Recent Advances in Photocatalytic CO2 Utilisation Over Multifunctional Metal–Organic Frameworks
Abstract
:1. Introduction
2. Photocatalytic CO2 Utilisation by MOF Hybrids
2.1. Noble-Metal-Based MOFs
2.1.1. Ag-Based Functionalised MOFs
2.1.2. Au-Based Functionalised MOFs
2.1.3. Pt- and Rh-Based Functionalised MOFs
2.2. Non-Noble-Metal-Based MOFs
2.3. MOFs Incorporated with Miscellaneous Species
3. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Izumi, Y. Recent advances in the photocatalytic conversion of carbon dioxide to fuels with water and/or hydrogen using solar energy and beyond. Coord. Chem. Rev. 2013, 257, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [PubMed]
- Gibbins, J.; Chalmers, H. Carbon capture and storage. Energy Policy 2008, 36, 4317–4322. [Google Scholar] [CrossRef] [Green Version]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar]
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Raman, S.K.; Raja, R.; Arnold, P.L.; Davidson, M.G.; Williams, C.K. Waste not, want not: CO2 (re)cycling into block polymers. Chem. Commun. 2019, 55, 7315–7318. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Hoivik, N.; Wang, K.; Jakobsen, H. Engineering TiO2 nanomaterials for CO2 conversion/solar fuels. Sol. Energy Mater. Sol. Cells 2012, 105, 53–68. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-Reduction activity of anatase TiO2 by Coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z-Scheme α-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2018, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. TiO2 -MnOx -Pt Hybrid Multiheterojunction Film Photocatalyst with Enhanced Photocatalytic CO2 -Reduction Activity. ACS Appl. Mater. Interfaces 2019, 11, 5581–5589. [Google Scholar] [CrossRef] [PubMed]
- Nie, N.; Zhang, L.; Fu, J.; Cheng, B.; Yu, J. Self-assembled hierarchical direct Z-scheme g-C3N4 /ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018, 441, 12–22. [Google Scholar]
- Choi, K.M.; Kim, D.; Rungtaweevoranit, B.; Trickett, C.A.; Barmanbek, J.T.D.; Alshammari, A.S.; Yang, P.; Yaghi, O.M. Plasmon-enhanced photocatalytic CO2 conversion within metal-organic frameworks under visible light. J. Am. Chem. Soc. 2017, 139, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Tu, W.; Yin, S.; Xing, W.; Wu, S.; Wang, H.; Wan, S.; Zhong, Q.; Xu, R. Amino-Assisted Anchoring of CsPbBr3 Perovskite Quantum Dots on Porous g-C3N4 for Enhanced Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2018, 130, 13758–13762. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar]
- Wang, C.C.; Zhang, Y.Q.; Li, J.; Wang, P. Photocatalytic CO2 reduction in metal-organic frameworks: A mini review. J. Mol. Struct. 2015, 1083, 127–136. [Google Scholar] [CrossRef]
- Zhu, Z.; Qin, J.; Jiang, M.; Ding, Z.; Hou, Y. Enhanced selective photocatalytic CO2 reduction into CO over Ag/CdS nanocomposites under visible light. Appl. Surf. Sci. 2017, 391, 572–579. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Gold-nanoparticle-modified TiO2 nanowires for plasmon-enhanced photocatalytic CO2 reduction with H2 under visible light irradiation. Appl. Surf. Sci. 2015, 356, 1289–1299. [Google Scholar] [CrossRef]
- Wan, S.; Chen, M.; Ou, M.; Zhong, Q. Plasmonic Ag nanoparticles decorated SrTiO3 nanocubes for enhanced photocatalytic CO2 reduction and H2 evolution under visible light irradiation. J. CO2 Util. 2019, 33, 357–364. [Google Scholar] [CrossRef]
- Kuriki, R.; Sekizawa, K.; Ishitani, O.; Maeda, K. Visible-light-driven CO2 reduction with carbon nitride: Enhancing the activity of ruthenium catalysts. Angew. Chem. Int. Ed. 2015, 54, 2406–2409. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamaguchi, A.; Umezawa, N.; Miyauchi, M. Photocatalytic CO2 Reduction Using a Pristine Cu2ZnSnS4 Film Electrode under Visible Light Irradiation. J. Phys. Chem. C 2018, 122, 21695–21702. [Google Scholar] [CrossRef]
- Huang, P.; Huang, J.; Pantovich, S.A.; Carl, A.D.; Fenton, T.G.; Caputo, C.A.; Grimm, R.L.; Frenkel, A.I.; Li, G. Selective CO2 Reduction Catalyzed by Single Cobalt Sites on Carbon Nitride under Visible-Light Irradiation. J. Am. Chem. Soc. 2018, 140, 16042–16047. [Google Scholar] [CrossRef]
- Potter, M.E.; Stewart, D.J.; Oakley, A.E.; Boardman, R.P.; Bradley, T.; Sazio, P.J.A.; Raja, R. Combining Photocatalysis and Optical Fiber Technology toward Improved Microreactor Design for Hydrogen Generation with Metallic Nanoparticles. ACS Photonics 2020, 7, 714–722. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Q.; Shi, R.; Waterhouse, G.I.N.; Zhang, X.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. FeO–CeO2 nanocomposites: An efficient and highly selective catalyst system for photothermal CO2 reduction to CO. NPG Asia Mater. 2020, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Wang, C.; Ma, H.; Song, M.; Li, D.; Li, Y.; Li, S.; Zhang, X.; Liu, Y. Revisiting Pt/TiO2 photocatalysts for thermally assisted photocatalytic reduction of CO2. Nanoscale 2020, 12, 7000–7010. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Ma, H.; Li, Y.; Liu, Y.; Suzuki, N.; Terashima, C.; Fujishima, A.; Zhang, X. Photothermal synergic enhancement of direct Z-scheme behavior of Bi4TaO8Cl/W18O49 heterostructure for CO2 reduction. Appl. Catal. B Environ. 2020, 268, 118401–118416. [Google Scholar] [CrossRef]
- Xu, M.; Hu, X.; Wang, S.; Yu, J.; Zhu, D.; Wang, J. Photothermal effect promoting CO2 conversion over composite photocatalyst with high graphene content. J. Catal. 2019, 377, 652–661. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Yan, T.; Hu, Z.; Jelle, A.A.; Meira, D.M.; Duchesne, P.N.; Loh, J.Y.Y.; Qiu, C.; Storey, E.E.; et al. Black indium oxide a photothermal CO2 hydrogenation catalyst. Nat. Commun. 2020, 11, 1–8. [Google Scholar]
- Mohan, A.; Ulmer, U.; Hurtado, L.; Loh, J.; Li, Y.F.; Tountas, A.A.; Krevert, C.; Chan, C.; Liang, Y.; Brodersen, P.; et al. Hybrid Photo-and Thermal Catalyst System for Continuous CO2 Reduction. ACS Appl. Mater. Interfaces 2020, 12, 33613–33620. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Morabito, J.V.; Tsung, C.K. Core-shell catalysts of metal nanoparticle core and metal-organic framework shell. ACS Catal. 2014, 4, 4409–4419. [Google Scholar] [CrossRef]
- Vermoortele, F.; Vimont, A.; Serre, C.; De Vos, D. An amino-modified Zr-terephthalate metal–organic framework as an acid–base catalyst for cross-aldol condensation. Chem. Commun. 2011, 47, 1521–1523. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar]
- Hinde, C.S.; Webb, W.R.; Chew, B.K.J.; Tan, H.R.; Zhang, W.H.; Hor, T.S.A.; Raja, R. Utilisation of gold nanoparticles on amine-functionalised UiO-66 (NH2-UiO-66) nanocrystals for selective tandem catalytic reactions. Chem. Commun. 2016, 52, 6557–6560. [Google Scholar] [CrossRef] [Green Version]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal-Organic Framework (MOF) Compounds: Photocatalysts for Redox Reactions and Solar Fuel Production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal-Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Wang, Q.; Zhou, J.; Lei, Y.; Chen, Z.; Wang, Z.; Pan, A.; Liang, S. Metal Organic Framework-Templated Synthesis of Bimetallic Selenides with Rich Phase Boundaries for Sodium-Ion Storage and Oxygen Evolution Reaction. ACS Nano 2019, 13, 5635–5645. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug deliveryand imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [PubMed]
- Zhuang, J.; Kuo, C.H.; Chou, L.Y.; Liu, D.Y.; Weerapana, E.; Tsung, C.K. Optimized metal-organic-framework nanospheres for drug delivery: Evaluation of small-molecule encapsulation. ACS Nano 2014, 8, 2812–2819. [Google Scholar] [PubMed]
- Ke, F.; Yuan, Y.P.; Qiu, L.G.; Shen, Y.H.; Xie, A.J.; Zhu, J.F.; Tian, X.Y.; Zhang, L.-D. Facile fabrication of magnetic metal-organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011, 21, 3843–3848. [Google Scholar] [CrossRef]
- Cai, W.; Chu, C.C.; Liu, G.; Wáng, Y.X.J. Metal-Organic Framework-Based Nanomedicine Platforms for Drug Delivery and Molecular Imaging. Small 2015, 11, 4806–4822. [Google Scholar]
- Wu, M.X.; Yang, Y.W. Metal–Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1–20. [Google Scholar] [CrossRef]
- Ma, L.; Wu, C.-D.; Wanderley, M.M.; Lin, W. Single-crystal to single-crystal cross-linking of an interpenetrating chiral metal-organic framework and implications in asymmetric catalysis. Angew. Chem. Int. Ed. 2010, 49, 8244–8248. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Kutzscher, C.; Drache, F.; Senkovska, I.; Kaskel, S. Chiral Functionalization of a Zirconium Metal-Organic Framework (DUT-67) as a Heterogeneous Catalyst in Asymmetric Michael Addition Reaction. Inorg. Chem. 2018, 57, 1483–1489. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Lin, W. Asymmetric catalysis with chiral porous metal-organic frameworks: Critical issues. J. Phys. Chem. Lett. 2011, 2, 1701–1709. [Google Scholar] [CrossRef]
- Song, F.; Wang, C.; Lin, W. A chiral metal-organic framework for sequential asymmetric catalysis. Chem. Commun. 2011, 47, 8256–8258. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Oda, S.; Shiro, M. A novel chiral porous metal-organic framework: Asymmetric ring opening reaction of epoxide with amine in the chiral open space. Chem. Commun. 2008, 7, 820–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-D.; Hu, A.; Zhang, L.; Lin, W. A homochiral porous metal-organic framework for highly enantioselective heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 2005, 127, 8940–8941. [Google Scholar] [CrossRef]
- Ma, L.; Falkowski, J.M.; Abney, C.; Lin, W. A series of isoreticular chiral metal–organic frameworks as a tunable platform for asymmetric catalysis. Nat. Chem. 2010, 2, 838–846. [Google Scholar] [CrossRef]
- Surblé, S.; Millange, F.; Serre, C.; Düren, T.; Latroche, M.; Bourrelly, S.; Llewellyn, P.L.; Férey, G. Synthesis of MIL-102, a chromium carboxylate metal-organic framework, with gas sorption analysis. J. Am. Chem. Soc. 2006, 128, 14889–14896. [Google Scholar] [CrossRef]
- Yoon, J.W.; Seo, Y.K.; Hwang, Y.K.; Chang, J.S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E.; et al. Controlled reducibility of a metal-organic framework with coordinatively unsaturated sites for preferential gas sorption. Angew. Chem. Int. Ed. 2010, 49, 5949–5952. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Chun, H.; Yoon, S.H.; Kim, D.; Kim, K. Microporous Manganese Formate: A Simple Metal-Organic Porous Material with High Framework Stability and Highly Selective Gas Sorption Properties. J. Am. Chem. Soc. 2004, 126, 32–33. [Google Scholar]
- Kondo, A.; Noguchi, H.; Carlucci, L.; Proserpio, D.M.; Ciani, G.; Kajiro, H.; Ohba, T.; Kanoh, H.; Kaneko, K. Double-step gas sorption of a two-dimensional metal-organic framework. J. Am. Chem. Soc. 2007, 129, 12362–12363. [Google Scholar] [CrossRef]
- Li, D.; Yu, S.H.; Jiang, H.L. From UV to Near-Infrared Light-Responsive Metal–Organic Framework Composites: Plasmon and Upconversion Enhanced Photocatalysis. Adv. Mater. 2018, 30, 1–7. [Google Scholar] [CrossRef]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A water-stable porphyrin-based metal-organic framework active for visible-light photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kuwahara, Y.; Mori, K.; Louis, C.; Yamashita, H. A hydrophobic titanium doped zirconium-based metal organic framework for photocatalytic hydrogen peroxide production in a two-phase system. J. Mater. Chem. A 2020, 8, 1904–1910. [Google Scholar]
- Chen, X.; Kondo, Y.; Kuwahara, Y.; Mori, K.; Louis, C.; Yamashita, H. Metal-organic framework-based nanomaterials for photocatalytic hydrogen peroxide production. Phys. Chem. Chem. Phys. 2020, 22, 14404–14414. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.P.; Gagliardi, L.; Truhlar, D.G. Cerium Metal-Organic Framework for Photocatalysis. J. Am. Chem. Soc. 2018, 140, 7904–7912. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Llabrés I Xamena, F.X.; Garcia, H. Semiconductor behavior of a metal-organic framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, S.; Lamberti, C.; Ricchiardi, G.; Regli, L.; Bonino, F.; Damin, A.; Lillerud, K.P.; Bjorgen, M.; Zecchina, A. Electronic and vibrational properties of a MOF-5 metal-organic framework: ZnO quantum dot behaviour. Chem. Commun. 2004, 5, 2300–2301. [Google Scholar] [CrossRef]
- Subudhi, S.; Rath, D.; Parida, K.M. A mechanistic approach towards the photocatalytic organic transformations over functionalised metal organic frameworks: A review. Catal. Sci. Technol. 2018, 8, 679–696. [Google Scholar]
- Beyzavi, M.H.; Stephenson, C.J.; Liu, Y.; Karagiaridi, O.; Hupp, J.T.; Farha, O.K. Metal–Organic Framework-Based Catalysts: Chemical Fixation of CO2 with Epoxides Leading to Cyclic Organic Carbonates. Front. Energy Res. 2015, 2, 63. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Perman, J.A.; Zhu, G.; Ma, S. Metal-Organic Frameworks for CO2 Chemical Transformations. Small 2016, 12, 6309–6324. [Google Scholar] [CrossRef]
- Luciani, G.; Imparato, C.; Vitiello, G. Photosensitive hybrid nanostructured materials: The big challenges for sunlight capture. Catalysts 2020, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, W.; Liu, B.; Qiao, J.; Lv, L.; Gao, X.; Zhang, X.; Xu, D.; Liu, W.; Liu, J.; et al. Recent Advances in MOF-based Nanocatalysts for Photo-Promoted CO2 Reduction Applications. Catalysts 2019, 9, 658. [Google Scholar] [CrossRef] [Green Version]
- Alkhatib, I.I.; Garlisi, C.; Pagliaro, M.; Al-Ali, K.; Palmisano, G. Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications. Catal. Today 2020, 340, 209–224. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, C.; Lin, W. Metal-organic frameworks for light harvesting and photocatalysis. ACS Catal. 2012, 2, 2630–2640. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J. Metal-organic frameworks for CO2 photoreduction. Front. Energy 2019, 13, 221–250. [Google Scholar]
- Kidanemariam, A.; Lee, J.; Park, J. Recent innovation of metal-organic frameworks for carbon dioxide photocatalytic reduction. Polymers 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, T.; Rizwan, K.; Bilal, M.; Iqbal, H.M.N. Metal-Organic Framework-Based Engineered Materials—Fundamentals and Applications. Molecules 2020, 25, 1598. [Google Scholar] [CrossRef] [Green Version]
- Crake, A. Metal-organic frameworks based materials for photocatalytic CO2 reduction. Mater. Sci. Technol. 2017, 33, 1737–1749. [Google Scholar] [CrossRef]
- Huang, C.W.; Nguyen, V.H.; Zhou, S.R.; Hsu, S.Y.; Tan, J.X.; Wu, K.C.W. Metal-organic frameworks: Preparation and applications in highly efficient heterogeneous photocatalysis. Sustain. Energy Fuels 2020, 4, 504–521. [Google Scholar]
- Zhang, H.; Wei, J.; Dong, J.; Liu, G.; Shi, L.; An, P.; Zhao, G.; Kong, J.; Wang, X.; Meng, X.; et al. Efficient Visible-Light-Driven Carbon Dioxide Reduction by a Single-Atom Implanted Metal–Organic Framework. Angew. Chem. Int. Ed. 2016, 55, 14310–14314. [Google Scholar] [CrossRef]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.Q.; Jiang, H.L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Design of silver-based controlled nanostructures for plasmonic catalysis under visible light irradiation. Bull. Chem. Soc. Jpn. 2019, 92, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Mori, K.; Kuwahara, Y.; Kamegawa, T.; Wen, M.; Verma, P.; Che, M. Single-site and nano-confined photocatalysts designed in porous materials for environmental uses and solar fuels. Chem. Soc. Rev. 2018, 47, 8072–8096. [Google Scholar] [PubMed]
- Verma, P.; Yuan, K.; Kuwahara, Y.; Mori, K.; Yamashita, H. Enhancement of plasmonic activity by Pt/Ag bimetallic nanocatalyst supported on mesoporous silica in the hydrogen production from hydrogen storage material. Appl. Catal. B Environ. 2018, 223, 10–15. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Enhancement of Ag-based plasmonic photocatalysis in hydrogen production from ammonia borane by the assistance of single-site Ti-oxide moieties within a silica framework. Chem. Eur. J. 2017, 23, 3616–3622. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Synthesis and characterization of a Pd/Ag bimetallic nanocatalyst on SBA-15 mesoporous silica as a plasmonic catalyst. J. Mater. Chem. A 2015, 3, 18889–18897. [Google Scholar] [CrossRef]
- Verma, P.; Mori, K.; Kuwahara, Y.; Cho, S.J.; Yamashita, H. Synthesis of plasmonic gold nanoparticles supported on morphology-controlled TiO2 for aerobic alcohol oxidation. Catal. Today 2020, 352, 255–261. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar]
- Langhammer, C.; Yuan, Z.; Zorić, I.; Kasemo, B. Plasmonic properties of supported Pt and Pd nanostructures. Nano Lett. 2006, 6, 833–838. [Google Scholar] [CrossRef]
- Jo, S.; Verma, P.; Kuwahara, Y.; Mori, K.; Choi, W.; Yamashita, H. Enhanced hydrogen production from ammonia borane using controlled plasmonic performance of Au nanoparticles deposited on TiO2. J. Mater. Chem. A 2017, 5, 21883–21892. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Synthesis of mesoporous silica-supported Ag nanorod-based bimetallic catalysts and investigation of their plasmonic activity under visible light irradiation. Catal. Sci. Technol. 2017, 7, 2551–2558. [Google Scholar] [CrossRef]
- Verma, P.; Navlani-garcía, M.; Kuwahara, Y.; Mori, K.; Yamashita, H. Mesoporous silica supported Pd/Ag bimetallic nanoparticles as a plasmonic catalyst for chemoselective hydrogenation of p-nitrostyrene under visible light irradiation. J. Chem. Sci. 2017, 129, 1661–1669. [Google Scholar] [CrossRef]
- Mori, K.; Verma, P.; Hayashi, R.; Fuku, K.; Yamashita, H. Color-Controlled Ag Nanoparticles and Nanorods within Confined Mesopores: Microwave-Assisted Rapid Synthesis and Application in Plasmonic Catalysis under Visible-Light Irradiation. Chem. Eur. J. 2015, 21, 11885–11893. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Pd/Ag and Pd/Au bimetallic nanocatalysts on mesoporous silica for plasmon-mediated enhanced catalytic activity under visible light irradiation. J. Mater. Chem. A 2016, 4, 10142–10150. [Google Scholar]
- Chen, M.; Han, L.; Zhou, J.; Sun, C.; Hu, C.; Wang, X.; Su, Z. Photoreduction of carbon dioxide under visible light by ultra-small Ag nanoparticles doped into Co-ZIF-9. Nanotechnology 2018, 29, 284003. [Google Scholar] [CrossRef]
- Guo, F.; Yang, S.; Liu, Y.; Wang, P.; Huang, J.; Sun, W.Y. Size Engineering of Metal-Organic Framework MIL-101(Cr)-Ag Hybrids for Photocatalytic CO2 Reduction. ACS Catal. 2019, 9, 8464–8470. [Google Scholar]
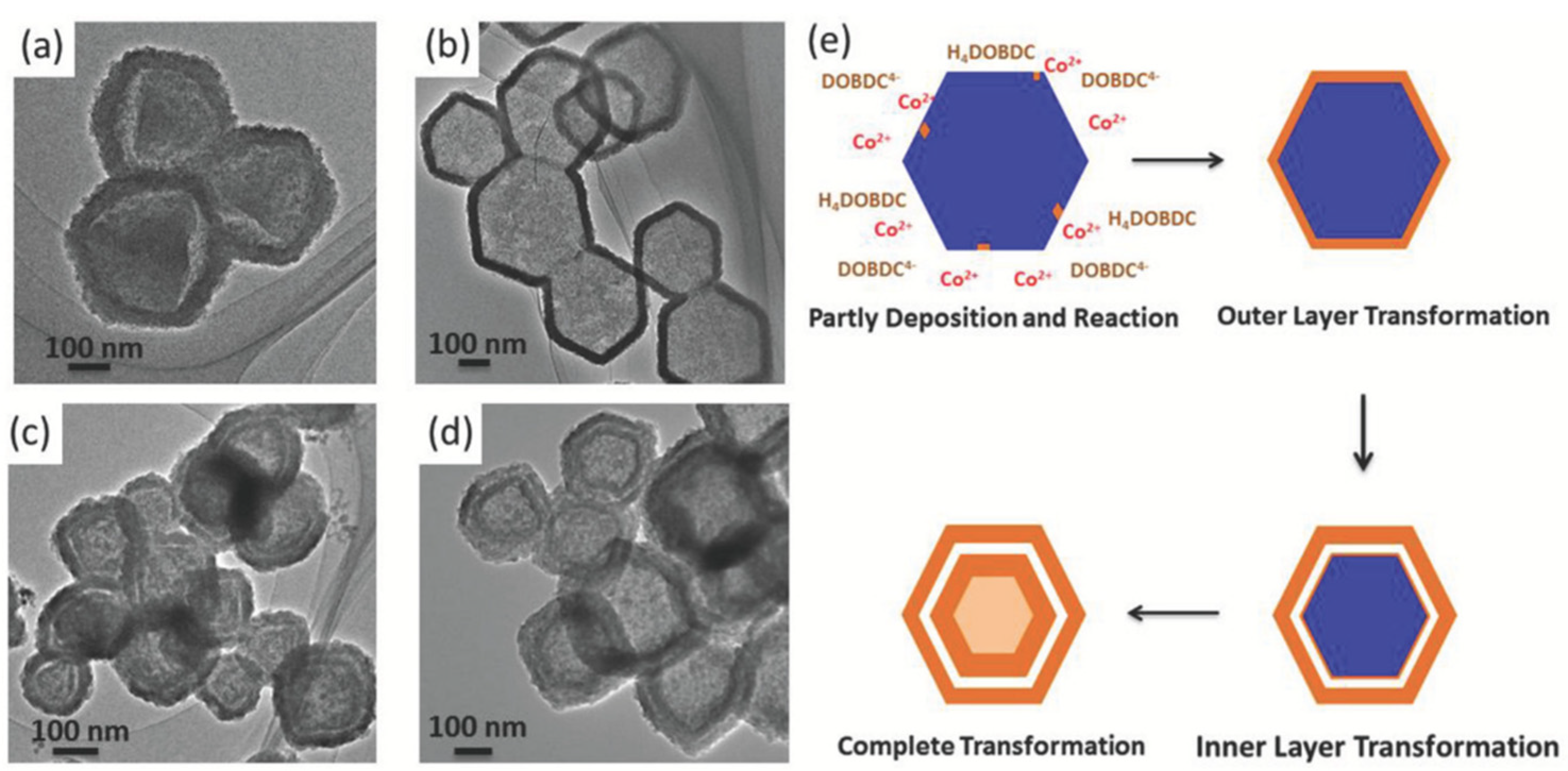
- Deng, X.; Yang, L.; Huang, H.; Yang, Y.; Feng, S.; Zeng, M.; Li, Q.; Xu, D. Shape-Defined Hollow Structural Co-MOF-74 and Metal Nanoparticles@Co-MOF-74 Composite through a Transformation Strategy for Enhanced Photocatalysis Performance. Small 2019, 15, 1–7. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Yu, F.; Shen, X.; Duan, C. A simple strategy for engineering heterostructures of Au nanoparticle-loaded metal-organic framework nanosheets to achieve plasmon-enhanced photocatalytic CO2 conversion under visible light. J. Mater. Chem. A 2019, 7, 11355–11361. [Google Scholar] [CrossRef]
- Khaletskaya, K.; Pougin, A.; Medishetty, R.; Rösler, C.; Wiktor, C.; Strunk, J.; Fischer, R.A. Fabrication of Gold/Titania Photocatalyst for CO2 Reduction Based on Pyrolytic Conversion of the Metal-Organic Framework NH2-MIL-125(Ti) Loaded with Gold Nanoparticles. Chem. Mater. 2015, 27, 7248–7257. [Google Scholar]
- Sun, D.; Liu, W.; Fu, Y.; Fang, Z.; Sun, F.; Fu, X.; Zhang, Y.; Li, Z. Noble metals can have different effects on photocatalysis over metal-organic frameworks (MOFs): A case study on M/NH2-MIL-125(Ti) (M=Pt and Au). Chem. Eur. J. 2014, 20, 4780–4788. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wei, Y.P.; Wang, S.Q.; Zhang, X.Y.; Wang, F.M.; Sun, W.Y. Pt nanoparticles embedded in flowerlike NH2-UiO-68 for enhanced photocatalytic carbon dioxide reduction. J. Mater. Chem. A 2019, 7, 26490–26495. [Google Scholar] [CrossRef]
- Chambers, M.B.; Wang, X.; Elgrishi, N.; Hendon, C.H.; Walsh, A.; Bonnefoy, J.; Canivet, J.; Quadrelli, E.A.; Farrusseng, D.; Mellot-Draznieks, C.; et al. Photocatalytic Carbon Dioxide Reduction with Rhodium-based Catalysts in Solution and Heterogenized within Metal-Organic Frameworks. ChemSusChem 2015, 8, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, W.; Lin, J.; Ding, Z.; Wang, X. Cobalt Imidazolate Metal-Organic Frameworks Photosplit CO2 under Mild Reaction Conditions. Angew. Chem. 2014, 53, 1034–1038. [Google Scholar]
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B Environ. 2015, 162, 494–500. [Google Scholar]
- Mei, B.; Pougin, A.; Strunk, J. Influence of photodeposited gold nanoparticles on the photocatalytic activity of titanate species in the reduction of CO2 to hydrocarbons. J. Catal. 2013, 306, 184–189. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A new photoactive crystalline highly porous titanium(IV) dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar]
- Sun, D.; Li, Z. Robust Ti- and Zr-Based Metal-Organic Frameworks for Photocatalysis. Chin. J. Chem. 2017, 35, 135–147. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti(IV) metal-organic framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar]
- Deria, P.; Mondloch, J.E.; Karagiaridi, O.; Bury, W.; Hupp, J.T.; Farha, O.K. Beyond post-synthesis modification: Evolution of metal-organic frameworks via building block replacement. Chem. Soc. Rev. 2014, 43, 5896–5912. [Google Scholar] [PubMed] [Green Version]
- Han, B.; Ou, X.; Deng, Z.; Song, Y.; Tian, C.; Deng, H.; Xu, Y.J.; Lin, Z. Nickel Metal–Organic Framework Monolayers for Photoreduction of Diluted CO2: Metal-Node-Dependent Activity and Selectivity. Angew. Chem. Int. Ed. 2018, 57, 16811–16815. [Google Scholar]
- Jiang, H.; Gong, S.; Xu, S.; Shi, P.; Fan, J.; Cecen, V.; Xu, Q.; Min, Y. Bimetal composites for photocatalytic reduction of CO2 to CO in the near-infrared region by the SPR effect. Dalt. Trans. 2020, 49, 5074–5086. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.C.; Liao, J.F.; Dong, Y.J.; Xu, Y.F.; Chen, H.Y.; Kuang, D.-B.; Su, C.Y. Core@shell CsPbBr3@zeolitic imidazolate framework nanocomposite for efficient photocatalytic CO2 reduction. ACS Energy Lett. 2018, 3, 2656–2662. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhou, Z.; Xu, M. Photocatalytic CO2 reduction catalyzed by metalloporphyrin: Understanding of cobalt and nickel sites in activity and adsorption. Appl. Surf. Sci. 2020, 513, 145801. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yang, M.Z.; Chen, B.X.; Wang, X.D.; Chen, H.Y.; Kuang, D.-B.; Su, C.Y. A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Z.; Li, Y.; Chu, X.; Li, Z.; Qu, Y.; Bai, L.; Jing, L. The synthesis of interface-modulated ultrathin Ni(ii) MOF/g-C3N4 heterojunctions as efficient photocatalysts for CO2 reduction. Nanoscale 2020, 12, 10010–10018. [Google Scholar] [CrossRef]
- Wang, D.; Huang, R.; Liu, W.; Sun, D.; Li, Z. Fe-based MOFs for photocatalytic CO2 reduction: Role of coordination unsaturated sites and dual excitation pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Dao, X.Y.; Guo, J.H.; Wei, Y.P.; Guo, F.; Liu, Y.; Sun, W.Y. Solvent-free photoreduction of CO2 to CO catalyzed by Fe-MOFs with superior selectivity. Inorg. Chem. 2019, 58, 8517–8524. [Google Scholar] [CrossRef]
- Wang, S.S.; Huang, H.H.; Liu, M.; Yao, S.; Guo, S.; Wang, J.W.; Zhang, Z.M.; Lu, T.B. Encapsulation of Single Iron Sites in a Metal-Porphyrin Framework for High-Performance Photocatalytic CO2 Reduction. Inorg. Chem. 2020, 59, 6301–6307. [Google Scholar] [CrossRef]
- Wu, L.Y.; Mu, Y.F.; Guo, X.X.; Zhang, W.; Zhang, Z.M.; Zhang, M.; Lu, T.B. Encapsulating Perovskite Quantum Dots in Iron-Based Metal–Organic Frameworks (MOFs) for Efficient Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 9491–9495. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Fu, Y.; Liu, W.; Ye, L.; Wang, D.; Yang, L.; Fu, X.; Li, Z. Studies on photocatalytic CO2 reduction over NH 2-uio-66(Zr) and its derivatives: Towards a better understanding of photocatalysis on metal-organic frameworks. Chem. Eur. J. 2013, 19, 14279–14285. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Zhu, W.; Li, X.; Zhang, C.; Su, Y.; Lian, Y.; Qi, P.; Deng, Z.; Zhang, D.; Wang, S.; et al. Electrostatic charge transfer for boosting the photocatalytic CO2 reduction on metal centers of 2D MOF/rGO heterostructure. Appl. Catal. B Environ. 2020, 262, 118144. [Google Scholar] [CrossRef]
- Li, S.; Ji, K.; Zhang, M.; He, C.; Wang, J.; Li, Z. Boosting the photocatalytic CO2 reduction of metal-organic frameworks by encapsulating carbon dots. Nanoscale 2020, 12, 9533–9540. [Google Scholar]
- Liu, S.; Chen, F.; Li, S.; Peng, X.; Xiong, Y. Enhanced photocatalytic conversion of greenhouse gas CO2 into solar fuels over g-C3N4 nanotubes with decorated transparent ZIF-8 nanoclusters. Appl. Catal. B Environ. 2017, 211, 1–10. [Google Scholar] [CrossRef]
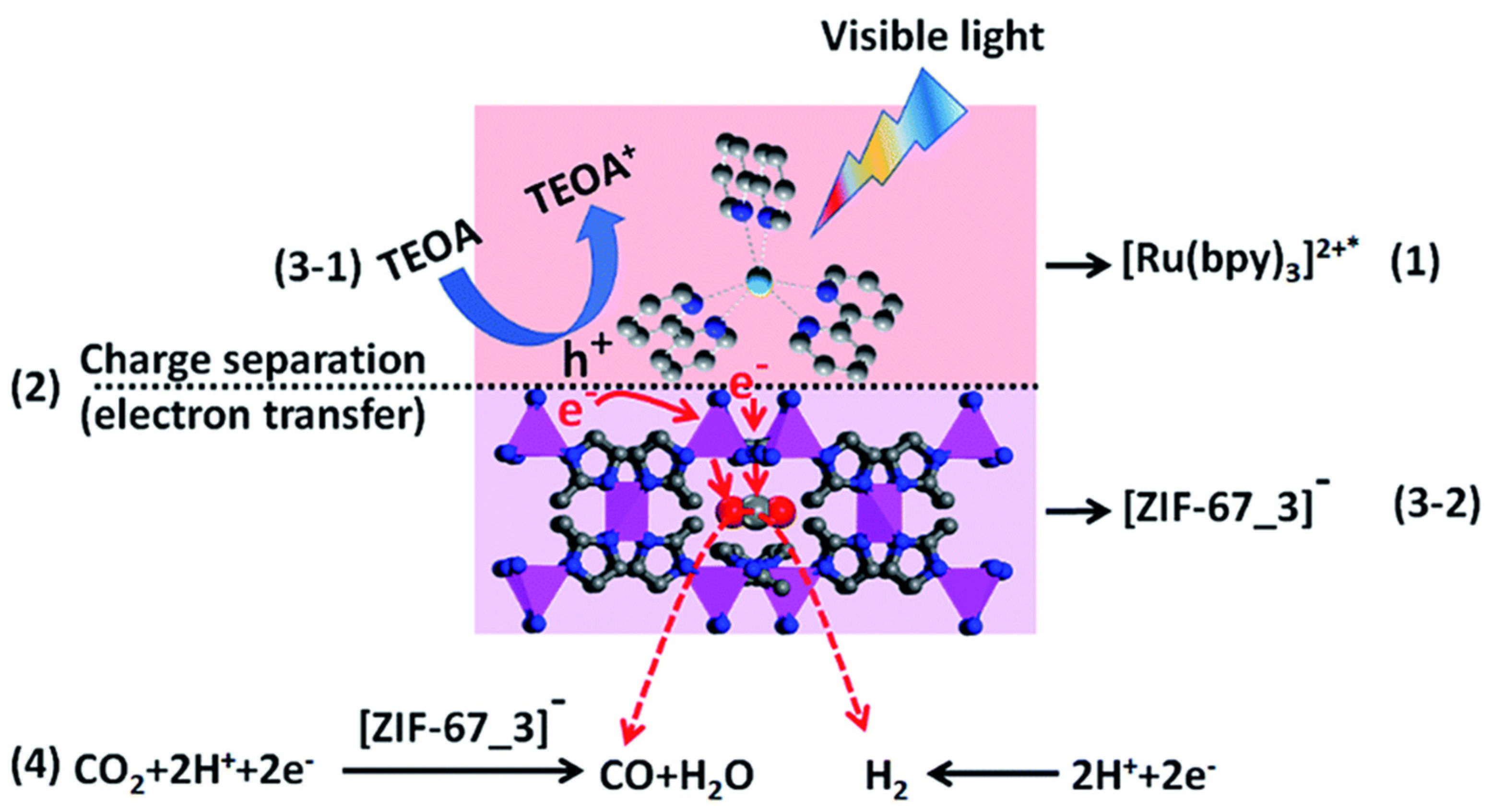
- Wang, M.; Liu, J.; Guo, C.; Gao, X.; Gong, C.; Wang, Y.; Liu, B.; Li, X.; Gurzadyan, G.G.; Sun, L. Metal-organic frameworks (ZIF-67) as efficient cocatalysts for photocatalytic reduction of CO2: The role of the morphology effect. J. Mater. Chem. A 2018, 6, 4768–4775. [Google Scholar] [CrossRef]
- Ren, J.T.; Zheng, Y.L.; Yuan, K.; Zhou, L.; Wu, K.; Zhang, Y.W. Self-templated synthesis of Co3O4 hierarchical nanosheets from a metal-organic framework for efficient visible-light photocatalytic CO2 reduction. Nanoscale 2020, 12, 755–762. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Zhang, J. Enhanced photocatalytic CO2 reduction activity of MOF-derived ZnO/NiO porous hollow spheres. J. CO2 Util. 2018, 24, 548–554. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; Dekrafft, K.E.; Lin, W. Doping metal-organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef]
- Benseghir, Y.; Lemarchand, A.; Duguet, M.; Mialane, P.; Gomez-Mingot, M.; Roch-Marchal, C.; Pino, T.; Ha-Thi, M.H.; Haouas, M.; Fontecave, M.; et al. Co-immobilization of a Rh Catalyst and a Keggin Polyoxometalate in the UiO-67 Zr-Based Metal-Organic Framework: In Depth Structural Characterization and Photocatalytic Properties for CO2 Reduction. J. Am. Chem. Soc. 2020, 142, 9428–9438. [Google Scholar] [CrossRef]
- Gao, X.; Guo, B.; Guo, C.; Meng, Q.; Liang, J.; Liu, J. Zirconium-Based Metal-Organic Framework for Efficient Photocatalytic Reduction of CO2 to CO: The Influence of Doped Metal Ions. ACS Appl. Mater. Interfaces 2020, 12, 24059–24065. [Google Scholar] [CrossRef] [PubMed]


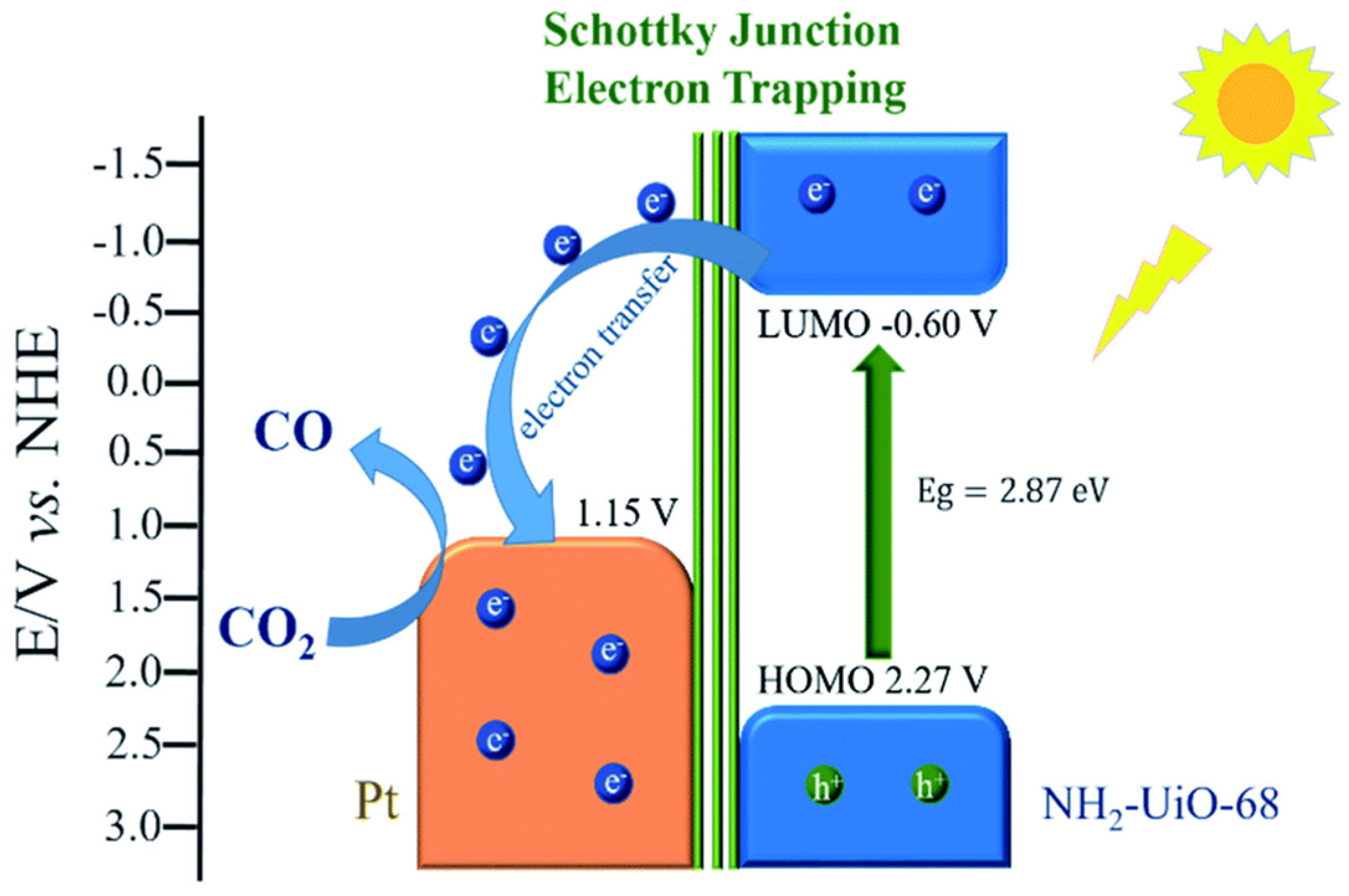
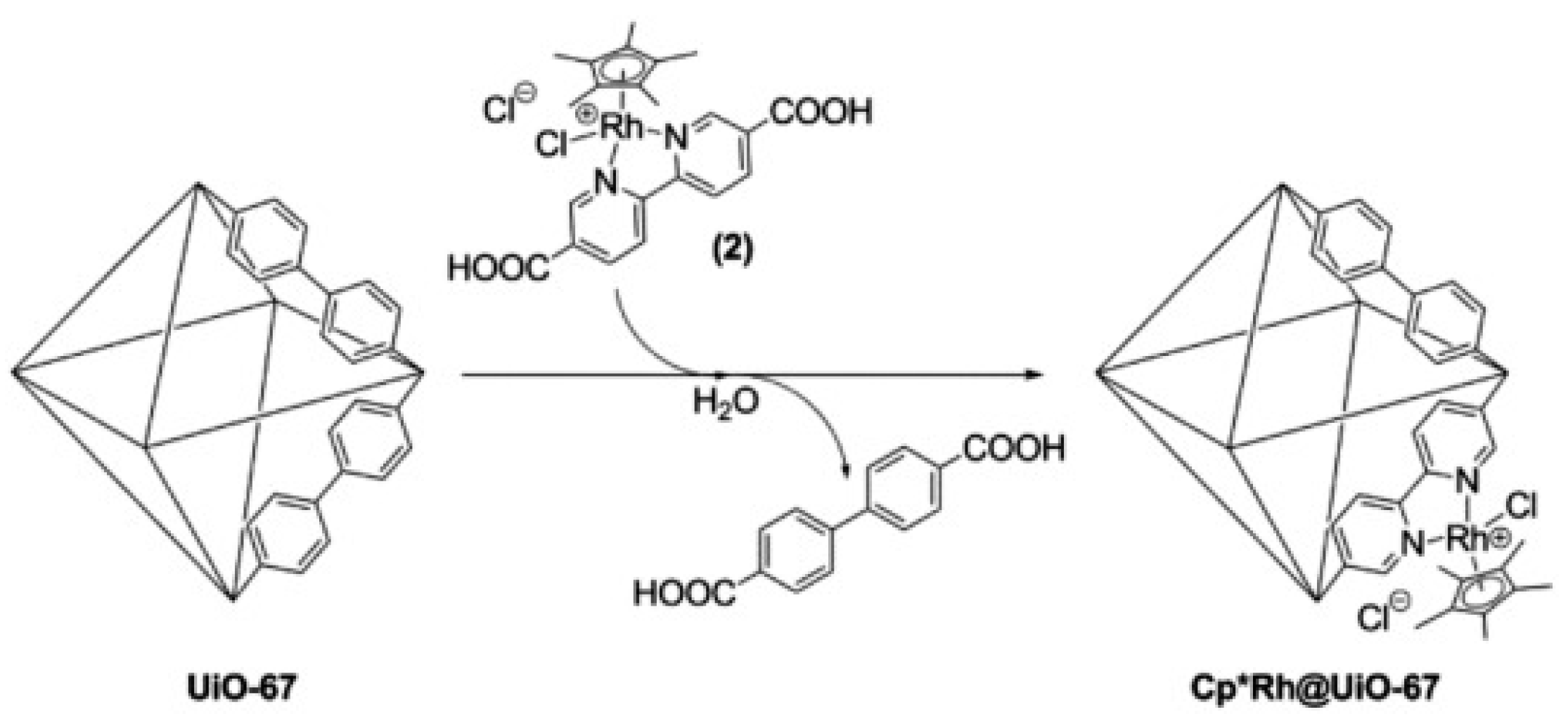

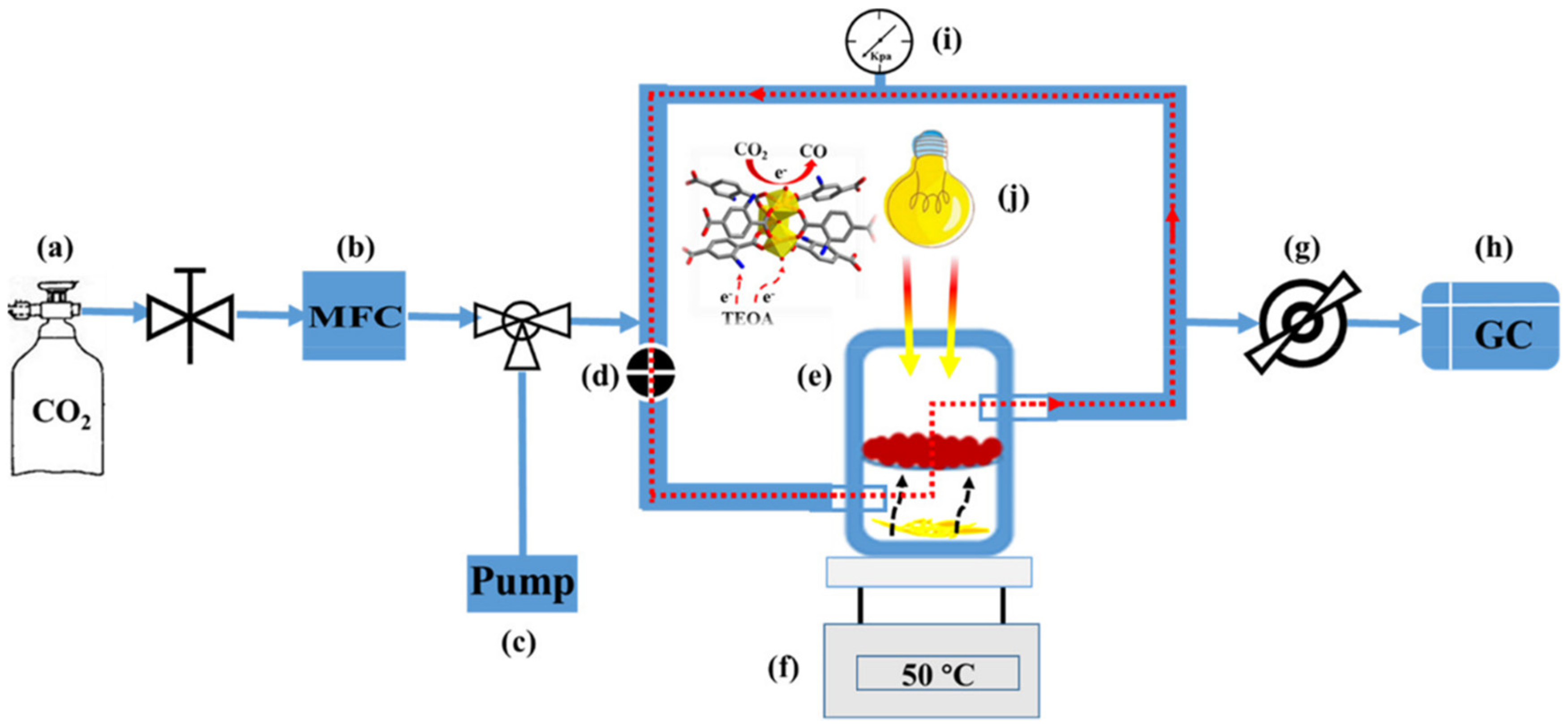

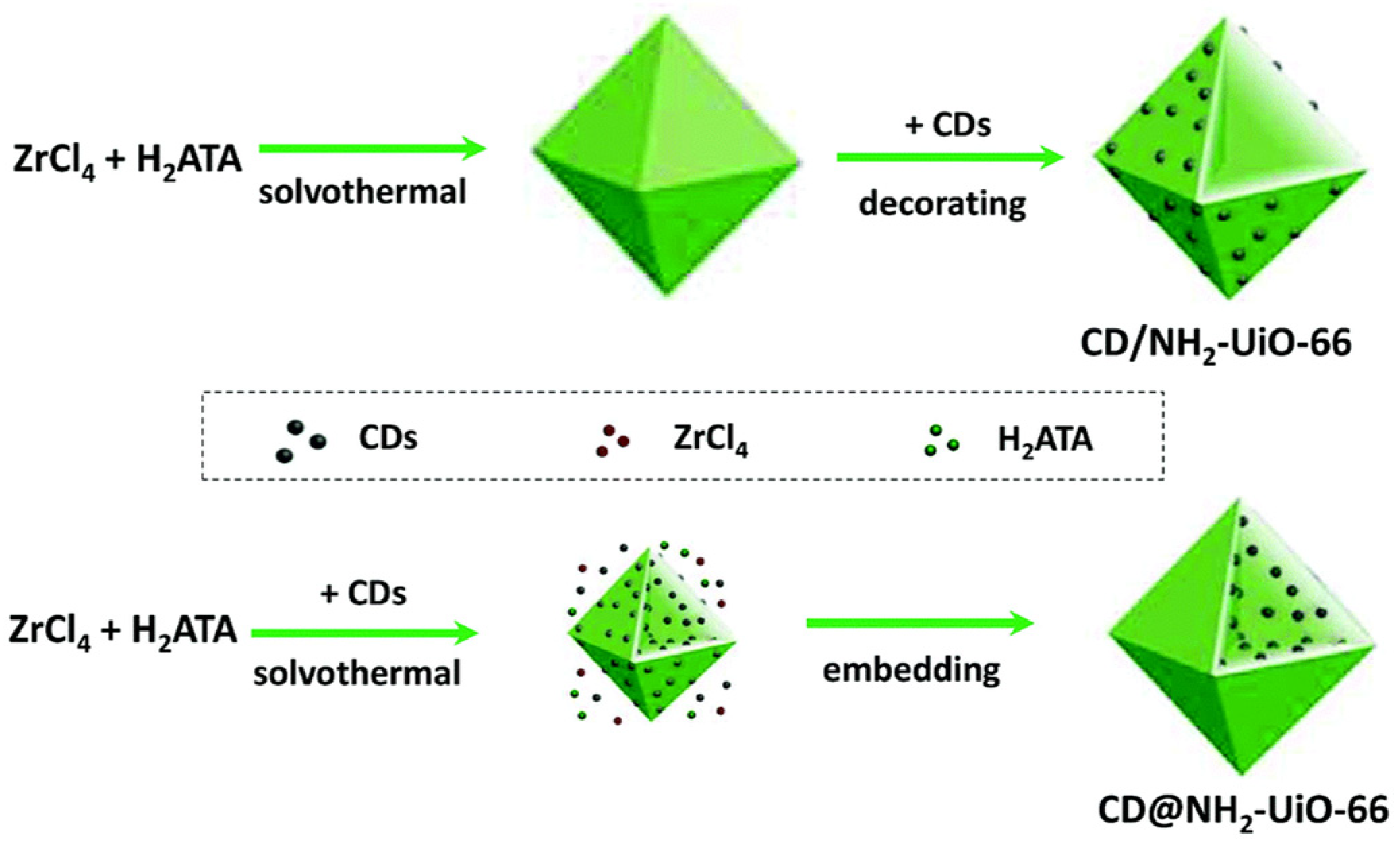

| Equation Number | Reaction | Reduction Potential (V) vs. NHE |
|---|---|---|
| 1 | CO2 + 2 + 2 HCOOH | −0.61 |
| 2 | CO2 + 4 + 4 HCHO + H2O | −0.53 |
| 3 | CO2 + 2 + 2 CO + H2O | −0.48 |
| 4 | CO2 + 6 + 6 CH3OH + H2O | −0.38 |
| 5 | CO2 + 8 + 8 CH4 + 2H2O | −0.24 |
| 6 | H2O ½ O2 + 2 + 2 | +0.82 |
| 7 | 2 + 2 H2 | −0.41 |
| MOF Hybrid Photocatalyst | Synthetic Methodology | Light Source | Photoreduction Product | Photocatalytic Performance | Reference |
|---|---|---|---|---|---|
| Ag@Co-ZIF-9 | In situ reduction | 200 W Xe ≥420 nm | CO | CO + H2 = 102.6 µmol h−1 | [96] |
| Ag@MIL-101-Cr | In situ reduction | 300 W Xe 400–780 nm | CO/CH4 | CO = 808.2 µmol g−1h−1 CH4 = 427.5 µmol g−1h−1 H2 = 82.1 µmol g−1h−1 | [97] |
| Ag@Co-MOF-74 | Solvothermal transformation | 300 W Xe 400–1000 nm | CO/H2 | CO ≈ 10 µmolh−1 H2 ≈ 17 µmolh−1 | [98] |
| Au@PPF-3 | Dispersion | 200 W Xe ≥400 nm | HCOOH | 42.3 µmol g−1h−1 | [99] |
| Au@TiO2 | Solvothermal/pyrolitic conversion | 200 W Hg/Xe 200–750 nm | CH4 | 10 ppm/h | [100] |
| Pt@MIL-125(Ti) | In situ reduction | 300 W Xe 420–800 nm | HCOO− | H2 = 235 µmol HCOO− = 13 µmol | [101] |
| Pt@NH2-UiO-68 | Solvothermal | 300 W Xe 400–780 nm | CO | 400 µmol g−1 | [102] |
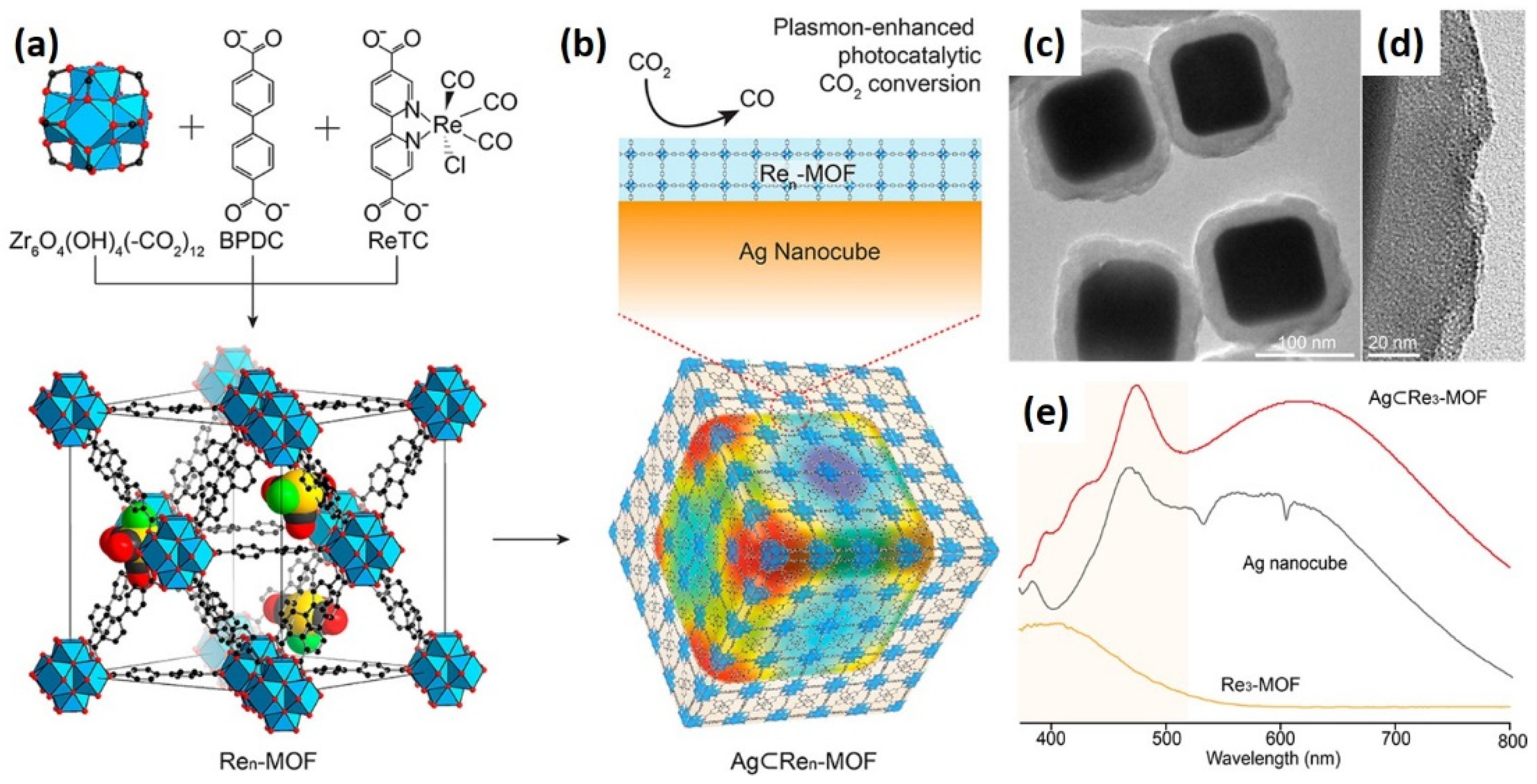
| Ag⊂Re3-MOF | Mixed linker Solvothermal | 300 W Xe 400–700 nm | CO | 0.093 h−1 | [15] |
| Cp*Rh@UiO-67 | Linker Exchange | 300 W Xe ≥415 nm | H2/HCOO− | TON HCOO− = 47 TON H2 = 36 HCOO− + H2 = 1.2 µmol h−1 | [103] |
| Descriptor | MOF Hybrid Photocatalyst | Photocatalytic Trend | Ref |
|---|---|---|---|
| Crystallite size | Ag@MIL-101-Cr | The smallest MIL-101 crystallite decorated with Ag provided superior activity | [97] |
| Organic linker | Pt@MIL-125(Ti) | The use of 2-aminoterephthalic acid instead of terephthalic acid organic linker enhanced the visible light absorption and CO2 adsorption capacity | [101] |
| Surface area and shell thickness | Ag@Co-MOF-74 | Large surface area and thin shell allowed exposure to active sites and easy diffusion of substrates, respectively | [98] |
| NP location | Pt@NH2-UiO-68 | Pt NPs embedded within the MOF displayed 3-fold enhanced activity compared with NPs present on surface | [102] |
| Length of organic linker | Pt@NH2-UiO-68 | NH2-UiO-68 gave higher product yields than NH2-UiO-66 due to its ability to accommodate more CO2 molecules | [102] |
| Thickness of framework | Au@PPF-3 | Au decorated thin PPF-3 sheets provided superior activity than with thicker sheets | [99] |
| Bifunctionality | Ag⊂Re3-MOF | The combination of Re3-MOF with Ag nanocubes enhanced light absorption and catalytic activity (five-fold) compared with bare Re3-MOF. | [15] |
| MOF Hybrid Photocatalyst | Synthetic Methodology | Light Source | Photoreduction Product | Photocatalytic Performance | Ref |
|---|---|---|---|---|---|
| NH2-Fe MOFs | Solvothermal | 300 W Xe 420–800 nm | HCOO− | 178 µmol (8 h) | [118] |
| Solvent-free NH2-Fe MOFs | Solvothermal | 300 W Xe 420–780 nm | CO | 87.6 μmol g−1 | [119] |
| LHP QDs@PCN-221(Fe) | Solvothermal/PSM | 300 W Xe ≥400 nm | CO/CH4 | 1559 µmolg−1 (80 h) CO (34%)/CH4 (66%) | [121] |
| In-FexTCPP-MOF | Solvothermal | 300 W Xe ≥400 nm | CO | 3469 μmolg−1 (24 h) | [120] |
| Descriptor | MOF Hybrid Photocatalyst | Photocatalytic Trend | Ref |
|---|---|---|---|
| Topology | NH2-Fe MOFs | Three different Fe-based MOFs, namely MIL-101, MIL-53, and MIL-88B, with different topologies and different photocatalytic performances | [118] |
| Excitation pathway | NH2-Fe MOFs | Difference in the excitation pathway for amino functionalised and unfunctionalised MOFs under visible light irradiation | [118] |
| Unsaturated Fe sites | Solvent-free NH2-Fe MOFs | Thermal treatment removes H2O ligands and exposes unsaturated Fe sites for enhanced activity | [119] |
| Proximity (QD to MOF) | LHP QDs@PCN-221(Fe) | Localisation of LHP QDs in close proximity to MOF imparts stability and superior photoreduction ability | [121] |
| Oxidation state | In-FexTCPP-MOF | The existence of Fe(III) oxidation state in MOF results in the superior photocatalytic reduction | [120] |
| MOF Hybrid Photocatalyst | Synthetic Methodology | Light Source | Photoreduction Product | Photocatalytic Performance | Ref |
|---|---|---|---|---|---|
| rGO- Ni3HITP2 | Ultrasonic dispersion | 100 W LED 420 nm | CO | TOF = 0.46 min−1; selectivity = 91% | [123] |
| CD@NH2-UiO-66 | Solvothermal and embedding | Xe Lamp ≥420 nm | CO | 16.6 µmol gcat −1h−1 | [124] |
| g-C3N4/ZIF-8 | In situ deposition | 300 W Xe Full spectrum | CO2/H2O to CH3OH | 0.75 µmol h−1 g−1 | [125] |
| Morphology-controlled ZIF-67 | Solvent-induced method | 300 W Xe ≥420 nm | CO | 3.89 µmol mg−1 h−1 | [126] |
| Single atom/Co-MOF | Wet impregnation | 300 W Xe 400–800 nm | CO and CH4 | CO = 200.6 µmol g−1 h−1 CH4 = 36.67 µmol g−1 h−1 | [80] |
| Co3O4 hierarchical nanosheets | Oil bath method and calcination | 300 W Xe ≥400 nm | CO and H2 | 39.7 µmol h−1 | [127] |
| MOF derived ZnO/NiO porous spheres | Thermal treatment from Zn-Ni MOFs | 300 W Xe Full spectrum | CH3OH | 1.57 µmol h−1 g−1 | [128] |
| Descriptor | Photocatalyst | Photocatalytic Trends | Ref |
|---|---|---|---|
| Surface area | rGO- Ni3HITP2 | rGO heterostructure creating high surface area and enhanced conductivity | [123] |
| Location of CDs (carbon dots) | CD@NH2-UiO-66 | An approximately 4 times higher catalytic yield with embedded CDs than surface decoration | [124] |
| Affinity sites | g-C3N4/ZIF-8 | Negatively charged g-C3N4 provided affinity to Zn ions for the growth of ZIF-8 nanoclusters | [125] |
| Single atom/Co-MOF | Co metallisation increasing the number of affinity sites in MOF-525 for CO2 adsorption | [80] | |
| Synergistic interaction | g-C3N4/ZIF-8 | Improved CO2 conversion due to synergistic tuning between g-C3N4 and ZIF-8 | [125] |
| Morphology vs. surface area | Morphology-controlled ZIF-67 | Smallest surface area for 2D leaf-like ZIF-67 with maximum CO2 adsorption because of flexible cavities absent in rhombic and pitaya-like morphologies | [126] |
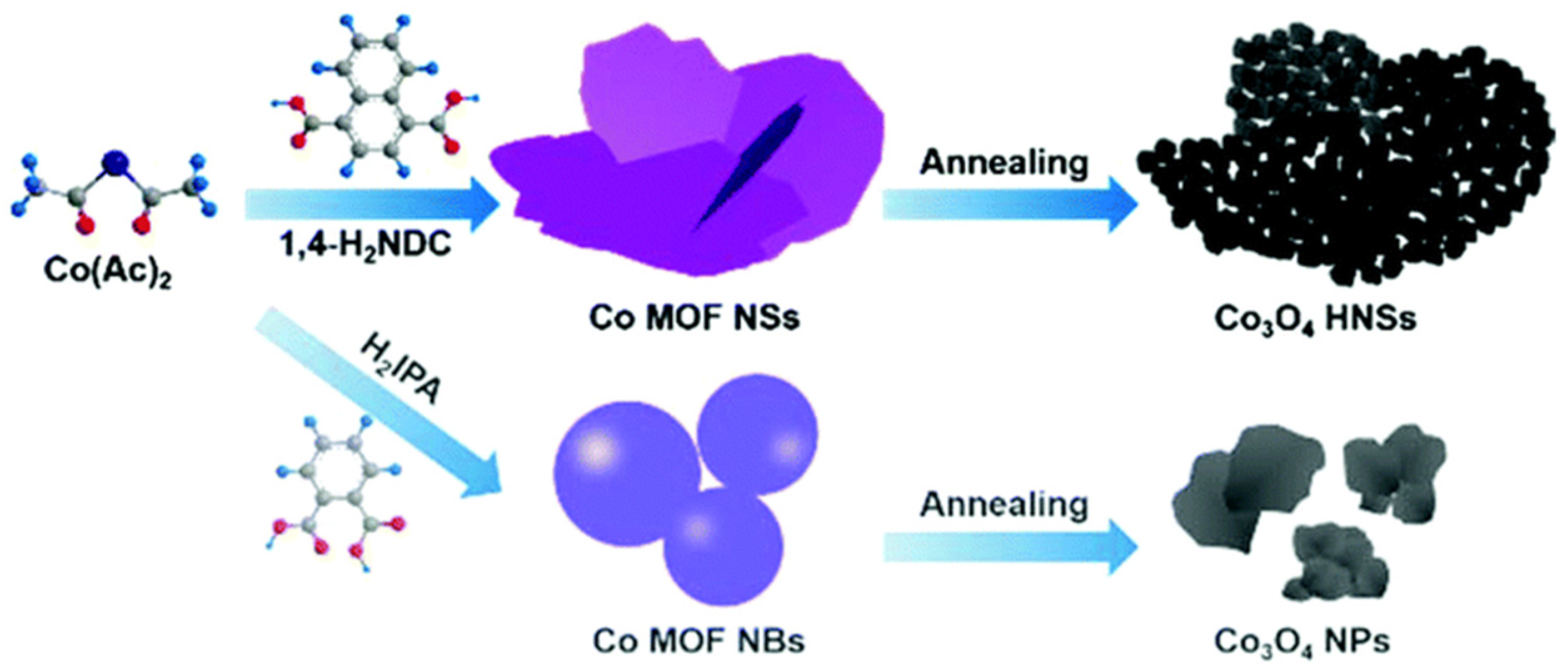
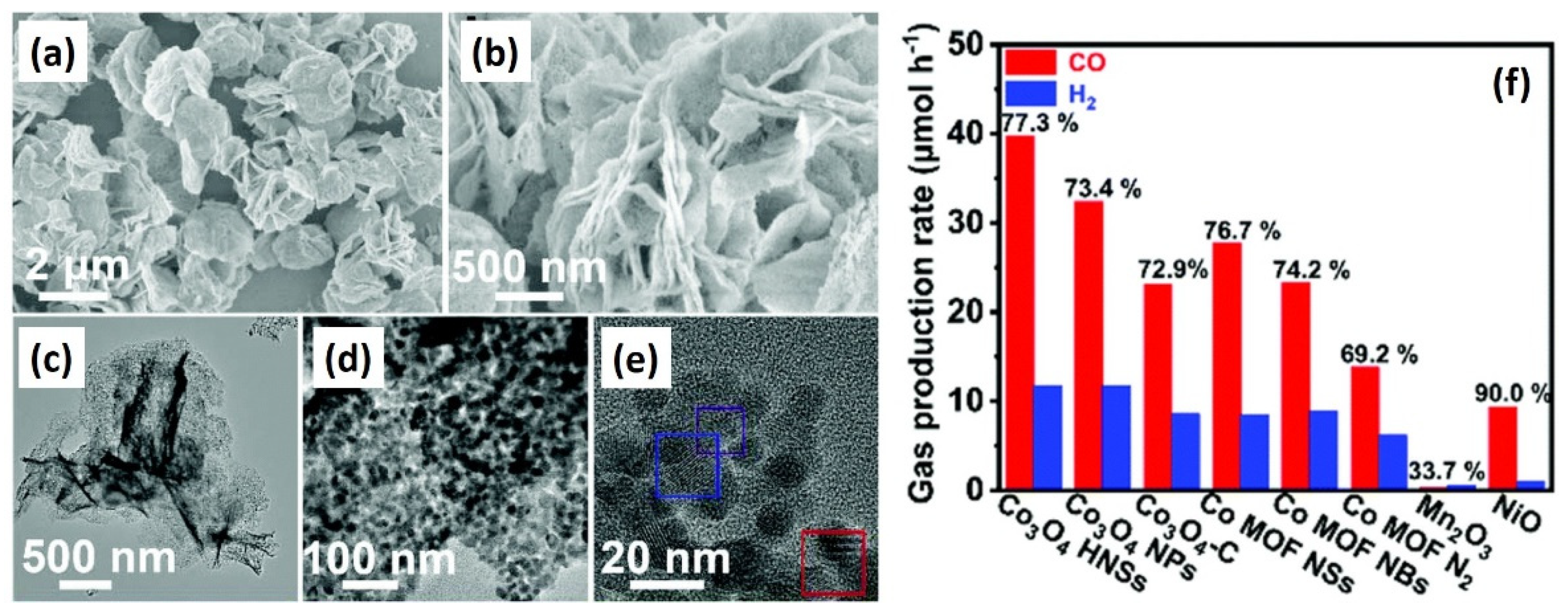
| Organic linker vs. morphology | Co3O4 hierarchical nanosheets (HNSs) | The use of 1,4-naphthalenedicarboxylic acid as organic linker formed Co3O4 HNSs and isophthalic acid formed Co3O4 NPs | [127] |
| NPs size vs. distribution | Co3O4 hierarchical nanosheets (HNSs) | Small NPs of HNSs with narrow size distribution enhanced migration efficiency | [127] |
| Monometallic vs. bimetallic | MOF derived ZnO/NiO porous spheres | Bimetallic ZnO/NiO MOFs exhibited three times greater yield than ZnO or NiO oxides | [128] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, P.; J. Stewart, D.; Raja, R. Recent Advances in Photocatalytic CO2 Utilisation Over Multifunctional Metal–Organic Frameworks. Catalysts 2020, 10, 1176. https://doi.org/10.3390/catal10101176
Verma P, J. Stewart D, Raja R. Recent Advances in Photocatalytic CO2 Utilisation Over Multifunctional Metal–Organic Frameworks. Catalysts. 2020; 10(10):1176. https://doi.org/10.3390/catal10101176
Chicago/Turabian StyleVerma, Priyanka, Daniel J. Stewart, and Robert Raja. 2020. "Recent Advances in Photocatalytic CO2 Utilisation Over Multifunctional Metal–Organic Frameworks" Catalysts 10, no. 10: 1176. https://doi.org/10.3390/catal10101176
APA StyleVerma, P., J. Stewart, D., & Raja, R. (2020). Recent Advances in Photocatalytic CO2 Utilisation Over Multifunctional Metal–Organic Frameworks. Catalysts, 10(10), 1176. https://doi.org/10.3390/catal10101176