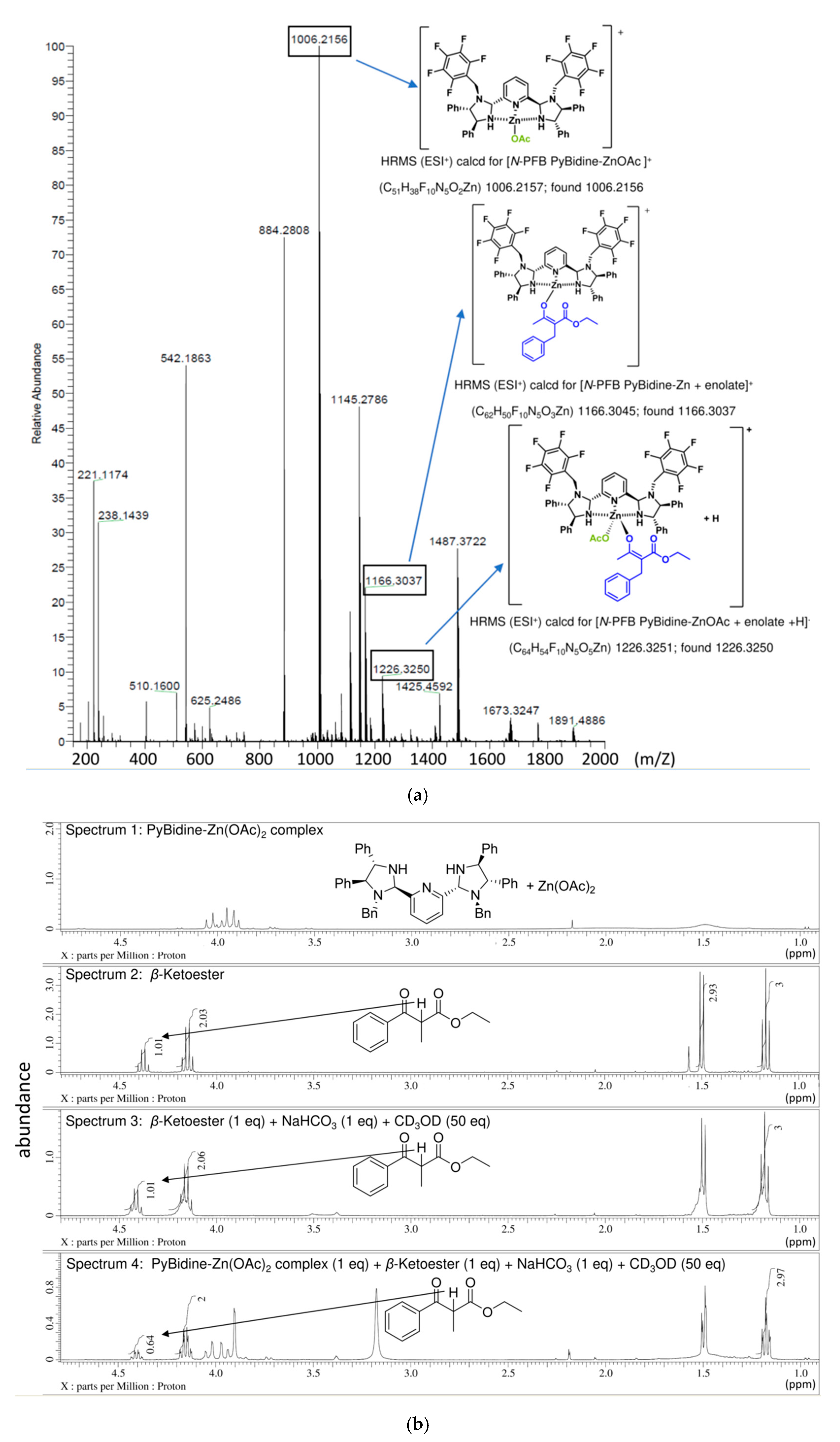
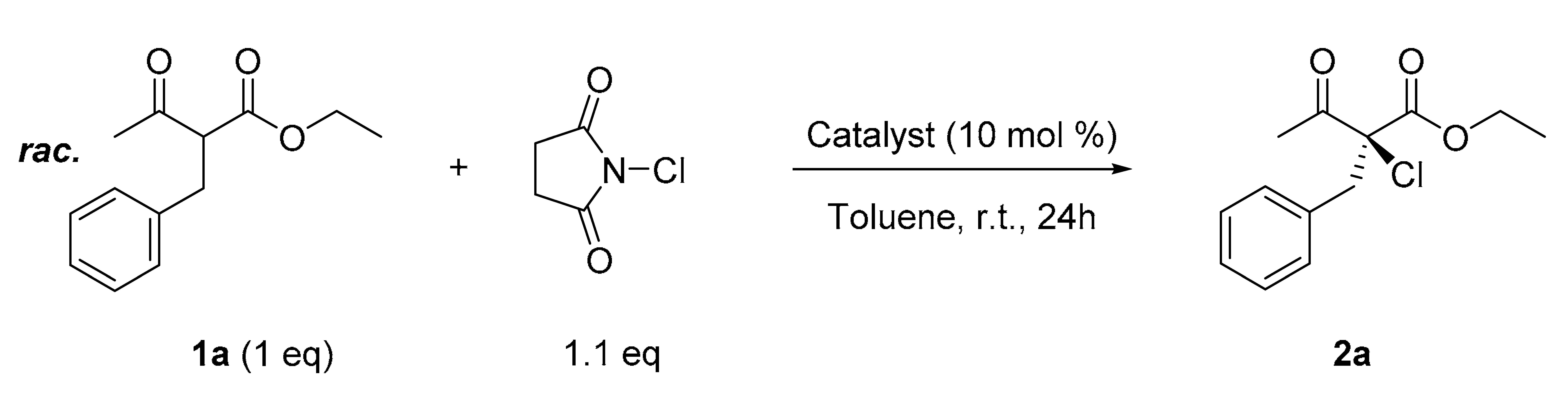
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Petrone, D.A.; Ye, J.; Lautens, M. Modern Transition-Metal-Catalyzed Carbon-Halogen Bond Formation. Chem. Rev. 2016, 116, 8003–8104. [Google Scholar] [CrossRef]
- France, S.; Weatherwax, A.; Lectka, T. Recent developments in catalytic, asymmetric α-halogenation: A new frontier in asymmetric catalysis. Eur. J. Org. Chem. 2005, 475–479. [Google Scholar] [CrossRef]
- Hintermann, L.; Togni, A. Catalytic enantioselective chlorination and bromination of β-keto esters. Helv. Chim. Acta 2000, 83, 2425–2435. [Google Scholar] [CrossRef]
- Ibrahim, H.; Kleinbeck, F.; Togni, A. Catalytic asymmetric chlorination of β-keto esters with hypervalent iodine compounds. Helv. Chim. Acta 2004, 87, 605–610. [Google Scholar] [CrossRef]
- Marigo, M.; Kumaragurubaran, N.; Jørgensen, K.A. Catalytic asymmetric bromination and chlorination of β-keto esters. Chem. Eur. J. 2004, 10, 2133–2137. [Google Scholar] [CrossRef]
- Frings, M.; Bolm, C. Enantioselective halogenation of β-oxo esters catalyzed by a chiral sulfoximine-copper complex. Eur. J. Org. Chem. 2009, 4085–4090. [Google Scholar] [CrossRef]
- Qi, M.-H.; Wang, F.-J.; Shi, M. Synthesis of novel chiral oxazoline-Schiff base ligands for the catalytic asymmetric chlorination of β-keto esters. Tetrahedron Asymmetry 2010, 21, 247–253. [Google Scholar] [CrossRef]
- Jiang, J.-J.; Huang, J.; Wang, D.; Yuan, Z.-L.; Zhao, M.-X.; Wang, F.-J.; Shi, M. Cu(I)-catalyzed asymmetric chlorination of β-keto esters in the presence of chiral phosphine-Schiff base type ligands. Chirality 2011, 23, 272–276. [Google Scholar] [CrossRef]
- Shibatomi, K.; Soga, Y.; Narayama, A.; Fujisawa, I.; Iwasa, S. Highly Enantioselective Chlorination of β-Keto Esters and Subsequent SN2 Displacement of Tertiary Chlorides: A Flexible Method for the Construction of Quaternary Stereogenic Centers. J. Am. Chem. Soc. 2012, 134, 9836–9839. [Google Scholar] [CrossRef]
- Li, J.; Pan, W.; Wang, Z.; Zhang, X.; Ding, K. Access to Both Enantiomers of α-Chloro-β-keto Esters with a Single Chiral Ligand: Highly Efficient Enantioselective Chlorination of Cyclic β-Keto Esters Catalyzed by Chiral Copper(II) and Zinc(II) Complexes of a Spiro-2,2’-bischroman-Based Bisoxazoline Ligand. Adv. Synth. Cat. 2012, 354, 1980–1986. [Google Scholar]
- Jia, S.-J.; Du, D.-M. Enantioselective chlorination of β-keto esters and amides catalyzed by chiral copper(II) complexes of squaramide-linked bisoxazoline ligand. Chin. Chem. Lett. 2014, 25, 1479–1484. [Google Scholar] [CrossRef]
- Shibatomi, K.; Kotozaki, M.; Sasaki, N.; Fujisawa, I.; Iwasa, S. Williamson Ether Synthesis with Phenols at a Tertiary Stereogenic Carbon: Formal Enantioselective Phenoxylation of β-Keto Esters. Chem. Eur. J. 2015, 21, 14095–14098. [Google Scholar] [CrossRef]
- Naganawa, Y.; Aoyama, T.; Kato, K.; Nishiyama, H. Cu(II)-catalyzed Enantioselective α-Hydroxylation and α-Chlorination of β-Ketoesters with N,N,O-Tridentate Chiral Phenanthroline Ligand. ChemistrySelect 2016, 1, 1938–1942. [Google Scholar] [CrossRef]
- Shibata, N.; Kohno, J.; Takai, K.; Ishimaru, T.; Nakamura, S.; Toru, T.; Kanemasa, S. Highly enantioselective catalytic fluorination and chlorination reactions of carbonyl compounds capable of two-point binding. Angew. Chem., Int. Ed. 2005, 44, 4204–4207. [Google Scholar] [CrossRef]
- Kawatsura, M.; Hayashi, S.; Komatsu, Y.; Hayase, S.; Itoh, T. Enantioselective α-fluorination and chlorination of β-keto esters by cobalt catalyst. Chem. Lett. 2010, 39, 466–467. [Google Scholar] [CrossRef]
- Kang, Y.K.; Kim, H.H.; Koh, K.O.; Kim, D.Y. Catalytic enantioselective chlorination of cyclic β-keto esters in the presence of chiral Pd(II) complexes. Tetrahedron Lett. 2012, 53, 3811–3814. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Ping, Y.-J.; Li, Z.-R.; Gu, X.; Xu, Z.-J.; Che, C.-M. Asymmetric Chlorination of Cyclic β-Keto Esters and N -Boc Oxindoles Catalyzed by an Iron(III)-BPsalan Complex. Synthesis 2018, 50, 1105–1112. [Google Scholar]
- Bartoli, G.; Bosco, M.; Carlone, A.; Locatelli, M.; Melchiorre, P.; Sambri, L. Organocatalytic Asymmetric α-Halogenation of 1,3-Dicarbonyl Compounds. Angew. Chem. Int. Ed. 2005, 44, 6219–6222. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, W.; Shen, K.; Wang, J.; Hu, X.; Lin, L.; Liu, X.; Feng, X. Highly enantioselective α-chlorination of cyclic β-ketoesters catalyzed by N,N’-Dioxide using NCS as the chlorine source. Chem. Commun. 2010, 46, 1250–1252. [Google Scholar] [CrossRef]
- Etayo, P.; Badorrey, R.; Diaz-de-Villegas, M.D.; Galvez, J.A. Chiral Amino Diol Derivatives as New Modular Organocatalysts for the Enantioselective α-Chlorination of Cyclic β-Keto Esters. Adv. Synth. Catal. 2010, 352, 3329–3338. [Google Scholar] [CrossRef]
- Luo, J.; Wu, W.; Xu, L.-W.; Meng, Y.; Lu, Y. Enantioselective direct fluorination and chlorination of cyclic β-ketoesters mediated by phase-transfer catalysts. Tetrahedron Lett. 2013, 54, 2623–2626. [Google Scholar] [CrossRef]
- Shirakawa, S.; Tokuda, T.; Kasai, A.; Maruoka, K. Design of Chiral Bifunctional Quaternary Phosphonium Bromide Catalysts Possessing an Amide Moiety. Org. Lett. 2013, 15, 3350–3353. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, R.; Liu, S.; Shi, L.; Li, X. Chiral 2-Amino Alcohol Derivatives Catalyze the Enantioselective α-Chlorination of β-Ketoesters. Bull. Korean Chem. Soc. 2014, 35, 1759–1762. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yang, F.; Meng, Q.; Gao, Z. Enantioselective α-chlorination of β-oxo esters catalyzed by chiral diterpenoid alkaloid derivatives. Tetrahedron Asymmetry 2014, 25, 1215–1220. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, S.-G.; Liang, X.-W.; Gao, D.-W.; Zheng, J.; You, S.-L. Organocatalytic asymmetric chlorinative dearomatization of naphthols. Chem. Sci. 2015, 6, 4179–4183. [Google Scholar] [CrossRef] [Green Version]
- Novacek, J.; Monkowius, U.; Himmelsbach, M.; Waser, M. Asymmetric α-chlorination of β-ketoesters using bifunctional ammonium salt catalysis. Monat. Chem. 2016, 147, 533–538. [Google Scholar] [CrossRef]
- Sakai, T.; Hirashima, S.; Nakashima, K.; Maeda, C.; Yoshida, A.; Koseki, Y.; Miura, T. Asymmetric chlorination of β-keto esters using diaminomethylenemalononitrile organocatalyst. Chem. Pharm. Bull. 2016, 64, 1781–1784. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Wu, D.; Karmaker, P.G.; Yin, H.; Chen, F.-X. Enantioselective Organocatalyzed Direct α-Thiocyanation of Cyclic β-Ketoesters by N-Thiocyanatophthalimide. Org. Lett. 2018, 20, 1600–1603. [Google Scholar] [CrossRef]
- Guan, X.; An, D.; Liu, G.; Zhang, H.; Gao, J.; Zhou, T.; Zhang, G.; Zhang, S. Enantioselective α-chlorination of β-keto esters and amides catalyzed by chiral imidodiphosphoric acids. Tetrahedron Lett. 2018, 59, 2418–2421. [Google Scholar] [CrossRef]
- Arai, T.; Kajikawa, S.; Matsumura, E. The Role of Ni-carboxylate During Catalytic Asymmetric Iodolactonization Using PyBidine-Ni(OAc)2. Synlett 2013, 24, 2045–2048. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Sugiyama, N.; Masu, H.; Kado, S.; Yabe, S.; Yamanaka, M. Trinuclear Zn3(OAc)4-3,3′-bis(aminoimino)binaphthoxide Complex for Highly Efficient Catalytic Asymmetric Iodolactonization. Chem. Commun. 2014, 42, 8287–8290. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Horigane, K.; Watanabe, O.; Kakino, J.; Sugiyama, N.; Makino, H.; Kamei, Y.; Yabe, S.; Yamanaka, M. Association of Halogen Bonding and Hydrogen Bonding in Metal Acetate-catalyzed Asymmetric Halolactonization. iScience 2019, 12, 280–292. [Google Scholar] [CrossRef]
- Arai, T.; Horigane, K.; Suzuki, T.K.; Itoh, R.; Yamanaka, M. Catalytic Asymmetric Iodoesterification of Simple Alkenes. Angew. Chem. Int. Ed. 2020, 59, 12680–12683. [Google Scholar] [CrossRef]
- Arai, T.; Mishiro, A.; Yokoyama, N.; Suzuki, K.; Sato, H. Chiral Bis(imidazolidine)pyridine-Cu(OTf)2: Catalytic Asymmetric Endo-Selective [3+2] Cycloaddition of Imino Esters with Nitroalkenes. J. Am. Chem. Soc. 2010, 132, 5338–5339. [Google Scholar] [CrossRef]
- Arai, T.; Ogawa, H.; Awata, A.; Sato, M.; Watabe, M.; Yamanaka, M. PyBidine-Cu(OTf)2-catalyzed asymmetric [3+2] cycloaddition using imino esters: Harmony of Cu-Lewis acid and imidazolidine-NH hydrogen-bonding in concerto catalysis. Angew. Chem. Int. Ed. 2015, 54, 1595–1599. [Google Scholar] [CrossRef]
- Arai, T.; Iimori, Y.; Shirasugi, M.; Shinohara, R.; Takagi, Y.; Suzuki, T.; Ma, J.; Kuwano, S.; Masu, H. Bis(imidazolidine)pyridine-CoCl2: A Novel, Catalytically Active Neutral Complex for Asymmetric Michael Reaction of 1,3-Carbonyl Compounds with Nitroalkenes. Adv. Synth. Catal. 2019, 361, 3704–3711. [Google Scholar] [CrossRef]
- Bhor, S.; Anilkumar, G.; Tse, M.K.; Klawonn, M.; Döbler, C.; Bitterlich, B.; Grotevendt, A.; Beller, M. Synthesis of a New Chiral N,N,N-Tridentate Pyridinebisimidazoline Ligand Library and Its Application in Ru-Catalyzed Asymmetric Epoxidation. Org. Lett. 2005, 7, 3393–3396. [Google Scholar] [CrossRef]




| Entry | Ligand | Metal Salt | Yield (%) | ee (%) |
|---|---|---|---|---|
| 1 | L1 | FeCl2 | 9 | 44 |
| 2 | L1 | Co(OAc)21 | 54 | 42 |
| 3 | L1 | CoCl2 | 34 | 27 |
| 4 | L1 | Ni(OAc)21 | 64 | 28 |
| 5 | L1 | Cu(OAc)2 | 57 | 10 |
| 6 | L1 | Cu(OTf)2 | 23 | 22 |
| 7 | L1 | Zn(OAc)2 | 60 | 60 |
| 8 | L1 | Zn(OBz)2 | 85 | 56 |
| 9 | L1 | ZnCl2 | 13 | −21 |
| 10 | L1 | Zn(OTf)2 | 22 | 21 |
| 11 | L2 | Zn(OAc)2 | 41 | 21 |
| 12 | L3 | Zn(OAc)2 | 90 | −6 |
| 13 | L4 | Zn(OAc)2 | 98 | 13 |
| 14 | L5 | Zn(OAc)2 | 52 | 15 |
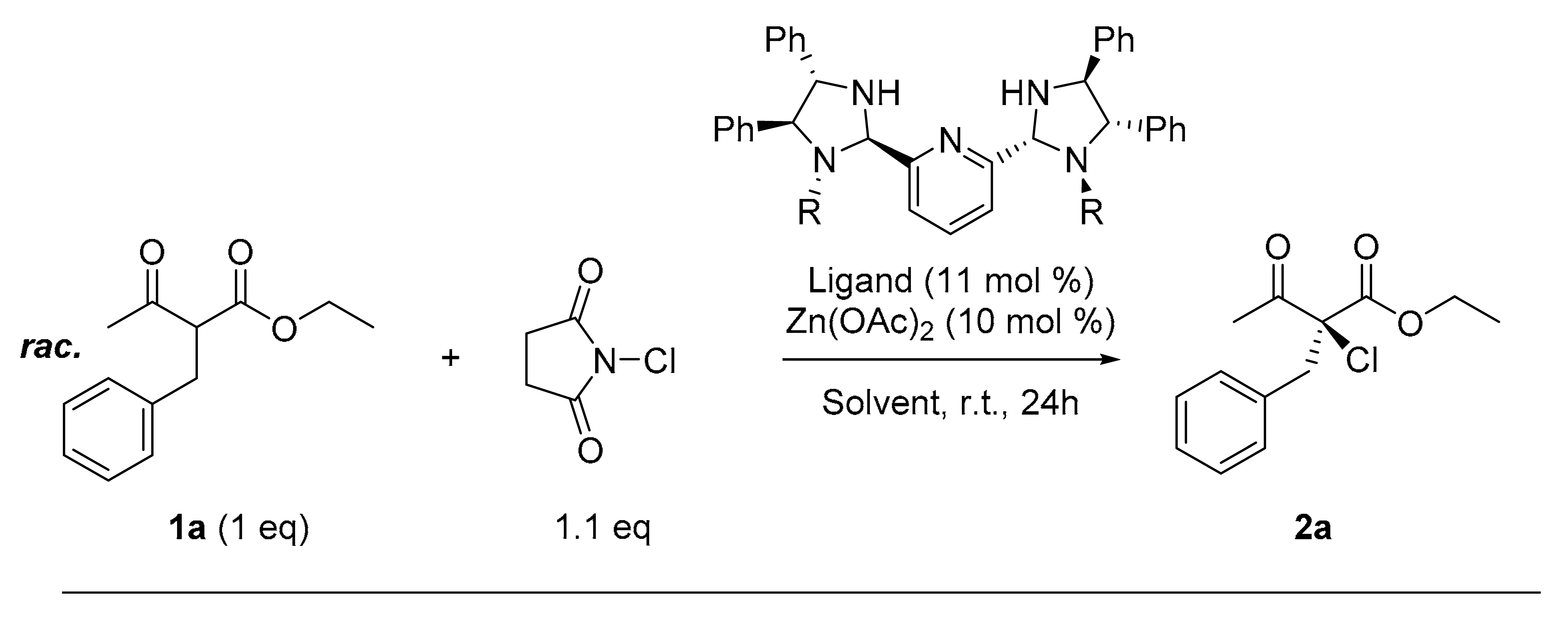
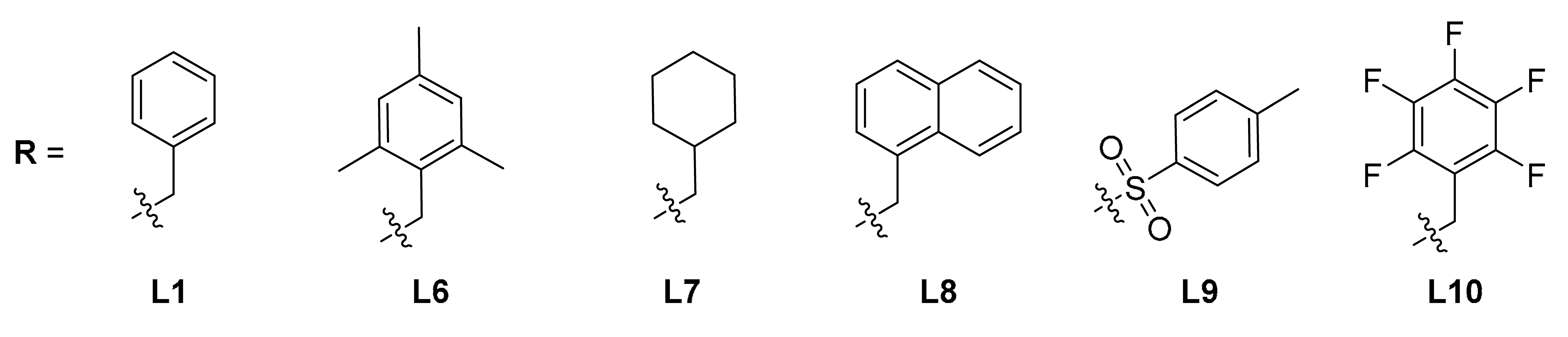
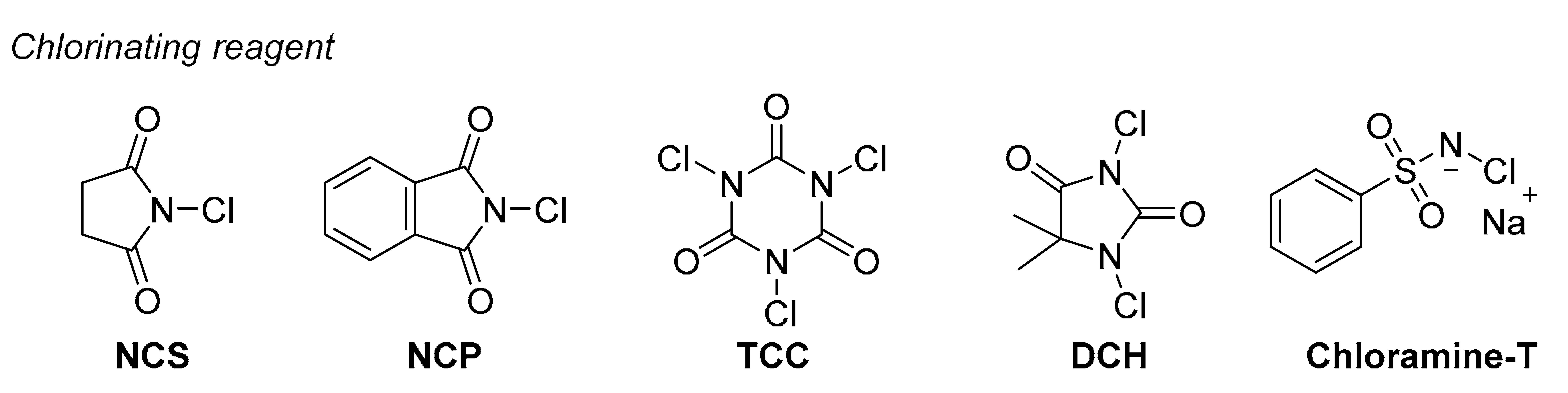
| Entry | Ligand | Solvent | Chlorinating Reagent | Additive 1 | Yield (%) | Ee (%) |
|---|---|---|---|---|---|---|
| 1 | L1 | toluene | NCS | - | 60 | 60 |
| 2 | L6 | toluene | NCS | - | 62 | 41 |
| 3 | L7 | toluene | NCS | - | 92 | 66 |
| 4 | L8 | toluene | NCS | - | 69 | 68 |
| 5 | L9 | toluene | NCS | - | 25 | 65 |
| 6 | L10 | toluene | NCS | - | 86 | 70 |
| 7 | L10 | EtOAc | NCS | - | 88 | 35 |
| 8 | L10 | Et2O | NCS | - | 67 | 57 |
| 9 | L10 | CH2Cl2 | NCS | - | 38 | 53 |
| 10 | L10 | CCl4 | NCS | - | 78 | 77 |
| 11 | L10 | cyclohexane | NCS | - | 85 | 79 |
| 12 | L10 | cyclohexane | NCP | - | 92 | 75 |
| 13 | L10 | cyclohexane | TCC | - | 42 | 26 |
| 14 | L10 | cyclohexane | DCH | - | 47 | 23 |
| 15 | L10 | cyclohexane | Chloramine-T | - | 95 | 9 |
| 16 | L10 | cyclohexane | NCS | K2CO3 | 96 | 59 |
| 17 | L10 | cyclohexane | NCS | TEA | 95 | 65 |
| 18 | L10 | cyclohexane | NCS | NaHCO3 | 99 | 78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Suzuki, T.; Kuwano, S.; Arai, T. Catalytic Asymmetric Chlorination of β-Ketoesters Using N-PFB-PyBidine-Zn(OAc)2. Catalysts 2020, 10, 1177. https://doi.org/10.3390/catal10101177
Ma J, Suzuki T, Kuwano S, Arai T. Catalytic Asymmetric Chlorination of β-Ketoesters Using N-PFB-PyBidine-Zn(OAc)2. Catalysts. 2020; 10(10):1177. https://doi.org/10.3390/catal10101177
Chicago/Turabian StyleMa, Junma, Takumi Suzuki, Satoru Kuwano, and Takayoshi Arai. 2020. "Catalytic Asymmetric Chlorination of β-Ketoesters Using N-PFB-PyBidine-Zn(OAc)2" Catalysts 10, no. 10: 1177. https://doi.org/10.3390/catal10101177
APA StyleMa, J., Suzuki, T., Kuwano, S., & Arai, T. (2020). Catalytic Asymmetric Chlorination of β-Ketoesters Using N-PFB-PyBidine-Zn(OAc)2. Catalysts, 10(10), 1177. https://doi.org/10.3390/catal10101177