Gold-Catalyzed Synthetic Strategies towards Four-Carbon Ring Systems
Abstract
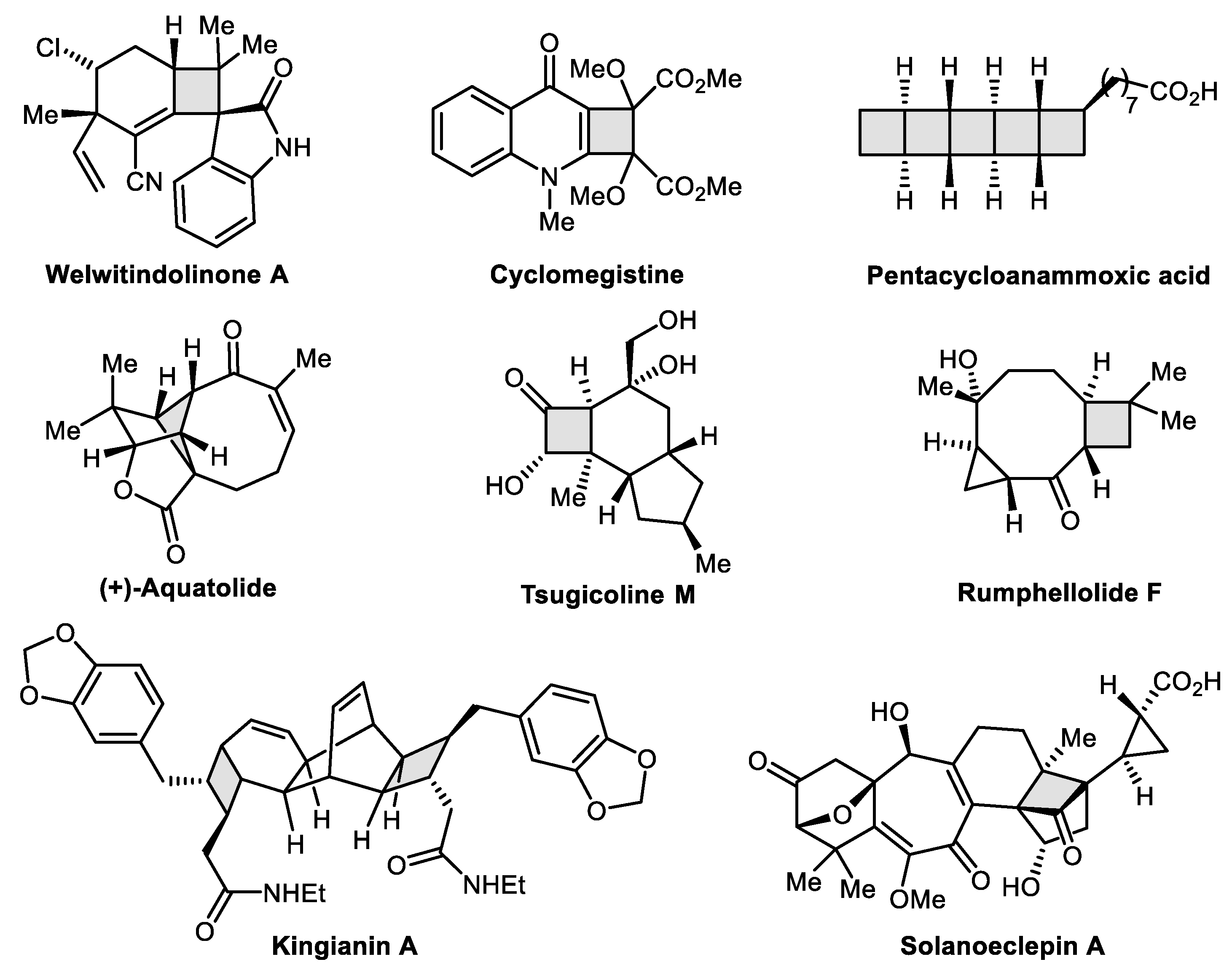
:1. Introduction
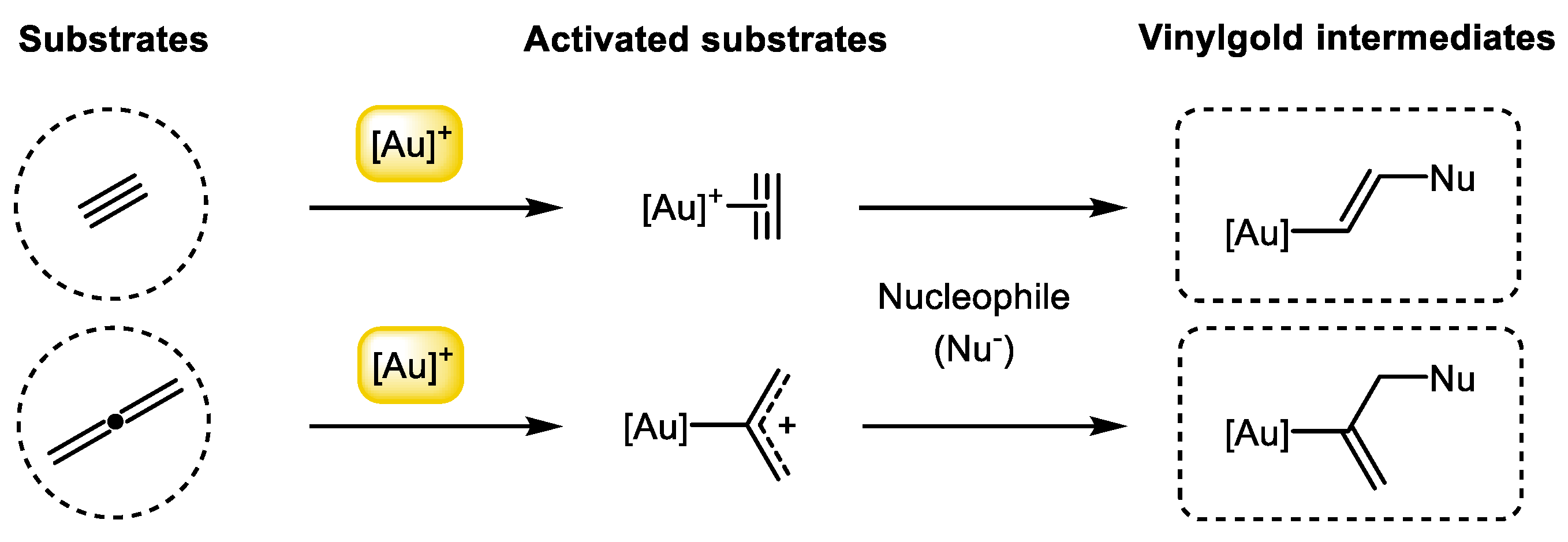
2. [2+2] Cycloadditions
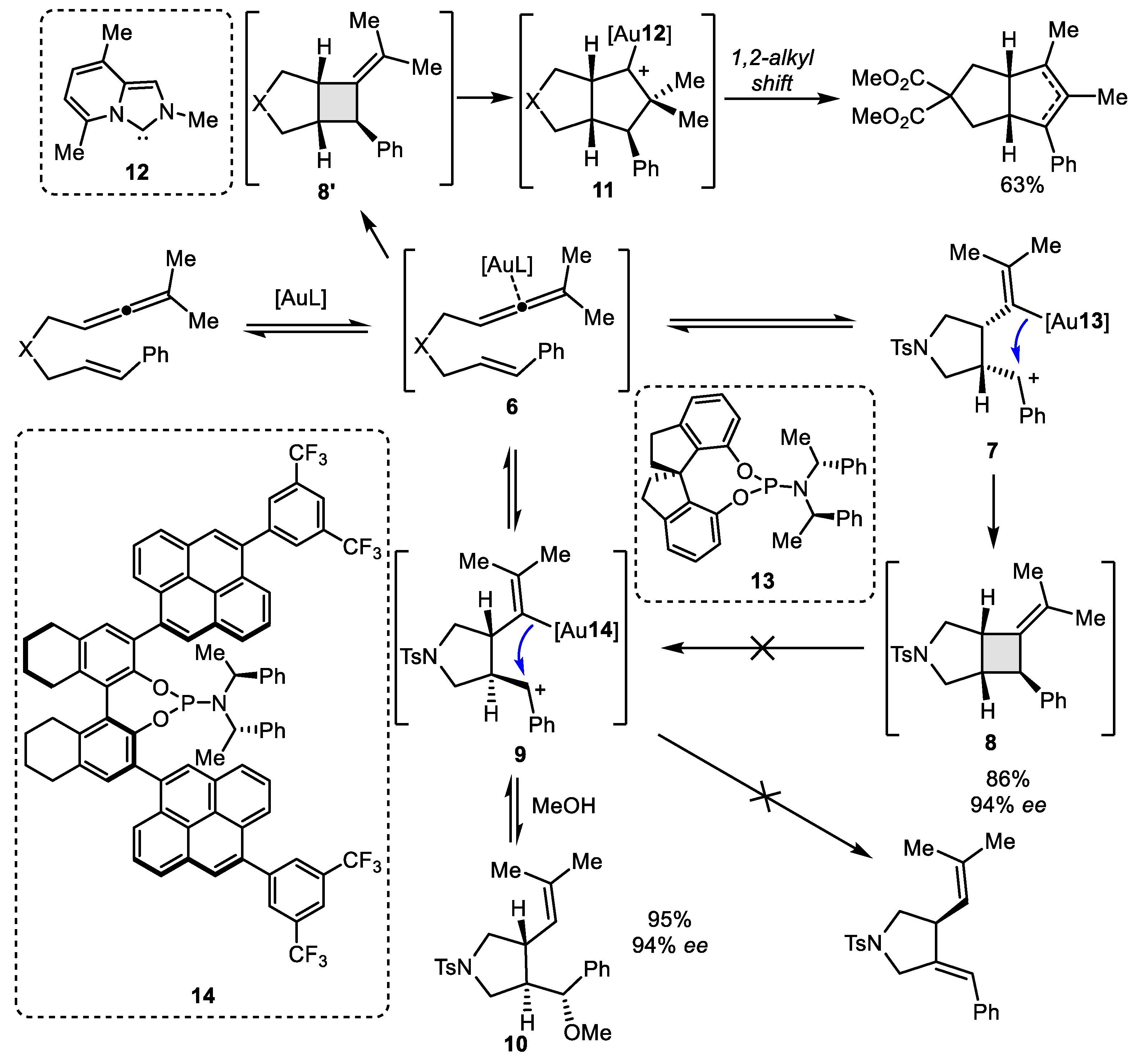
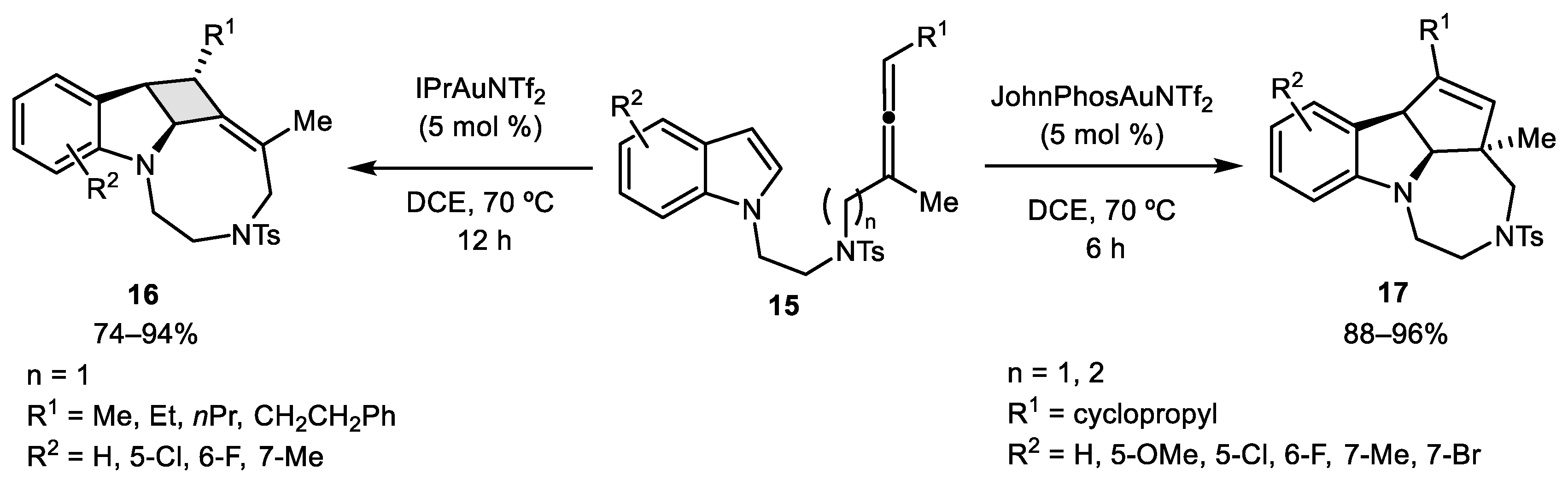
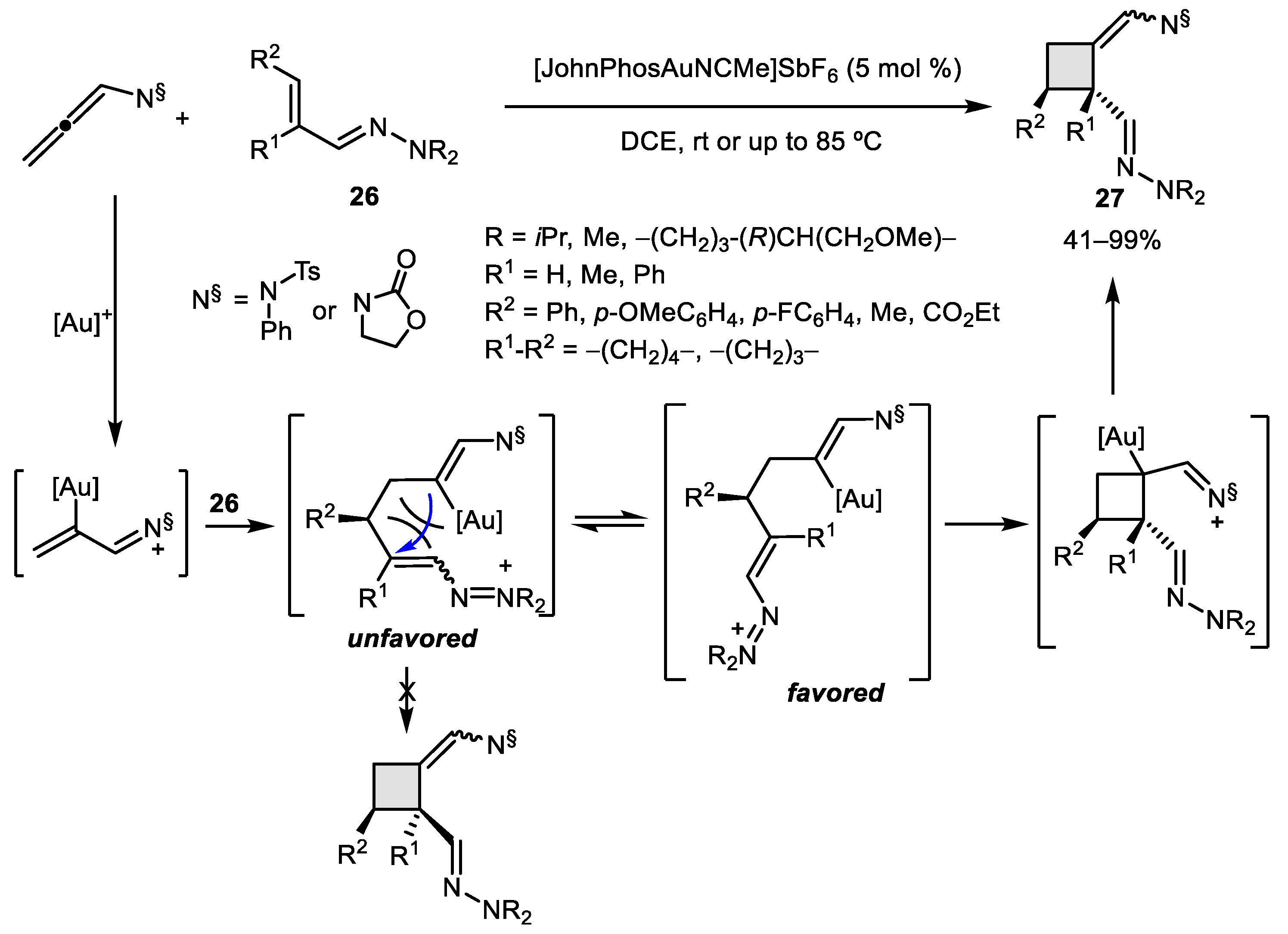
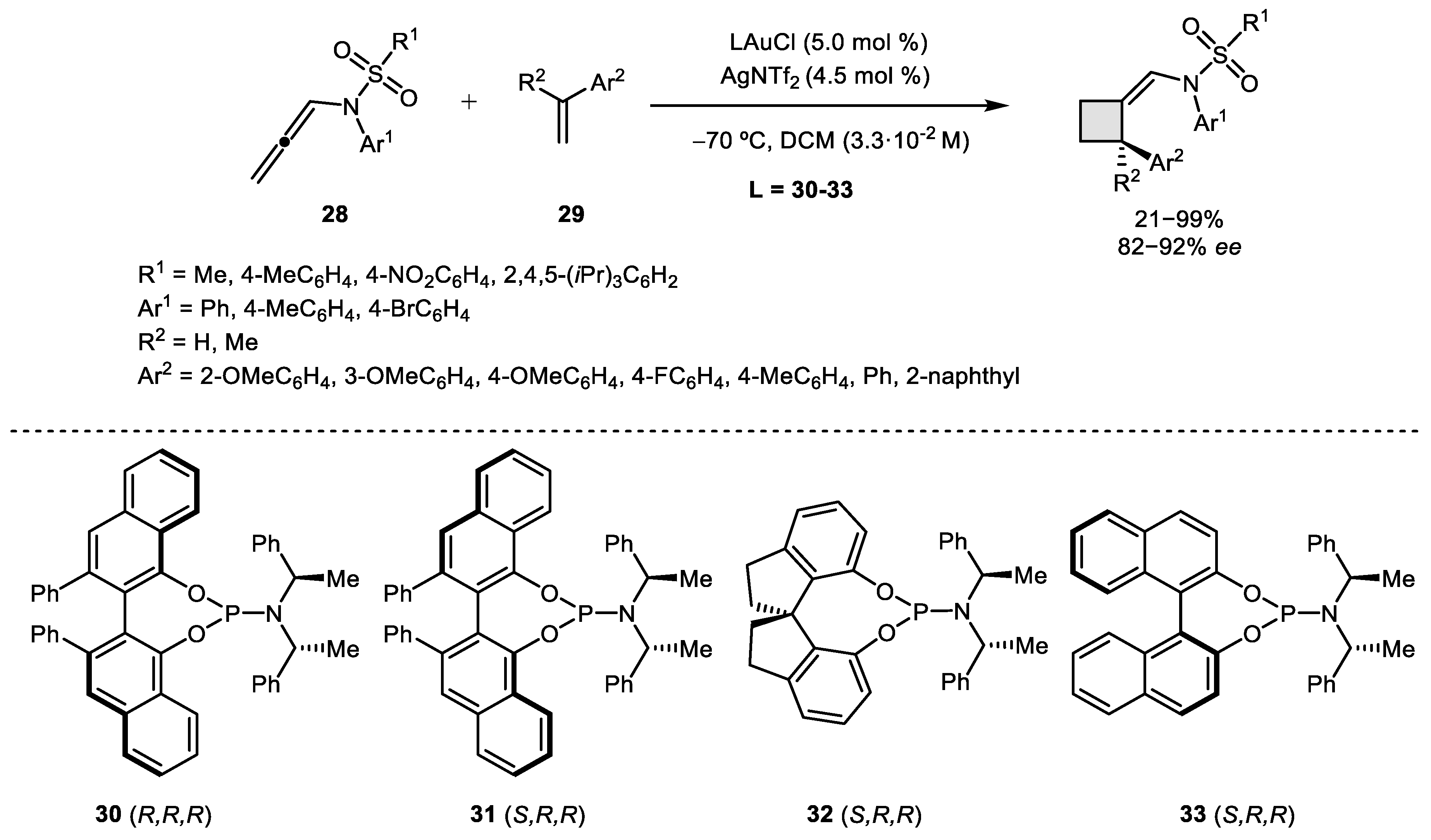
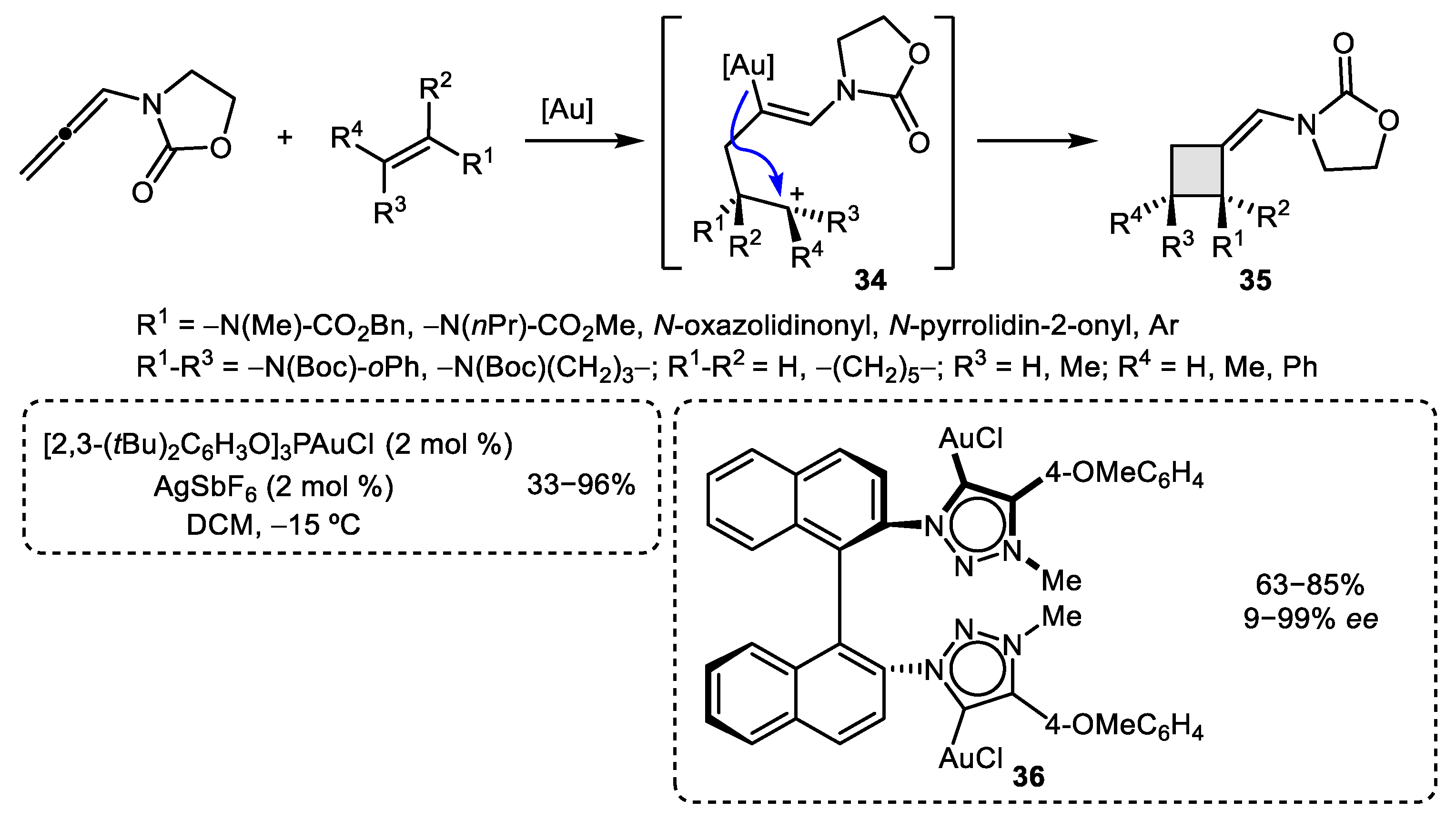
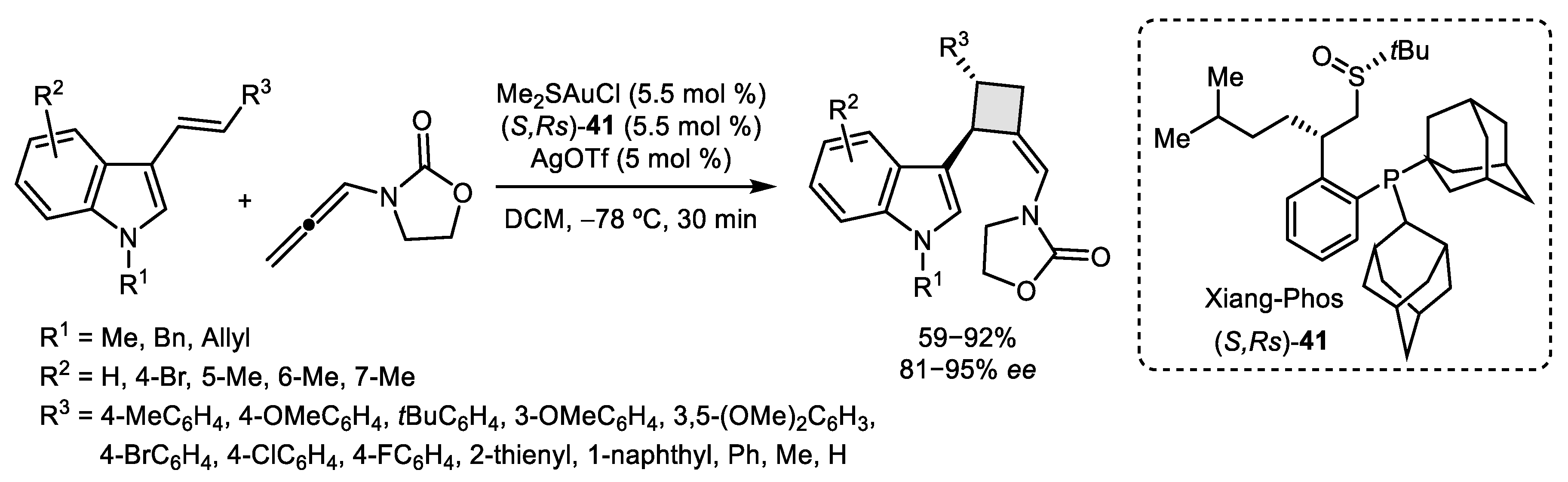
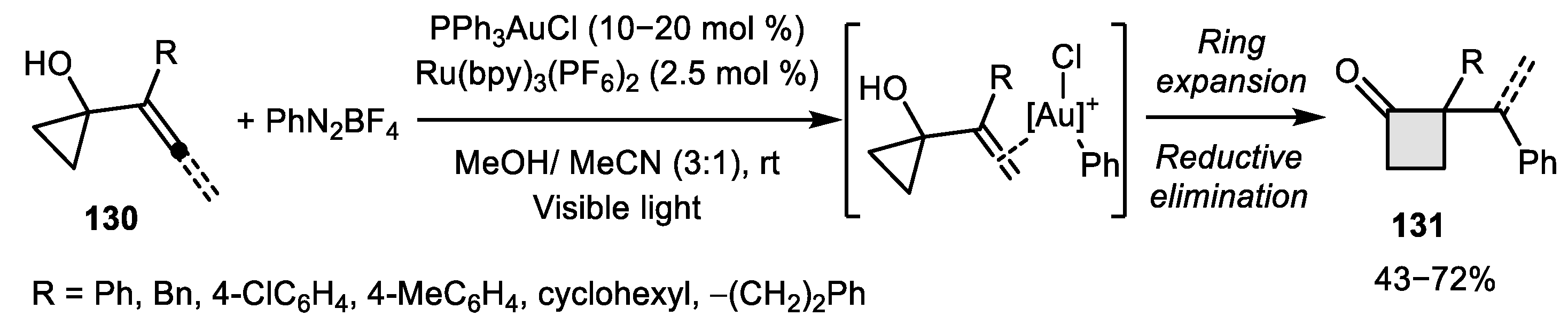
2.1. Allene–Alkene
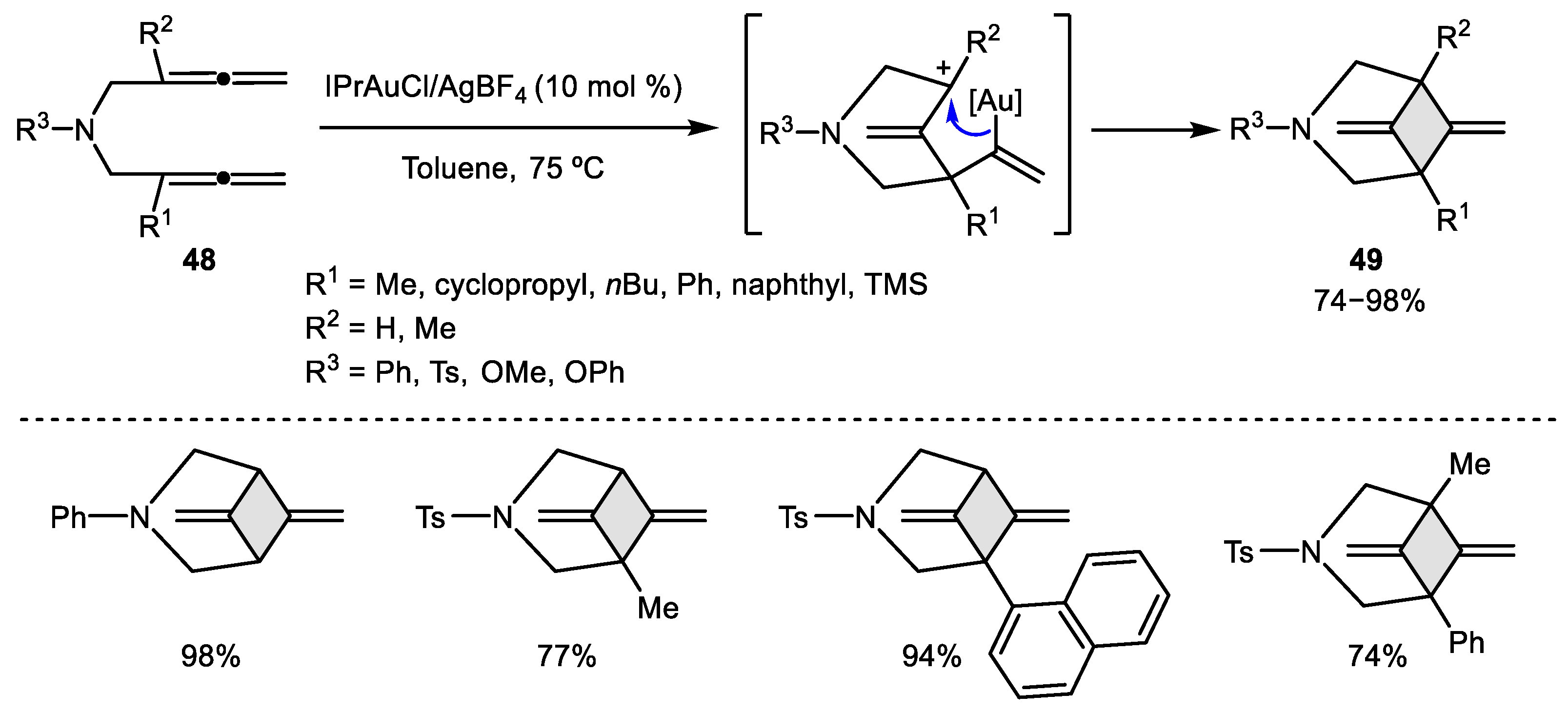
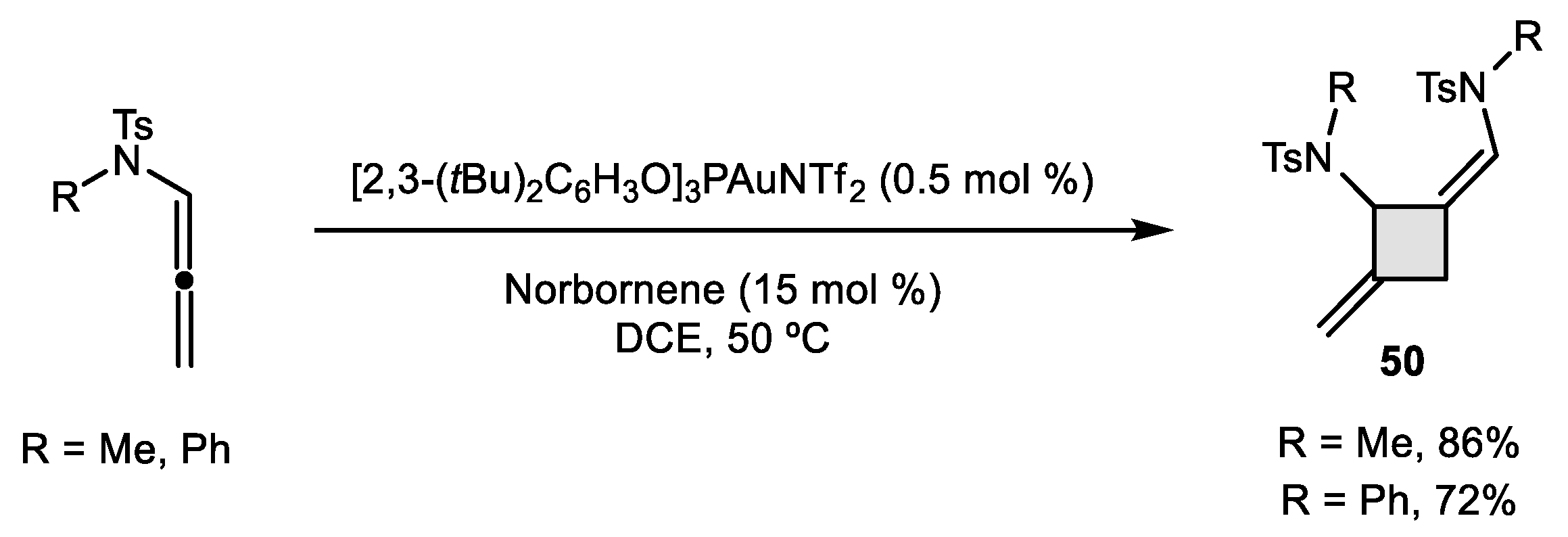
2.2. Allene–Allene
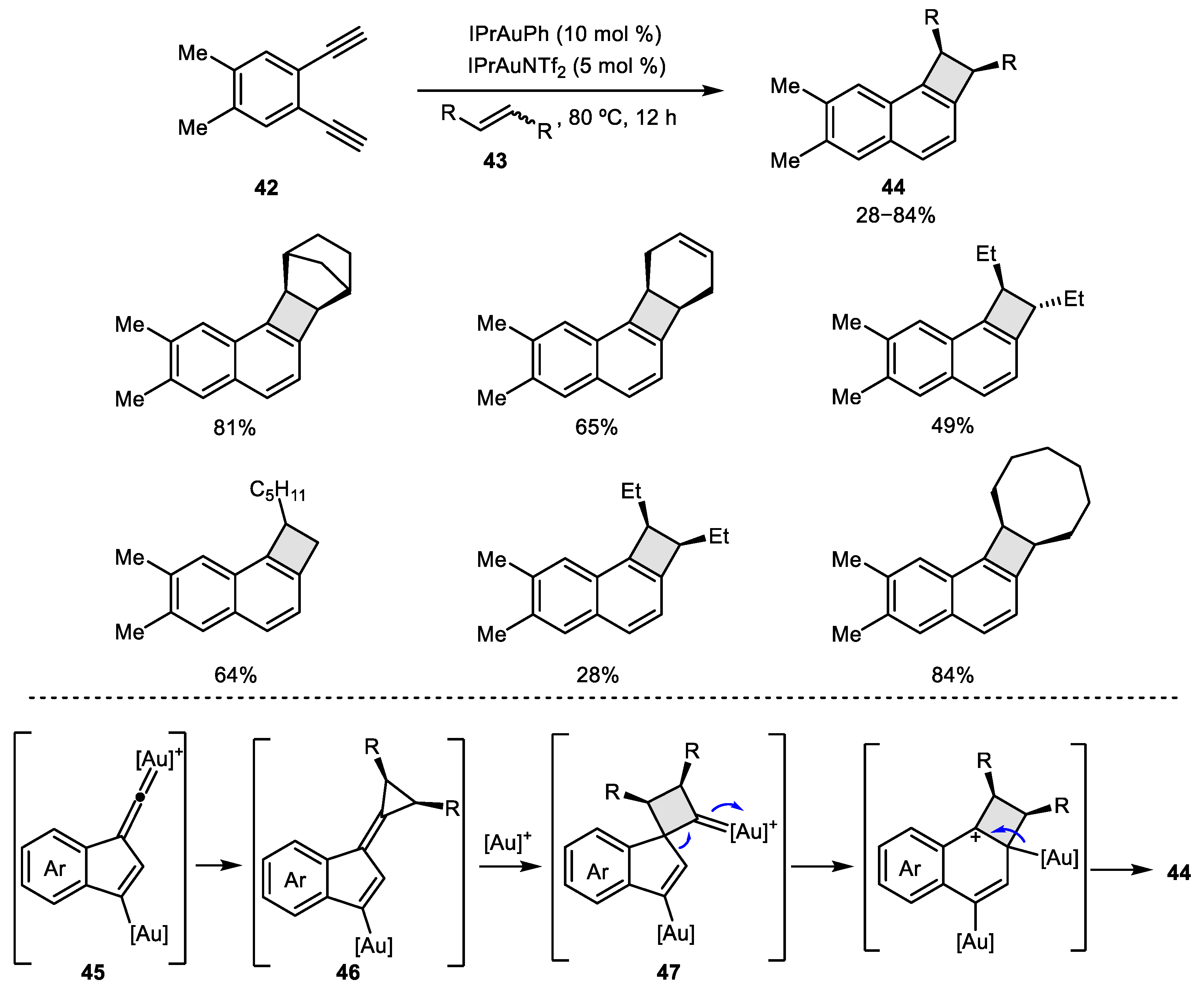
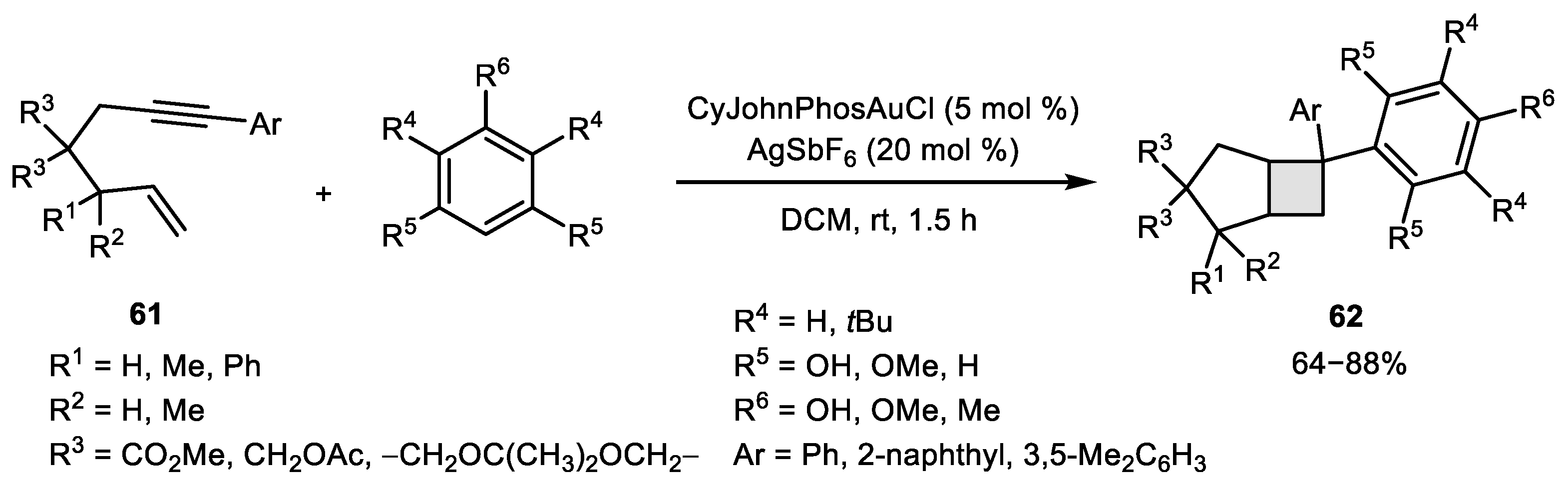
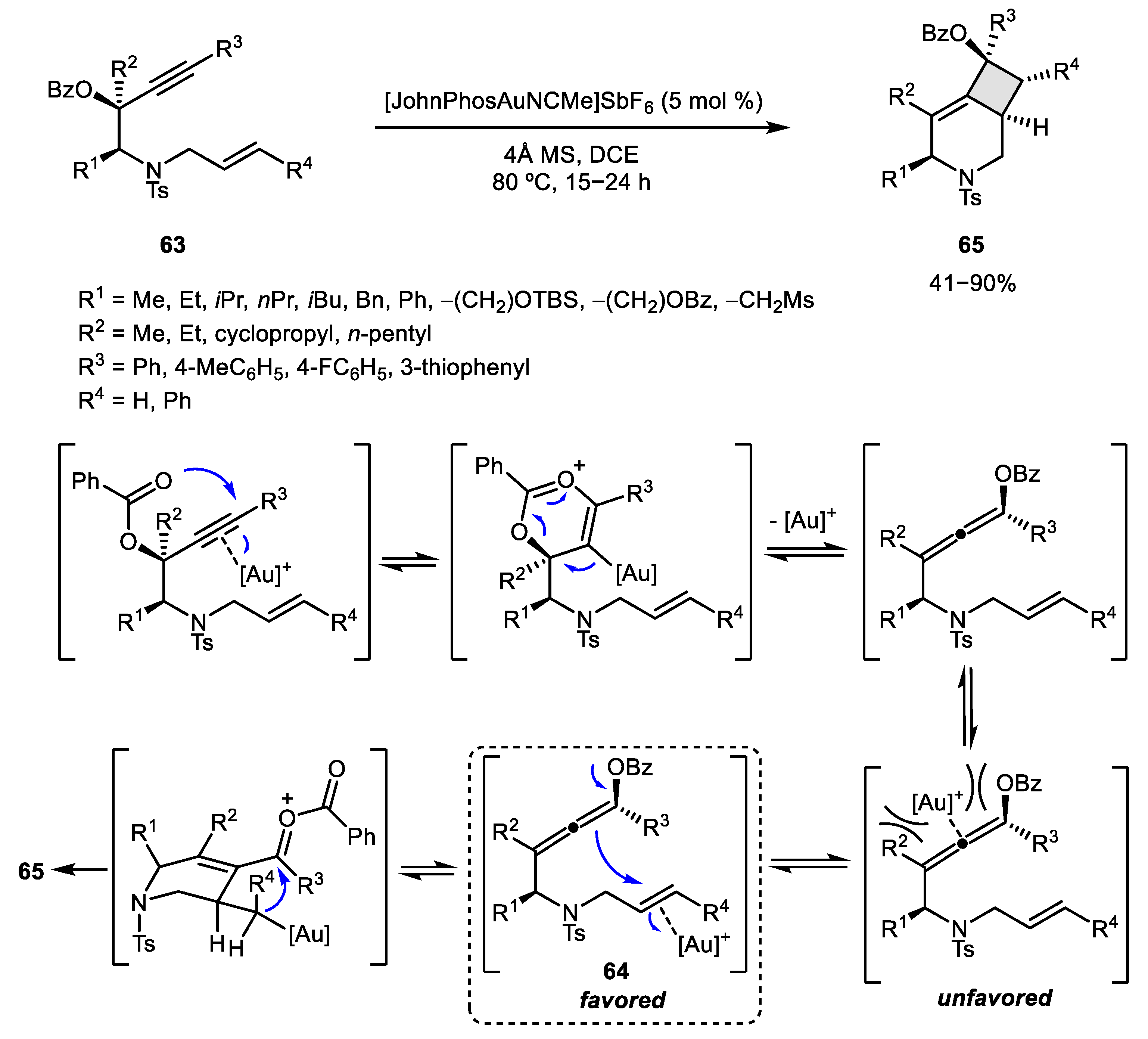
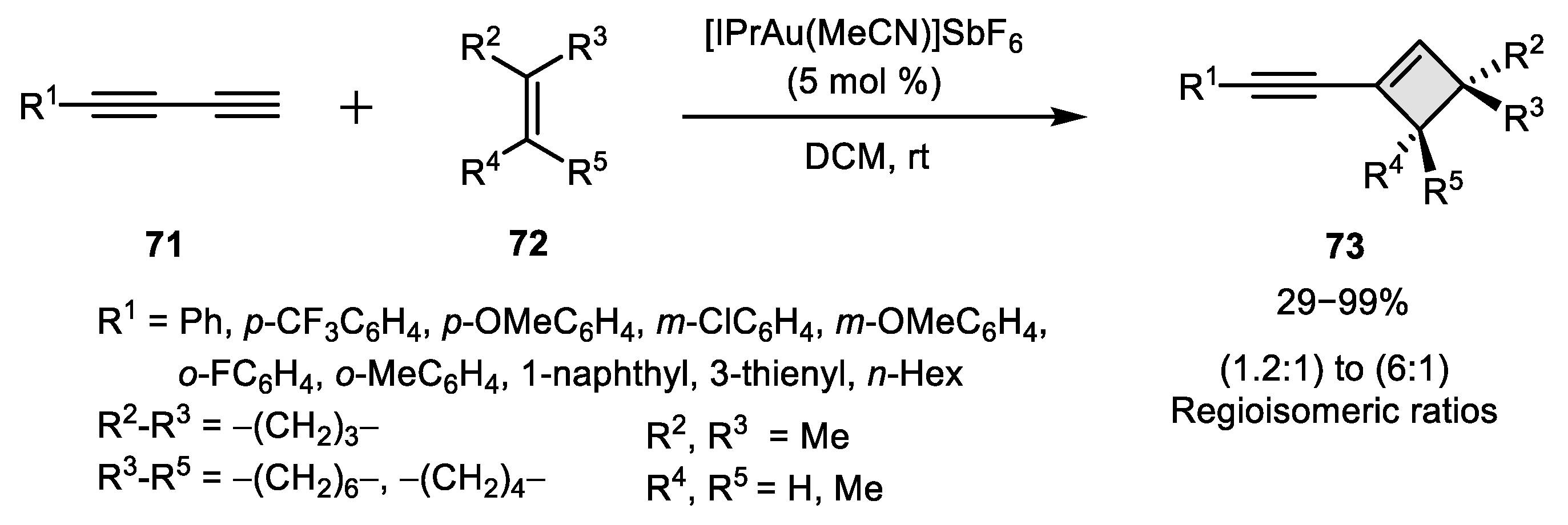
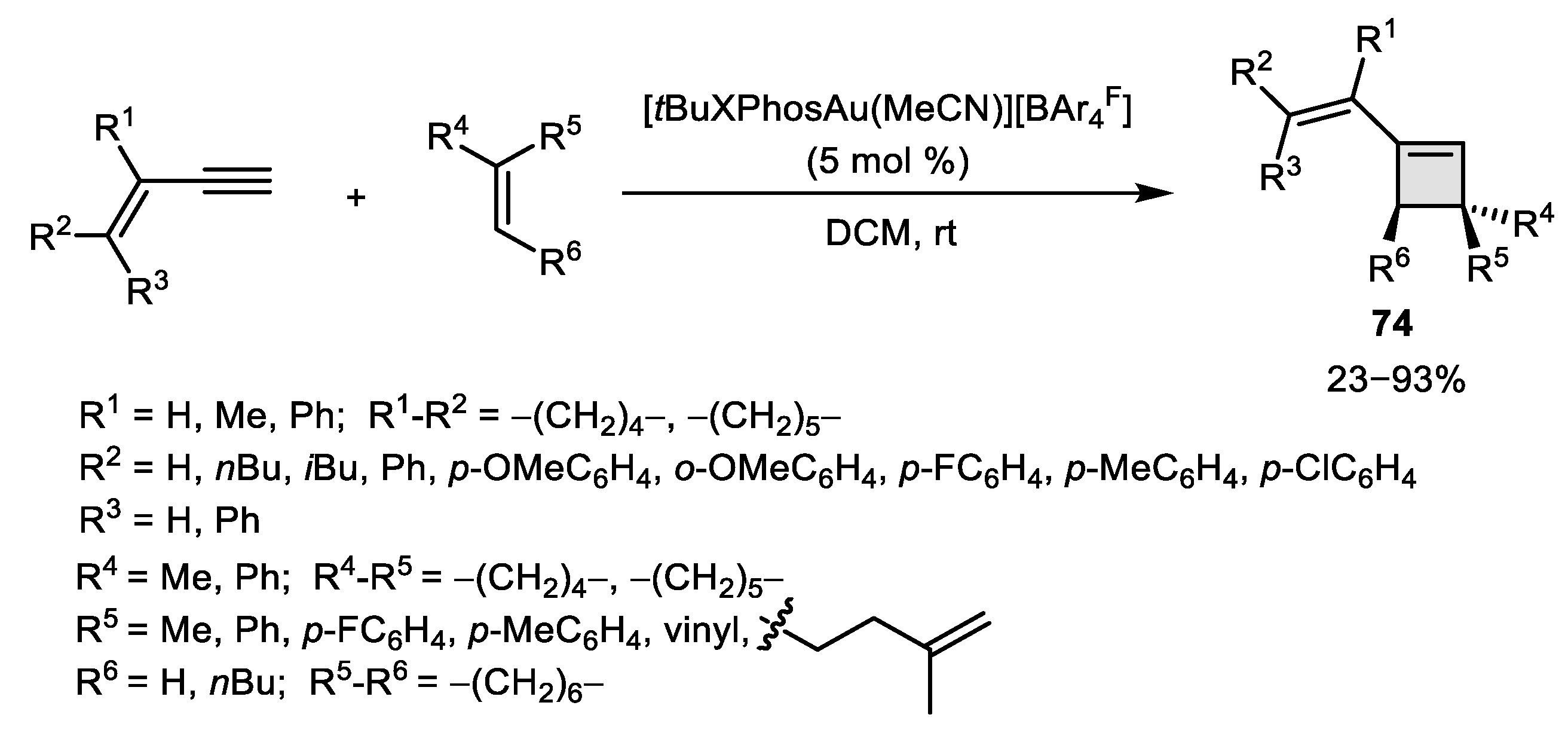
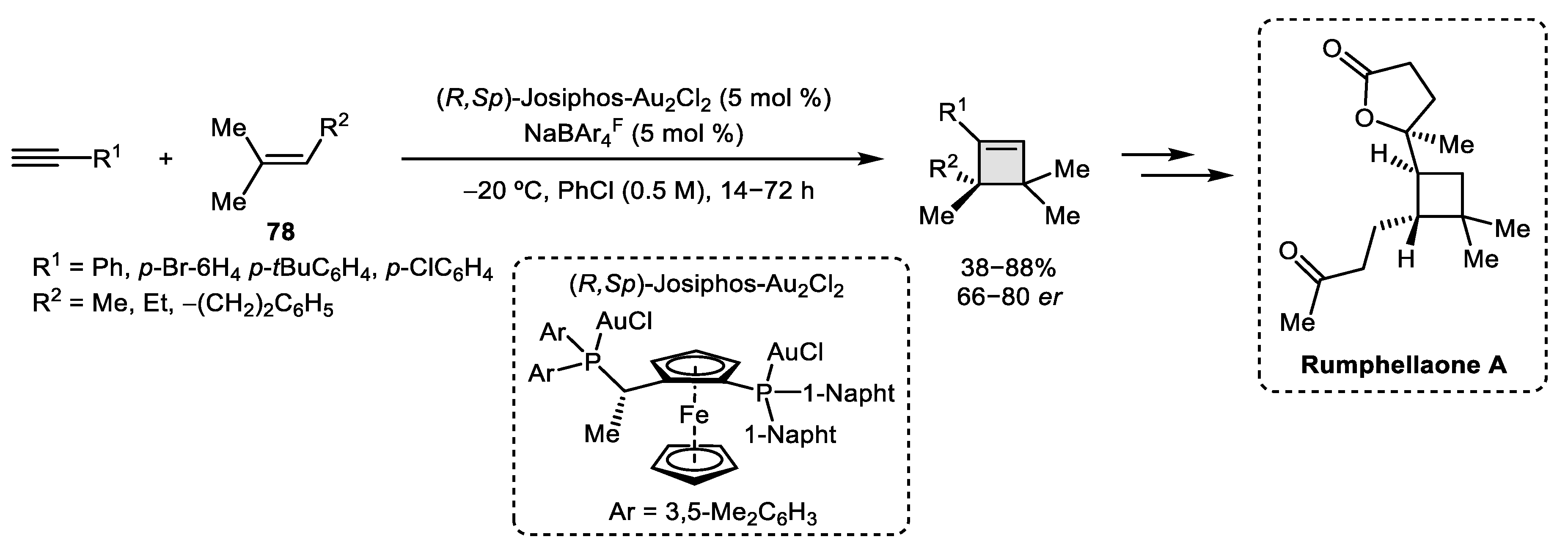
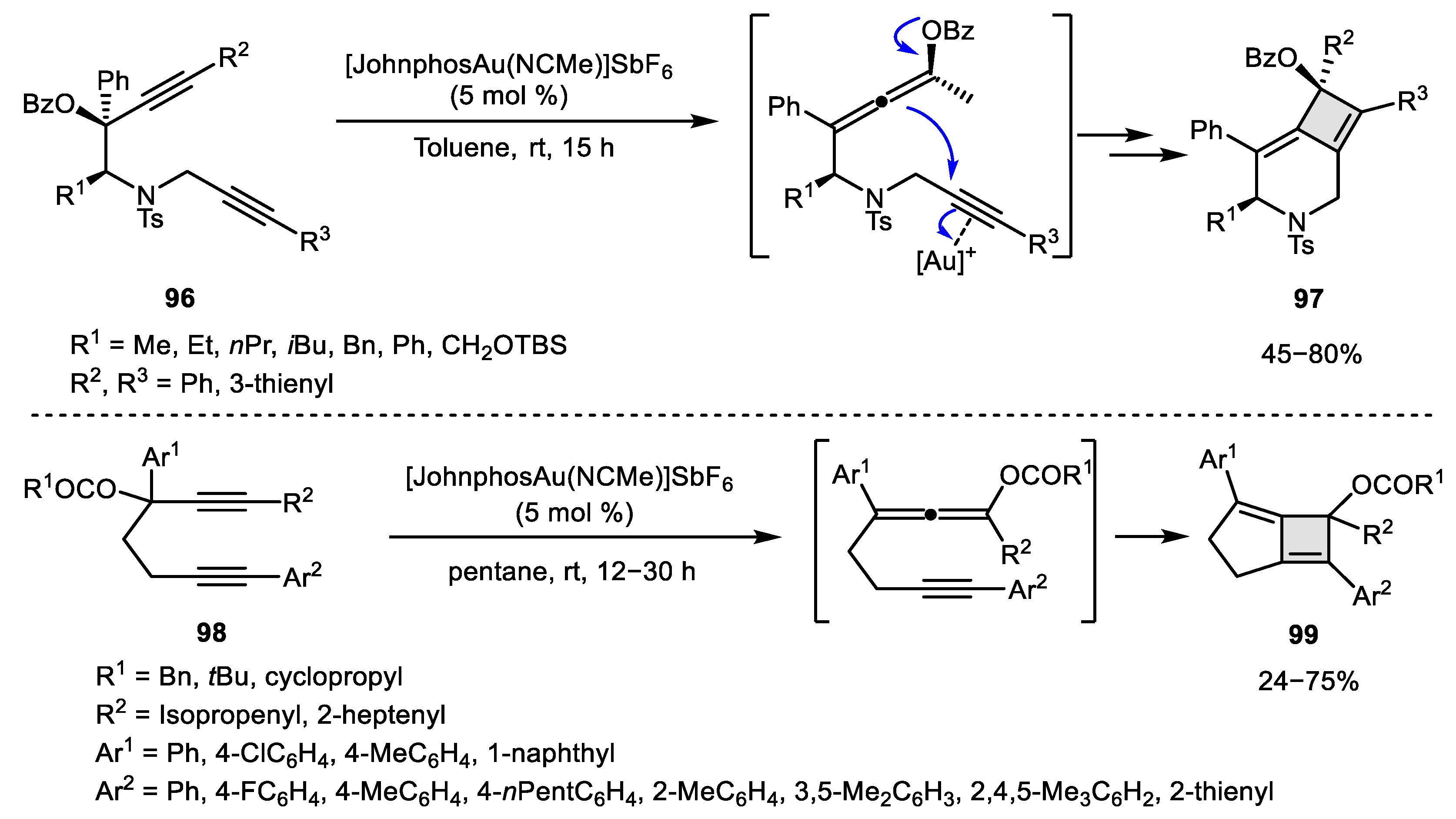
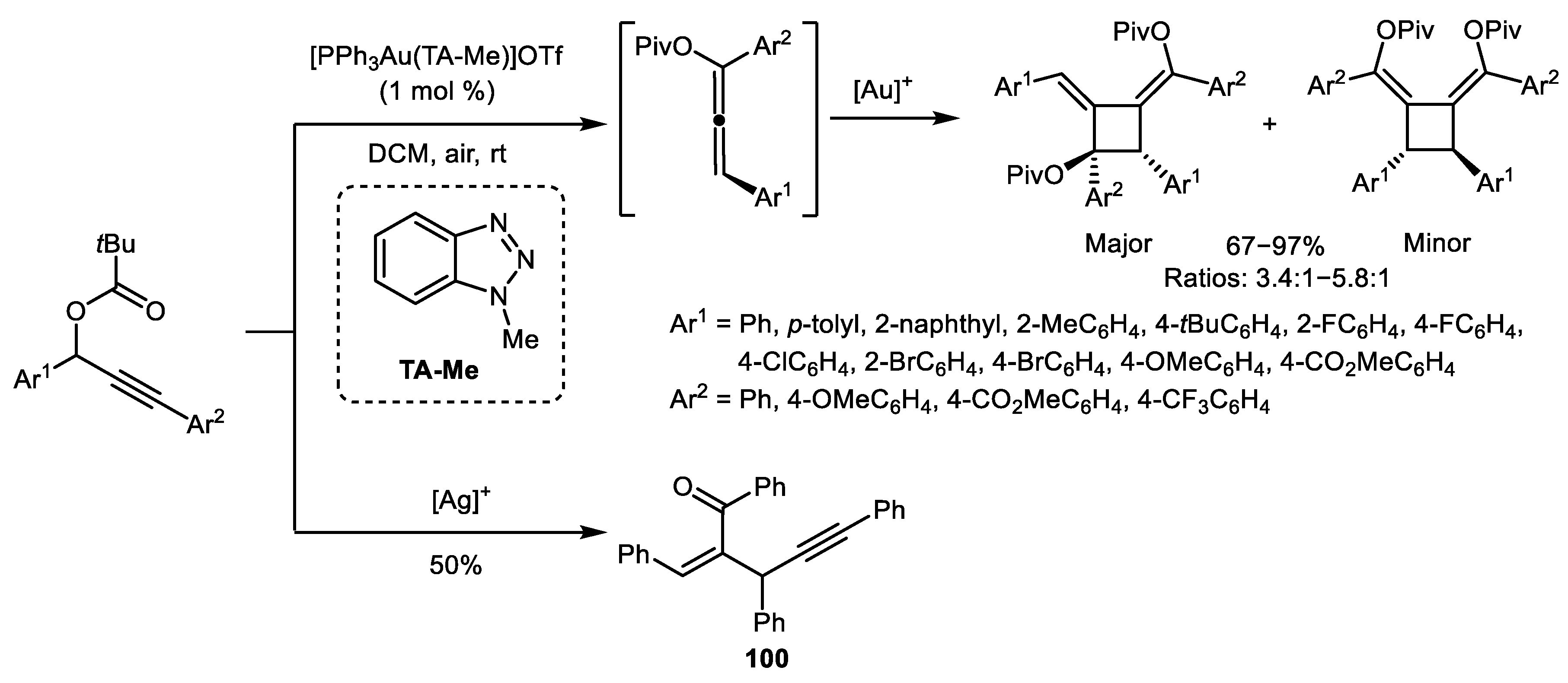
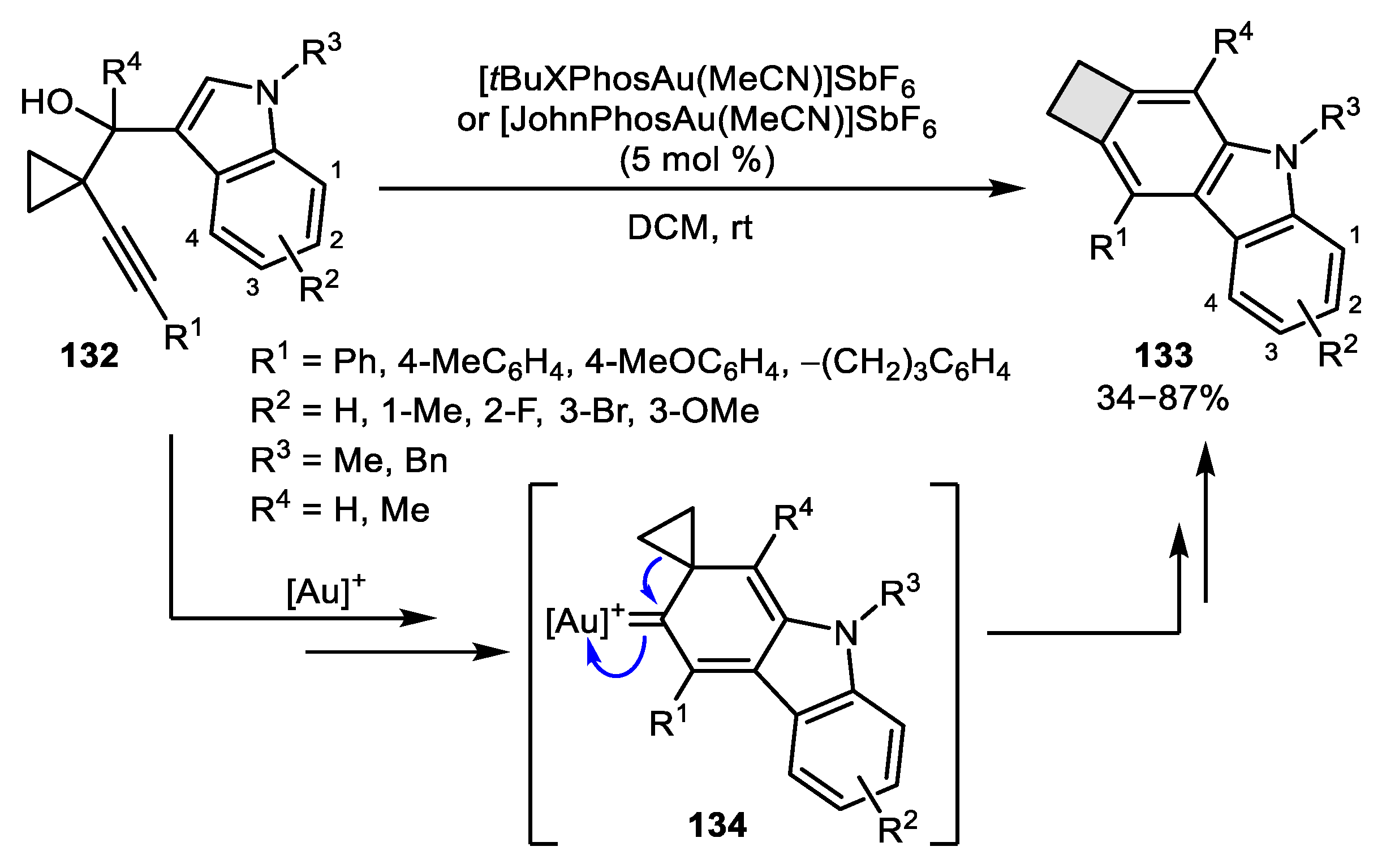
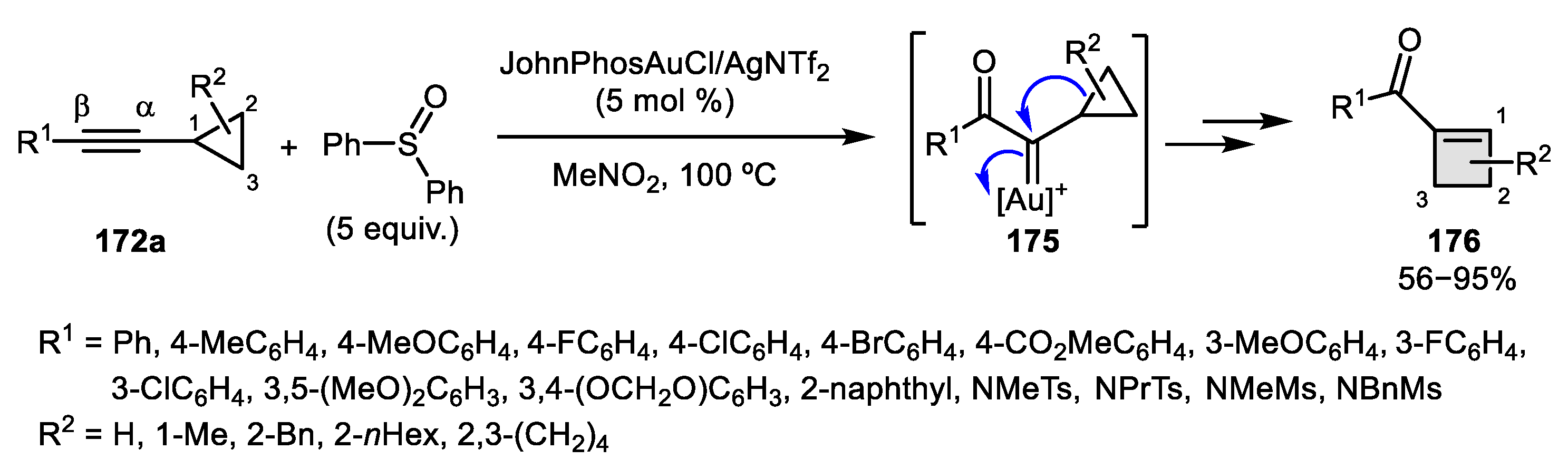
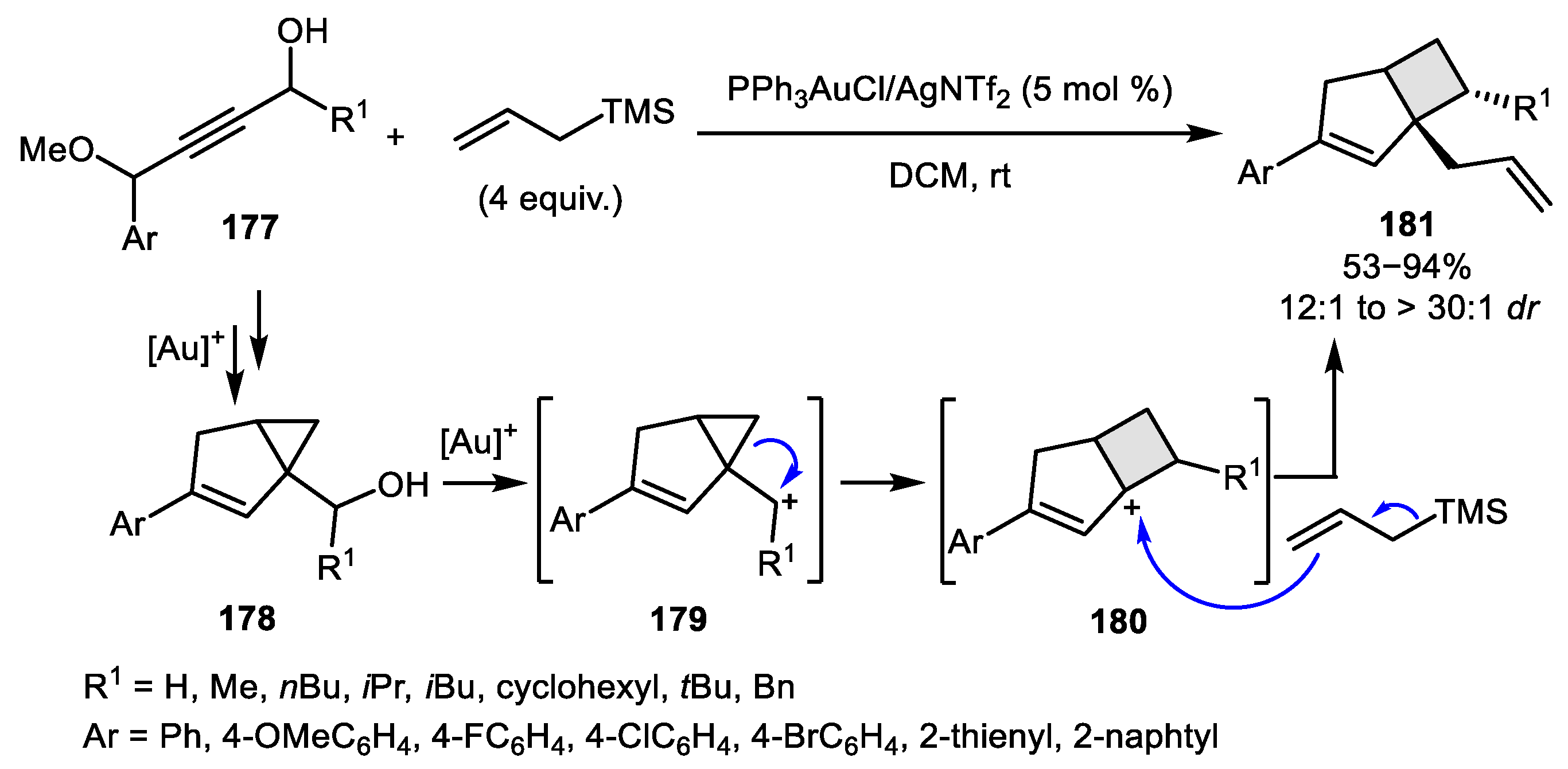
2.3. Alkene–Alkyne
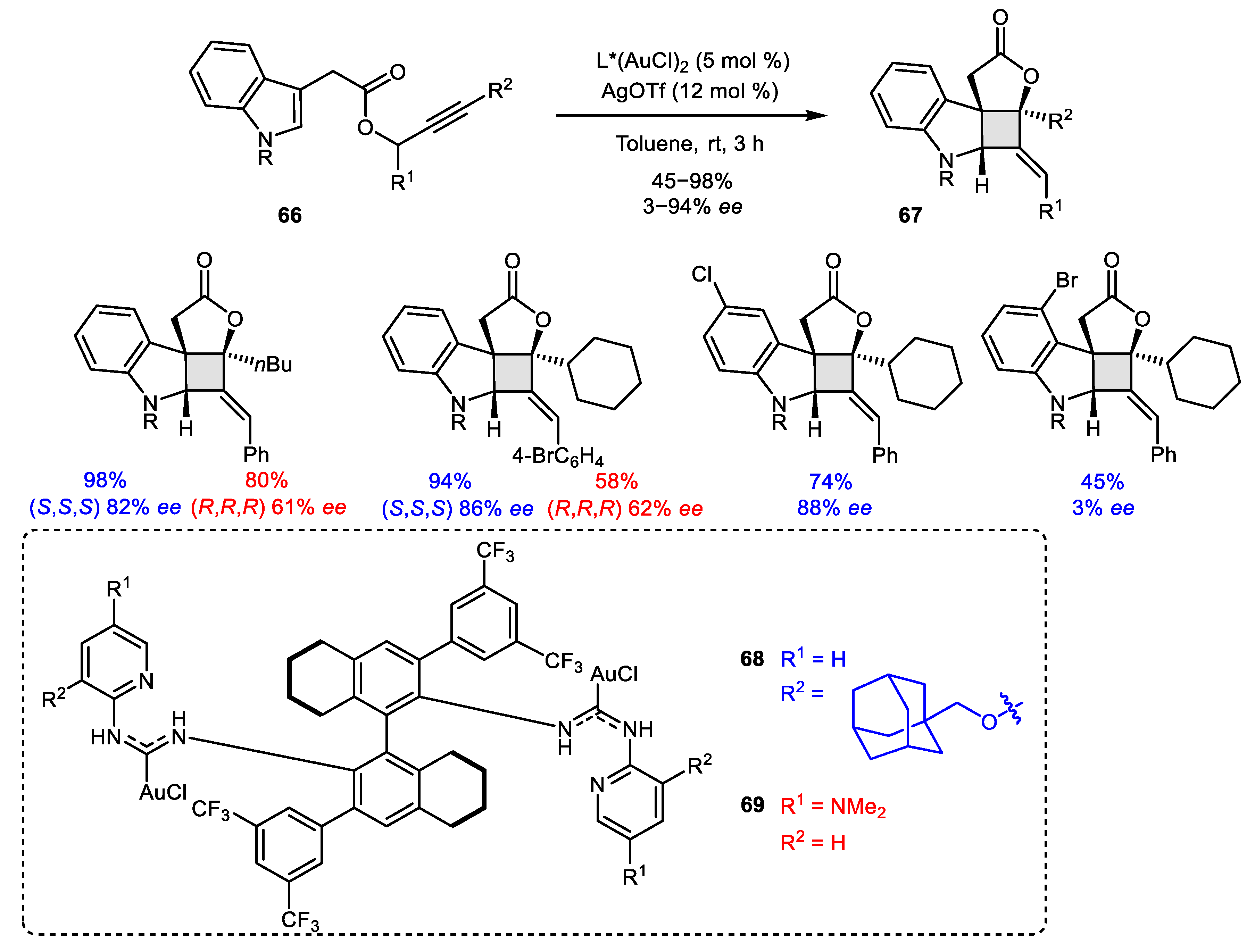
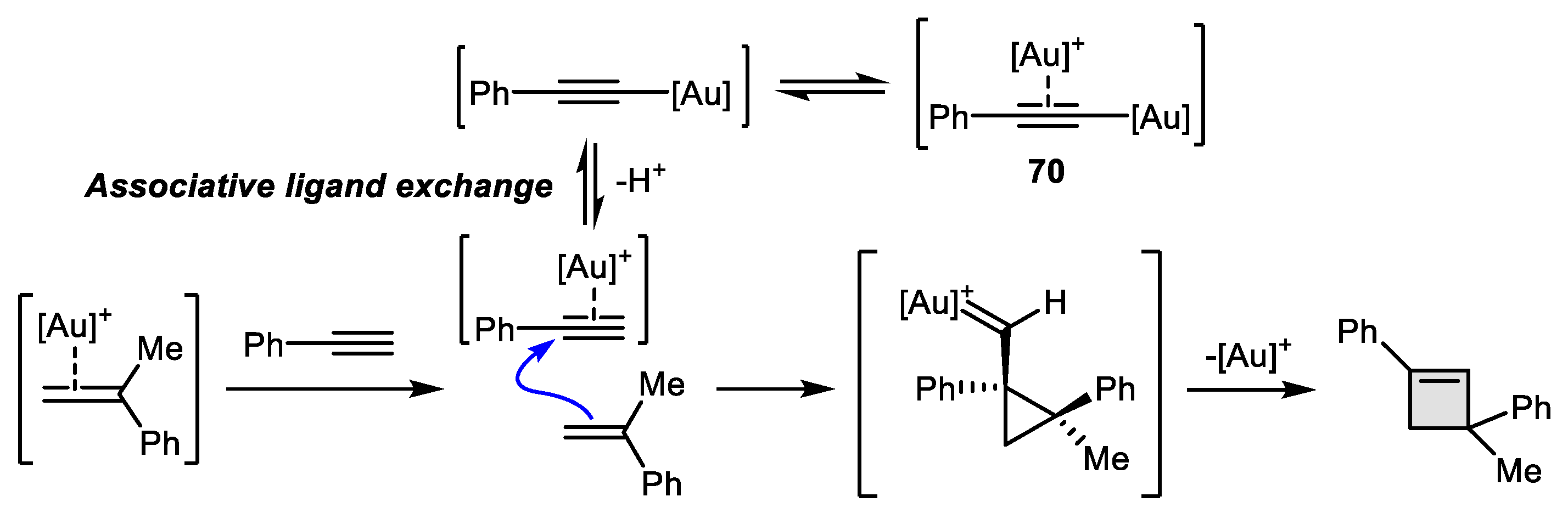
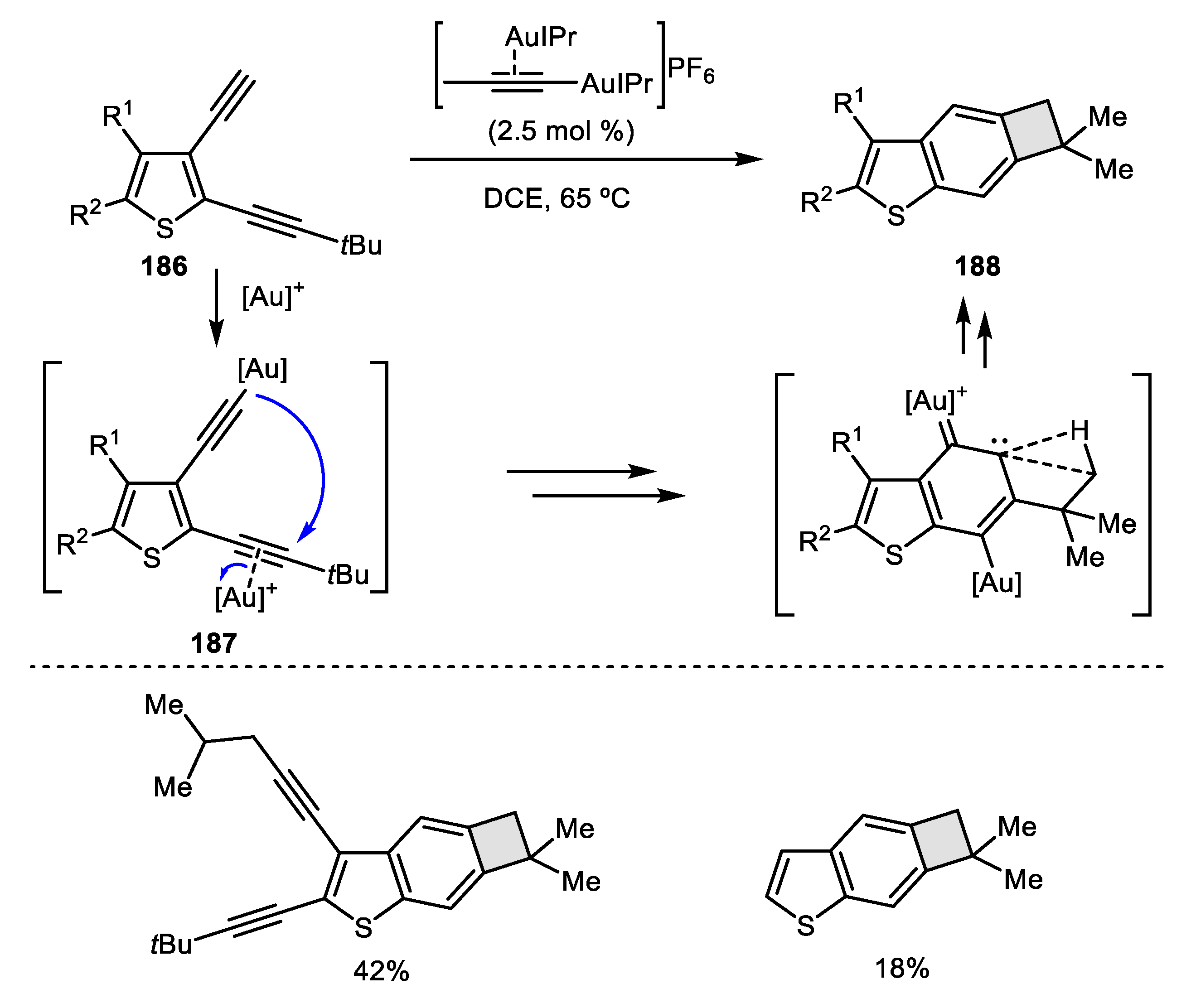
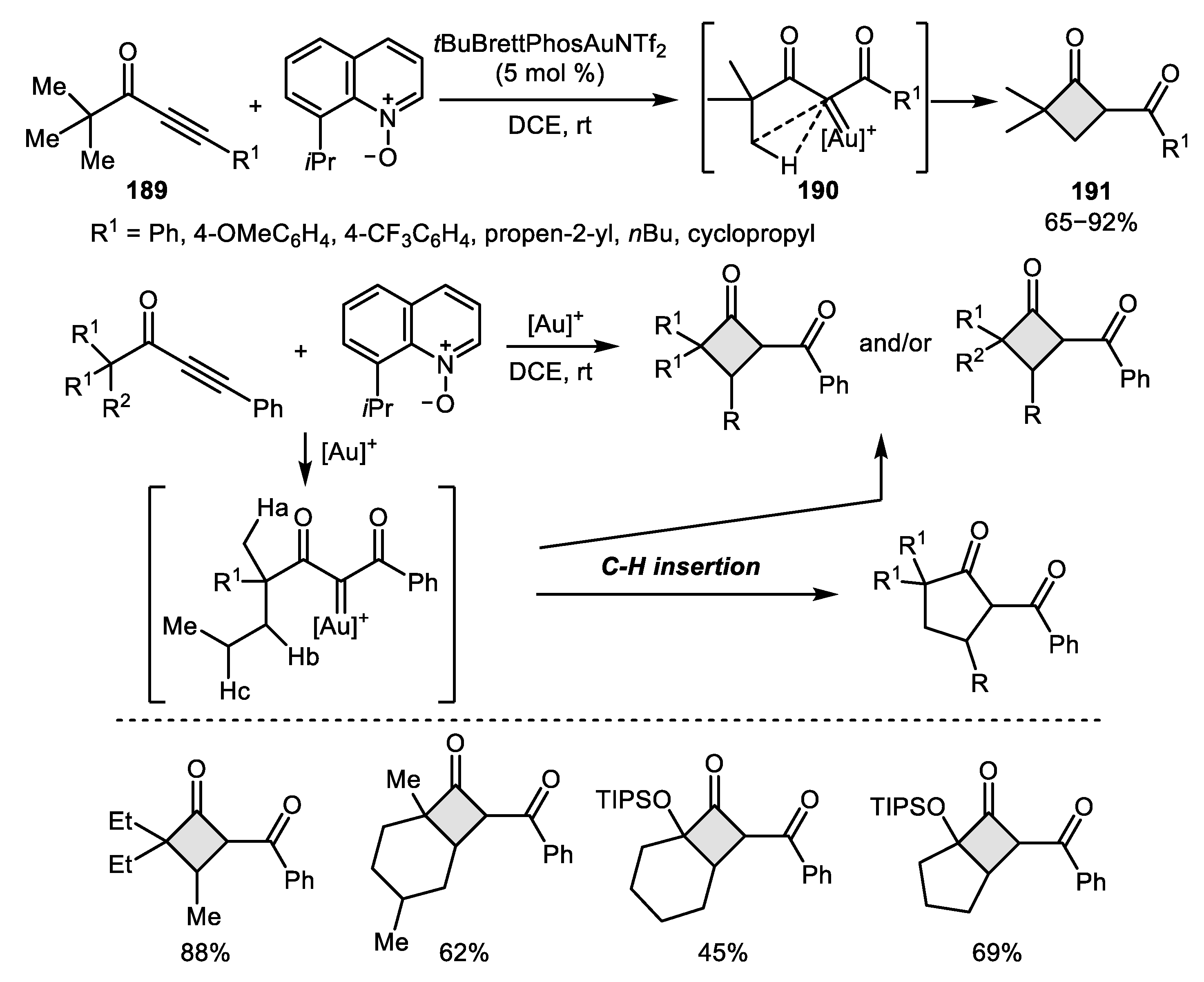
2.4. Alkyne-Alkyne
3. Ring Expansion
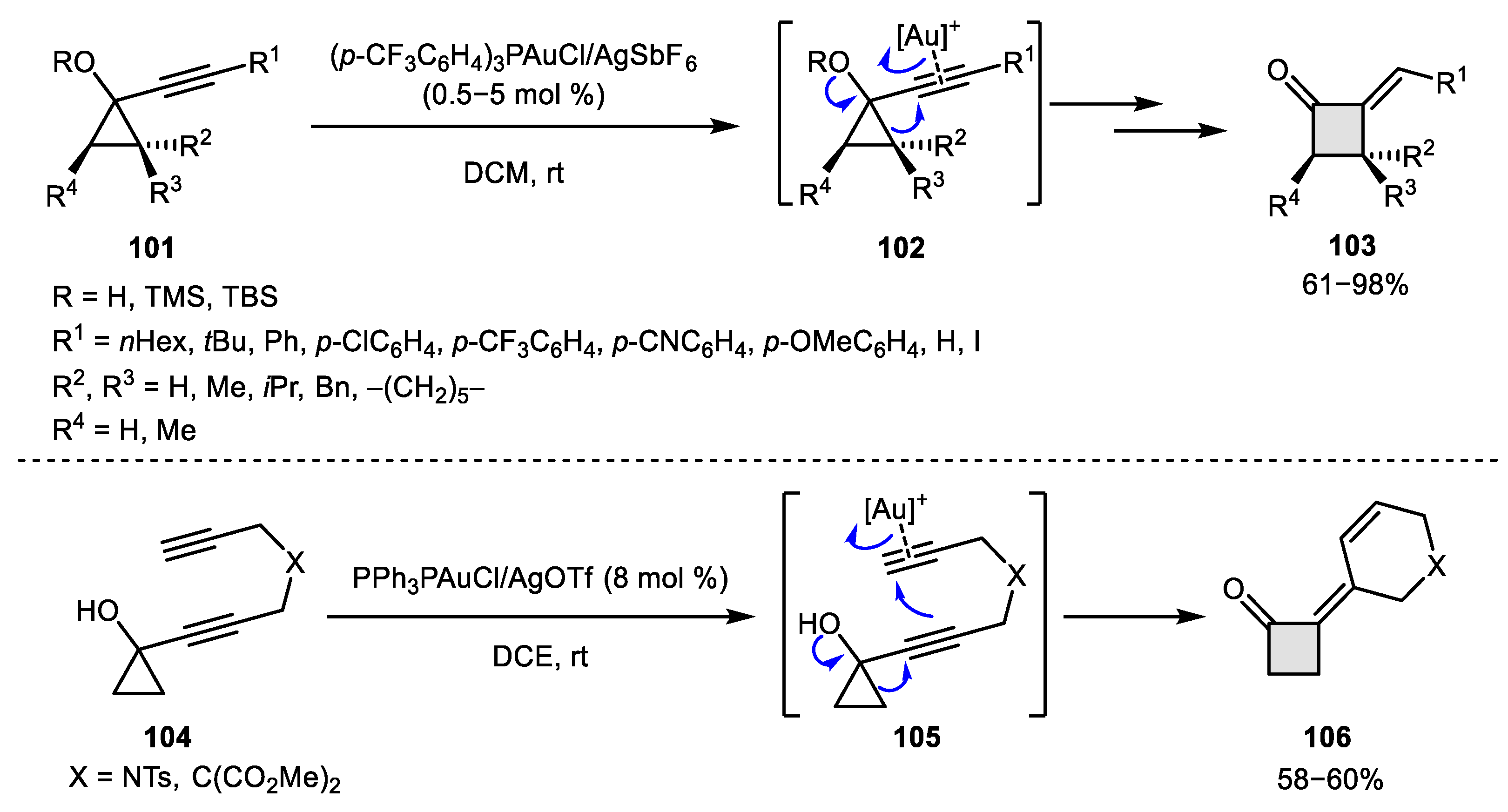
3.1. Pinacol-Like Transformations
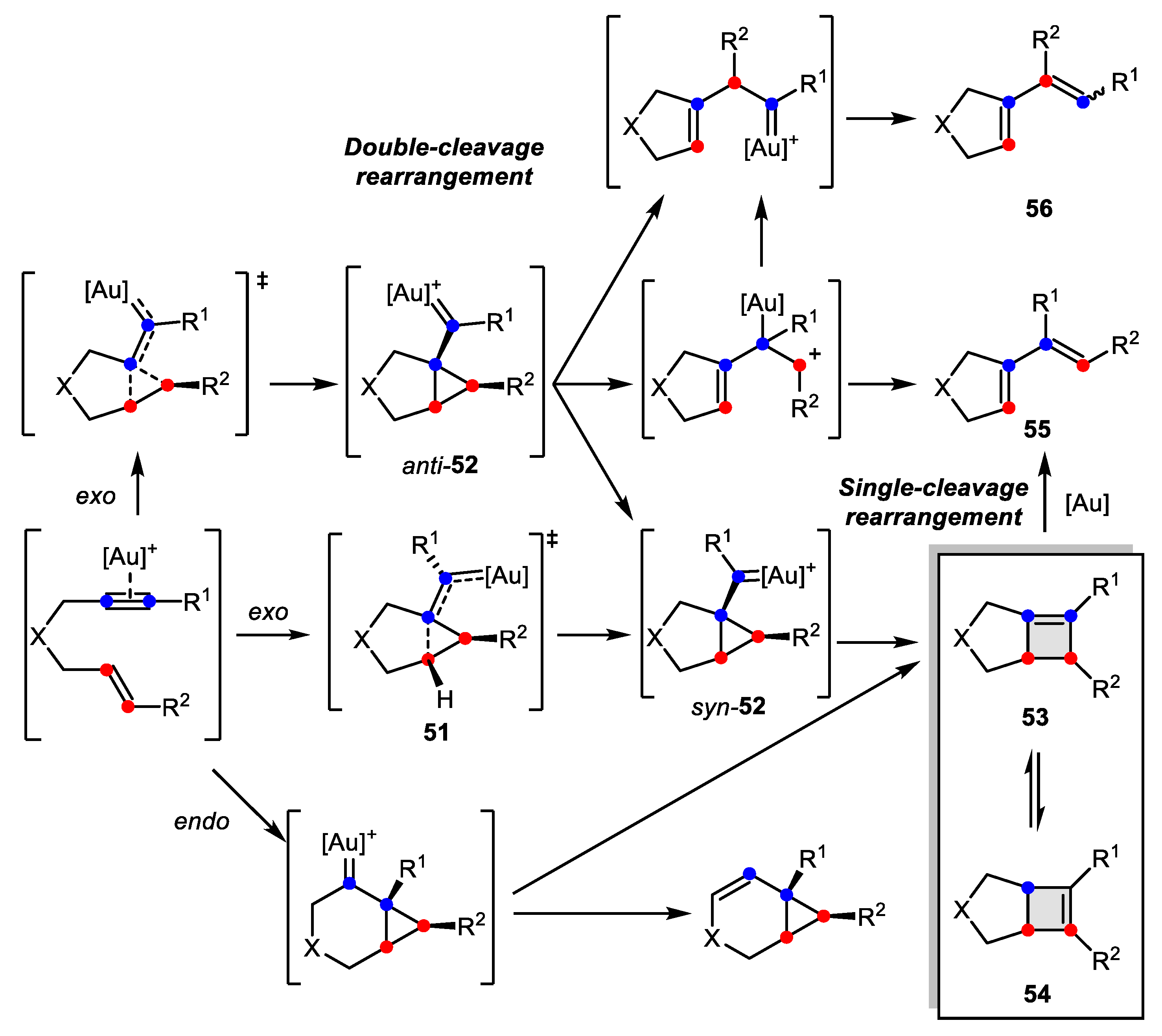
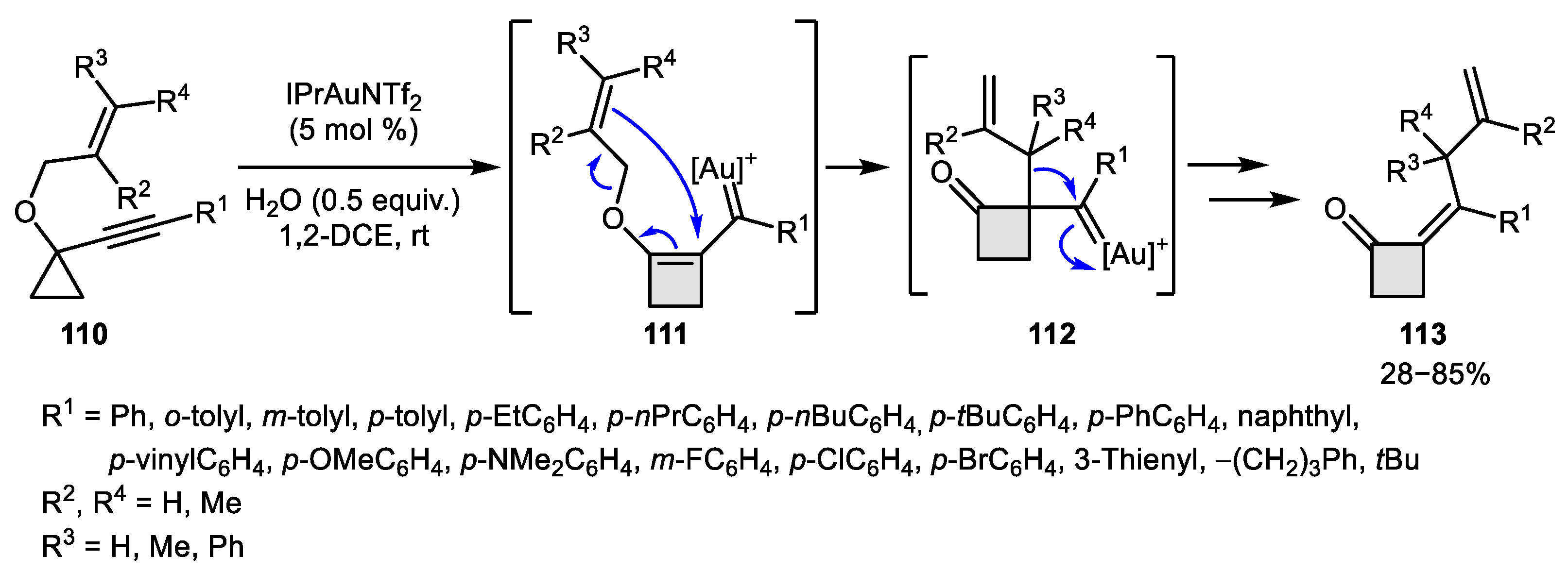
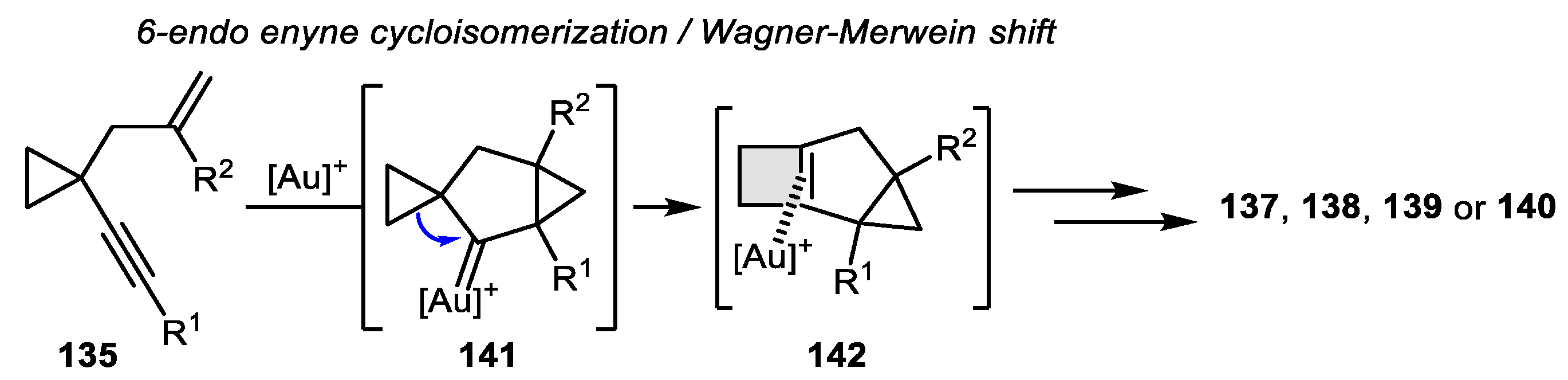
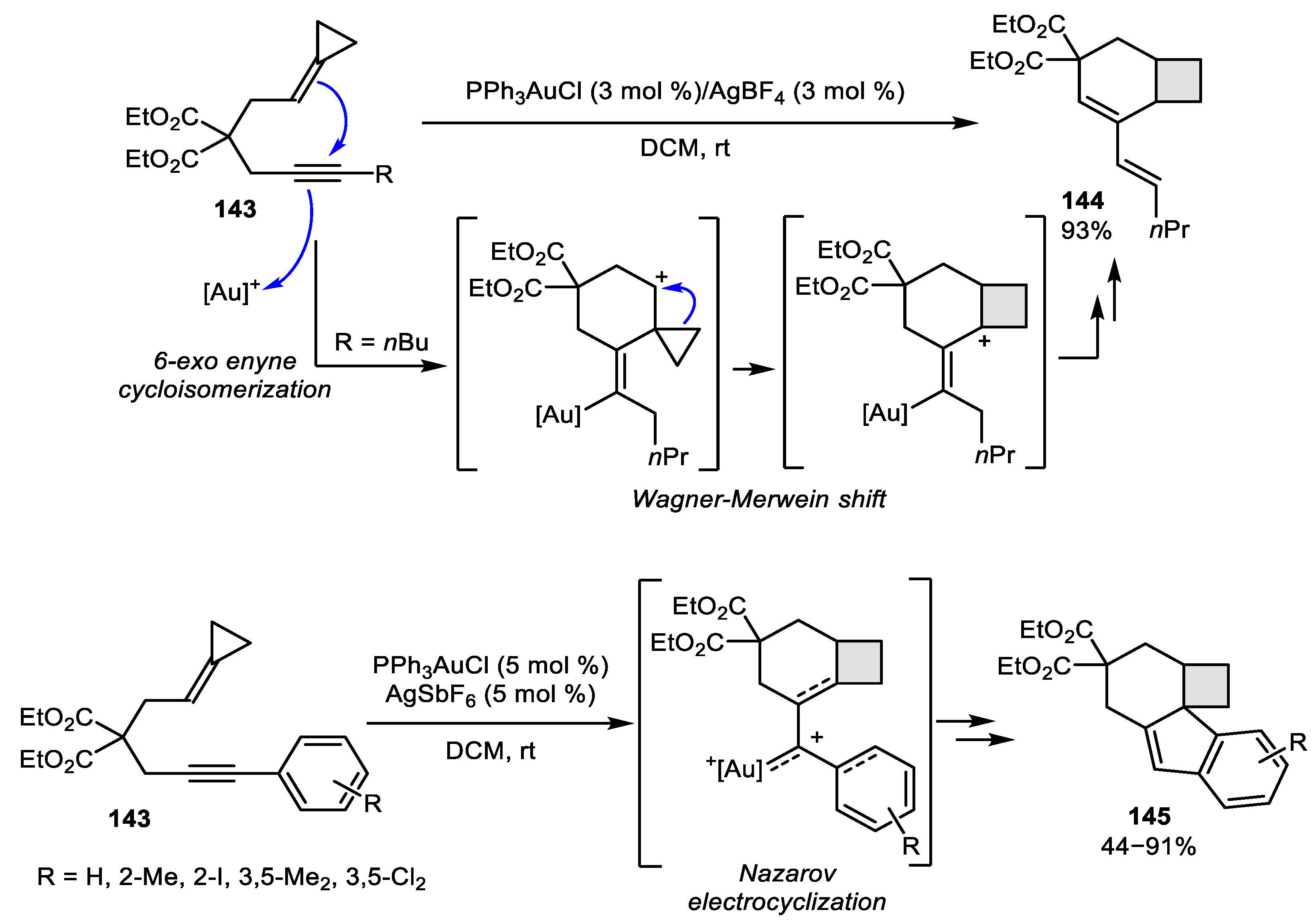
3.2. Wagner-Meerwein-Like Transformations
4. Other Cyclization Approaches
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nouri, D.H.; Tantillo, D.J. They Came From the Deep: Syntheses, Applications, and Biology of Ladderanes. Curr. Org. Chem. 2006, 10, 2055–2074. [Google Scholar] [CrossRef]
- Iriondo-Alberdi, J.; Greaney, M.F. Photocycloaddition in Natural Product Synthesis. Eur. J. Org. Chem. 2007. [Google Scholar] [CrossRef]
- Bach, T.; Hehn, J.P. Photochemical Reactions as Key Steps in Natural Product Synthesis. Angew. Chem. Int. Ed. 2011, 50, 1000–1045. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M. Natural Sesquiterpenoids. Nat. Prod. Rep. 2013, 30, 1226–1264. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lu, P. Catalytic Approaches to Assemble Cyclobutane Motifs in Natural Product Synthesis. Org. Chem. Front. 2018, 5, 254–259. [Google Scholar] [CrossRef]
- Li, J.; Gao, K.; Bian, M.; Ding, H. Recent Advances in the Total Synthesis of Cyclobutane-containing Natural Products. Org. Chem. Front. 2020, 7, 136–154. [Google Scholar] [CrossRef]
- Davis, E.M.; Croteau, R. Cyclization Enzymes in the Biosynthesis of Monoterpenes, Sesquiterpenes, and Diterpenes. Top. Curr. Chem. 2000, 209, 53–95. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of Terrestrial Origin. Nat. Prod. Rep. 2013, 30, 1346–1356. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Gao, X.-H.; Yue, J.-M. Attractive Natural Products with Strained Cyclopropane and/or Cyclobutane Ring Systems. Sci. China Chem. 2016, 59, 1126–1141. [Google Scholar] [CrossRef]
- Sergeiko, A.; Poroikov, V.V.; Hanus, L.O.; Dembitsky, V.M. Cyclobutane-containing Alkaloids: Origin, Synthesis, and Biological Activities. Open Med. Chem. J. 2008, 2, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Dembitsky, V.M. Naturally Occurring Bioactive Cyclobutane-Containing (CBC) Alkaloids in Fungi, Fungal Endophytes, and Plants. Phytomedicine 2014, 21, 1559–1581. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, A.; Awang, K.; Guéritte, F.; Litaudon, M. Pentacyclic Polyketides From Endiandra Kingiana as Inhibitors of the Bcl-xL/Bak Interaction. Phytochemistry 2011, 72, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P.; Aragoncillo, C. Exploiting [2 + 2] Cycloaddition Chemistry: Achievements with Allenes. Chem. Soc. Rev. 2010, 39, 783–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, N. Formation of a Four-Membered Ring. In Handbook of Synthetic Photochemistry; Albini, A., Fagnoni, M., Eds.; Wiley-VCH: Sttutgart, Germany, 2010; pp. 137–169. [Google Scholar]
- Hehn, J.P.; Müller, C.; Bach, T. Formation of a Four-Membered Ring: From a Carbonyl-Conjugated Alkene. In Handbook of Synthetic Photochemistry; Albini, A., Fagnoni, M., Eds.; Wiley-VCH: Sttutgart, Germany, 2010; pp. 171–215. [Google Scholar] [CrossRef]
- Alcaide, B.; Aragoncillo, C.; Almendros, P. 5.02 Thermal Cyclobutane Ring Formation. In Comprehensive Organic Synthesis II (Second Edition); Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 66–84. [Google Scholar] [CrossRef]
- Poplata, S.; Tröster, A.; Zou, Y.-Q.; Bach, T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Bera, N.; Ghosh, S. [2 + 2] Photochemical Cycloaddition in Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 1310–1326. [Google Scholar] [CrossRef]
- Takeda, K.; Haraguchi, H.; Okamoto, Y. A New Strategy for Construction of Eight-Membered Carbocycles by Brook Rearrangement Mediated [6 + 2] Annulation. Org. Lett. 2003, 5, 3705–3707. [Google Scholar] [CrossRef]
- Hussain, M.M.; Li, H.; Hussain, N.; Ureña, M.; Carroll, P.J.; Walsh, P.J. Applications of 1-Alkenyl-1, 1-Heterobimetallics in the Stereoselective Synthesis of Cyclopropylboronate Esters, Trisubstituted Cyclopropanols and 2, 3-Disubstituted Cyclobutanones. J. Am. Chem. Soc. 2009, 131, 6516–6524. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Yu, Z.-X. TfOH-catalyzed Tandem Cyclopropane Ring Enlargement/C–C Formation/Etherification of Alkynylcyclopropanes and 1,3-Diketones to Cyclobutane-Fused Dihydrofurans. Chem. Commun. 2011, 47, 794–796. [Google Scholar] [CrossRef]
- Barluenga, J.; Riesgo, L.; López, L.A.; Rubio, E.; Tomás, M. Discrimination of Diazo Compounds Toward Carbenoids: Copper (I)-Catalyzed Synthesis of Substituted Cyclobutenes. Angew. Chem. Int. Ed. 2009, 48, 7569–7572. [Google Scholar] [CrossRef]
- Ito, H.; Toyoda, T.; Sawamura, M. Stereospecific Synthesis of Cyclobutylboronates through Copper (I)-Catalyzed Reaction of Homoallylic Sulfonates and a Diboron Derivative. J. Am. Chem. Soc. 2010, 132, 5990–5992. [Google Scholar] [CrossRef]
- Han, Y.T.; Kim, N.-J.; Jung, J.-W.; Yun, H.; Lee, S.; Suh, Y.-G. A Versatile Synthetic Approach to Grandisol Monoterpene Pheromone. Arch. Pharm. Res. 2011, 34, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Parker, G.D.; Tei, T.; Paquette, L.A. In Pursuit of Pestalotiopsin A via Zirconocene-Mediated Ring Contraction. Org. Lett. 2006, 8, 2429–2431. [Google Scholar] [CrossRef] [PubMed]
- Chaumontet, M.; Piccardi, R.; Audic, N.; Hitce, J.; Peglion, J.-L.; Clot, E.; Baudoin, O. Synthesis of Benzocyclobutenes by Palladium-Catalyzed C–H Activation of Methyl Groups: Method and Mechanistic Study. J. Am. Chem. Soc. 2008, 130, 15157–15166. [Google Scholar] [CrossRef] [PubMed]
- William, T.; Jordan, G.; Neil, C. Transition Metal-Catalyzed [2 + 2] Cycloaddition Reactions between Bicyclic Alkenes and Alkynes. Curr. Org. Synth. 2009, 6, 219–238. [Google Scholar] [CrossRef]
- Carreira, E.M.; Fessard, T.C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. [Google Scholar] [CrossRef]
- Parthasarathy, K.; Cheng, C.-H. 5.07 Metal-Mediated and Metal-Catalyzed [2 + 2] Cycloadditions. In Comprehensive Organic Synthesis II, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 222–272. [Google Scholar] [CrossRef]
- Fructos, M.R.; Prieto, A. [2 + 2] Cycloaddition Reactions Promoted by Group 11 Metal-Based Catalysts. Tetrahedron 2016, 72, 355–369. [Google Scholar] [CrossRef]
- Greene, A.E.; Charbonnier, F. Asymmetric Induction in the Cycloaddition Reaction of Dichloroketene with Chiral Enol Ethers. A Versatile Approach to Optically Active Cyclopentenone Derivatives. Tetrahedron Lett. 1985, 26, 5525–5528. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Ghosez, L. Study Of Chiral Auxiliaries for the Intramolecular [2 + 2] Cycloaddition of a Keteniminium Salt to an Olefinic Double Bond. A New Asymmetric Synthesis of Cyclobutanones. Tetrahedron Lett. 1990, 31, 4467–4470. [Google Scholar] [CrossRef]
- Yujiro, H.; Shigeo, N.; Koichi, N. [2 + 2] Cycloaddition Reaction between Allenyl Sulfides and Electron Deficient Olefins Promoted by Lewis Acids. Chem. Lett. 1990, 19, 2091–2094. [Google Scholar] [CrossRef]
- Nemoto, H.; Fukumoto, K. A Novel Domino Route to Chiral Cyclobutanones and its Function as Cornerstone in the Synthesis of Versatile Natural Products. Synlett 1997. [Google Scholar] [CrossRef]
- Lee-Ruff, E.; Mladenova, G. Enantiomerically Pure Cyclobutane Derivatives and Their Use in Organic Synthesis. Chem. Rev. 2003, 103, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Ueno, M.; Inanaga, K.; Ihara, M. Catalytic (2 + 2)-Cycloaddition Reactions of Silyl Enol Ethers. A Convenient and Stereoselective Method for Cyclobutane Ring Formation. J. Org. Chem. 2004, 69, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Canales, E.; Corey, E.J. Highly Enantioselective [2 + 2]-Cycloaddition Reactions Catalyzed by a Chiral Aluminum Bromide Complex. J. Am. Chem. Soc. 2007, 129, 12686–12687. [Google Scholar] [CrossRef] [PubMed]
- Secci, F.; Frongia, A.; Piras, P.P. Stereocontrolled Synthesis and Functionalization of Cyclobutanes and Cyclobutanones. Molecules 2013, 18, 15541–15572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Conner, M.L.; Brown, M.K. Cyclobutane and Cyclobutene Synthesis: Catalytic Enantioselective [2 + 2] Cycloadditions. Angew. Chem. Int. Ed. 2015, 54, 11918–11928. [Google Scholar] [CrossRef]
- Oppolzer, W.; Godel, T. A New and Efficient Total Synthesis of (±)-Longifolene. J. Am. Chem. Soc. 1978, 100, 2583–2584. [Google Scholar] [CrossRef]
- Wender, P.A.; Lechleiter, J.C. Total Synthesis of (+)-Isabelin. J. Am. Chem. Soc. 1980, 102, 6340–6341. [Google Scholar] [CrossRef]
- Danheiser, R.L.; Gee, S.K. A Regiocontrolled Annulation Approach to Highly Substituted Aromatic Compounds. J. Org. Chem. 1984, 49, 1672–1674. [Google Scholar] [CrossRef]
- Krysan, D.J.; Gurski, A.; Liebeskind, L.S. A Synthesis of Highly Substituted Aromatics Through Regiocontrolled Construction of Cyclobutenones Bearing Unsaturated Substituents at the 4-Position. J. Am. Chem. Soc. 1992, 114, 1412–1418. [Google Scholar] [CrossRef]
- Dowd, P.; Zhang, W.; Geib, S.J. Free Radical Annulation of Dichlorocyclobutanones with Sequential Ring Expansion. Tetrahedron 1995, 51, 3435–3454. [Google Scholar] [CrossRef]
- Dowd, P.; Zhang, W.; Mahmood, K. Cyclobutanone-Based Tandem Free Radical Rearrangements: Formation of Bicyclic and Tricyclic Ketones. Tetrahedron 1995, 51, 39–50. [Google Scholar] [CrossRef]
- Kakiuchi, K.; Horiguchi, T.; Minato, K.; Tobe, Y.; Kurosawa, H. Skeletal Rearrangement of 8-Methylenebicyclo [4.2.0] octan-2-ones with Mercury (II) Perchlorate. J. Org. Chem. 1995, 60, 6557–6562. [Google Scholar] [CrossRef]
- Kakiuchi, K.; Nakamura, I.; Matsuo, F.; Nakata, M.; Ogura, M.; Tobe, Y.; Kurosawa, H. Total Synthesis of (±)-Tetramethylmediterraneol B. J. Org. Chem. 1995, 60, 3318–3333. [Google Scholar] [CrossRef]
- Crimmins, M.T.; Pace, J.M.; Nantermet, P.G.; Kim-Meade, A.S.; Thomas, J.B.; Watterson, S.H.; Wagman, A.S. Total Synthesis of (±)-Ginkgolide B. J. Am. Chem. Soc. 1999, 121, 10249–10250. [Google Scholar] [CrossRef]
- Randall, M.L.; Lo, P.C.-K.; Bonitatebus, P.J.; Snapper, M.L. [2 + 2] Photocycloaddition/Thermal Retrocycloaddition. A New Entry into Functionalized 5-8-5 Ring Systems. J. Am. Chem. Soc. 1999, 121, 4534–4535. [Google Scholar] [CrossRef]
- Crimmins, M.T.; Hauser, E.B. Synthesis of Crossed [2 + 2] Photocycloadducts: A Novel Approach to the Synthesis of Bridged Bicyclic Alkenes. Org. Lett. 2000, 2, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Tsuruta, T.; Ito, Y. Lactone Formation by Rhodium-Catalyzed C–C Bond Cleavage of Cyclobutanone. Angew. Chem. Int. Ed. 2000, 39, 2484–2486. [Google Scholar] [CrossRef]
- White, J.D.; Kim, J.; Drapela, N.E. Enantiospecific Synthesis of (+)-Byssochlamic Acid, a Nonadride from the Ascomycete Byssochlamys fulva. J. Am. Chem. Soc. 2000, 122, 8665–8671. [Google Scholar] [CrossRef]
- Inoue, M.; Sato, T.; Hirama, M. Total Synthesis of Merrilactone A. J. Am. Chem. Soc. 2003, 125, 10772–10773. [Google Scholar] [CrossRef]
- Namyslo, J.C.; Kaufmann, D.E. The Application of Cyclobutane Derivatives in Organic Synthesis. Chem. Rev. 2003, 103, 1485–1538. [Google Scholar] [CrossRef]
- Inoue, M.; Sato, T.; Hirama, M. Asymmetric Total Synthesis of (−)-Merrilactone A: Use of a Bulky Protecting Group as Long-Range Stereocontrolling Element. Angew. Chem. Int. Ed. 2006, 45, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Shipe, W.D.; Sorensen, E.J. Convergent, Enantioselective Syntheses of Guanacastepenes A and E Featuring a Selective Cyclobutane Fragmentation. J. Am. Chem. Soc. 2006, 128, 7025–7035. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.T.; Johnson, J.S. Formal [4 + 2] Cycloaddition of Donor–Acceptor Cyclobutanes and Aldehydes: Stereoselective Access to Substituted Tetrahydropyrans. J. Am. Chem. Soc. 2009, 131, 14202–14203. [Google Scholar] [CrossRef] [PubMed]
- Seiser, T.; Saget, T.; Tran, D.N.; Cramer, N. Cyclobutanes in Catalysis. Angew. Chem. Int. Ed. 2011, 50, 7740–7752. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Rainey, T.J. Pd (II)/Brønsted Acid Catalyzed Enantioselective Allylic C–H Activation for the Synthesis of Spirocyclic Rings. J. Am. Chem. Soc. 2012, 134, 3615–3618. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.J.; Njardarson, J.T. Recent Advances in the Metal-Catalyzed Ring Expansions of Three- and Four-Membered Rings. ACS Catal. 2013, 3, 272–286. [Google Scholar] [CrossRef]
- De Nanteuil, F.; De Simone, F.; Frei, R.; Benfatti, F.; Serrano, E.; Waser, J. Cyclization and Annulation Reactions of Nitrogen-Substituted Cyclopropanes and Cyclobutanes. Chem. Commun. 2014, 50, 10912–10928. [Google Scholar] [CrossRef] [Green Version]
- Cramer, N. 5.23 Anion- and Metal-Promoted Rearrangements of Small-Ring Systems. In Comprehensive Organic Synthesis II, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1077–1105. [Google Scholar] [CrossRef]
- Marek, I.; Masarwa, A.; Delaye, P.-O.; Leibeling, M. Selective Carbon–Carbon Bond Cleavage for the Stereoselective Synthesis of Acyclic Systems. Angew. Chem. Int. Ed. 2015, 54, 414–429. [Google Scholar] [CrossRef]
- Vemula, N.; Pagenkopf, B.L. Synthesis and Reactivity of Alkoxy-Activated Cyclobutane-1,1-Dicarboxylates. Org. Chem. Front. 2016, 3, 1205–1212. [Google Scholar] [CrossRef]
- Muratore, M.E.; Homs, A.; Obradors, C.; Echavarren, A.M. Meeting the Challenge of Intermolecular Gold(I)-Catalyzed Cycloadditions of Alkynes and Allenes. Chem. Asian J. 2014, 9, 3066–3082. [Google Scholar] [CrossRef] [Green Version]
- Ahlsten, N.; Cambeiro, X.C.; Perry, G.J.P.; Larrosa, I. C–H Functionalisation of Heteroaromatic Compounds via Gold Catalysis. In Au-Catalyzed Synthesis and Functionalization of Heterocycles; Bandini, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–226. [Google Scholar] [CrossRef]
- Arcadi, A. Gold-Catalyzed Synthesis of Nitrogen Heterocyclic Compounds via Hydroamination Reactions. In Au-Catalyzed Synthesis and Functionalization of Heterocycles; Bandini, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 53–85. [Google Scholar] [CrossRef]
- Asiri, A.M.; Hashmi, A.S.K. Gold-Catalysed Reactions of Diynes. Chem. Soc. Rev. 2016, 45, 4471–4503. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, M.N.; Tlahuext-Aca, A.; Glorius, F. Merging Visible Light Photoredox and Gold Catalysis. Acc. Chem. Res. 2016, 49, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S. Recent Advances in Gold-Catalyzed Intermolecular Aryl C–H Functionalization. Chem. Eur. J. 2016, 22, 15584–15598. [Google Scholar] [CrossRef] [PubMed]
- Mascareñas, J.L.; López, F. Synthesis of Oxygenated Heterocyclic Compounds via Gold-Catalyzed Functionalization of π-Systems. In Au-Catalyzed Synthesis and Functionalization of Heterocycles; Bandini, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–52. [Google Scholar] [CrossRef]
- Miró, J.; del Pozo, C. Fluorine and Gold: A Fruitful Partnership. Chem. Rev. 2016, 116, 11924–11966. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Cao, Z.-Y.; Zhou, J. Gold Catalysis in the Synthesis of Natural Products: Heterocycle Construction via Direct C–X-Bond-Forming Reactions. In Au-Catalyzed Synthesis and Functionalization of Heterocycles; Bandini, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 249–283. [Google Scholar] [CrossRef]
- Shahzad, S.A.; Sajid, M.A.; Khan, Z.A.; Canseco-González, D. Gold Catalysis in Organic Transformations: A Review. Synth. Commun. 2017, 47, 735–755. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, M. Synthesis of Cyclic and Heterocyclic Compounds via Gold-Catalyzed Reactions. Synlett 2017, 28, 2230–2240. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, C.; Ye, L.-W. Recent Advances in Dual Visible Light Photoredox and Gold-Catalyzed Reactions. Synthesis 2017, 49, 1150–1157. [Google Scholar] [CrossRef]
- Fang, W.; Shi, M. Recent Advances in the Cycloisomerizations of Methylenecyclopropanes using Gold Catalysis. Chem. Eur. J. 2018, 24, 9998–10005. [Google Scholar] [CrossRef]
- Fürstner, A. Gold Catalysis for Heterocyclic Chemistry: A Representative Case Study on Pyrone Natural Products. Angew. Chem. Int. Ed. 2018, 57, 4215–4233. [Google Scholar] [CrossRef]
- Murakami, R.; Inagaki, F. Recent Topics of Gold Catalyst Featuring Z-Type Ligands. Tetrahedron Lett. 2019, 60, 151231. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L. Gold-Catalyzed Cascade Reactions. In Catalytic Cascade Reactions; Peng-Fei, X., Wang, W., Eds.; Wiley-VCH: Sttutgart, Germany, 2013; pp. 145–177. [Google Scholar] [CrossRef]
- Brenzovich, W.E., Jr. Gold in Total Synthesis: Alkynes as Carbonyl Surrogates. Angew. Chem. Int. Ed. 2012, 51, 8933–8935. [Google Scholar] [CrossRef] [PubMed]
- Bellavance, G.; Barriault, L. Total Syntheses of Hyperforin and Papuaforins A–C, and Formal Synthesis of Nemorosone through a Gold (I)-Catalyzed Carbocyclization. Angew. Chem. Int. Ed. 2014, 53, 6701–6704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, T.; Yang, Z. Strategic Innovation in the Total Synthesis of Complex Natural Products Using Gold Catalysis. Nat. Prod. Rep. 2014, 31, 489–503. [Google Scholar] [CrossRef]
- Hoffmann-Röder, A.; Krause, N. The Golden Gate to Catalysis. Org. Biomol. Chem. 2005, 3, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Krause, N.; Belting, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Hoffmann-Röder, A.; Morita, N.; Volz, F. Golden opportunities in catalysis. Pure Appl. Chem. 2008, 80, 1063–1069. [Google Scholar] [CrossRef]
- Melchiorre, P.; Marigo, M.; Carlone, A.; Bartoli, G. Asymmetric Aminocatalysis—Gold Rush in Organic Chemistry. Angew. Chem. Int. Ed. 2008, 47, 6138–6171. [Google Scholar] [CrossRef]
- Yanagisawa, A. Ag (I), Au (I) Lewis Acids. In Lewis Acids in Organic Synthesis; Yamamoto, H., Ed.; Wile-VCH: Sttutgart, Germany, 2008; pp. 575–596. [Google Scholar] [CrossRef]
- Sengupta, S.; Shi, X. Recent Advances in Asymmetric Gold Catalysis. ChemCatChem 2010, 2, 609–619. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, Y.; Liu, H. Recent Advances in the Gold-Catalyzed Additions to C–C Multiple Bonds. Beilstein J. Org. Chem. 2011, 7, 897–936. [Google Scholar] [CrossRef] [Green Version]
- Pradal, A.; Toullec, P.Y.; Michelet, V. Recent Developments in Asymmetric Catalysis in the Presence of Chiral Gold Complexes. Synthesis 2011, 1501–1514. [Google Scholar] [CrossRef]
- Barbazanges, M.; Fensterbank, L. Chiral Acyclic Diaminocarbene Complexes: A New Opportunity for Gold Asymmetric Catalysis. ChemCatChem 2012, 4, 1065–1066. [Google Scholar] [CrossRef]
- Huguet, N.; Echavarren, A.M. Asymmetric Gold-Catalyzed Reactions. In Asymmetric Synthesis II; Christmann, M., Brase, S., Eds.; Wiley-VCH: Sttutgart, Germany, 2012; pp. 205–211. [Google Scholar] [CrossRef]
- Cera, G.; Bandini, M. Enantioselective Gold (I) Catalysis with Chiral Monodentate Ligands. Isr. J. Chem. 2013, 53, 848–855. [Google Scholar] [CrossRef]
- Gu, P.; Xu, Q.; Shi, M. Development and Outlook of Chiral Carbene–Gold (I) Complexes Catalyzed Asymmetric Reactions. Tetrahedron Lett. 2014, 55, 577–584. [Google Scholar] [CrossRef]
- Jia, M.; Bandini, M. Counterion Effects in Homogeneous Gold Catalysis. ACS Catal. 2015, 5, 1638–1652. [Google Scholar] [CrossRef]
- Miles, D.H.; Toste, F.D. Enantioselective Gold-Catalyzed Synthesis of Heterocyclic Compounds. In Au-Catalyzed Synthesis and Functionalization of Heterocycles; Bandini, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 227–248. [Google Scholar] [CrossRef]
- Michon, C.; Abadie, M.-A.; Medina, F.; Agbossou-Niedercorn, F. Recent Metal-Catalysed Asymmetric Hydroaminations of Alkenes. J. Organomet. Chem. 2017, 847, 13–27. [Google Scholar] [CrossRef]
- Rodriguez, J.; Bourissou, D. Well-Defined Chiral Gold (III) Complexes: New Opportunities in Asymmetric Catalysis. Angew. Chem. Int. Ed. 2018, 57, 386–388. [Google Scholar] [CrossRef] [PubMed]
- López, F.; Mascareñas, J.L. Gold (I)-Catalyzed Enantioselective Cycloaddition Reactions. Beilstein J. Org. Chem. 2013, 9, 2250–2264. [Google Scholar] [CrossRef]
- Hong, Y.J.; Tantillo, D.J. How Cyclobutanes are Assembled in Nature—Insights from Quantum Chemistry. Chem. Soc. Rev. 2014, 43, 5042–5050. [Google Scholar] [CrossRef]
- Wang, D.; Cai, R.; Sharma, S.; Jirak, J.; Thummanapelli, S.K.; Akhmedov, N.G.; Zhang, H.; Liu, X.; Petersen, J.L.; Shi, X. “Silver Effect” in Gold (I) Catalysis: An Overlooked Important Factor. J. Am. Chem. Soc. 2012, 134, 9012–9019. [Google Scholar] [CrossRef]
- Landor, S.R. The Chemistry of Allenes; Academic Press: London, United Kingdom, 1982; Volume 1. [Google Scholar]
- Pasto, D.J. Recent Developments in Allene Chemistry. Tetrahedron 1984, 40, 2805–2827. [Google Scholar] [CrossRef]
- Zimmer, R.; Dinesh, C.U.; Nandanan, E.; Khan, F.A. Palladium-Catalyzed Reactions of Allenes. Chem. Rev. 2000, 100, 3067–3126. [Google Scholar] [CrossRef]
- Reissig, H.-U.; Schade, W.; Amombo, G.M.O.; Pulz, R.; Hausherr, A. Stereoselective syntheses of heterocycles via metallated alkoxyallenes. Pure Appl. Chem. 2002, 74, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Krause, N.; Hashmi, A.S.K. (Eds.) Modern Allene Chemistry. In Modern Allene Chemistry; Wiley-VCH: Sttutgart, Germany, 2004; pp. 1–995. [Google Scholar]
- Hoffmann-Röder, A.; Krause, N. Synthesis and Properties of Allenic Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2004, 43, 1196–1216. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Some Typical Advances in the Synthetic Applications of Allenes. Chem. Rev. 2005, 105, 2829–2872. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Palladium-Catalyzed Two- or Three-Component Cyclization of Functionalized Allenes. In Palladium in Organic Synthesis; Tsuji, J., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2005; pp. 183–210. [Google Scholar] [CrossRef]
- Krause, N. Cumulenes and Allenes. In Science of Synthesis; Thieme Chemistry: Stuttgart, Germany, 2008; Volume 44, p. 1. [Google Scholar] [CrossRef]
- Brasholz, M.; Reissig, H.-U.; Zimmer, R. Sugars, Alkaloids, and Heteroaromatics: Exploring Heterocyclic Chemistry with Alkoxyallenes. Acc. Chem. Res. 2009, 42, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Mascareñas, J.L.; Varela, I.; López, F. Allenes and Derivatives in Gold (I)- and Platinum (II)-Catalyzed Formal Cycloadditions. Acc. Chem. Res. 2019, 52, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Tius, M.A. Variation 1: Intermolecular Reactions to form Carbocyclic Products. In Science of Synthesis; Thieme Chemistry: Stuttgart, Germany, 2008; Volume 44, p. 353. [Google Scholar] [CrossRef]
- Hoffmann-Röder, A.; Krause, N. Enantioselective Synthesis of and with Allenes. Angew. Chem. Int. Ed. 2002, 41, 2933–2935. [Google Scholar] [CrossRef]
- Yu, S.; Ma, S. How Easy are the Syntheses of Allenes? Chem. Commun. 2011, 47, 5384–5418. [Google Scholar] [CrossRef]
- Yu, S.; Ma, S. Allenes in Catalytic Asymmetric Synthesis and Natural Product Syntheses. Angew. Chem. Int. Ed. 2012, 51, 3074–3112. [Google Scholar] [CrossRef]
- Luzung, M.R.; Mauleón, P.; Toste, F.D. Gold (I)-Catalyzed [2 + 2]-Cycloaddition of Allenenes. J. Am. Chem. Soc. 2007, 129, 12402–12403. [Google Scholar] [CrossRef]
- González, A.Z.; Benitez, D.; Tkatchouk, E.; Goddard, W.A.; Toste, F.D. Phosphoramidite Gold (I)-Catalyzed Diastereo- and Enantioselective Synthesis of 3,4-Substituted Pyrrolidines. J. Am. Chem. Soc. 2011, 133, 5500–5507. [Google Scholar] [CrossRef] [Green Version]
- Teller, H.; Corbet, M.; Mantilli, L.; Gopakumar, G.; Goddard, R.; Thiel, W.; Fürstner, A. One-Point Binding Ligands for Asymmetric Gold Catalysis: Phosphoramidites with a TADDOL-Related but Acyclic Backbone. J. Am. Chem. Soc. 2012, 134, 15331–15342. [Google Scholar] [CrossRef] [PubMed]
- Teller, H.; Flügge, S.; Goddard, R.; Fürstner, A. Enantioselective Gold Catalysis: Opportunities Provided by Monodentate Phosphoramidite Ligands with an Acyclic TADDOL Backbone. Angew. Chem. Int. Ed. 2010, 49, 1949–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcarazo, M.; Stork, T.; Anoop, A.; Thiel, W.; Fürstner, A. Steering the Surprisingly Modular π-Acceptor Properties of N-Heterocyclic Carbenes: Implications for Gold Catalysis. Angew. Chem. Int. Ed. 2010, 49, 2542–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, I.; Trillo, B.; López, F.; Montserrat, S.; Ujaque, G.; Castedo, L.; Lledós, A.; Mascareñas, J.L. Gold-Catalyzed [4C + 2C] Cycloadditions of Allenedienes, including an Enantioselective Version with New Phosphoramidite-Based Catalysts: Mechanistic Aspects of the Divergence between [4C + 3C] and [4C + 2C] Pathways. J. Am. Chem. Soc. 2009, 131, 13020–13030. [Google Scholar] [CrossRef] [PubMed]
- Christian, A.H.; Niemeyer, Z.L.; Sigman, M.S.; Toste, F.D. Uncovering Subtle Ligand Effects of Phosphines Using Gold (I) Catalysis. ACS Catal. 2017, 7, 3973–3978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-F.; Loh, T.-P. Acid-Catalyzed Intramolecular [2 + 2] Cycloaddition of Ene-allenones: Facile Access to Bicyclo [n.2.0] Frameworks. Angew. Chem. Int. Ed. 2009, 48, 7232–7235. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.-Y.; Wei, Y.; Tang, X.-Y.; Shi, M. Catalyst-Dependent Stereodivergent and Regioselective Synthesis of Indole-Fused Heterocycles through Formal Cycloadditions of Indolyl-Allenes. J. Am. Chem. Soc. 2015, 137, 8131–8137. [Google Scholar] [CrossRef]
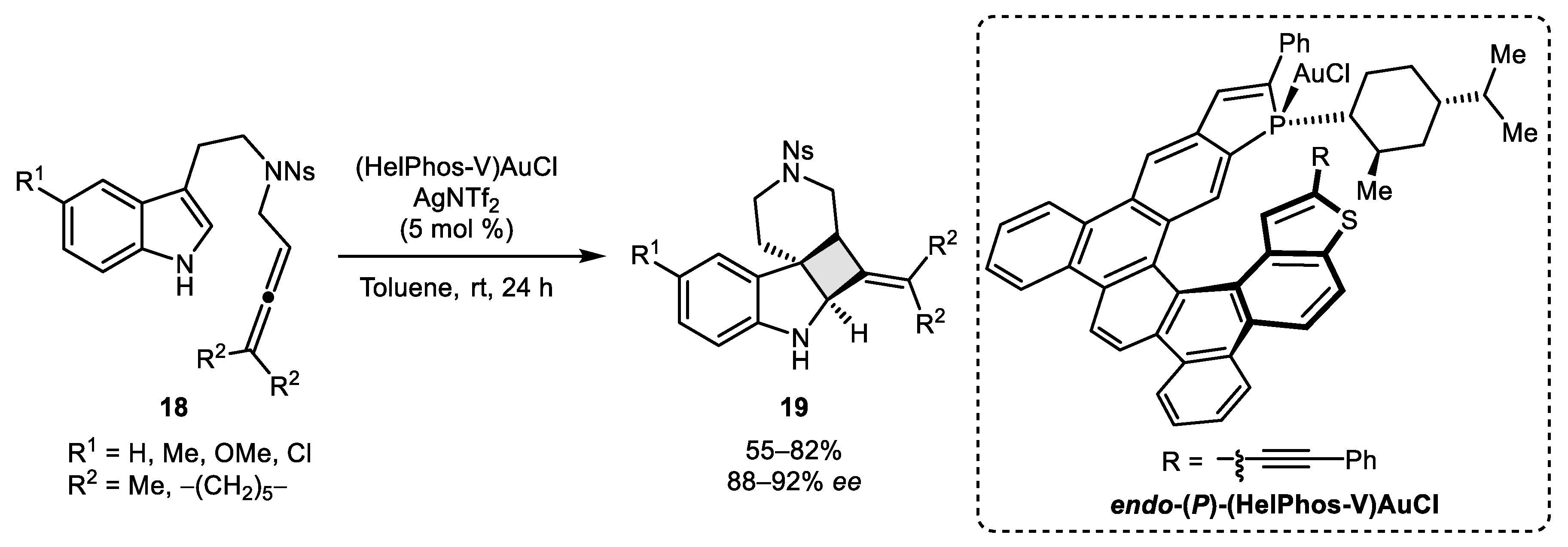
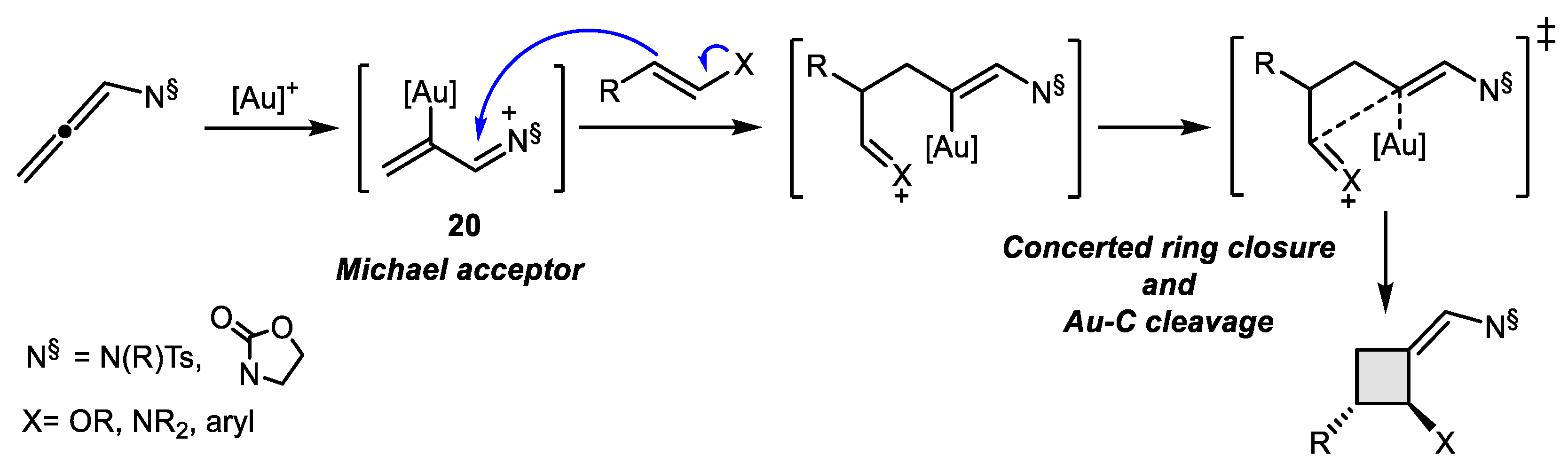
- Magné, V.; Sanogo, Y.; Demmer, C.S.; Retailleau, P.; Marinetti, A.; Guinchard, X.; Voituriez, A. Chiral Phosphathiahelicenes: Improved Synthetic Approach and Uses in Enantioselective Gold (I)-Catalyzed [2 + 2] Cycloadditions of N-Homoallenyl Tryptamines. ACS Catal. 2020. [Google Scholar] [CrossRef]
- Aillard, P.; Retailleau, P.; Voituriez, A.; Marinetti, A. Synthesis of New Phosphahelicene Scaffolds and Development of Gold (I)-Catalyzed Enantioselective Allenene Cyclizations. Chem. Eur. J. 2015, 21, 11989–11993. [Google Scholar] [CrossRef]
- Faustino, H.; Bernal, P.; Castedo, L.; López, F.; Mascareñas, J.L. Gold (I)-Catalyzed Intermolecular [2 + 2] Cycloadditions between Allenamides and Alkenes. Adv. Synth. Catal. 2012, 354, 1658–1664. [Google Scholar] [CrossRef]
- Li, X.-X.; Zhu, L.-L.; Zhou, W.; Chen, Z. Formal Intermolecular [2 + 2] Cycloaddition Reaction of Alleneamides with Alkenes via Gold Catalysis. Org. Lett. 2012, 14, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Pantiga, S.; Hernández-Díaz, C.; Piedrafita, M.; Rubio, E.; González, J.M. Phosphite-Gold (I)-Catalyzed [2 + 2] Intermolecular Cycloaddition of Enol Ethers with N-Allenylsulfonamides. Adv. Synth. Catal. 2012, 354, 1651–1657. [Google Scholar] [CrossRef]
- Mauleón, P. Gold-Catalyzed Intermolecular Enantioselective [2 + 2] Cycloadditions of Sulfonylallenamides and Styrenes. ChemCatChem 2013, 5, 2149–2151. [Google Scholar] [CrossRef]
- Bernal-Albert, P.; Faustino, H.; Gimeno, A.; Asensio, G.; Mascareñas, J.L.; López, F. Gold (I)-Catalyzed Intermolecular Cycloaddition of Allenamides with α,β-Unsaturated Hydrazones: Efficient Access to Highly Substituted Cyclobutanes. Org. Lett. 2014, 16, 6196–6199. [Google Scholar] [CrossRef]
- Suárez-Pantiga, S.; Hernández-Díaz, C.; Rubio, E.; González, J.M. Intermolecular [2 + 2] Reaction of N-Allenylsulfonamides with Vinylarenes: Enantioselective Gold (I)-Catalyzed Synthesis of Cyclobutane Derivatives. Angew. Chem. Int. Ed. 2012, 51, 11552–11555. [Google Scholar] [CrossRef]
- Faustino, H.; Alonso, I.; Mascareñas, J.L.; López, F. Gold (I)-Catalyzed Cascade Cycloadditions between Allenamides and Carbonyl-Tethered Alkenes: An Enantioselective Approach to Oxa-Bridged Medium-Sized Carbocycles. Angew. Chem. Int. Ed. 2013, 52, 6526–6530. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.-C.; Jin, R.; Chen, B.-L.; Chen, Z. Synthesis of Axially Chiral 1,2,3-Triazol-5-ylidene–Au (I) Complex and Its Application in Enantioselective [2 + 2] Cycloaddition of Alleneamides with Alkenes. Organometallics 2018, 37, 3196–3209. [Google Scholar] [CrossRef]
- Jia, M.; Monari, M.; Yang, Q.-Q.; Bandini, M. Enantioselective Gold Catalyzed Dearomative [2 + 2]-Cycloaddition Between Indoles and Allenamides. Chem. Commun. 2015, 51, 2320–2323. [Google Scholar] [CrossRef]
- Ocello, R.; De Nisi, A.; Jia, M.; Yang, Q.-Q.; Monari, M.; Giacinto, P.; Bottoni, A.; Miscione, G.P.; Bandini, M. Gold (I)-Catalyzed Dearomative [2 + 2]-Cycloaddition of Indoles with Activated Allenes: A Combined Experimental–Computational Study. Chem. Eur. J. 2015, 21, 18445–18453. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Liu, Y.; Xia, F.; Zhang, J. Enantioselective Gold-Catalyzed Intermolecular [2 + 2] versus [4 + 2]-Cycloadditions of 3-Styrylindoles with N-Allenamides: Observation of Interesting Substituent Effects. Chem. Sci. 2015, 6, 5564–5570. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Wang, Y.; Qian, D.; Zhang, Z.-M.; Liu, L.; Zhang, J. Enantioselective Gold-Catalyzed Intermolecular [2 + 2]-Cycloadditions of 3-Styrylindoles with N-Allenyl Oxazolidinone. Org. Chem. Front. 2016, 3, 759–763. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Wieteck, M.; Braun, I.; Rudolph, M.; Rominger, F. Gold Vinylidene Complexes: Intermolecular C (sp3)-H Insertions and Cyclopropanations Pathways. Angew. Chem. Int. Ed. 2012, 51, 10633–10637. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Park, J.H.; Kang, Y.K.; Chung, Y.K. N-Heterocyclic Carbene Gold (I) Catalyzed Transformation of N-Tethered 1, 5-Bisallenes to 6, 7-Dimethylene-3-azabicyclo [3.1.1] heptanes. Angew. Chem. Int. Ed. 2009, 48, 4532–4535. [Google Scholar] [CrossRef] [PubMed]
- Obradors, C.; Echavarren, A.M. Gold-Catalyzed Rearrangements and Beyond. Acc. Chem. Res. 2014, 47, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Mézailles, N.; Ricard, L.; Gagosz, F. Phosphine Gold (I) Bis- (trifluoromethanesulfonyl) imidate Complexes as New Highly Efficient and Air-Stable Catalysts for the Cycloisomerization of Enynes. Org. Lett. 2005, 7, 4133–4136. [Google Scholar] [CrossRef]
- Nieto-Oberhuber, C.; López, S.; Echavarren, A.M. Intramolecular [4 + 2] Cycloadditions of 1, 3-Enynes or Arylalkynes with Alkenes with Highly Reactive Cationic Phosphine Au (I) Complexes. J. Am. Chem. Soc. 2005, 127, 6178–6179. [Google Scholar] [CrossRef]
- Couty, S.; Meyer, C.; Cossy, J. Diastereoselective Gold-Catalyzed Cycloisomerizations of Ene-Ynamides. Angew. Chem. Int. Ed. 2006, 45, 6726–6730. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, S.M.; Choi, M.R.; Kim, S.Y.; Chung, Y.K.; Han, W.-S.; Kang, S.O. Au (I)-Catalyzed Cyclization of Enynes Bearing an Olefinic Cycle. J. Org. Chem. 2006, 71, 9366–9372. [Google Scholar] [CrossRef]
- Nieto-Oberhuber, C.; Pérez-Galán, P.; Herrero-Gómez, E.; Lauterbach, T.; Rodríguez, C.; López, S.; Bour, C.; Rosellón, A.; Cárdenas, D.J.; Echavarren, A.M. Gold (I)-Catalyzed Intramolecular [4 + 2] Cycloadditions of Arylalkynes or 1, 3-Enynes with Alkenes: Scope and Mechanism. J. Am. Chem. Soc. 2008, 130, 269–279. [Google Scholar] [CrossRef]
- Couty, S.; Meyer, C.; Cossy, J. Gold-Catalyzed Cycloisomerizations of Ene-Ynamides. Tetrahedron 2009, 65, 1809–1832. [Google Scholar] [CrossRef]
- Escribano-Cuesta, A.; Pérez-Galán, P.; Herrero-Gómez, E.; Sekine, M.; Braga, A.A.C.; Maseras, F.; Echavarren, A.M. The Role of Cyclobutenes in Gold (I)-Catalysed Skeletal Rearrangement of 1, 6-Enynes. Org. Biomol. Chem. 2012, 10, 6105–6111. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, F.; Matsumoto, C.; Okada, Y.; Maruyama, N.; Mukai, C. Air-Stable Cationic Gold (I) Catalyst Featuring a Z-Type Ligand: Promoting Enyne Cyclizations. Angew. Chem. Int. Ed. 2015, 54, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.D.; Brooner, R.E.M.; Widenhoefer, R.A. Tandem Gold/Silver-Catalyzed Cycloaddition/Hydroarylation of 7-Aryl-1, 6-enynes to Form 6,6-Diarylbicyclo [3.2.0] heptanes. Chem. Eur. J. 2015, 21, 5714–5717. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.-Z.; Tung, P.-T.; Chao, T.-H.; Yeh, M.-C.P. Gold-Catalyzed Stereoselective Synthesis of Bicyclic Lactams and Ketones from N-Tosylynamidomethyl-Tethered Cyclohexenes. J. Org. Chem. 2017, 82, 481–501. [Google Scholar] [CrossRef]
- Nieto-Oberhuber, C.; López, S.; Muñoz, M.P.; Cárdenas, D.J.; Buñuel, E.; Nevado, C.; Echavarren, A.M. Divergent Mechanisms for the Skeletal Rearrangement and [2 + 2] Cycloaddition of Enynes Catalyzed by Gold. Angew. Chem. Int. Ed. 2005, 44, 6146–6148. [Google Scholar] [CrossRef]
- Huple, D.B.; Liu, R.-S. Gold-Catalyzed Diastereoselective [2 + 2 + 2] -Cycloaddition of 1, 7-Enynes with Carbonyl Compounds. Chem. Commun. 2012, 48, 10975–10977. [Google Scholar] [CrossRef]
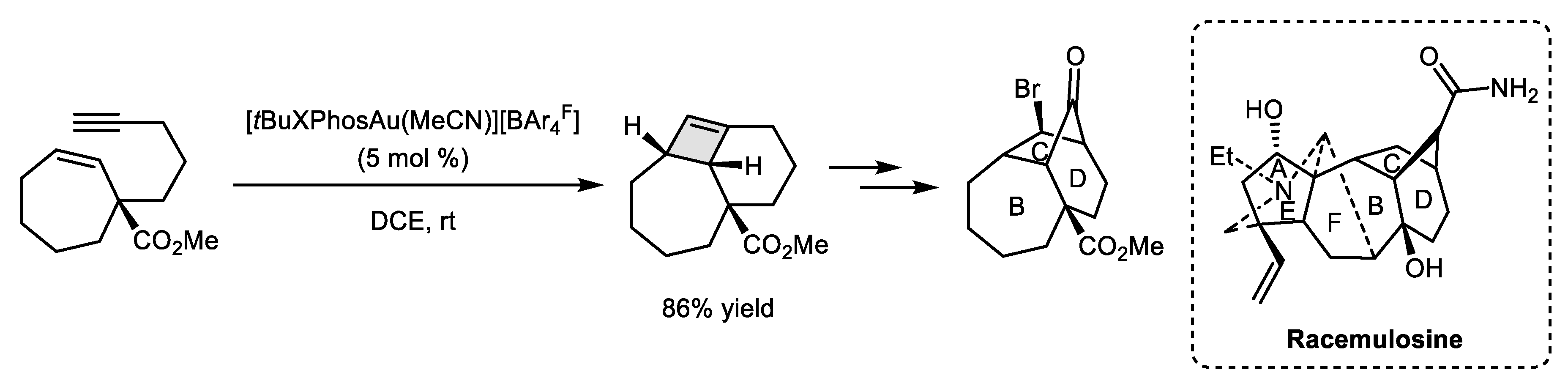
- Cao, S.-Y.; Yue, H.-J.; Zhu, M.-Q.; Xu, L. Synthesis of Tricyclo [7.2.1.09,10] dodecan-11-one Core Ring Systems of Norditerpenoid Alkaloids and Racemulosine. Org. Chem. Front. 2020, 7, 933–937. [Google Scholar] [CrossRef]
- Zang, W.; Wei, Y.; Shi, M. Gold (I)-Catalyzed Cascade Cyclization of O-Tethered 1, 7-Enynes Bearing a Cyclopropane Moiety: Construction of Multi-Substituted Furans. Chem. Commun. 2019, 55, 8126–8129. [Google Scholar] [CrossRef]
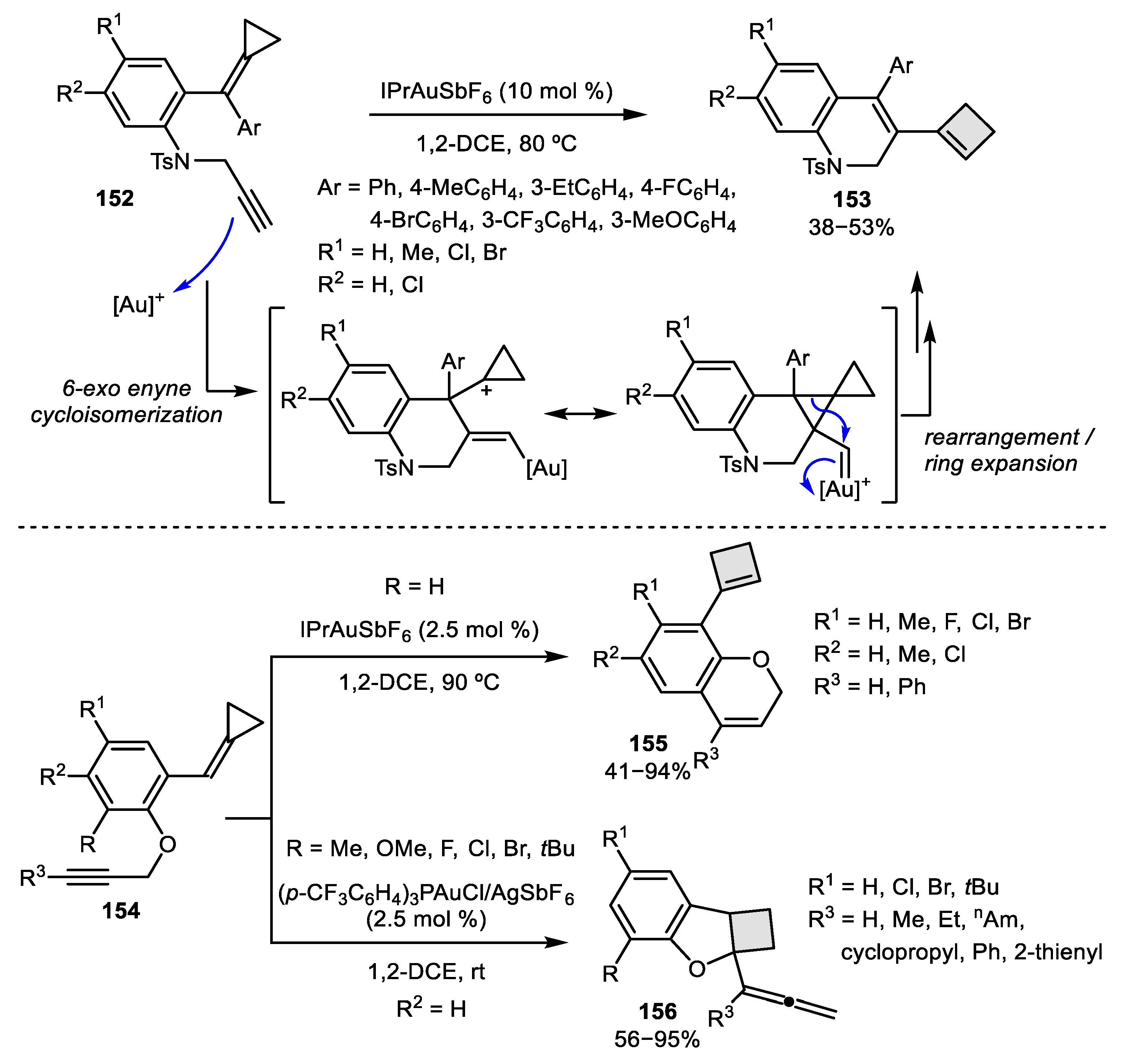
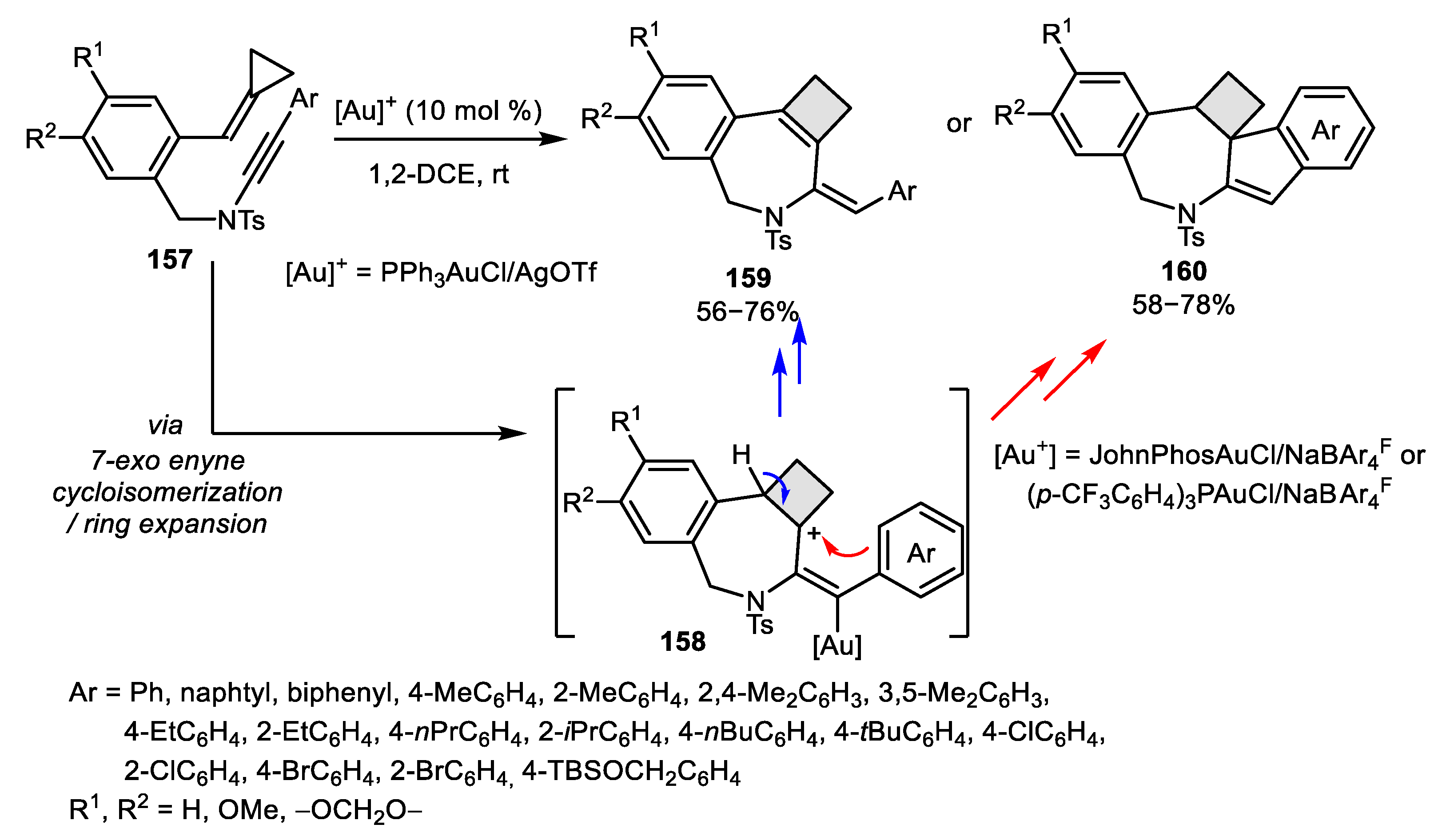
- Odabachian, Y.; Gagosz, F. Cyclobutenes as Isolable Intermediates in the Gold (I)-Catalysed Cycloisomerisation of 1, 8-Enynes. Adv. Synth. Catal. 2009, 351, 379–386. [Google Scholar] [CrossRef]
- Iwai, T.; Ueno, M.; Okochi, H.; Sawamura, M. Synthesis of Cyclobutene-Fused Eight-Membered Carbocycles through Gold-Catalyzed Intramolecular Enyne [2 + 2] Cycloaddition. Adv. Synth. Catal. 2018, 360, 670–675. [Google Scholar] [CrossRef]
- Obradors, C.; Leboeuf, D.; Aydin, J.; Echavarren, A.M. Gold (I)-Catalyzed Macrocyclization of 1, n-Enynes. Org. Lett. 2013, 15, 1576–1579. [Google Scholar] [CrossRef]
- Ranieri, B.; Obradors, C.; Mato, M.; Echavarren, A.M. Synthesis of Rumphellaone A and Hushinone by a Gold-Catalyzed [2 + 2] Cycloaddition. Org. Lett. 2016, 18, 1614–1617. [Google Scholar] [CrossRef] [Green Version]
- García-Morales, C.; Echavarren, A.M. From Straightforward Gold (I)-Catalyzed Enyne Cyclizations to more Demanding Intermolecular Reactions of Alkynes with Alkenes. Synlett 2018, 29, 2225–2237. [Google Scholar] [CrossRef]
- Kim, N.; Brooner, R.E.M.; Widenhoefer, R.A. Unexpected Skeletal Rearrangement in the Gold (I)/Silver (I) -Catalyzed Conversion of 7-Aryl-1, 6-enynes to Bicyclo [3.2.0] hept-6-enes via Hidden Brønsted Acid Catalysis. Organometallics 2017, 36, 673–678. [Google Scholar] [CrossRef]
- Brooner, R.E.M.; Brown, T.J.; Widenhoefer, R.A. Direct Observation of a Cationic Gold (I) –Bicyclo [3.2.0] hept-1 (7) -ene Complex Generated in the Cycloisomerization of a 7-Phenyl-1, 6-enyne. Angew. Chem. Int. Ed. 2013, 52, 6259–6261. [Google Scholar] [CrossRef]
- Brooner, R.E.M.; Robertson, B.D.; Widenhoefer, R.A. Mechanism of 1,3-Hydrogen Migration in a Gold Bicyclo [3.2.0] heptene Complex: The Role of Brønsted Acid in the Gold-Catalyzed Cycloisomerization of 7-Aryl-1, 6-Enynes. Organometallics 2014, 33, 6466–6473. [Google Scholar] [CrossRef]
- Zhang, L. Tandem Au-Catalyzed 3, 3-Rearrangement–[2 + 2] Cycloadditions of Propargylic Esters: Expeditious Access to Highly Functionalized 2, 3-Indoline-Fused Cyclobutanes. J. Am. Chem. Soc. 2005, 127, 16804–16805. [Google Scholar] [CrossRef]
- Rao, W.; Susanti, D.; Chan, P.W.H. Gold-Catalyzed Tandem 1, 3-Migration/[2 + 2] Cycloaddition of 1, 7-Enyne Benzoates to Azabicyclo [4.2.0] oct-5-enes. J. Am. Chem. Soc. 2011, 133, 15248–15251. [Google Scholar] [CrossRef]
- Rao, W.; Koh, M.J.; Kothandaraman, P.; Chan, P.W.H. Gold-Catalyzed Cycloisomerization of 1, 7-Diyne Benzoates to Indeno [1, 2-c] azepines and Azabicyclo [4.2.0] octa-1 (8), 5-dines. J. Am. Chem. Soc. 2012, 134, 10811–10814. [Google Scholar] [CrossRef]
- Niemeyer, Z.L.; Pindi, S.; Khrakovsky, D.A.; Kuzniewski, C.N.; Hong, C.M.; Joyce, L.A.; Sigman, M.S.; Toste, F.D. Parameterization of Acyclic Diaminocarbene Ligands Applied to a Gold (I)-Catalyzed Enantioselective Tandem Rearrangement/Cyclization. J. Am. Chem. Soc. 2017, 139, 12943–12946. [Google Scholar] [CrossRef] [Green Version]
- López-Carrillo, V.; Echavarren, A.M. Gold (I)-Catalyzed Intermolecular [2 + 2] Cycloaddition of Alkynes with Alkenes. J. Am. Chem. Soc. 2010, 132, 9292–9294. [Google Scholar] [CrossRef]
- Grirrane, A.; García, H.; Corma, A.; Álvarez, E. Intermolecular [2 + 2] Cycloaddition of Alkyne-Alkene Catalyzed by Au (I) Complexes. What Are the Catalytic Sites Involved? ACS Catal. 2011, 1, 1647–1653. [Google Scholar] [CrossRef]
- Homs, A.; Obradors, C.; Lebœuf, D.; Echavarren, A.M. Dissecting Anion Effects in Gold (I)-Catalyzed Intermolecular Cycloadditions. Adv. Synth. Catal. 2014, 356, 221–228. [Google Scholar] [CrossRef] [Green Version]
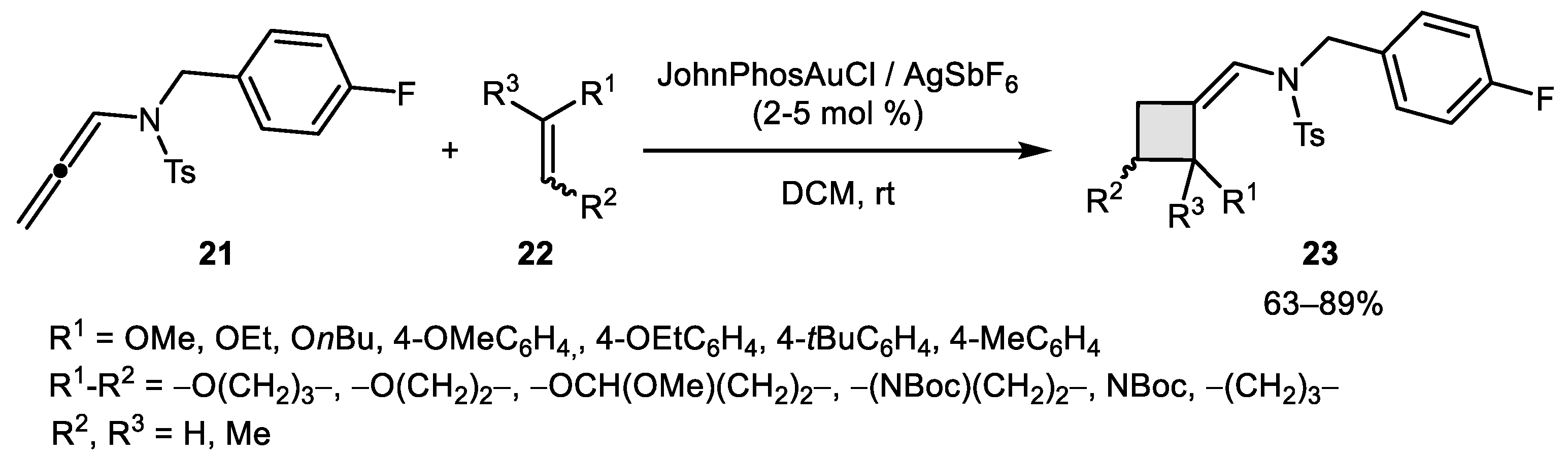
- De Orbe, M.E.; Amenós, L.; Kirillova, M.S.; Wang, Y.; López-Carrillo, V.; Maseras, F.; Echavarren, A.M. Cyclobutene vs 1,3-Diene Formation in the Gold-Catalyzed Reaction of Alkynes with Alkenes: The Complete Mechanistic Picture. J. Am. Chem. Soc. 2017, 139, 10302–10311. [Google Scholar] [CrossRef]
- De Orbe, M.E.; Echavarren, A.M. Broadening the Scope of the Gold-Catalyzed [2 + 2] Cycloaddition Reaction: Synthesis of Vinylcyclobutenes and Further Transformations. Eur. J. Org. Chem. 2018, 2740–2752. [Google Scholar] [CrossRef]
- Bai, Y.-B.; Luo, Z.; Wang, Y.; Gao, J.-M.; Zhang, L. Au-Catalyzed Intermolecular [2 + 2] Cycloadditions between Chloroalkynes and Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 5860–5865. [Google Scholar] [CrossRef]
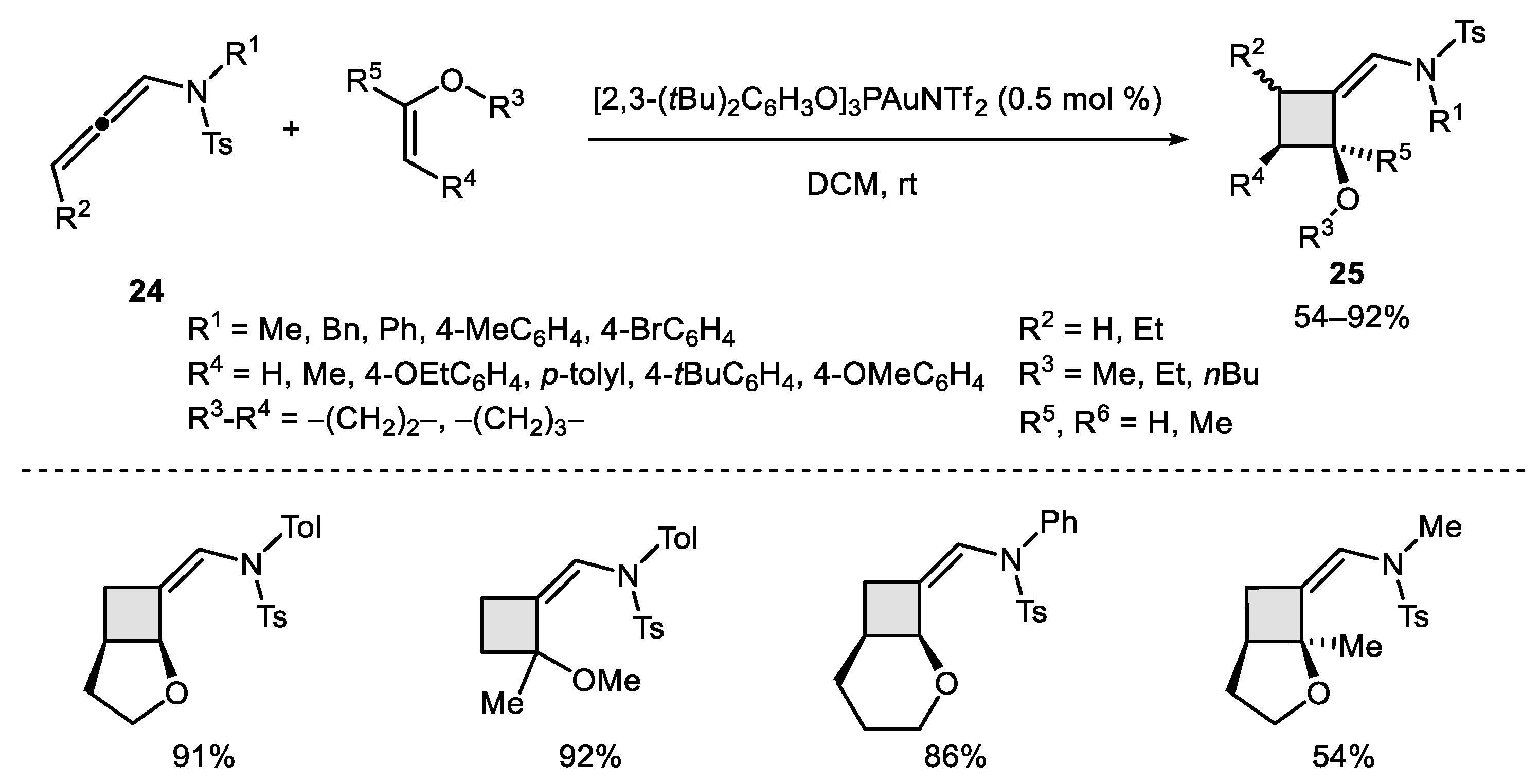
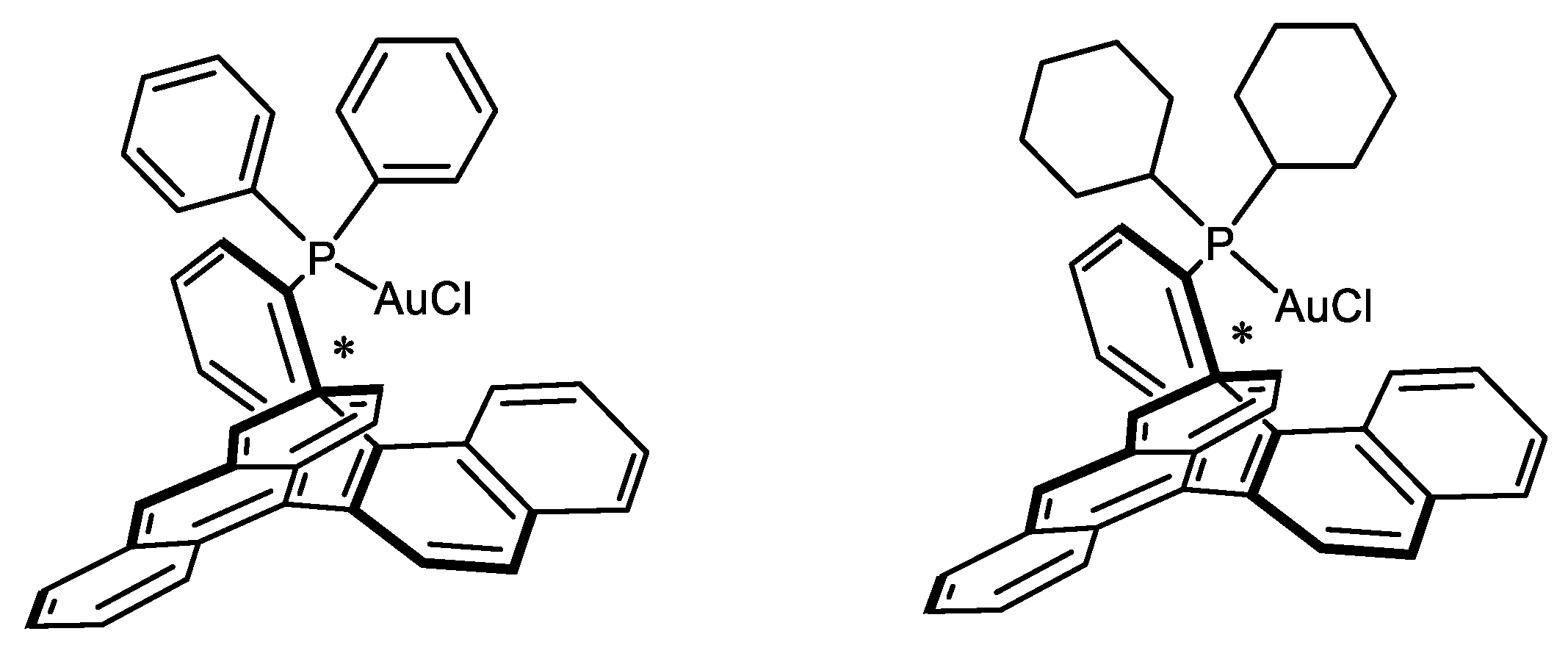
- García-Morales, C.; Ranieri, B.; Escofet, I.; López-Suarez, L.; Obradors, C.; Konovalov, A.I.; Echavarren, A.M. Enantioselective Synthesis of Cyclobutenes by Intermolecular [2 + 2] Cycloaddition with Non-C2 Symmetric Digold Catalysts. J. Am. Chem. Soc. 2017, 139, 13628–13631. [Google Scholar] [CrossRef]
- Castrogiovanni, A.; Lotter, D.; Bissegger, F.R.; Sparr, C. JoyaPhos: An Atropisomeric Teraryl Monophosphine Ligand. Chem. Eur. J. 2020, 26. [Google Scholar] [CrossRef]
- Oh, C.H.; Kim, A. Gold-Catalyzed [2 + 2] Cyclization of Alkyne-propargylic Pivaloates to Fused Bicyclic Compounds. Synlett 2008. [Google Scholar] [CrossRef]
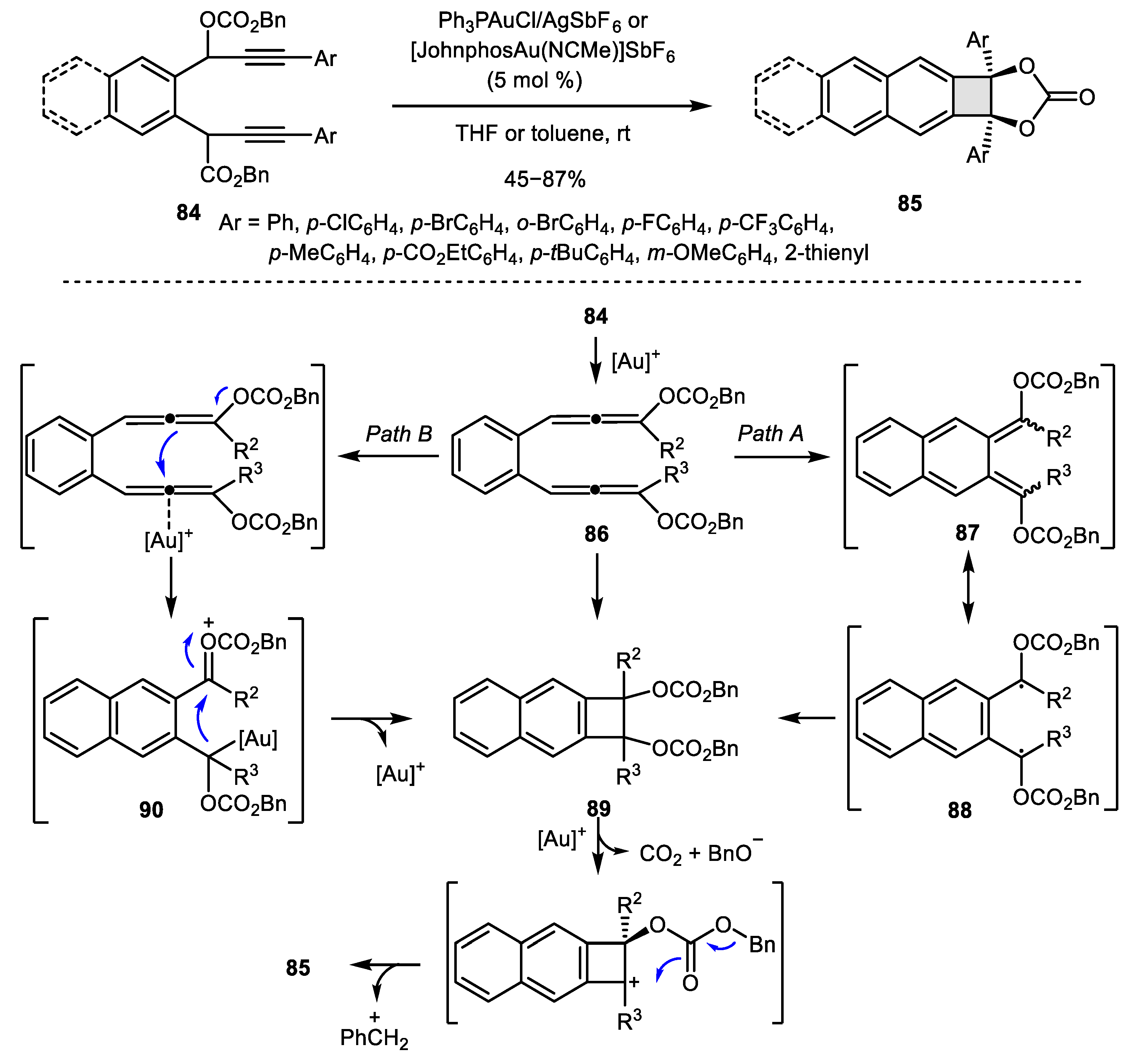
- Chen, M.; Liu, J.; Wang, L.; Zhou, X.; Liu, Y. Gold-Catalyzed Cascade Cycloisomerization of 1, 7-Diyn-3, 6-bis (propargyl carbonate) s: Stereoselective Synthesis of Naphtho [b] cyclobutenes. Chem. Commun. 2013, 49, 8650–8652. [Google Scholar] [CrossRef]
- Nayak, S.; Ghosh, N.; Sahoo, A.K. Access to Cyclobutene-Fused Azepines through Au-Catalyzed Cycloisomerization of Stable Alkyne Tethered Ketene N, N-Acetals. Org. Lett. 2014, 16, 2996–2999. [Google Scholar] [CrossRef]
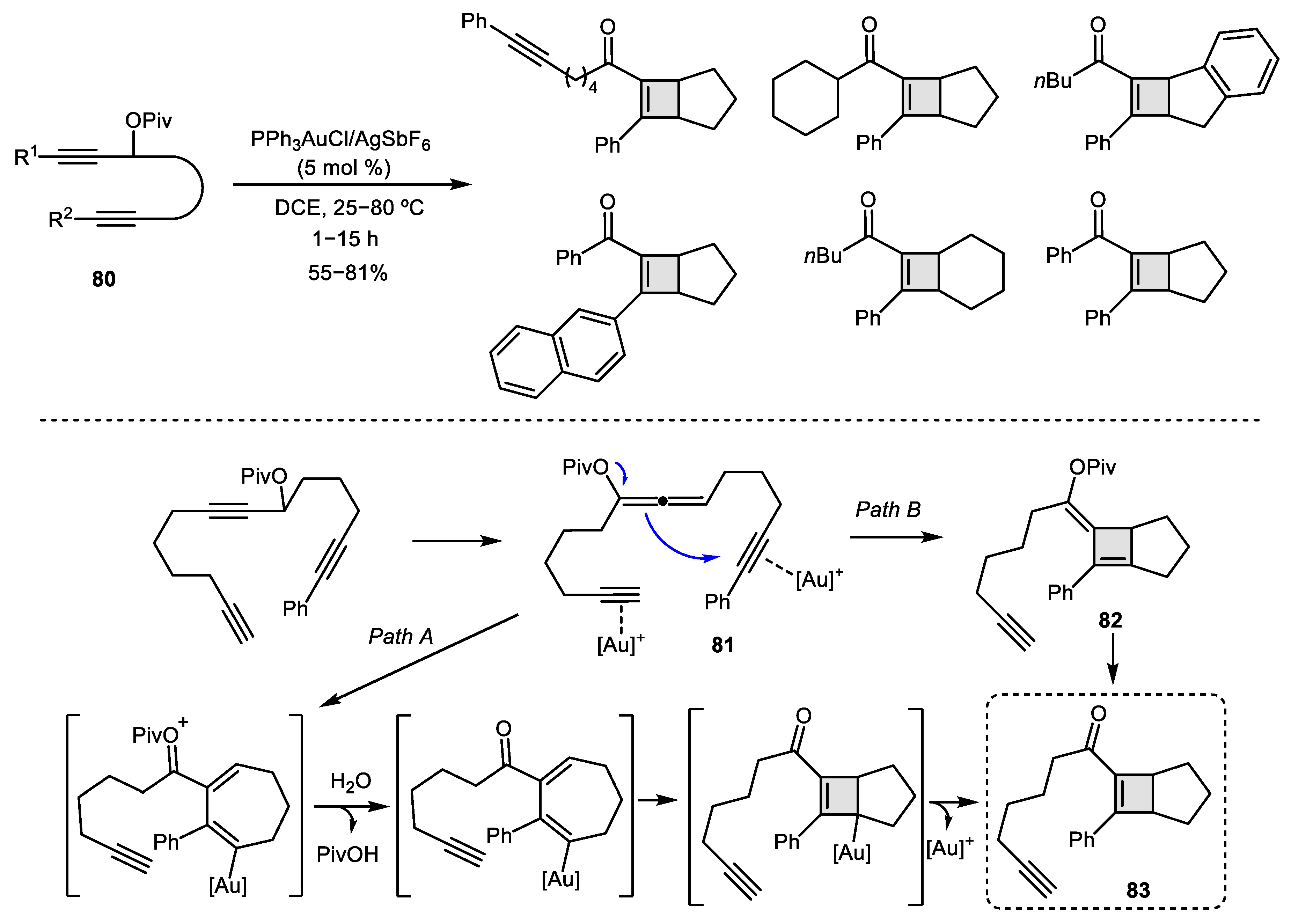
- Li, D.; Rao, W.; Tay, G.L.; Ayers, B.J.; Chan, P.W.H. Gold-Catalyzed Cycloisomerization of 1, 6-Diyne Esters to 1H-Cyclopenta [b] naphthalenes, cis-Cyclopenten-2-yl δ-Diketones, and Bicyclo [3.2.0] hepta-1, 5-dienes. J. Org. Chem. 2014, 79, 11301–11315. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Akhmedov, N.G.; Petersen, J.L.; Shi, X. Ambient Intermolecular [2 + 2] Cycloaddition: An Example of Carbophilicity and Oxophilicity Competition in Au/Ag Catalysis. Org. Lett. 2014, 16, 2478–2481. [Google Scholar] [CrossRef]
- Markham, J.P.; Staben, S.T.; Toste, F.D. Gold (I)-Catalyzed Ring Expansion of Cyclopropanols and Cyclobutanols. J. Am. Chem. Soc. 2005, 127, 9708–9709. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Guo, L.; Wang, Y.-H.; Zhu, L.-L.; Chen, Z.-L. An Efficient Method to Construct 2-vinylidenyl Cyclobutanone Derivatives and its Structural Determination. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2010, 40, 241–245. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Li, X.-X.; Zhou, W.; Li, X.; Chen, Z. Divergent Synthetic Routes for Ring Expansion or Cyclization from 1, 4-Allylic Diol Derivatives via Gold (I) Catalysis or Zinc (II) Mediation. J. Org. Chem. 2011, 76, 8814–8823. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Wang, T.; Shi, S.; Rudolph, M. Regioselectivity Switch: Gold (I)-Catalyzed Oxidative Rearrangement of Propargyl Alcohols to 1, 3-Diketones. J. Org. Chem. 2012, 77, 7761–7767. [Google Scholar] [CrossRef]
- Zang, W.; Wang, L.; Wei, Y.; Shi, M.; Guo, Y. Gold (I)-Catalyzed Ring Expansion of Alkynylcyclopropyl Allyl Ethers to Construct Tetrasubstituted Methylenecyclobutanones: A Mechanistic Investigation about the Character of Catalytic Amount of Water. Adv. Synth. Catal. 2019, 361, 2321–2328. [Google Scholar] [CrossRef]
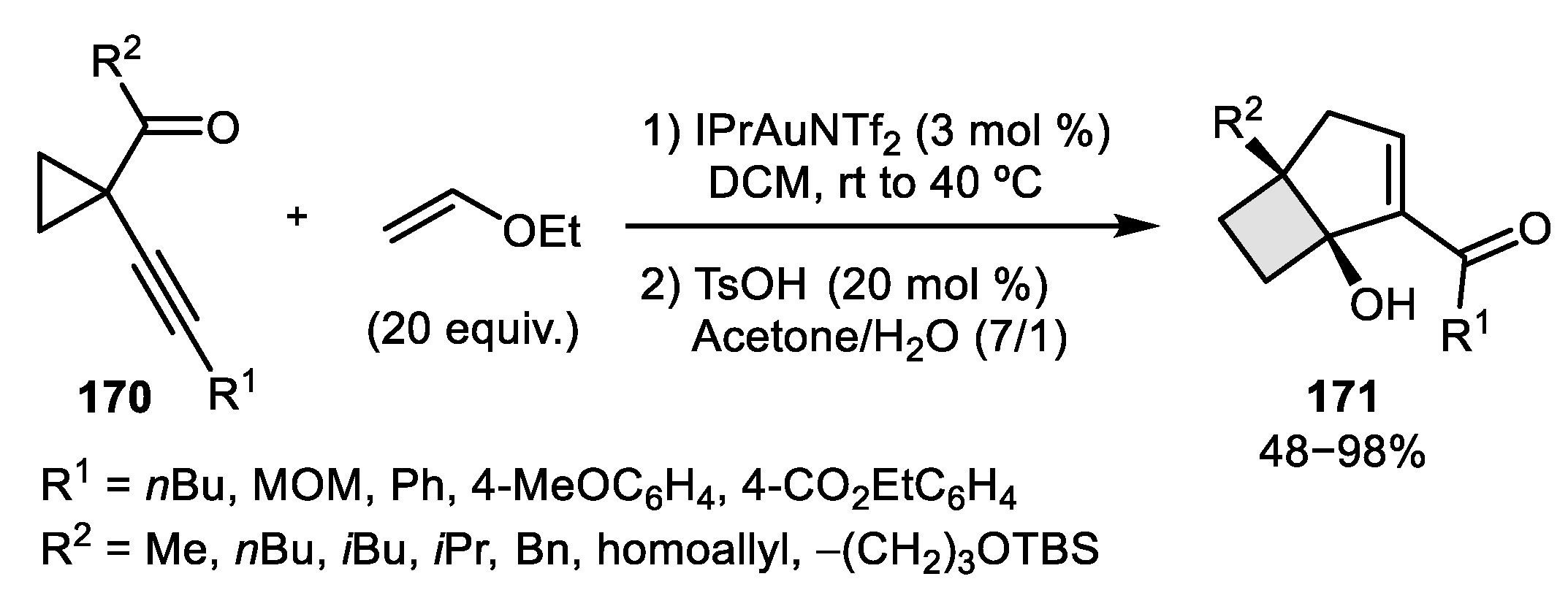
- Jiménez-Núñez, E.; Claverie, C.K.; Nieto-Oberhuber, C.; Echavarren, A.M. Prins Cyclizations in Au-Catalyzed Reactions of Enynes. Angew. Chem. Int. Ed. 2006, 45, 5452–5455. [Google Scholar] [CrossRef]
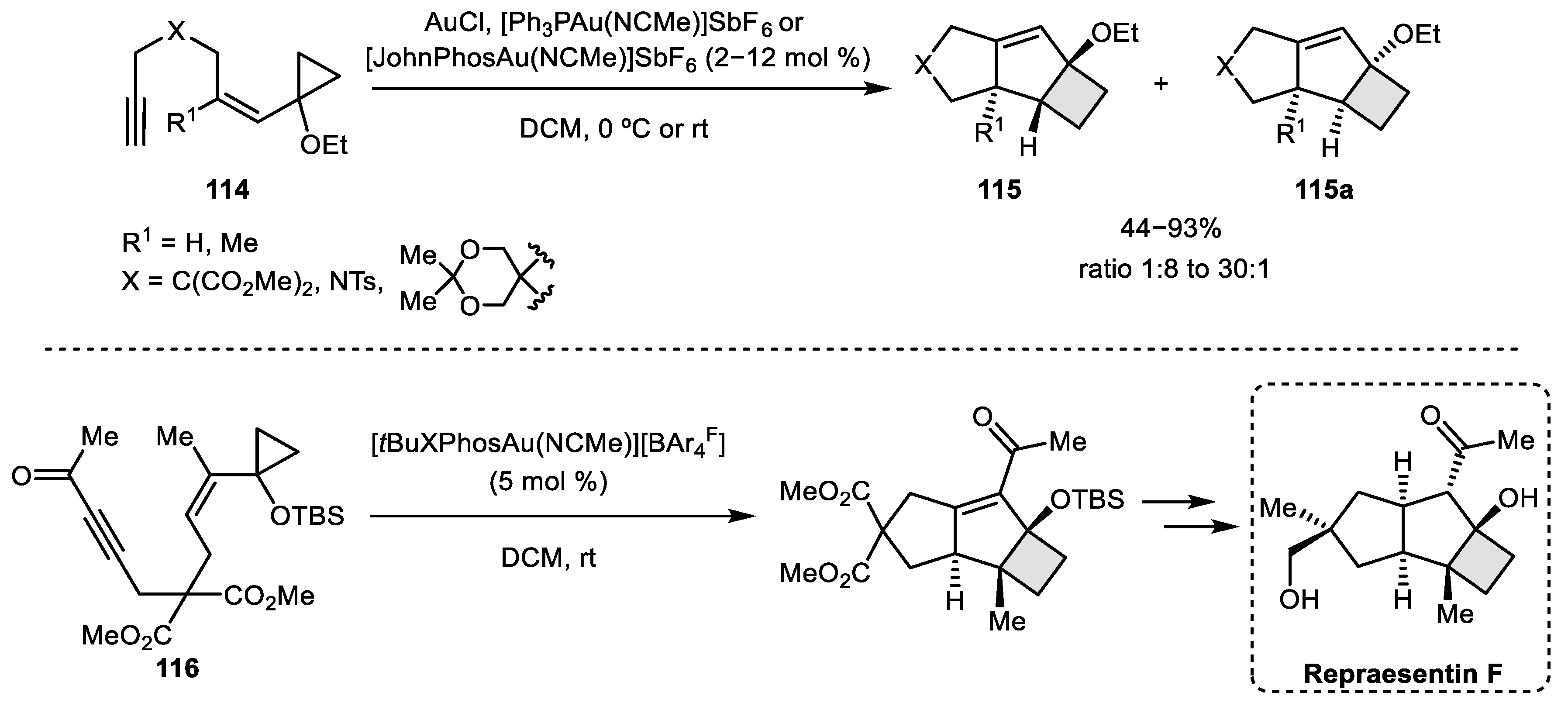
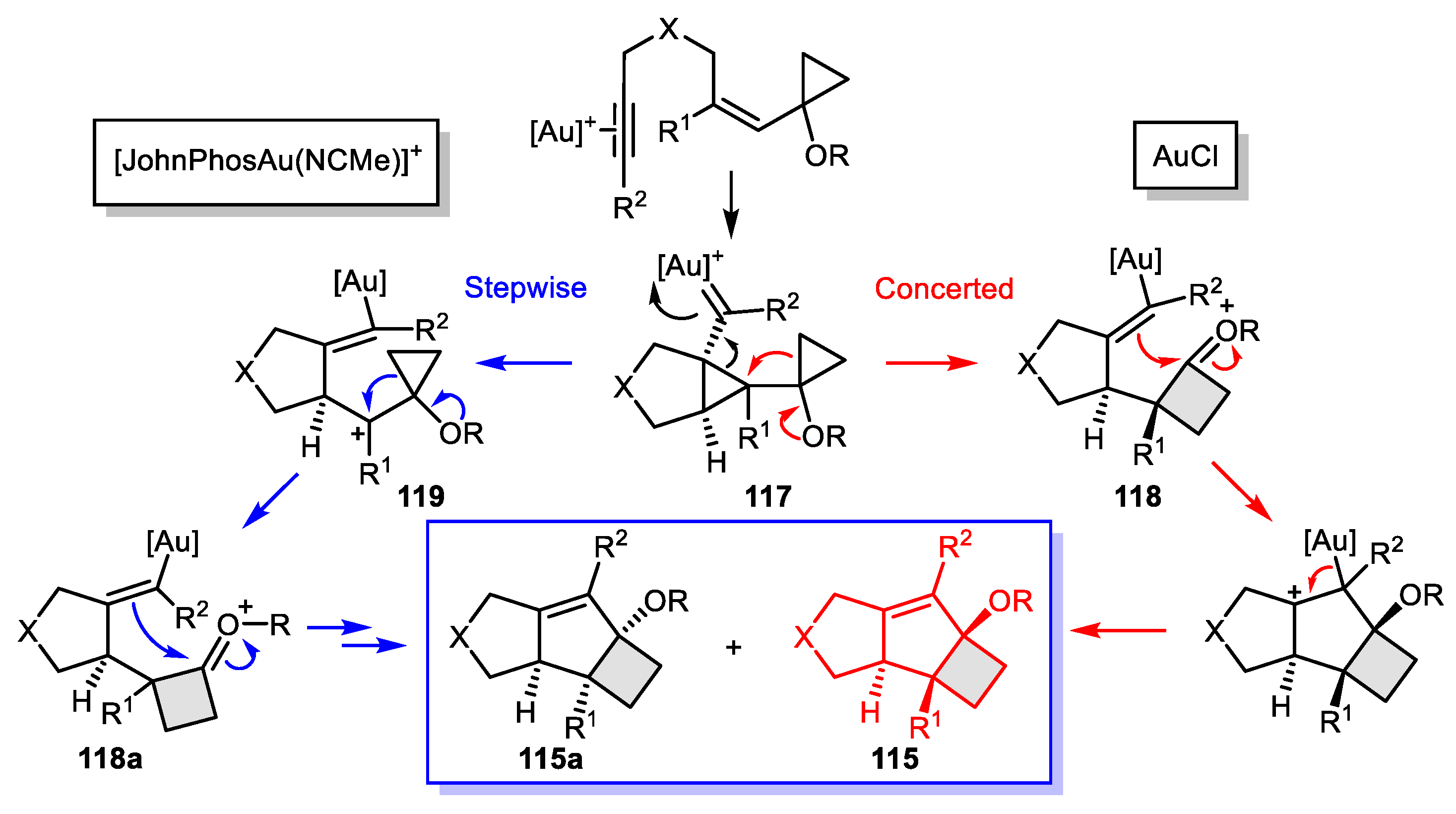
- Ferrer, S.; Echavarren, A.M. Total Synthesis of Repraesentin F and Configuration Reassignment by a Gold (I)-Catalyzed Cyclization Cascade. Org. Lett. 2018, 20, 5784–5788. [Google Scholar] [CrossRef]
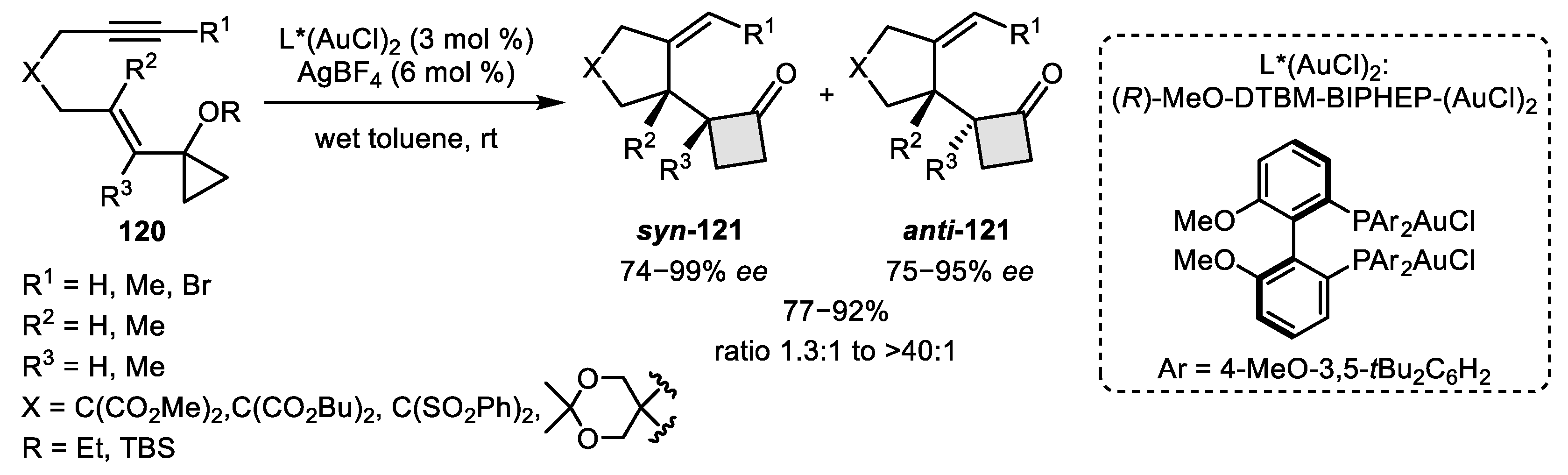
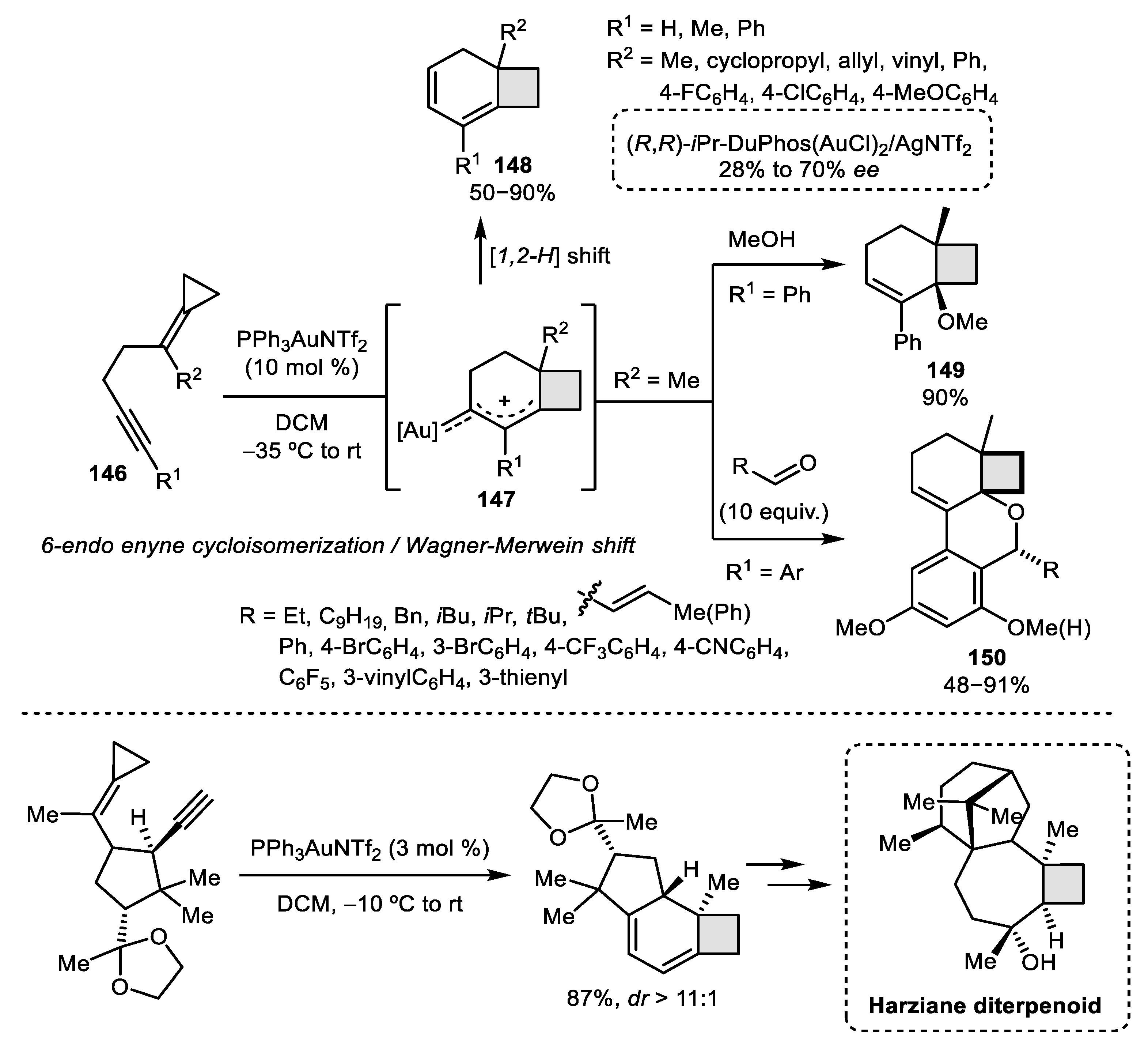
- Wu, Z.; Lebœuf, D.; Retailleau, P.; Gandon, V.; Marinetti, A.; Voituriez, A. Enantioselective Gold (I)-Catalyzed Rearrangement of Cyclopropyl-substituted 1,6-Enynes into 2-Oxocyclobutyl-cyclopentanes. Chem. Commun. 2017, 53, 7026–7029. [Google Scholar] [CrossRef]
- Sethofer, S.G.; Staben, S.T.; Hung, O.Y.; Toste, F.D. Au (I)-Catalyzed Ring Expanding Cycloisomerizations: Total Synthesis of Ventricosene. Org. Lett. 2008, 10, 4315–4318. [Google Scholar] [CrossRef] [Green Version]
- Pitaval, A.; Leboeuf, D.; Ceccon, J.; Echavarren, A.M. Access to the Protoilludane Core by Gold-Catalyzed Allene-vinylcyclopropane Cycloisomerization. Org. Lett. 2013, 15, 4580–4583. [Google Scholar] [CrossRef]
- Kleinbeck, F.; Toste, F.D. Gold (I)-Catalyzed Enantioselective Ring Expansion of Allenylcyclopropanols. J. Am. Chem. Soc. 2009, 131, 9178–9179. [Google Scholar] [CrossRef] [Green Version]
- Shu, X.-Z.; Zhang, M.; He, Y.; Frei, H.; Toste, F.D. Dual Visible Light Photoredox and Gold-Catalyzed Arylative Ring Expansion. J. Am. Chem. Soc. 2014, 136, 5844–5847. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, X.; Xu, Q.; Shi, M. Gold-Catalyzed Cyclization of 1-(Indol-3-yl)-3-alkyn-1-ols: Facile Synthesis of Diversified Carbazoles. Chem. Eur. J. 2013, 19, 10625–10631. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Fang, W.; Wei, Y.; Tang, X.-Y.; Shi, M. Divergent Reaction Pathways in Gold-catalyzed Cycloisomerization of 1, 5-enynes Containing a Cyclopropane Ring: Dramatic Ortho Substituent and Temperature Effects. Chem. Sci. 2016, 7, 4318–4328. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-Q.; Fang, W.; Wei, Y.; Tang, X.-Y.; Shi, M. Gold (I)-catalyzed Dehydrogenative Cycloisomerization of 1, 5-enynes. Chem. Commun. 2016, 52, 10799–10802. [Google Scholar] [CrossRef]
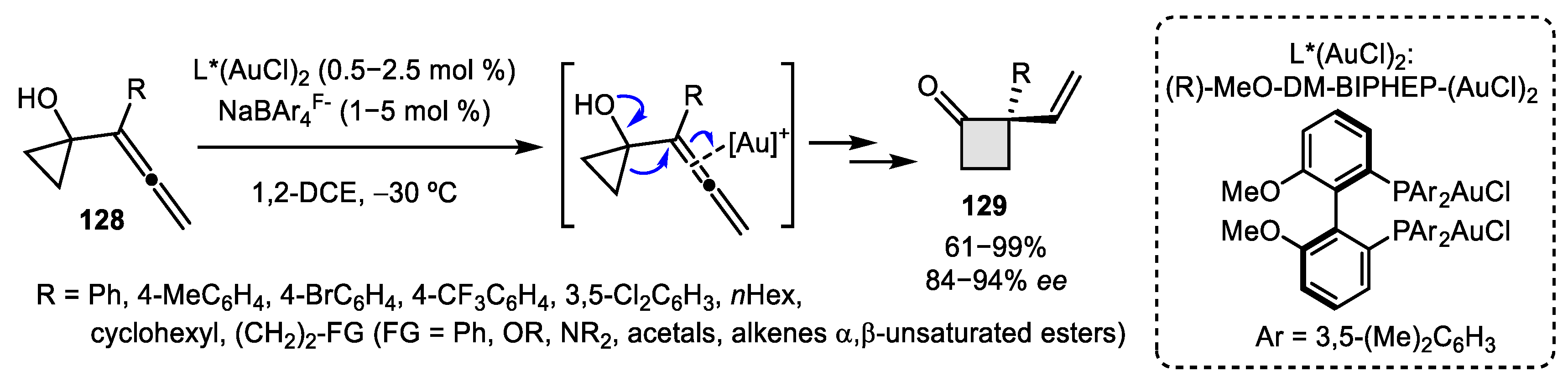
- Zheng, H.; Felix, R.J.; Gagné, M.R. Gold-Catalyzed Enantioselective Ring-Expanding Cycloisomerization of Cyclopropylidene Bearing 1,5-Enynes. Org. Lett. 2014, 16, 2272–2275. [Google Scholar] [CrossRef]
- Hönig, M.; Carreira, E.M. Total Synthesis and Structural Revision of a Harziane Diterpenoid. Angew. Chem. Int. Ed. 2020, 59, 1192–1196. [Google Scholar] [CrossRef]
- Felix, R.J.; Gutierrez, O.; Tantillo, D.J.; Gagné, M.R. Gold (I)-Catalyzed Formation of Bicyclo [4.2.0] oct-1-enes. J. Org. Chem. 2013, 78, 5685–5690. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.A.; Gagné, M.R. Gold (I)-catalyzed Addition of Aldehydes to Cyclopropylidene Bearing 6-Aryl-1, 5-enynes. Org. Biomol. Chem. 2016, 14, 11261–11265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Adduci, L.L.; Felix, R.J.; Gagné, M.R. Gold-Catalyzed Diastereoselective Cycloisomerization of Alkylidene-Cyclopropane-Bearing 1, 6-Diynes. Angew. Chem. Int. Ed. 2014, 53, 7904–7907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Wei, Y.; Shi, M. Gold- and Silver-catalyzed Intramolecular Annulation and Rearrangement of Aniline-linked 1, 6-enynes Containing Methylenecyclopropanes. Org. Chem. Front. 2018, 5, 2091–2097. [Google Scholar] [CrossRef]
- Fang, W.; Tang, X.-Y.; Shi, M. Gold (I)-catalyzed Intramolecular Hydroarylation and the Subsequent Ring Enlargement of Methylenecyclopropanes to Cyclobutenes. RSC Adv. 2016, 6, 40474–40479. [Google Scholar] [CrossRef]
- Fang, W.; Wei, Y.; Tang, X.-Y.; Shi, M. Gold (I)-Catalyzed Cycloisomerization of ortho-(Propargyloxy) arenemethylenecyclopropanes Controlled by Adjacent Substituents at Aromatic Rings. Chem. Eur. J. 2017, 23, 6845–6852. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Wei, Y.; Shi, M. Gold-catalyzed Ring Enlargement and Cycloisomerization of Alkynylamide Tethered Alkylidenecyclopropanes. Org. Chem. Front. 2018, 5, 2980–2985. [Google Scholar] [CrossRef]
- Li, C.-L.; Yu, Z.-X. Asymmetric Synthesis of Azepine-Fused Cyclobutanes from Yne-Methylenecyclopropanes Involving Cyclopropanation/C–C Cleavage/Wagner–Meerwein Rearrangement and Reaction Mechanism. J. Org. Chem. 2019, 84, 9913–9928. [Google Scholar] [CrossRef]
- Li, D.-Y.; Wei, Y.; Marek, I.; Tang, X.-Y.; Shi, M. Gold (I)-catalyzed Cycloisomerization of Vinylidenecyclopropane-enes Via Carbene or Non-carbene Processes. Chem. Sci. 2015, 6, 5519–5525. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-Y.; Fang, W.; Wei, Y.; Shi, M. C (sp3)–H Functionalizations Promoted by the Gold Carbene Generated from Vinylidenecyclopropanes. Chem. Eur. J. 2016, 22, 18080–18084. [Google Scholar] [CrossRef]
- Li, D.-Y.; Wei, Y.; Shi, M. Gold (I)-Catalyzed Intramolecular Carbon-Oxygen Bond Cleavage Reaction via Gold Carbenes Derived from Vinylidenecyclopropanes. Adv. Synth. Catal. 2016, 358, 3002–3009. [Google Scholar] [CrossRef]
- Yuan, W.; Dong, X.; Wei, Y.; Shi, M. An Efficient Method for the Synthesis of Alkylidenecyclobutanones by Gold-Catalyzed Oxidative Ring Enlargement of Vinylidenecyclopropanes. Chem. Eur. J. 2012, 18, 10501–10505. [Google Scholar] [CrossRef]
- Li, G.; Huang, X.; Zhang, L. Au (I)-Catalyzed Efficient Synthesis of Functionalized Bicyclo [3.2.0] heptanes. J. Am. Chem. Soc. 2008, 130, 6944–6945. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Lin, M.-S.; Liao, H.-H.; Liu, R.-S. Diversity of Products in the Gold-Catalyzed Cyclization of 1-Epoxy-1-alkynylcyclopropanes by Using 1-Oxyallyl Cations. Chem. Eur. J. 2010, 16, 2696–2699. [Google Scholar] [CrossRef]
- Liao, H.-H.; Liu, R.-S. Effects of Haloniums on Gold-catalyzed Ring Expansion of 1-Oxiranyl-1-alkynylcyclopropanes. Chem. Commun. 2011, 47, 1339–1341. [Google Scholar] [CrossRef]
- Ye, S.; Yu, Z.-X. Gold (I)-Catalyzed Ring Expansions of Unactivated Alkynylcyclopropanes to (E)-2-Alkylidenecyclobutanamines in the Presence of Sulfonamides. Org. Lett. 2010, 12, 804–807. [Google Scholar] [CrossRef]
- Yi, F.; Huang, B.; Nie, Q.; Cai, M. A Heterogeneous Gold (I)-catalyzed Ring Expansion of Unactivated Alkynylcyclopropanes with Sulfonamides Leading to (E)-2-Alkylidenecyclobutanamines. Tetrahedron Lett. 2016, 57, 4405–4410. [Google Scholar] [CrossRef]
- Li, C.-W.; Pati, K.; Lin, G.-Y.; Sohel, S.M.A.; Hung, H.-H.; Liu, R.-S. Gold-Catalyzed Oxidative Ring Expansions and Ring Cleavages of Alkynylcyclopropanes by Intermolecular Reactions Oxidized by Diphenylsulfoxide. Angew. Chem. Int. Ed. 2010, 49, 9891–9894. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Wang, C.-D.; Tian, S.-F.; Liu, R.-S. Gold-Catalyzed Synthesis of Bicyclo [3.2.0] heptenes via a Formal [3 + 2]/[2 + 2] -Annulation of Allylsilane with 4-Methoxybut-2-yn-1-ols. Adv. Synth. Catal. 2010, 352, 1605–1609. [Google Scholar] [CrossRef]
- Xu, M.; Ren, T.-T.; Wang, K.-B.; Li, C.-Y. Synthesis of Cyclobutanones via Gold-Catalyzed Oxidative Rearrangement of Homopropargylic Ethers. Adv. Synth. Catal. 2013, 355, 2488–2494. [Google Scholar] [CrossRef]
- Xu, M.; Ren, T.-T.; Li, C.-Y. Gold-Catalyzed Oxidative Rearrangement of Homopropargylic Ether via Oxonium Ylide. Org. Lett. 2012, 14, 4902–4905. [Google Scholar] [CrossRef] [PubMed]
- Tšupova, S.; Hansmann, M.M.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. New Pathways for the Dual Gold-Catalyzed Cyclization of Diynes. Chem. Eur. J. 2016, 22, 16286–16291. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, Y.; Ma, X.; Li, Y.; Zhang, L. Non-Diazo C-H Insertion Approach to Cyclobutanones via Oxidative Gold Catalysis. Angew. Chem. Int. Ed. 2020, 59, 17398–17402. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |





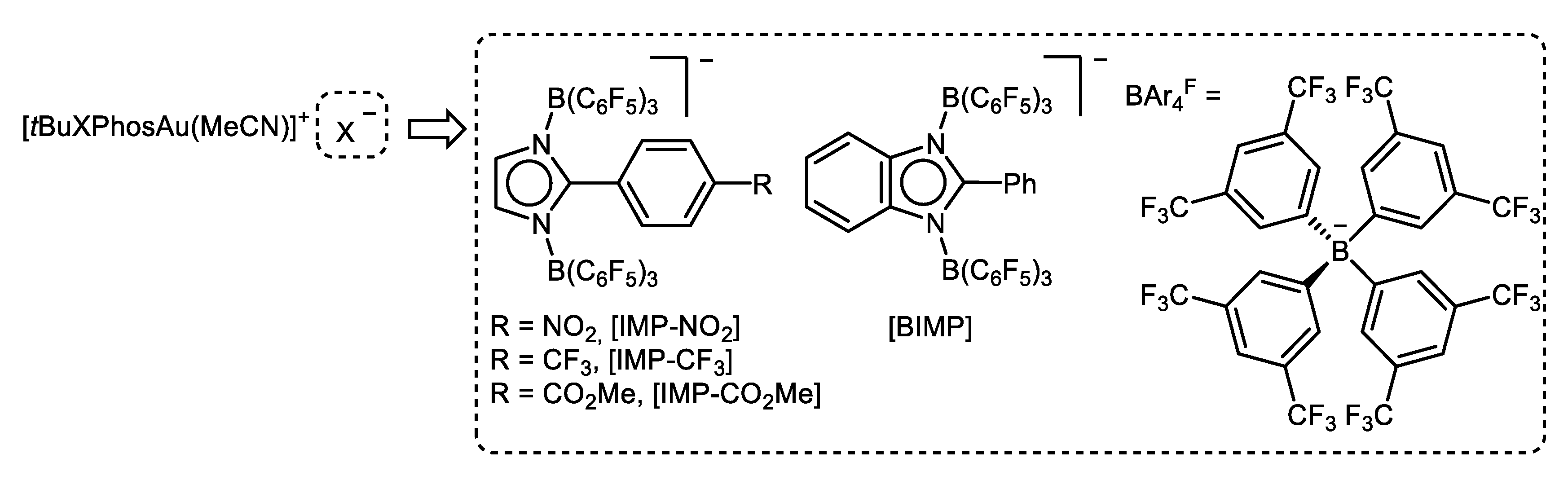












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otárola, G.; Vaquero, J.J.; Merino, E.; Fernández-Rodríguez, M.A. Gold-Catalyzed Synthetic Strategies towards Four-Carbon Ring Systems. Catalysts 2020, 10, 1178. https://doi.org/10.3390/catal10101178
Otárola G, Vaquero JJ, Merino E, Fernández-Rodríguez MA. Gold-Catalyzed Synthetic Strategies towards Four-Carbon Ring Systems. Catalysts. 2020; 10(10):1178. https://doi.org/10.3390/catal10101178
Chicago/Turabian StyleOtárola, Guillermo, Juan J. Vaquero, Estíbaliz Merino, and Manuel A. Fernández-Rodríguez. 2020. "Gold-Catalyzed Synthetic Strategies towards Four-Carbon Ring Systems" Catalysts 10, no. 10: 1178. https://doi.org/10.3390/catal10101178
APA StyleOtárola, G., Vaquero, J. J., Merino, E., & Fernández-Rodríguez, M. A. (2020). Gold-Catalyzed Synthetic Strategies towards Four-Carbon Ring Systems. Catalysts, 10(10), 1178. https://doi.org/10.3390/catal10101178






