In Situ Generated Nanosized Sulfide Ni-W Catalysts Based on Zeolite for the Hydrocracking of the Pyrolysis Fuel Oil into the BTX Fraction
Abstract
:1. Introduction
2. Results
2.1. Catalytic Properties
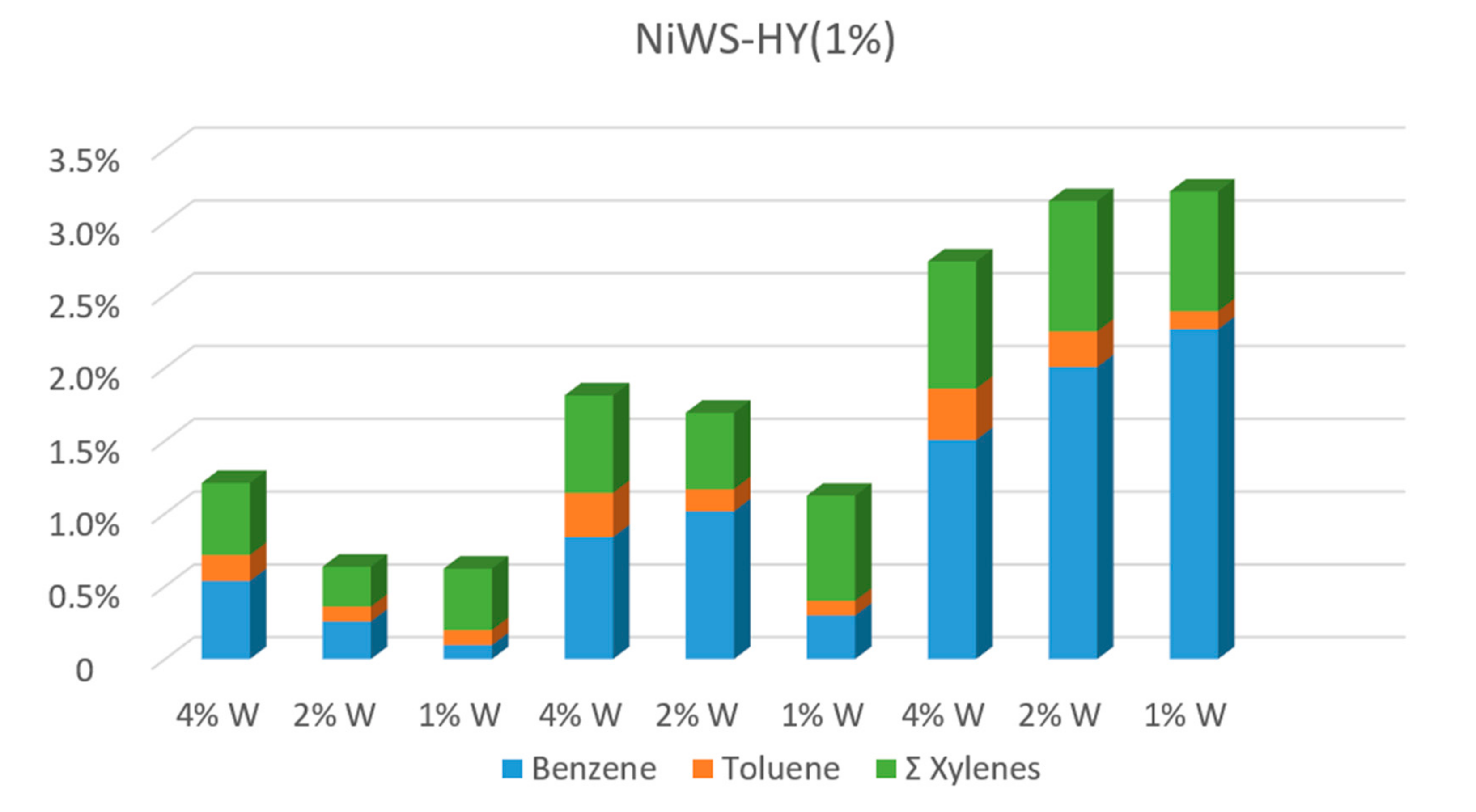
2.1.1. Naphthalene Hydrocracking with NiWS-HY Catalysts
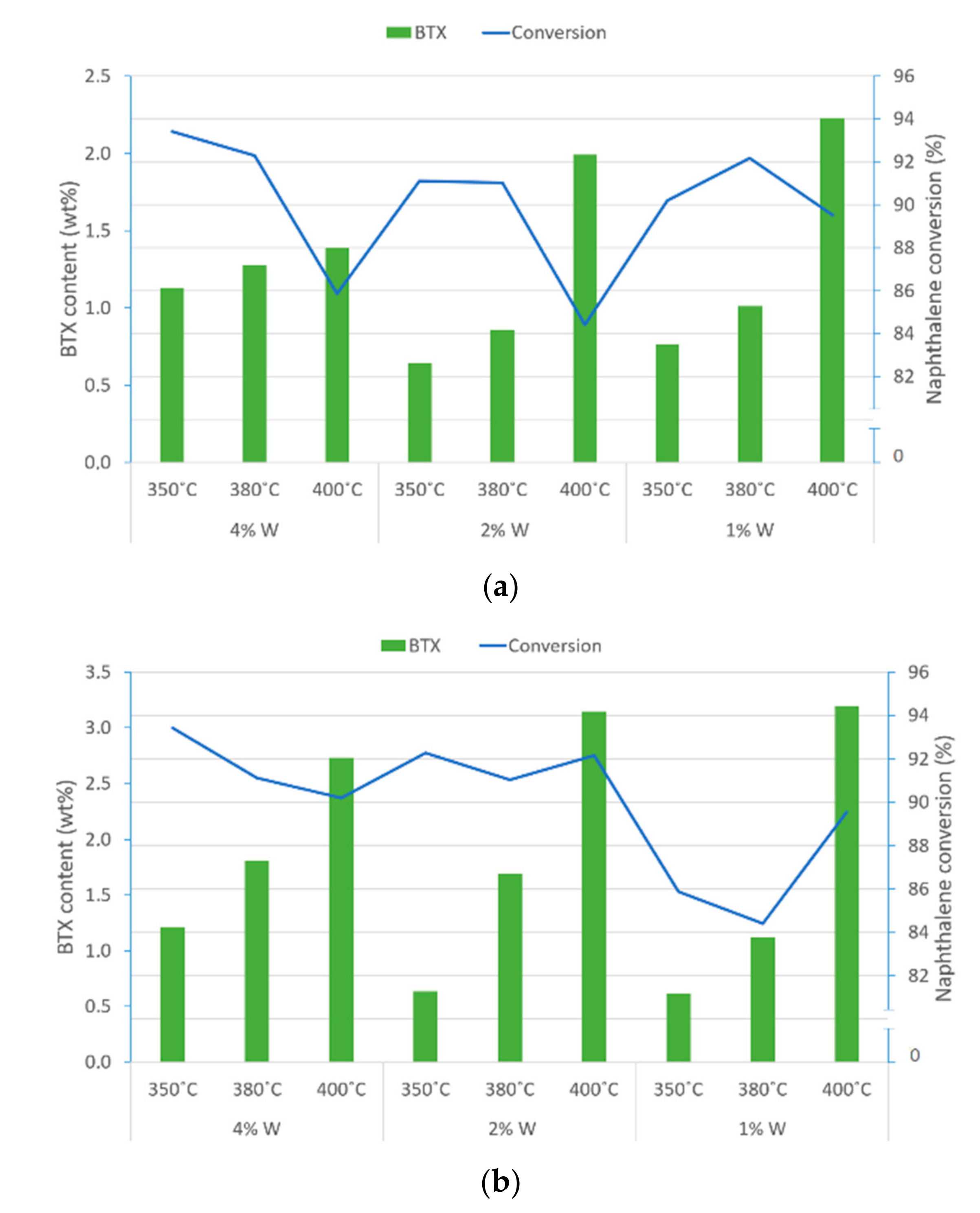
2.1.2. Pyrolysis Fuel Oil Hydrocracking with NiWS-HY Catalysts
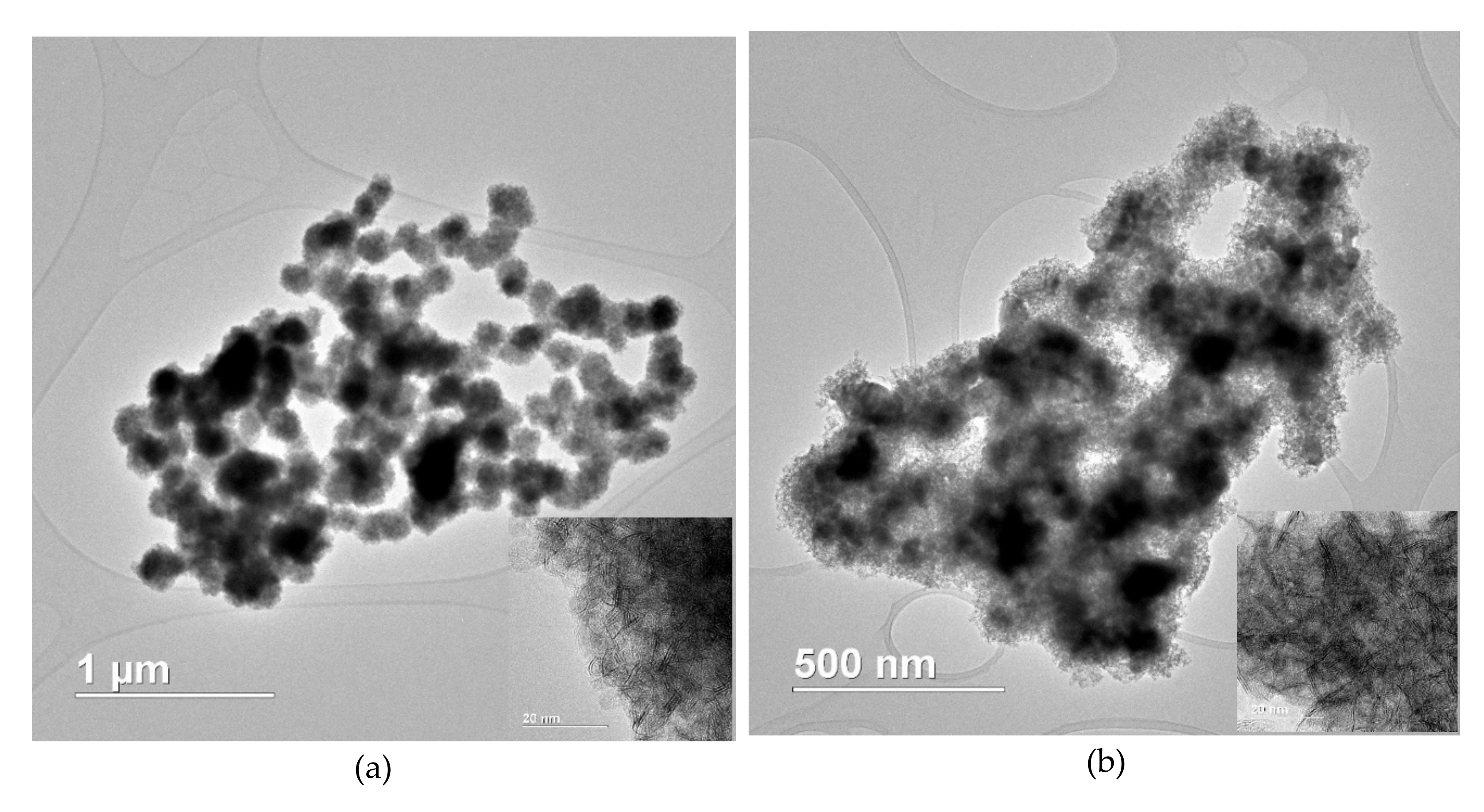
2.2. Catalyst Properties
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.3. Catalytic Tests
3.4. Products Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
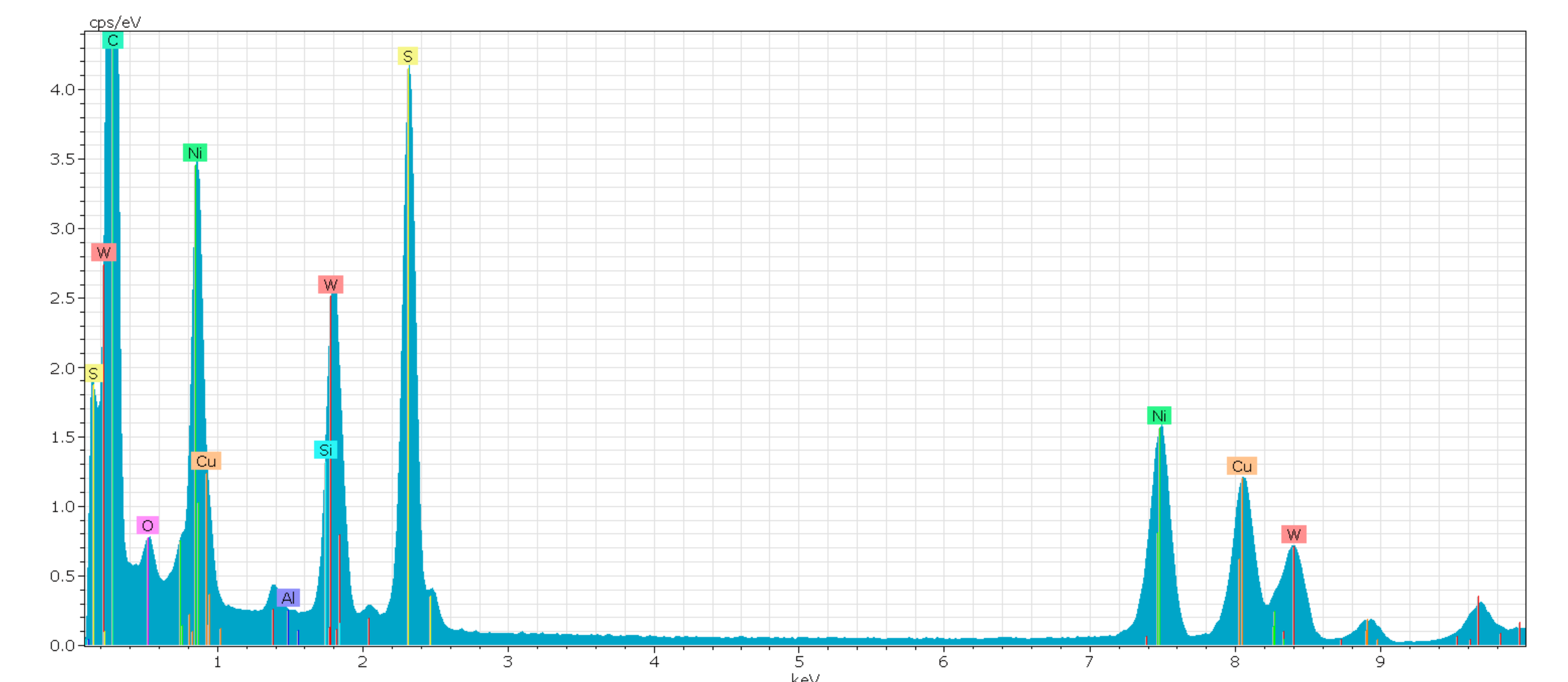
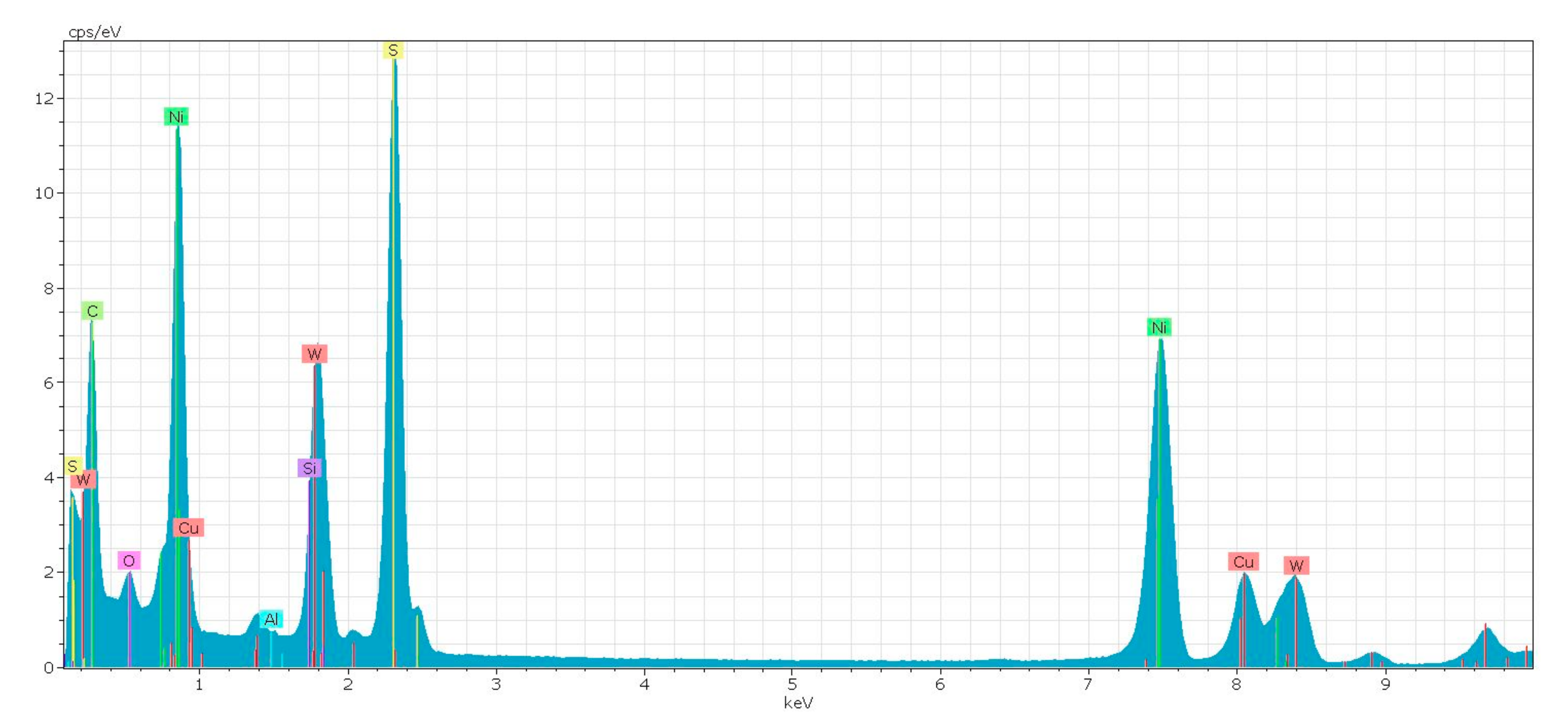
References
- Yan, J.D. Biomass to energy in china: Development status and strategic consideration. Chem. Ind. For. Prod. 2014, 34, 151–158. [Google Scholar]
- Cho, M.H.; Jung, S.H.; Kim, J.S. Pyrolysis of mixed plastic wastes for the recovery of benzene, toluene, and xylene (BTX) aromatics in a fluidized bed and chlorine removal by applying various additives. Energy Fuels 2010, 24, 1389–1395. [Google Scholar] [CrossRef]
- Xu, X.W.; Jiang, E.C. “BTX” from guaiacol HDO under atmospheric pressure: The effect of support and the carbon deposition. Energy Fuels 2017, 31, 2855–2864. [Google Scholar] [CrossRef]
- Xu, N.; Pan, D.; Wu, Y.; Xu, S.; Gao, L.; Zhang, J.; Xiao, G. Preparation of nano-sized HZSM-5 zeolite with sodium alginate for glycerol aromatization. React. Kinet. Mech. Catal. 2019, 127, 449–467. [Google Scholar] [CrossRef]
- Song, W.; Zhou, S.; Hu, S.; Lai, W.; Lian, Y.; Wang, J.; Jiang, X. Surface engineering of CoMoS nano-sulfide for hydrodeoxygenation of lignin-derived phenols to arenes. ACS Catal. 2019, 9, 259–268. [Google Scholar] [CrossRef]
- Jung, S.H.; Cho, M.H.; Kang, B.S.; Kim, J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Xu, X.W.; Jiang, E.C.; Li, Z.Y.; Sun, Y. BTX from anisole by hydrodeoxygenation and transalkylation at ambient pressure with zeolite catalysts. Fuel 2018, 221, 440–446. [Google Scholar] [CrossRef]
- Tamiyakul, S.; Ubolcharoen, W.; Tungasmita, D.N.; Jongpatiwut, S. Conversion of glycerol to aromatic hydrocarbons over Zn-promoted HZSM-5 catalysts. Catal. Today 2015, 256, 325–335. [Google Scholar] [CrossRef]
- Laredo, G.C.; Pérez-Romo, P.; Escobar, J.; Garcia-Gutierrez, J.L.; Vega-Merino, P.M. Light cycle oil upgrading to benzene, toluene, and xylenes by hydrocracking: Studies using model mixtures. Ind. Eng. Chem. Res. 2017, 56, 10939–10948. [Google Scholar] [CrossRef]
- Polliotto, V.; Livraghi, S.; Agnoli, S.; Granozzi, G.; Giamello, E. Reversible adsorption of oxygen as superoxide ion on cerium doped zirconium titanate. Appl. Catal. A Gen. 2019, 580, 140–148. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.; Shin, J.; Lee, S.; Kim, D.; Lee, J.K. Selective hydroconversion of naphthalenes into light alkyl-aromatic hydrocarbons. Appl. Catal. A Gen. 2015, 492, 140–150. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Cho, K.-S.; Lee, Y.-K. Structure and activity of Ni2P/desilicated zeolite β catalysts for hydrocracking of pyrolysis fuel oil into benzene, toluene, and xylene. Catalyst 2020, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Upare, D.P.; Park, S.; Kim, M.S.; Jeon, Y.-P.; Kim, J.; Lee, D.; Lee, C.W. Selective hydrocracking of pyrolysis fuel oil into benzene, toluene and xylene over CoMo/beta zeolite catalyst. J. Ind. Eng. Chem. 2017, 46, 356–363. [Google Scholar] [CrossRef]
- Sato, K.; Iwata, Y.; Miki, Y.; Shimad, H. Hydrocracking of TEtralin over NiW/USY zeolite catalysts: For the improvement of heavy-oil upgrading catalysts. J. Catal. 1999, 186, 45–56. [Google Scholar] [CrossRef]
- Ishihara, A.; Itoh, T.; Nasu, H.; Hashimoto, T.; Doi, T. Hydrocracking of 1-methylnaphthalene/decahydronaphthalene mixture catalyzed by zeolite-alumina composite supported NiMo catalysts. Fuel Process. Technol. 2013, 116, 222–227. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, J.K.; Miyawaki, J.; Kim, Y.K.; Yoon, S.H.; Mochida, I. Hydro-conversion of 1-methyl naphthalene into (alkyl)benzenes over alumina-coated USY zeolite-supported NiMoS catalysts. Fuel 2011, 90, 182–189. [Google Scholar] [CrossRef]
- Ali, M.A.; Tatsumi, T.; Masuda, T. Development of heavy oil hydrocracking catalysts using amorphous silica-alumina and zeolites as catalyst supports. Appl. Cataly. A Gen. 2002, 233, 77–90. [Google Scholar] [CrossRef]
- Silva-Rodrigo, R.; Calderón-Salas, C.; Melo-Banda, J.A.; Domínguez, J.M.; Vázquez-Rodríguez, A. Synthesis, characterization and comparison of catalytic properties of NiMo- and NiW/Ti-MCM-41 catalysts for HDS of thiophene and HVGO. Catal. Today 2004, 98, 123–129. [Google Scholar] [CrossRef]
- Leyva, C.; Rana, M.S.; Trejo, F.; Ancheyta, J. On the use of acid-base-supported catalysts for hydroprocessing of heavy petroleum. Ind. Eng. Chem. Res. 2007, 46, 7448–7466. [Google Scholar] [CrossRef]
- Leyva, C.; Rana, M.S.; Trejo, F.; Ancheyta, J. NiMo supported acidic catalysts for heavy oil hydroprocessing. Catal. Today 2009, 141, 168–175. [Google Scholar] [CrossRef]
- Tiwari, R.; Rana, B.S.; Kumar, R.; Verma, D.; Kumar, R.; Joshi, R.K.; Garg, M.O.; Sinha, A.K. Hydrotreating and hydrocracking catalysts for processing of waste soya-oil and refinery-oil mixtures. Catal. Commun. 2011, 12, 559–562. [Google Scholar] [CrossRef]
- Looi, P.Y.; Mohamed, A.R.; Tye, C.T. Hydrocracking of residual oil using molybdenum supported over mesoporous alumina as a catalyst. Chem. Eng. J. 2012, 181, 717–724. [Google Scholar] [CrossRef]
- Nishijima, A.; Shimada, H.; Sato, T.; Yoshimur, Y.; Hiraishi, J. Support effects on hydrocarcking and hydrogenation activities of molybdenum catlysts used for upgrading coal-derived liquids. Polyhedron 1986, 5, 243–247. [Google Scholar] [CrossRef]
- Shimada, H. Morphology, dispersion and catalytic functions of supported molybdenum sulfide catalysts for hydrotreating petroleum fractions. J. Jpn. Pet. Inst. 2016, 59, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Villalón, P.; Ramírez, J.; Cuevas, R.; Vázquez, P.; Castañeda, R. Influence of the support on the catalytic performance of Mo, CoMo, and NiMo catalysts supported on Al2O3 and TiO2 during the HDS of thiophene, dibenzothiophene, or 4,6-dimethyldibenzothiophene. Catal. Today 2016, 259, 140–149. [Google Scholar] [CrossRef]
- Laredo, G.C.; Pérez-Romo, P.; Vega-Merino, P.M.; Arzate-Barbosa, E.; García-López, A.; Agueda-Rangel, R.; Martínez-Moreno, V.H. Effect of the catalytic system and operating conditions on BTX formation using tetralin as a model molecule. Appl. Petrochem. Res. 2019, 9, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Upare, D.P.; Park, S.; Kim, M.S.; Kim, J.; Lee, D.; Lee, J.; Lee, C.W. Cobalt promoted Mo/beta zeolite for selective hydrocracking of tetralin and pyrolysis fuel oil into monocyclic aromatic hydrocarbons. J. Ind. Eng. Chem. 2016, 35, 99–107. [Google Scholar] [CrossRef]
- Yi, Y.; Jin, X.; Wang, L.; Zhang, Q.; Xiong, G.; Liang, C. Preparation of unsupported Ni-Mo-S catalysts for hydrodesulfurization of dibenzothiophene by thermal decomposition of tetramethylammonium thiomolybdates. Catal. Today 2011, 175, 460. [Google Scholar] [CrossRef]
- Olivas, A.; Zepeda, T.A.; Villalpando, I.; Fuentes, S. Performance of unsupported Ni(Co,Fe)/MoS2 catalysts in hydrotreating reactions. Catal. Commun. 2008, 9, 1317–1328. [Google Scholar] [CrossRef]
- Beller, M.; Renken, A.; van Santen, R.A. Catalysis: From Principles to Applications; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Furimsky, E.; Massoth, F.E. Deactivation of hydroprocessing catalysts. Catal. Today 1999, 52, 381–495. [Google Scholar] [CrossRef]
- Hagenbach, G.; Courty, P.; Delmon, B. Physicochemical investigations and catalytic activity measurements on crystallized molydbenum sulfide-cobalt sulfide mixed catalysts. Catalyst 1973, 31, 264–273. [Google Scholar] [CrossRef]
- Tops⊘e, H.; Clausen, B.S. Importance of Co-Mo-S type structures in hydrodesulfurization. Catal. Rev. 1984, 26, 395–420. [Google Scholar] [CrossRef]
- Hur, Y.G.; Kim, M.-S.; Lee, D.-W.; Kim, S.; Eom, H.-J.; Jeong, G.; Lee, K.-Y. Hydrocracking of vacuum residue into lighter fuel oils using nanosheet-structured WS 2 catalyst. Fuel 2014, 137, 237–244. [Google Scholar] [CrossRef]
- Bellussi, G.; Rispoli, G.; Landoni, A.; Millini, R.; Molinari, D.; Montanari, E.; Moscotti, D.; Pollesel, P. Hydroconversion of heavy residues in slurry reactors: Developments and perspectives. J. Catal. 2013, 308, 189–200. [Google Scholar] [CrossRef]
- Knyazeva, M.I.; Panyukova, D.I.; Kuchinskaya, T.S.; Kulikov, A.B.; Maximov, A.L. Effect of composition of cobalt-molybdenum-containing sulfonium thiosalts on the hydrogenation activity of nanosized catalysts in situ synthesized on their basis. Pet. Chem. 2019, 59, 1285–1292. [Google Scholar] [CrossRef]
- Kennepohl, D.; Sanford, E. Conversion of athabasca bitumen with dispersed and supported mo-based catalysts as a function of dispersed catalyst concentration. Energy Fuels 1996, 10, 229–234. [Google Scholar] [CrossRef]
- Alonso, G.; Chianelli, R.R. WS2 catalysts from tetraalkyl thiotungstate precursors and their concurrent in situ activation during HDS of DBT. J. Catal. 2004, 221, 657–661. [Google Scholar] [CrossRef]
- Jeong, H.-R.; Lee, Y.K. Comparison of unsupported WS2 and MoS2 catalysts for slurry phase hydrocracking of vacuum residue. Appl. Catal. A Gen. 2019, 572, 90–96. [Google Scholar] [CrossRef]
- Sizova, I.A.; Panyukova, D.I.; Maksimov, A.L. Hydrotreating of high-aromatic waste of coke and by-product processes in the presence of in situ synthesized sulfide nanocatalysts. Pet. Chem. 2017, 57, 1304–1309. [Google Scholar] [CrossRef]
- Onishchenko, M.I.; Kulikov, A.B.; Maksimov, A.L. Application of zeolite Y-based Ni-W supported and in situ prepared catalysts in the process of vacuum gas oil hydrocracking. Pet. Chem. 2017, 57, 1287–1294. [Google Scholar] [CrossRef]
- Hur, Y.G.; Lee, D.-W.; Lee, K.-Y. Hydrocracking of vacuum residue using NiWS(x) dispersed catalysts. Fuel 2016, 185, 794–803. [Google Scholar] [CrossRef]
- Jeon, S.G.; Na, J.-G.; Ko, C.H.; Lee, K.B.; Rho, N.S.; Park, S.B. A new approach for preparation of oil-soluble bimetallic dispersed catalyst from layered ammonium nickel molybdate. Mater. Sci. Eng. B. 2011, 176, 606–610. [Google Scholar] [CrossRef]
- Ben Tayeb, K.; Lamonier, C.; Lancelot, C.; Fournier, M.; Payen, E.; Bonduelle, A.; Bertoncini, F. Study of the active phase of NiW hydrocracking sulfided catalysts obtained from an innovative heteropolyanion based preparation. Catal. Today 2010, 150, 207–212. [Google Scholar] [CrossRef]
- McDonald, W.; Friesen, G.D.; Rosenhein, L.D.; Newton, W.E. Syntheses and characterization of ammonium and tetraalkylammonium thiomolybdates and thiotungstates. Inorg. Chim. Acta 1983, 72, 205–210. [Google Scholar] [CrossRef]
- Furimsky, E. Catalyst for Upgrading Heavy Petroleum Feed; Elsevier: Amsterdam, The Netherlands, 2007; p. 404. [Google Scholar]
- Kniazeva, M.; Maximov, A. Effect of additives on the activity of Nickel-Tungsten sulfide hydroconversion catalysts prepared in situ from oil-soluble precursors. Catalysts 2018, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Sun, H.; Fu, H.; Zheng, J.; Zhang, N.; Li, Y.; Chen, B.H. Effect of ruthenium nickelbimetallic composition on the catalytic performance for benzene hydrogenation tocyclohexane. Appl. Catal. A Gen. 2015, 49, 124–132. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; van der Meer, Y.; van Veen, J.A.R.; Niemantsverdriet, J.W. Insight into the formation of theactive phases in supported NiW hydrotreating catalysts. Appl. Catal. A Gen. 2007, 322, 16–32. [Google Scholar] [CrossRef]
- Díaz de León, J.N.; Antunes-García, J.; Alonso-Nuñez, G.; Zepeda, T.A.; Galvan, D.H.; de los Reyes, J.A.; Fuentes, S. Support effects of NiW hydrodesulfurization catalysts from experiments and DFT calculations. Appl. Catal. B Environ. 2018, 238, 480–490. [Google Scholar] [CrossRef]
- Eijsbouts, S.; Li, X.; Bergwerff, J.; Louwen, J.; Woning, L.; Loos, J. Nickel sulfide crystals in Ni-Mo and Ni-W catalysts: Eye-catching inactive feature or an active phase in its own right? Catal. Today 2017, 292, 38–50. [Google Scholar] [CrossRef]
- Voorhoeve, R. The mechanism of the hydrogenation of cyclohexene and benzene on nickel-tungsten sulfide catalysts. J. Catal. 1971, 23, 243–252. [Google Scholar] [CrossRef]
- Kasztelan, S.; Toulhoat, H.; Grimblot, J.; Bonnelle, J.P. A geometrical model of the active phase of hydrotreating catalysts. Appl. Catal. 1984, 13, 127–159. [Google Scholar] [CrossRef]



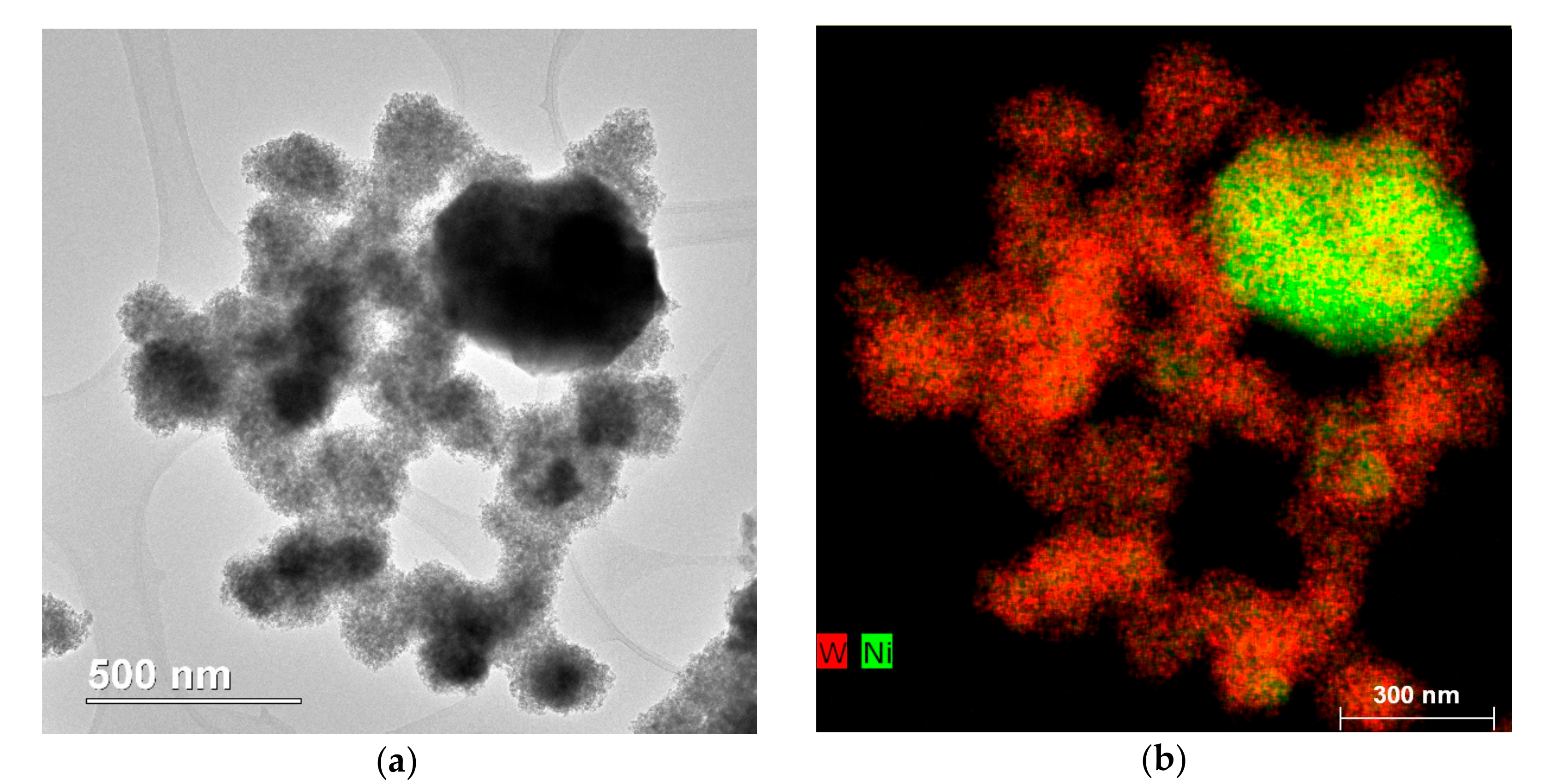
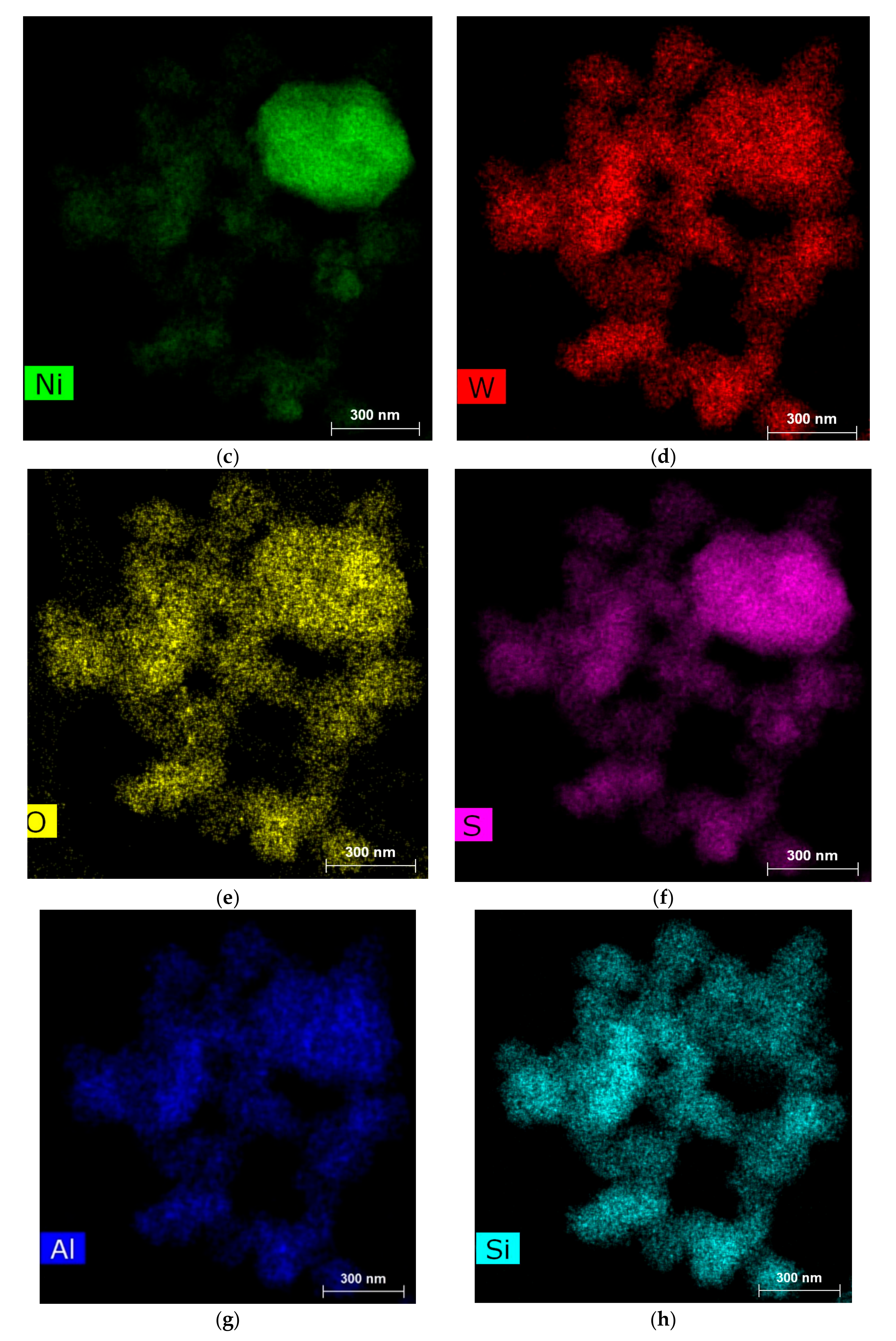
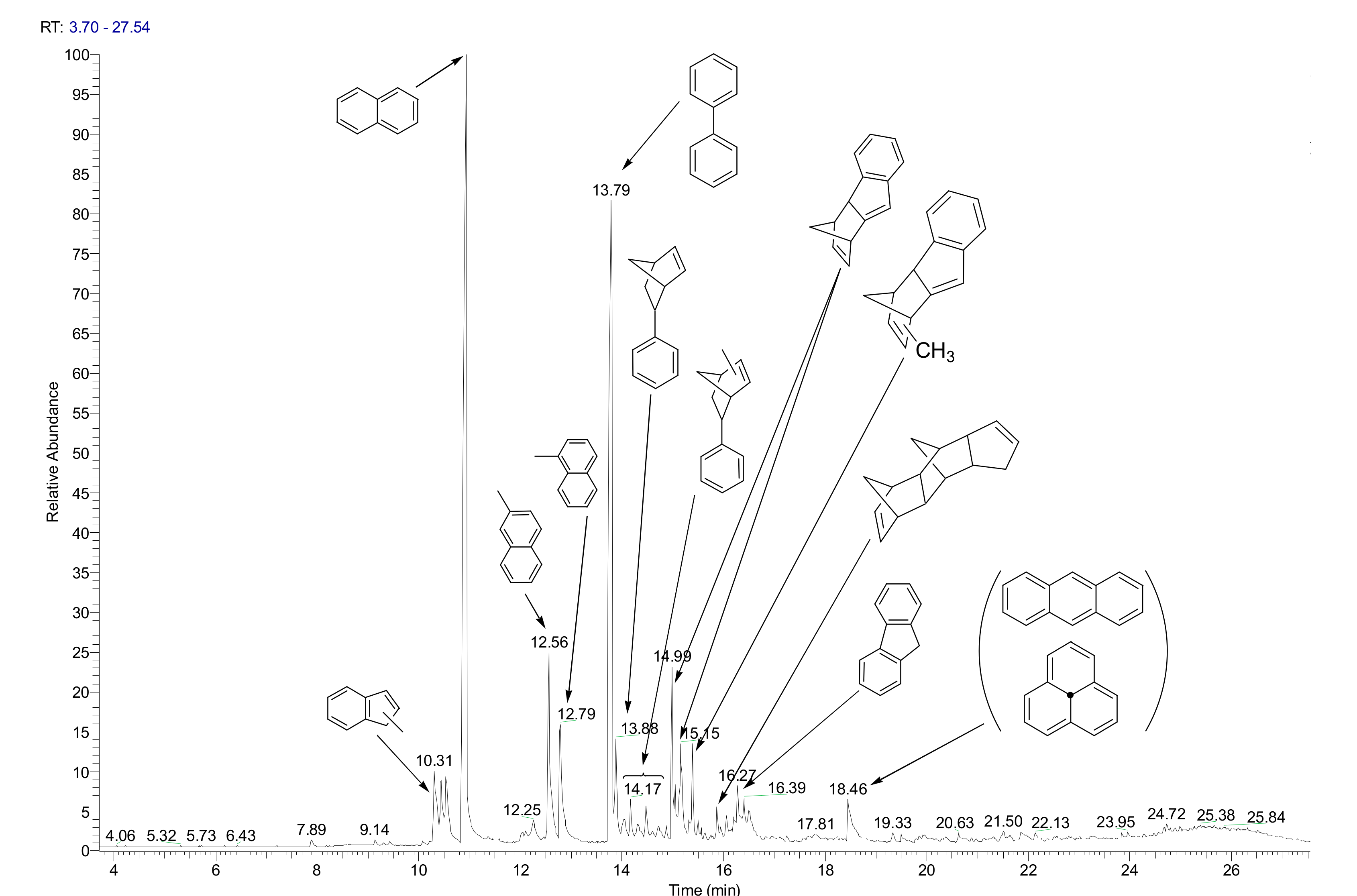
| Compound | Amount (wt %) | Compound | Amount (wt %) |
|---|---|---|---|
| Methylcyclopentane | 0.47 | Benzene | 2.84 |
| 3-Methylhexane | 0.39 | Heptane | 0.24 |
| Methylcyclohexane | 0.37 | Dimethylhexane | 0.17 |
| 4-Methylheptane | 0.5 | Toluene | 0.15 |
| 3-Methylheptane | 0.92 | Octane | 0.12 |
| m-Xynene | 0.72 | p-Xynen | 0.06 |
| o-Xynene | 0.04 | Decals | 3.4 |
| Butylbenzene | 0.2 | Methylindanes | 13.14 |
| Tetralin | 63.81 | - | - |
| Catalyst | Process Conditions | W (wt %) | BTX Composition (wt %) | |||
|---|---|---|---|---|---|---|
| Benzene | Toluene | Xylenes | ΣBTX | |||
| NiWS-HY(0.5%) | 350 °C | 4.0 | 3.3 | 2.5 | 0.8 | 6.6 |
| 380 °C | 7.7 | 2.2 | 0.95 | 10.85 | ||
| 400 °C | 5 | 0.7 | 1.1 | 6.8 | ||
| NiWS-HY(1%) | 350 °C | 5.4 | 2.2 | 1.5 | 9.1 | |
| 380 °C | 9.5 | 3.7 | 1.8 | 15 | ||
| 400 °C | 3.3 | 4.1 | 2.3 | 9.7 | ||
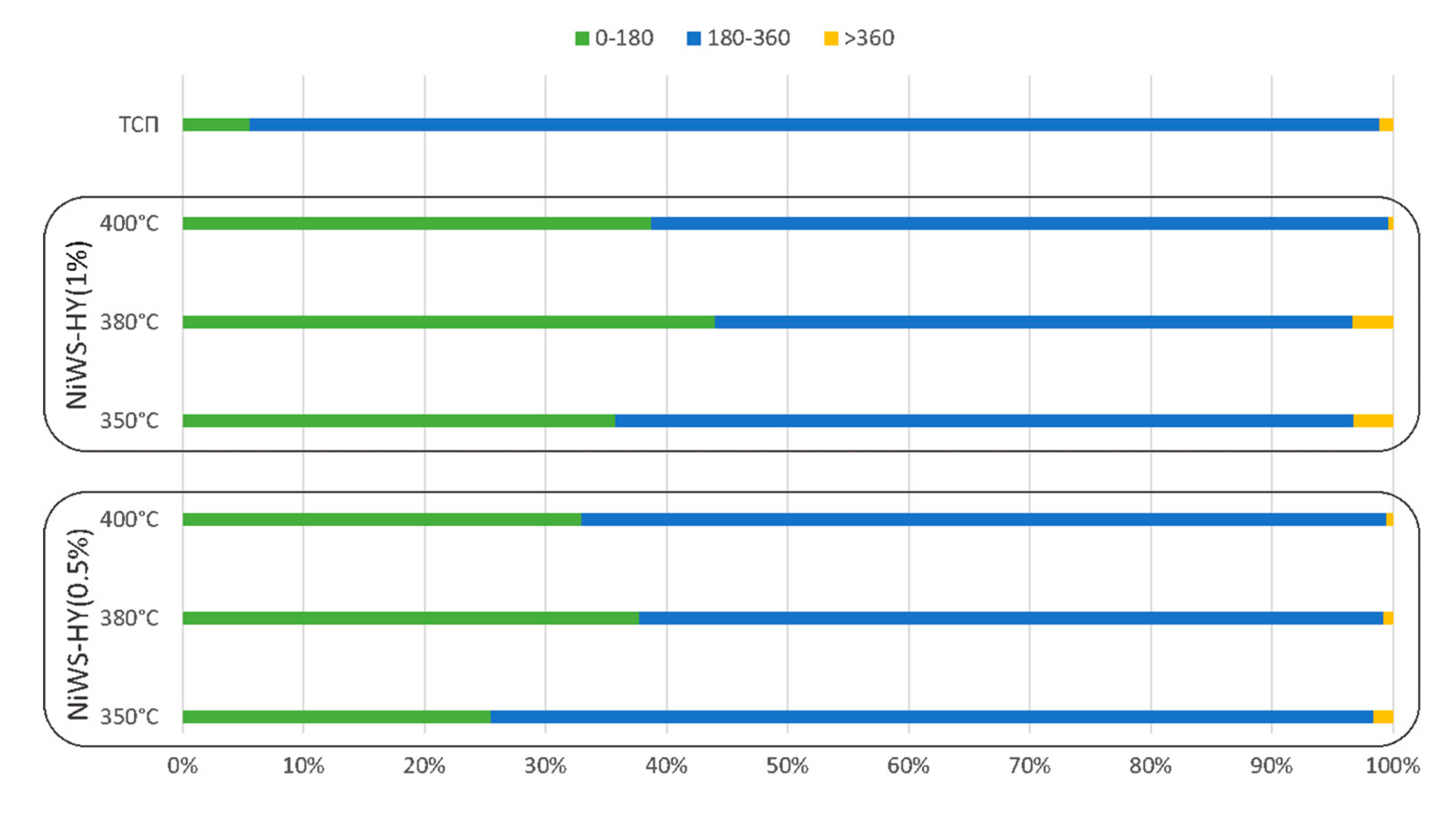
| Catalyst | W (wt %) | Fractional Composition (wt %) | BTX Composition (wt %) | |||||
|---|---|---|---|---|---|---|---|---|
| 0 to 180 | 180 to 360 | >360 | Benzene | Toluene | Xylenes | ΣBTX | ||
| NiWS-HY(1%) | 4.0 | 44 | 52.7 | 3.3 | 9.5 | 3.7 | 1.8 | 15 |
| 2.0 | 28.9 | 67.5 | 3.6 | 5.9 | 2.6 | 1.8 | 10.3 | |
| 1.0 | 24.2 | 72.4 | 3.34 | 3.9 | 2.5 | 2.1 | 8.5 | |
| NiWS-HY(0.5%) | 4.0 | 37.7 | 61.5 | 0.8 | 7.7 | 2.2 | 1 | 10.9 |
| 2.0 | 33 | 65.2 | 1.8 | 7.3 | 1 | 1.2 | 9.5 | |
| 1.0 | 31.8 | 68% | 0.2 | 5.4 | 1.8 | 2.1 | 9.3 | |
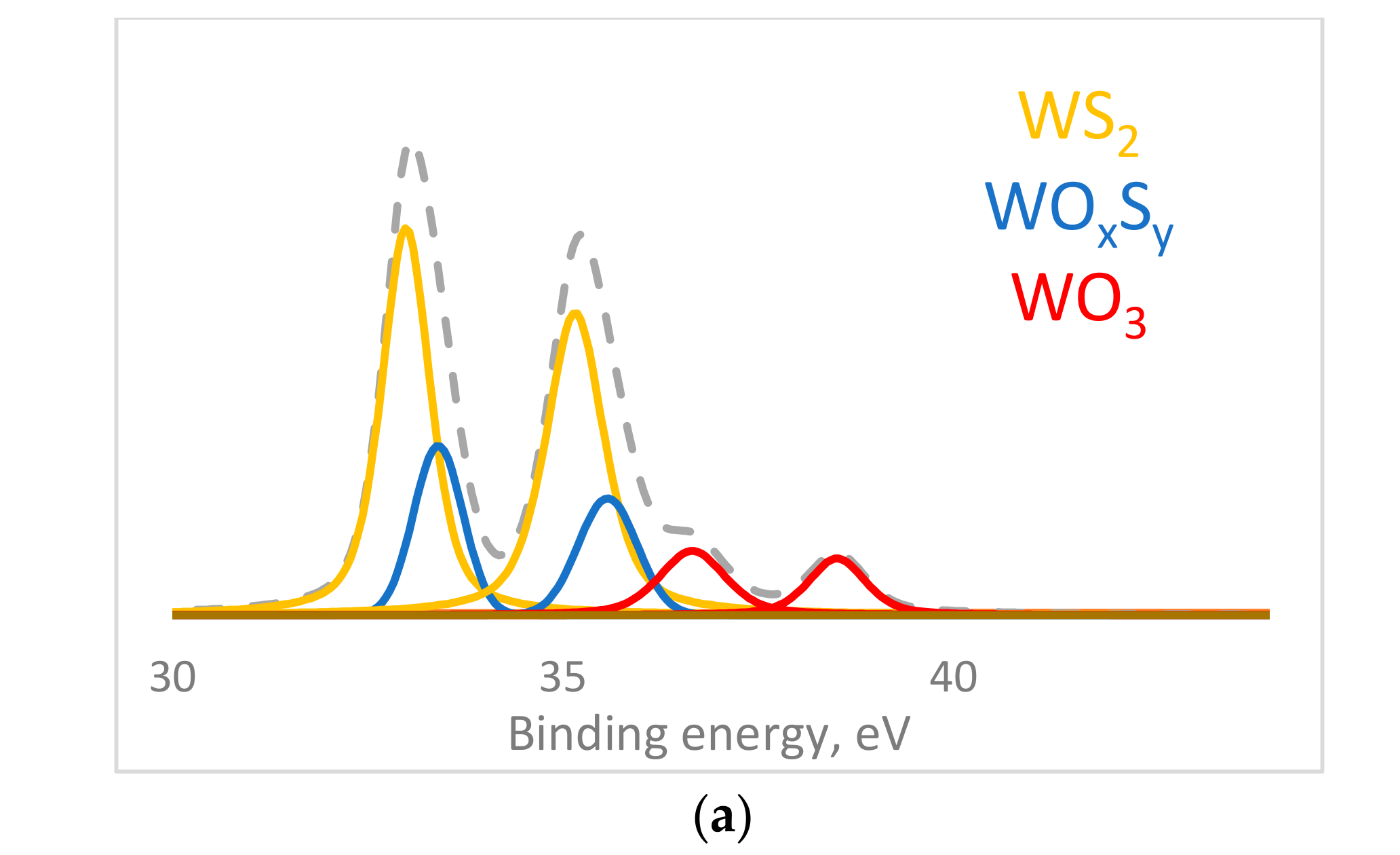
| Element | Status | |||||||
|---|---|---|---|---|---|---|---|---|
| NiWS-HY(0.5%) | NiWS-HY(1%) | |||||||
| Binding Energy, eV | Weight % | Atomic % | Binding Energy, eV | Weight % | Atomic % | Status | ||
| W4f | 4f 7/2 | 33 | 65.6 | 3.5 | 33.2 | 52.53 | 2.77 | WS2 |
| 4f 5/2 | 35.1 | 35.3 | ||||||
| 4f 7/2 | 33.4 | 21.7 | 1.2 | 33.5 | 30.01 | 1.58 | WOxSy | |
| 4f 5/2 | 35.5 | 35.8 | ||||||
| 4f 7/2 | 36.7 | 12.8 | 0.7 | 37 | 13.88 | 0.73 | WO3 | |
| 4f 5/2 | 38.5 | 38.7 | ||||||
| Ni2p | 2p3/2 | 854.9 | 48.3 | 1.64 | 854.7 | 42.25 | 0.80 | NiS |
| 2p1/2 | 874.8 | 874.7 | ||||||
| 2p3/2 | 857 | 34.5 | 1.17 | 856.6 | 28.09 | 0.53 | Ni-W-S | |
| 2p1/2 | 877.6 | 877 | ||||||
| 2p3/2 | 858.7 | 17.3 | 0.59 | 858.4 | 29.67 | 0.5% | NiO | |
| 2p1/2 | 879.8 | 880 | ||||||
| S2p | 2p3/2 | 162.6 | 74.6 | 12.6 | 162.7 | 77.80 | 13.04 | S2− |
| 2p3/2 | 163.9 | 14.2 | 2.4 | 164 | 12.94 | 2.17 | S22− | |
| 2p3/2 | 167.9 | 10.4 | 1.8 | 168 | 9.26 | 1.55 | S6+ | |
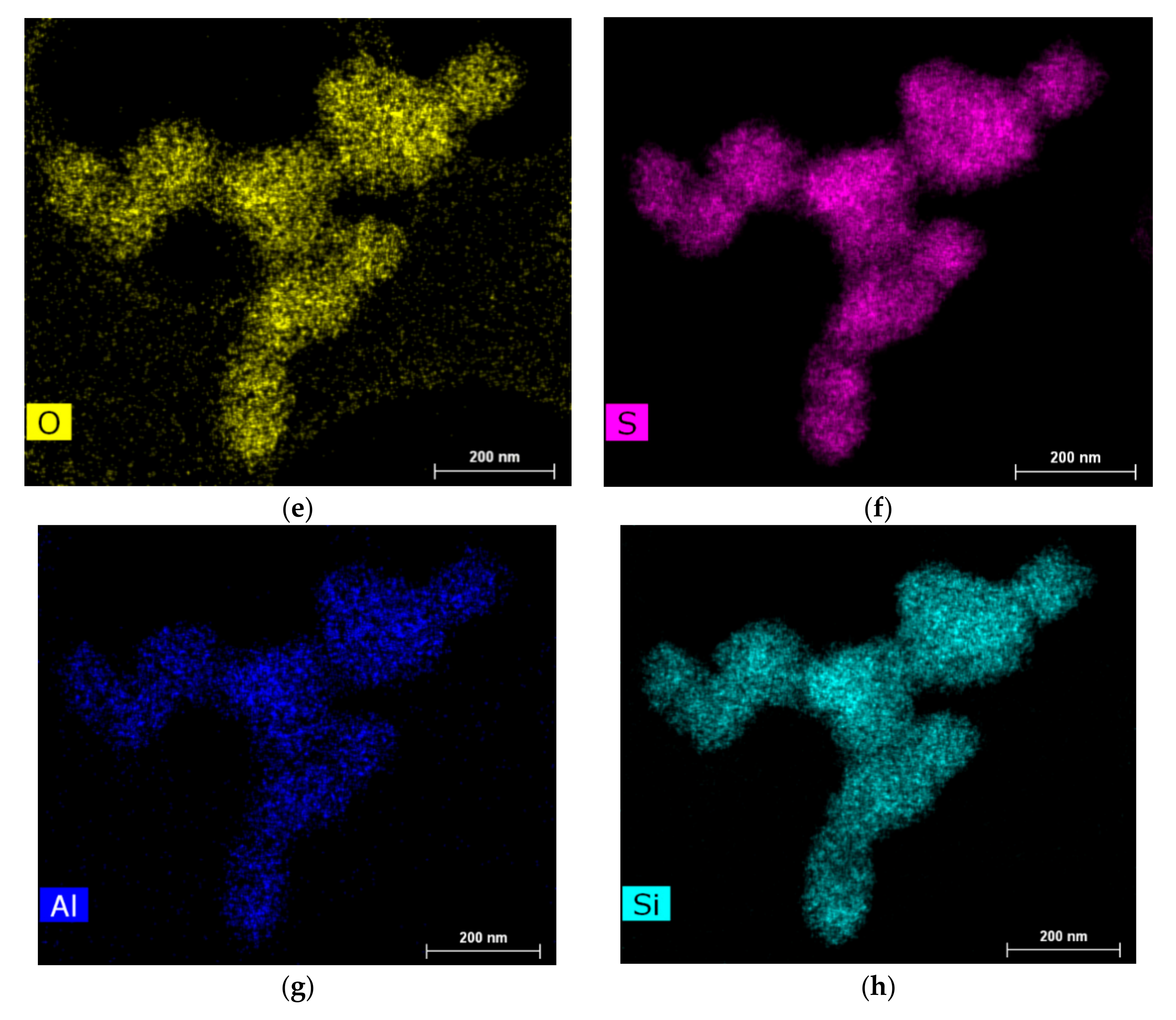
| Catalyst | Atomic % | |||||
|---|---|---|---|---|---|---|
| C | W | Ni | S | O | N | |
| NiWS-HY(0.5%) | 63.146 | 5.311 | 3.390 | 16.922 | 11.232 | - |
| NiWS-HY(1%) | 66.637 | 5.273 | 1.891 | 16.763 | 9.436 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchinskaya, T.; Kniazeva, M.; Samoilov, V.; Maximov, A. In Situ Generated Nanosized Sulfide Ni-W Catalysts Based on Zeolite for the Hydrocracking of the Pyrolysis Fuel Oil into the BTX Fraction. Catalysts 2020, 10, 1152. https://doi.org/10.3390/catal10101152
Kuchinskaya T, Kniazeva M, Samoilov V, Maximov A. In Situ Generated Nanosized Sulfide Ni-W Catalysts Based on Zeolite for the Hydrocracking of the Pyrolysis Fuel Oil into the BTX Fraction. Catalysts. 2020; 10(10):1152. https://doi.org/10.3390/catal10101152
Chicago/Turabian StyleKuchinskaya, Tatiana, Mariia Kniazeva, Vadim Samoilov, and Anton Maximov. 2020. "In Situ Generated Nanosized Sulfide Ni-W Catalysts Based on Zeolite for the Hydrocracking of the Pyrolysis Fuel Oil into the BTX Fraction" Catalysts 10, no. 10: 1152. https://doi.org/10.3390/catal10101152





