Palladium Catalysts Based on Porous Aromatic Frameworks, Modified with Ethanolamino-Groups, for Hydrogenation of Alkynes, Alkenes and Dienes
Abstract
:1. Introduction
2. Results and Discussion
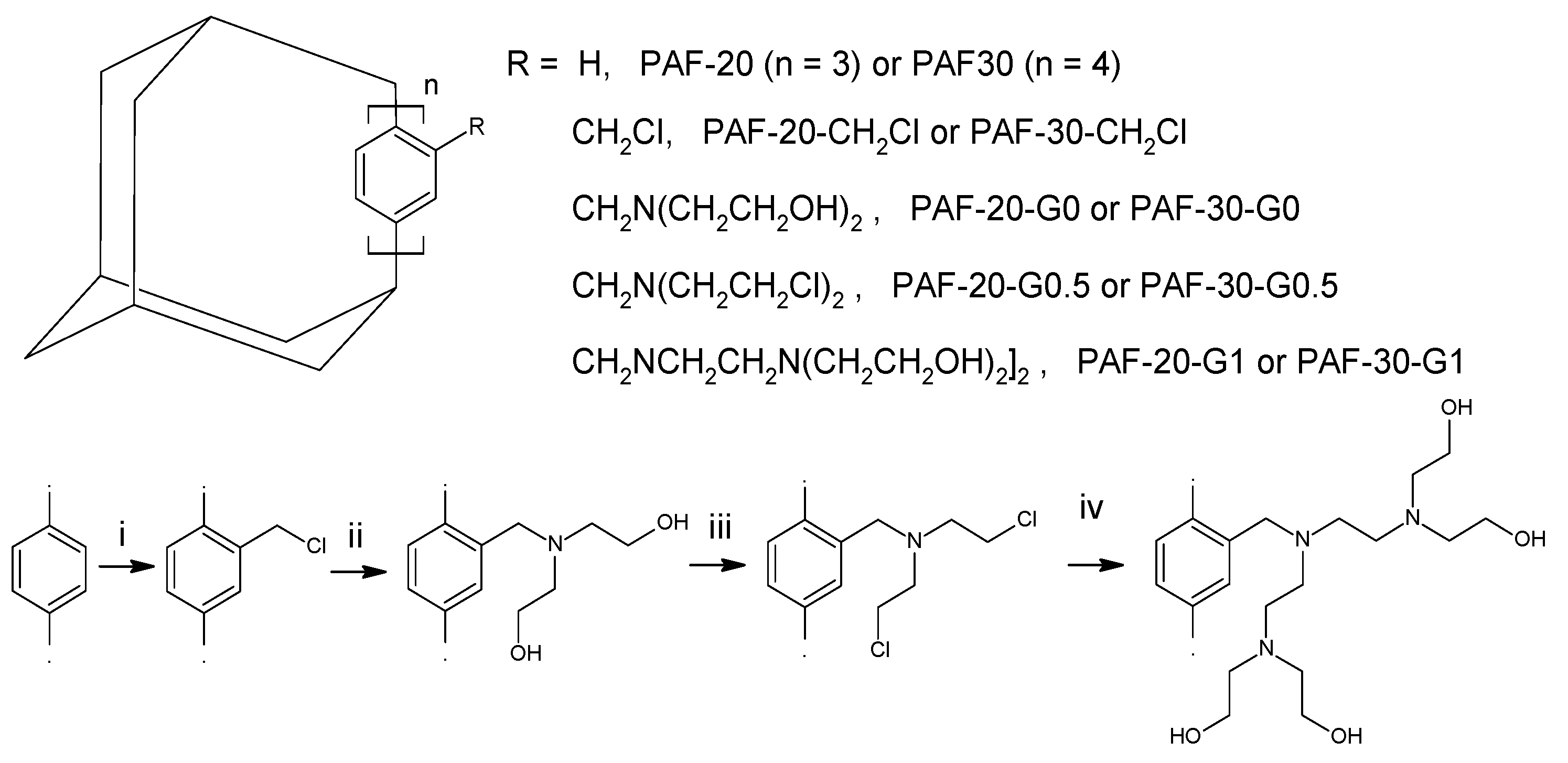
2.1. Synthesis and Characterization of Supports
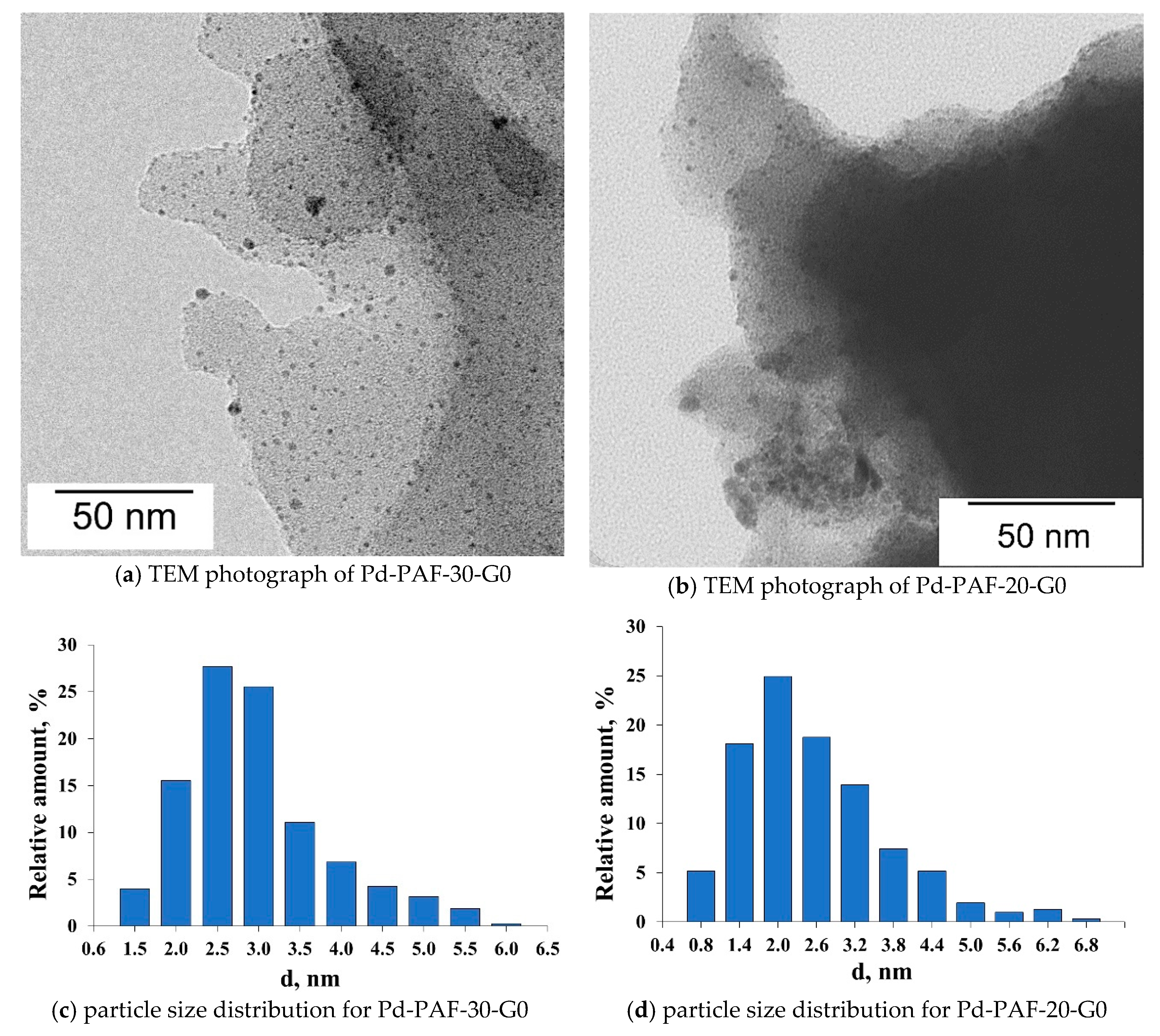
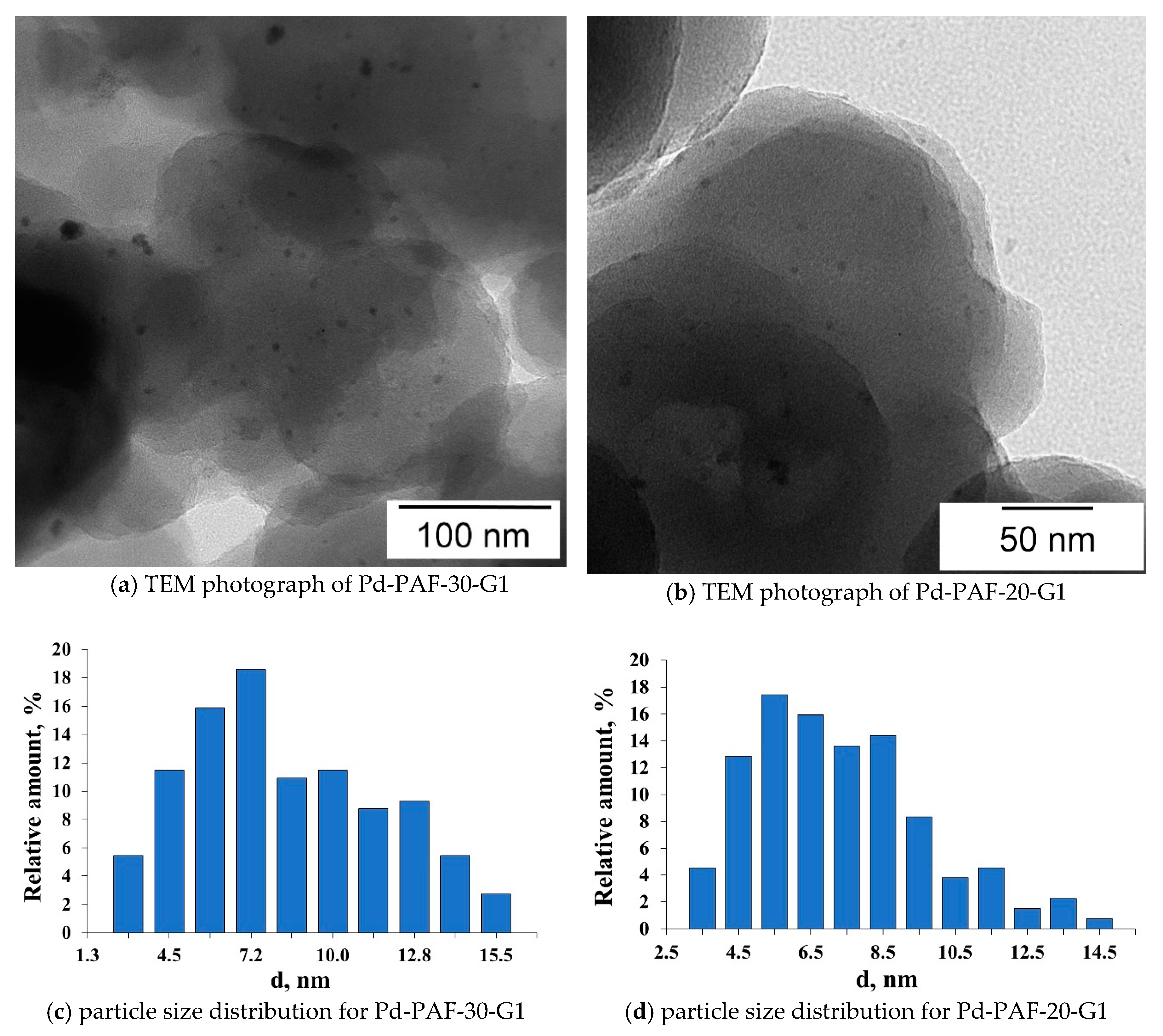
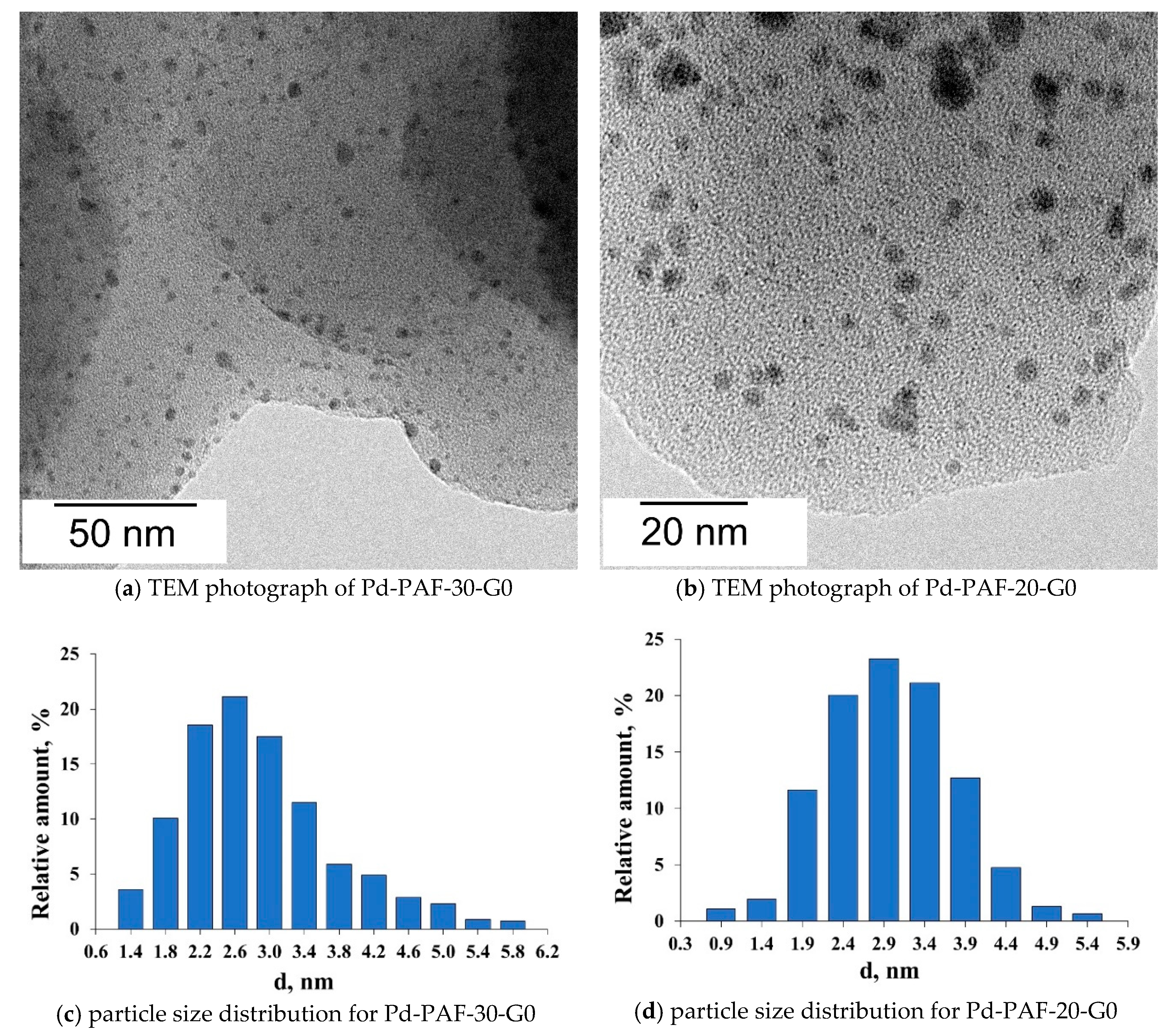
2.2. Characterization of Palladium Catalysts
2.3. Catalytic Activity
3. Materials and Methods
3.1. Used Reagents
3.2. Synthesis of PAF-20-CH2Cl
3.3. Synthesis of PAF-20-G0
3.4. Synthesis of PAF-20-G1
3.5. Synthesis of Catalyst Pd-PAF-G0 and Pd-PAF-G1
3.6. Catalytic Experiments
3.7. Characterization
3.7.1. Low Temperature Nitrogen Adsorption
3.7.2. Transmission Electron Microscopy (TEM)
3.7.3. X-ray Photoelectron Spectroscopy (XPS)
3.7.4. Gas-Liquid Chromatography
3.7.5. Atomic Absorption Spectroscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, L.; Zhou, C.; Zhao, H.; Wang, R. Spatial control of palladium nanoparticles in flexible click-based porous organic polymers for hydrogenation of olefins and nitrobenzene. Nano Res. 2015, 8, 709–721. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Wang, R. Tailorable synthesis of porous organic polymers decorating ultrafine palladium nanoparticles for hydrogenation of olefins. ACS Catal. 2015, 5, 948–955. [Google Scholar] [CrossRef]
- Garg, G.; Foltran, S.; Favier, I.; Pla, D.; Medina-González, Y.; Gómez, M. Palladium nanoparticles stabilized by novel choline-based ionic liquids in glycerol applied in hydrogenation reactions. Catal. Today 2020, 346, 69–75. [Google Scholar] [CrossRef]
- Chung, J.; Kim, C.; Jeong, H.; Yu, T.; Binh, D.H.; Jang, J.; Lee, J.; Kim, B.M.; Lim, B. Selective semihydrogenation of alkynes on shape-controlled palladium nanocrystals. Chem. Asian J. 2013, 8, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Zhang, S. Novel functionalized microporous organic networks based on triphenylphosphine. Chem. Eur. J. 2013, 19, 10024–10029. [Google Scholar] [CrossRef]
- Hausoul, P.J.C.; Eggenhuisen, T.M.; Nand, D.; Baldus, M.; Weckhuysen, B.M.; Klein Gebbink, R.J.M.; Bruijnincx, P.C.A. Development of a 4,4′-biphenyl/phosphine-based COF for the heterogeneous Pd-catalysed telomerisation of 1,3-butadiene. Catal. Sci. Technol. 2013, 3, 2571–2579. [Google Scholar] [CrossRef] [Green Version]
- Karakhanov, E.A.; Maksimov, A.L.; Zolotukhina, A.V.; Kardasheva, Y.S. Hydrogenation catalysts based on metal nanoparticles stabilized by organic ligands. Russ. Chem. Bull. 2013, 62, 1465–1492. [Google Scholar]
- Karakhanov, E.; Maximov, A.; Kardasheva, Y.; Semernina, V.; Zolotukhina, A.; Ivanov, A.; Abbott, G.; Rosenberg, E.; Vinokurov, V. Pd nanoparticles in dendrimers immobilized on silica-polyamine composites as catalysts for selective hydrogenation. ACS Appl. Mater. Interfaces 2014, 6, 8807–8816. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Maximov, A.L.; Zolotukhina, A.V. Selective semi-hydrogenation of phenyl acetylene by Pd nanocatalysts encapsulated into dendrimer networks. Mol. Catal. 2019, 469, 98–110. [Google Scholar] [CrossRef]
- Chen, H.; He, Y.; Pfefferle, L.D.; Pu, W.; Wu, Y.; Qi, S. Phenol Catalytic Hydrogenation over Palladium Nanoparticles Supported on Metal-Organic Frameworks in the Aqueous Phase. ChemCatChem 2018, 10, 2558–2570. [Google Scholar] [CrossRef]
- Jansen, J.F.G.A.; De Brabander-Van Den Berg, E.M.M.; Meijer, E.W. Encapsulation of guest molecules into a dendritic box. Science 1994, 266, 1226–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.; Crooks, R.M. Dendrimer-encapsulated metal nanoparticles and their applications to catalysis. C. R. Chim. 2003, 6, 1049–1059. [Google Scholar] [CrossRef]
- Yates, C.R.; Hayes, W. Synthesis and applications of hyperbranched polymers. Eur. Polym. J. 2004, 40, 1257–1281. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Maximov, A.L.; Skorkin, V.A.; Zolotukhina, A.V.; Smerdov, A.S.; Tereshchenko, A.Y. Nanocatalysts based on dendrimers. Pure Appl. Chem. 2009, 81, 2013–2023. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Maximov, A.L.; Zakharyan, E.M.; Zolotukhina, A.V.; Ivanov, A.O. Palladium nanoparticles on dendrimer-containing supports as catalysts for hydrogenation of unsaturated hydrocarbons. Mol. Catal. 2017, 440, 107–119. [Google Scholar] [CrossRef]
- Karakanov, E.A.; Zolotukhina, A.V.; Ivanov, A.O.; Maximov, A.L. Dendrimer-Encapsulated Pd Nanoparticles, Immobilized in Silica Pores, as Catalysts for Selective Hydrogenation of Unsaturated Compounds. Chem. Open 2019, 8, 358–381. [Google Scholar] [CrossRef]
- Krishnan, G.R.; Sreekumar, K. Synthesis and Characterization of Polystyrene Supported Catalytically Active Poly(amidoamine) Dendrimer-Palladium Nanoparticle Conjugates. Soft Mater. 2010, 8, 114–129. [Google Scholar] [CrossRef]
- Alvarez, J.; Sun, L.; Crooks, R.M. Electroactive composite dendrimer films containing thiophene-terminated poly(amidoamine) dendrimers cross-linked by poly(3-methylthiophene). Chem. Mater. 2002, 14, 3995–4001. [Google Scholar] [CrossRef]
- Murugan, E.; Rangasamy, R. Synthesis, characterization, and heterogeneous catalysis of polymer-supported poly(propyleneimine) dendrimer stabilized gold nanoparticle catalyst. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2525–2532. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Wang, J.; Wang, R. Facile fabrication of ultrafine palladium nanoparticles with size- and location-control in click-based porous organic polymers. ACS Nano 2014, 8, 5352–5364. [Google Scholar]
- Karakhanov, E.; Kardasheva, Y.; Kulikov, L.; Maximov, A.; Zolotukhina, A.; Vinnikova, M.; Ivanov, A. Sulfide catalysts supported on porous aromatic frameworks for naphthalene hydroprocessing. Catalysts 2016, 6, 1–11. [Google Scholar]
- Yuan, Y.; Zhu, G. Porous Aromatic Frameworks as a Platform for Multifunctional Applications. ACS Cent. Sci. 2019, 5, 409–418. [Google Scholar] [PubMed] [Green Version]
- Maximov, A.; Zolotukhina, A.; Kulikov, L.; Kardasheva, Y.; Karakhanov, E. Ruthenium catalysts based on mesoporous aromatic frameworks for the hydrogenation of arenes. React. Kinet. Mech. Catal. 2016, 117, 729–743. [Google Scholar]
- Wang, F.; Mielby, J.; Richter, F.H.; Wang, G.; Prieto, G.; Kasama, T.; Weidenthaler, C.; Bongard, H.J.; Kegnæs, S.; Fürstner, A.; et al. A polyphenylene support for pd catalysts with exceptional catalytic activity. Angew. Chem. Int. Ed. 2014, 53, 8645–8648. [Google Scholar]
- Kulikov, L.A.; Terenina, M.V.; Kryazheva, I.Y.; Karakhanov, E.A. Unsaturated-compound hydrogenation nanocatalysts based on palladium and platinum particles immobilized in pores of mesoporous aromatic frameworks. Pet. Chem. 2017, 57, 222–229. [Google Scholar]
- Su, J.; Chen, J.S. Synthetic porous materials applied in hydrogenation reactions. Microporous Mesoporous Mater. 2017, 237, 246–259. [Google Scholar]
- Ben, T.; Ren, H.; Shengqian, M.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar]
- Garibay, S.J.; Weston, M.H.; Mondloch, J.E.; Colón, Y.J.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. Accessing functionalized porous aromatic frameworks (PAFs) through a de novo approach. CrystEngComm 2013, 15, 1515–1519. [Google Scholar]
- Tian, Y.; Song, J.; Zhu, Y.; Zhao, H.; Muhammad, F.; Ma, T.; Chen, M.; Zhu, G. Understanding the desulphurization process in an ionic porous aromatic framework. Chem. Sci. 2019, 10, 606–613. [Google Scholar] [PubMed] [Green Version]
- Vilian, A.T.E.; Puthiaraj, P.; Kwak, C.H.; Hwang, S.K.; Huh, Y.S.; Ahn, W.S.; Han, Y.K. Fabrication of Palladium Nanoparticles on Porous Aromatic Frameworks as a Sensing Platform to Detect Vanillin. ACS Appl. Mater. Interfaces 2016, 8, 12740–12747. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, T.; Jing, X.; Zhu, G. Phosphine-based porous aromatic frameworks for gold nanoparticle immobilization with superior catalytic activities. J. Mater. Chem. A 2019, 7, 10004–10009. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Zanaveskin, L.N.; Smirnov, V.V.; Averyanov, V.A.; Zanaveskin, K.L. Catalytic hydrogenation of alkyne and alkadiene impurities from alkenes. Practical and theoretical aspects. Russ. Chem. Rev. 2009, 78, 231–247. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Selectivity enhancement in heterogeneous catalysis induced by reaction modifiers. Appl. Catal. A Gen. 2000, 200, 3–22. [Google Scholar] [CrossRef]
- Xing, R.; Liu, Y.; Wu, H.; Li, X.; He, M.; Wu, P. Preparation of active and robust palladium nanoparticle catalysts stabilized by diamine-functionalized mesoporous polymers. Chem. Commun. 2008, 47, 6297–6299. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Terenina, M.; Vinokurov, V.; Kulikov, L.; Makeeva, D.; Glotov, A. Selective hydrogenation of terminal alkynes over palladium nanoparticles within the pores of amino-modified porous aromatic frameworks. Catal. Today 2019. [Google Scholar] [CrossRef]
- Verde-Sesto, E.; Merino, E.; Rangel-Rangel, E.; Corma, A.; Iglesias, M.; Sánchez, F. Postfunctionalized Porous Polymeric Aromatic Frameworks with an Organocatalyst and a Transition Metal Catalyst for Tandem Condensation-Hydrogenation Reactions. ACS Sustain. Chem. Eng. 2016, 4, 1078–1084. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Zhong, H.; Wang, R. Urea-based porous organic frameworks: Effective supports for catalysis in neat water. Chem. Eur. J. 2014, 20, 3050–3060. [Google Scholar] [CrossRef]
- Zhong, H.; Gong, Y.; Zhang, F.; Li, L.; Wang, R. Click-based porous organic framework containing chelating terdentate units and its application in hydrogenation of olefins. J. Mater. Chem. A 2014, 2, 7502–7508. [Google Scholar] [CrossRef]
- Tang, D.; Sun, X.; Zhao, D.; Zhu, J.; Zhang, W.; Xu, X.; Zhao, Z. Nitrogen-Doped Carbon Xerogels Supporting Palladium Nanoparticles for Selective Hydrogenation Reactions: The Role of Pyridine Nitrogen Species. ChemCatChem 2018, 10, 1291–1299. [Google Scholar] [CrossRef]
- Méry, D.; Astruc, D. Dendritic catalysis: Major concepts and recent progress. Coord. Chem. Rev. 2006, 250, 1965–1979. [Google Scholar] [CrossRef]
- Lu, S.; Hu, Y.; Wan, S.; McCaffrey, R.; Jin, Y.; Gu, H.; Zhang, W. Synthesis of Ultrafine and Highly Dispersed Metal Nanoparticles Confined in a Thioether-Containing Covalent Organic Framework and Their Catalytic Applications. J. Am. Chem. Soc. 2017, 139, 17082–17088. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tsumori, N.; Kitta, M.; Xu, Q. Fast Dehydrogenation of Formic Acid over Palladium Nanoparticles Immobilized in Nitrogen-Doped Hierarchically Porous Carbon. ACS Catal. 2018, 8, 12041–12045. [Google Scholar] [CrossRef]
- Neeli, C.K.P.; Puthiaraj, P.; Lee, Y.R.; Chung, Y.M.; Baeck, S.H.; Ahn, W.S. Transfer hydrogenation of nitrobenzene to aniline in water using Pd nanoparticles immobilized on amine-functionalized UiO-66. Catal. Today 2018, 303, 227–234. [Google Scholar] [CrossRef]
- Sadjadi, S.; Koohestani, F. Pd immobilized on polymeric network containing imidazolium salt, cyclodextrin and carbon nanotubes: Efficient and recyclable catalyst for the hydrogenation of nitroarenes in aqueous media. J. Mol. Liq. 2020, 301, 112414. [Google Scholar] [CrossRef]
- Zhou, S.; Shang, L.; Zhao, Y.; Shi, R.; Waterhouse, G.I.N.; Huang, Y.; Zheng, L.; Zhang, T. Pd Single-Atom Catalysts on Nitrogen-Doped Graphene for the Highly Selective Photothermal Hydrogenation of Acetylene to Ethylene. Adv. Mater. 2019, 31, 1900509. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Boronoev, M.P.; Zolotukhina, A.V.; Ignatyeva, V.I.; Terenina, M.V.; Maximov, A.L.; Karakhanov, E.A. Palladium Catalysts Based on Mesoporous Organic Materials in Semihydrogenation of Alkynes. Macromol. Symp. 2016, 363, 57–63. [Google Scholar] [CrossRef]
- King, A.S.H.; Twyman, L.J. Heterogeneous and solid supported dendrimer catalysts. J. Chem. Soc. Perkin 2002, 2, 2209–2218. [Google Scholar] [CrossRef]
- Soğukömeroğulları, H.G.; Karataş, Y.; Celebi, M.; Gülcan, M.; Sönmez, M.; Zahmakiran, M. Palladium nanoparticles decorated on amine functionalized graphene nanosheets as excellent nanocatalyst for the hydrogenation of nitrophenols to aminophenol counterparts. J. Hazard. Mater. 2019, 369, 96–107. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, M.; Xu, D.; Zhao, H.; Zhu, Y.; Fan, M.; Zhang, F.; Dong, Z. Highly dispersed ultrafine palladium nanoparticles encapsulated in a triazinyl functionalized porous organic polymer as a highly efficient catalyst for transfer hydrogenation of aldehydes. J. Mater. Chem. A 2018, 6, 18242–18251. [Google Scholar] [CrossRef]
- Xu, D.; Wang, F.; Yu, G.; Zhao, H.; Yang, J.; Yuan, M.; Zhang, X.; Dong, Z. Aminal-based Hypercrosslinked Polymer Modified with Small Palladium Nanoparticles for Efficiently Catalytic Reduction of Nitroarenes. ChemCatChem 2018, 10, 4569–4577. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, F.; Ren, H.; Jing, X.; Wang, W.; Ma, H.; Zhao, H.; Zhu, G. Targeted synthesis of a porous aromatic framework with a high adsorption capacity for organic molecules. J. Mater. Chem. 2011, 21, 13498–13502. [Google Scholar] [CrossRef]
- Gangadharan, D.; Dhandhala, N.; Dixit, D.; Thakur, R.S.; Popat, K.M.; Anand, P.S. Investigation of solid supported dendrimers for water disinfection. J. Appl. Polym. Sci. 2012, 124, 1384–1391. [Google Scholar] [CrossRef]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Wei, Z.; Zhou, H.C. Polyamine-tethered porous polymer networks for carbon dioxide capture from flue gas. Angew. Chem. Int. Ed. 2012, 51, 7480–7484. [Google Scholar] [CrossRef]
- Law, R.V.; Sherrington, D.C.; Snape, C.E.; Ando, I.; Korosu, H. Solid State 13C MAS NMR Studies of Anion Exchange Resins and Their Precursors. Ind. Eng. Chem. Res. 1995, 34, 2740–2749. [Google Scholar] [CrossRef]
- Rangel-Rangel, E.; Verde-Sesto, E.; Rasero-Almansa, A.M.; Iglesias, M.; Sánchez, F. Porous aromatic frameworks (PAFs) as efficient supports for N-heterocyclic carbene catalysts. Catal. Sci. Technol. 2016, 6, 6037–6045. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Jeromenok, J.; Weber, J. Restricted access: On the nature of adsorption/desorption hysteresis in amorphous, microporous polymeric materials. Langmuir 2013, 29, 12982–12989. [Google Scholar] [CrossRef]

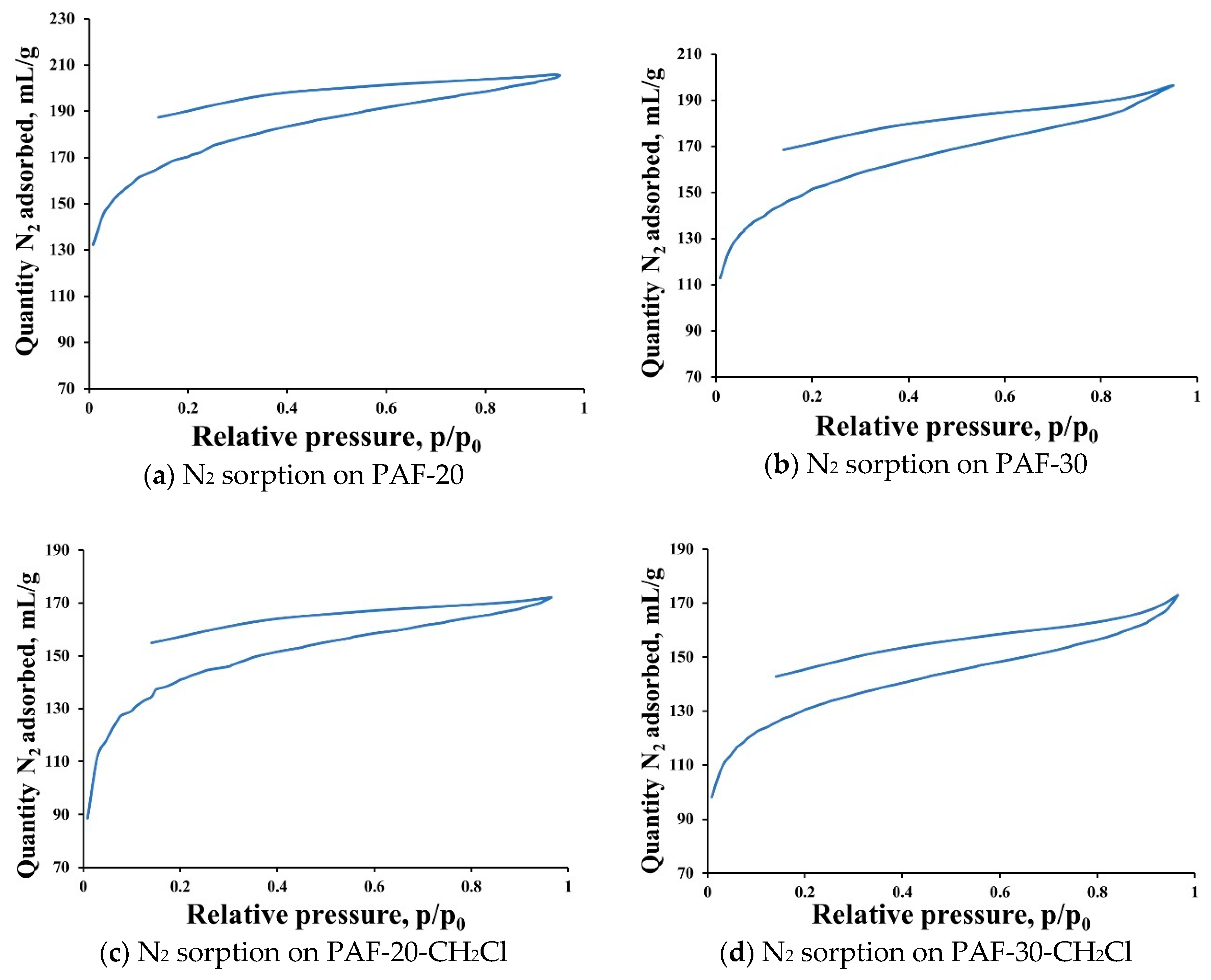
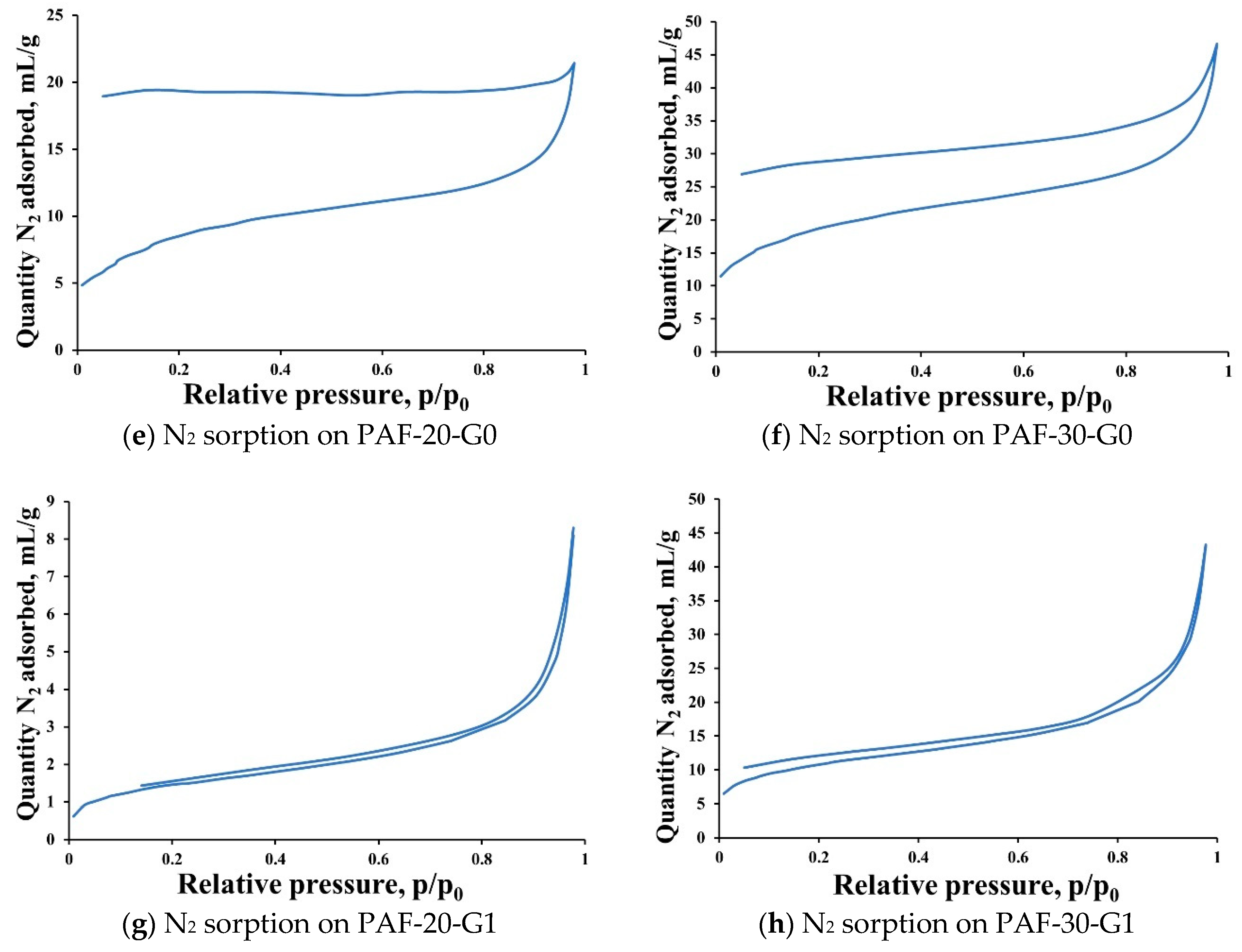

| Samples | Materials Based on PAF-20 | Materials Based on PAF-30 | ||
|---|---|---|---|---|
| SBET, m2/g | Total Pore Volume (BJH), cm3/g | SBET, m2/g | Total Pore Volume (BJH), cm3/g | |
| PAF | 579 | 0.316 | 506 | 0.311 |
| PAF-CH2Cl | 472 | 0.264 | 436 | 0.262 |
| PAF-G0 | 29 | 0.026 | 61 | 0.054 |
| PAF-G1 | 5 | 0.001 | 38 | 0.007 |
| Material | Element Content, Mass. % | |
|---|---|---|
| Cl | N | |
| PAF-20-CH2Cl | 3.08% | - |
| PAF-30-CH2Cl | 3.00% | - |
| PAF-20-G0 | 0.91% | 1.84% |
| PAF-30-G0 | 1.19% | 1.68% |
| PAF-20-G1 | 2.43% | 1.88% |
| PAF-30-G1 | 3.30% | 2.16% |
| Material | Pd-PAF-20-G0 | Pd-PAF-30-G0 | Pd-PAF-20-G1 | Pd-PAF-30-G1 |
|---|---|---|---|---|
| Pd, mass % | 2.4 | 1.0 | 0.6 | 1.8 |
| Catalysts | C | O | N | Pd | Cl |
|---|---|---|---|---|---|
| Pd-PAF-20-G0 | 82.2 at.% | 13.3 at.% | 1.5 at.% | 2.8 at.% | 0.1 at.% |
| Pd-PAF-30-G0 | 85.6 at.% | 11.2 at.% | 1.7 at.% | 1.3 at.% | 0.2 at.% |
| Pd-PAF-20-G1 | 87.9 at.% | 6.8 at.% | 3.4 at.% | 0.3 at.% | 0.6 at.% |
| Pd-PAF-30-G1 | 87.0 at.% | 9.2 at.% | 3.0 at.% | 0.3 at.% | 0.5 at.% |
| Catalyst | Parameter | Pd0 | PdOx |
|---|---|---|---|
| Pd-PAF-20-G0 | Binding energy, eV | Pd 3d5/2 334.85 eV | Pd 3d5/2 336.58 eV |
| Pd 3d3/2 340.10 eV | Pd 3d3/2 341.82 eV | ||
| Content, % | 67 | 33 | |
| Pd-PAF-30-G0 | Binding energy, eV | Pd 3d5/2 334.65 eV | Pd 3d5/2 336.75 eV |
| Pd 3d3/2 339.96 eV | Pd 3d3/2 341.91 eV | ||
| Content, % | 56 | 44 | |
| Pd-PAF-20-G1 | Binding energy, eV | Pd 3d5/2 335.11 eV | Pd 3d5/2 336.8 eV |
| Pd 3d3/2 340.47 eV | Pd 3d3/2 342.43 eV | ||
| Content, % | 48 | 51 | |
| Pd-PAF-30-G1 | Binding energy, eV | Pd 3d5/2 335.45 eV | Pd 3d5/2 337.07 eV |
| Pd 3d3/2 340.89 eV | Pd 3d3/2 342.56 eV | ||
| Content, % | 56 | 44 |
| Substrate | Reaction Products | Product Yield, % | |||
|---|---|---|---|---|---|
| Pd-PAF-20-G0 | Pd-PAF-30-G0 | Pd-PAF-20-G1 | Pd-PAF-30-G1 | ||
| Hexyne-1 | Hexene-1 | 85 | 94 | 9 | 35 |
| Hexane | 4 | 6 | - | - | |
| Hexene-1 | Hexane | 34 | 100 | <1 | <1 |
| Cyclohexene | Cyclohexane | 11 | 12 | - | - |
| 1,3-cyclohexadiene | Cyclohexadiene | 7 | 9 | - | - |
| Octyne-1 | Octene-1 | 6 | 99 | - | - |
| Octyne-4 | Octene-4 | 3 | 4 | - | - |
| Octene-1 | Octane | 7 | 99 | <1 | 1 |
| Isomerization products | 85 | <1 | 5 | 5 | |
| 2,5-dimethyl-2,4-hexadiene | 2,5-dimethyl-3-hexene | 8 | 5 | <1 | <1 |
| 2,5-dimethylhexane | <1 | 5 | <1 | 1 | |
| 2,5-dimethyl-2-hexene | 18 | 82 | - | - | |
| Phenylacetylene | Styrene | 21 | 37 | - | - |
| Styrene | Ethylbenzene | 10 | 26 | - | - |
| 4-methoxystyrene | 4-methoxyethylbenzene | 3 | 4 | - | - |
| Substrate | Pd-PAF-20-G0 | Pd-PAF-30-G0 | Pd-PAF-20-G1 | Pd-PAF-30-G1 |
|---|---|---|---|---|
| Hexyne-1 | 94,600 | 323,400 | 100,700 | 189,800 |
| Hexene-1 | 34,600 | 305,100 | - | - |
| Cyclohexene | 11,200 | 36,600 | - | - |
| 1,3-cyclohexadiene | 7100 | 27,400 | ||
| Octyne-1 | 7100 | 302,000 | - | - |
| Octyne-4 | 5600 | 12,200 | ||
| Octene-1 | 7100 | 305,100 | - | - |
| 2,5-dimethyl-2,4-hexadiene | 27,500 | 294,900 | - | - |
| Phenylacetylene | 22,400 | 115,900 | - | - |
| Styrene | 10,200 | 79,300 | - | - |
| 4-methoxystyrene | 5600 | 12,200 |
| Catalyst | Product Yield, % | ||||
|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | |
| Pd-PAF-20-G0 | 34 | 28 | 16 | 16 | 17 |
| Pd-PAF-30-G0 | 99 | 98 | 86 | 84 | 83 |
| Sample | C | O | N | Cl | Pd | |
|---|---|---|---|---|---|---|
| Pd-PAF-20-G0 | Before reaction | 82.2 at.% | 13.3 at.% | 1.5 at.% | 0.1 at.% | 2.8 at.% |
| After 5 runs | 79.4 at.% | 16.1 at.% | 2.1 at.% | 0.2 at.% | 2.1 at.% | |
| Pd-PAF-30-G0 | Before reaction | 85.6 at.% | 11.2 at.% | 1.7 at.% | 0.2 at.% | 1.3 at.% |
| After 5 runs | 85.6 at.% | 12.3 at.% | 0.9 at.% | 0.2 at.% | 1.0 at.% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikov, L.; Kalinina, M.; Makeeva, D.; Maximov, A.; Kardasheva, Y.; Terenina, M.; Karakhanov, E. Palladium Catalysts Based on Porous Aromatic Frameworks, Modified with Ethanolamino-Groups, for Hydrogenation of Alkynes, Alkenes and Dienes. Catalysts 2020, 10, 1106. https://doi.org/10.3390/catal10101106
Kulikov L, Kalinina M, Makeeva D, Maximov A, Kardasheva Y, Terenina M, Karakhanov E. Palladium Catalysts Based on Porous Aromatic Frameworks, Modified with Ethanolamino-Groups, for Hydrogenation of Alkynes, Alkenes and Dienes. Catalysts. 2020; 10(10):1106. https://doi.org/10.3390/catal10101106
Chicago/Turabian StyleKulikov, Leonid, Maria Kalinina, Daria Makeeva, Anton Maximov, Yulia Kardasheva, Maria Terenina, and Eduard Karakhanov. 2020. "Palladium Catalysts Based on Porous Aromatic Frameworks, Modified with Ethanolamino-Groups, for Hydrogenation of Alkynes, Alkenes and Dienes" Catalysts 10, no. 10: 1106. https://doi.org/10.3390/catal10101106
APA StyleKulikov, L., Kalinina, M., Makeeva, D., Maximov, A., Kardasheva, Y., Terenina, M., & Karakhanov, E. (2020). Palladium Catalysts Based on Porous Aromatic Frameworks, Modified with Ethanolamino-Groups, for Hydrogenation of Alkynes, Alkenes and Dienes. Catalysts, 10(10), 1106. https://doi.org/10.3390/catal10101106







