Recent Advancement of Ullmann Condensation Coupling Reaction in the Formation of Aryl-Oxygen (C-O) Bonding by Copper-Mediated Catalyst
Abstract
:1. Introduction
2. Aryl C-O Bond Formation Catalyzed by Copper Metal
2.1. Heterogeneous Catalyst
2.1.1. The Mono-Element of Cu Nanoparticle
2.1.2. Cu-Nanoparticles in the Presence of Ligands (A)
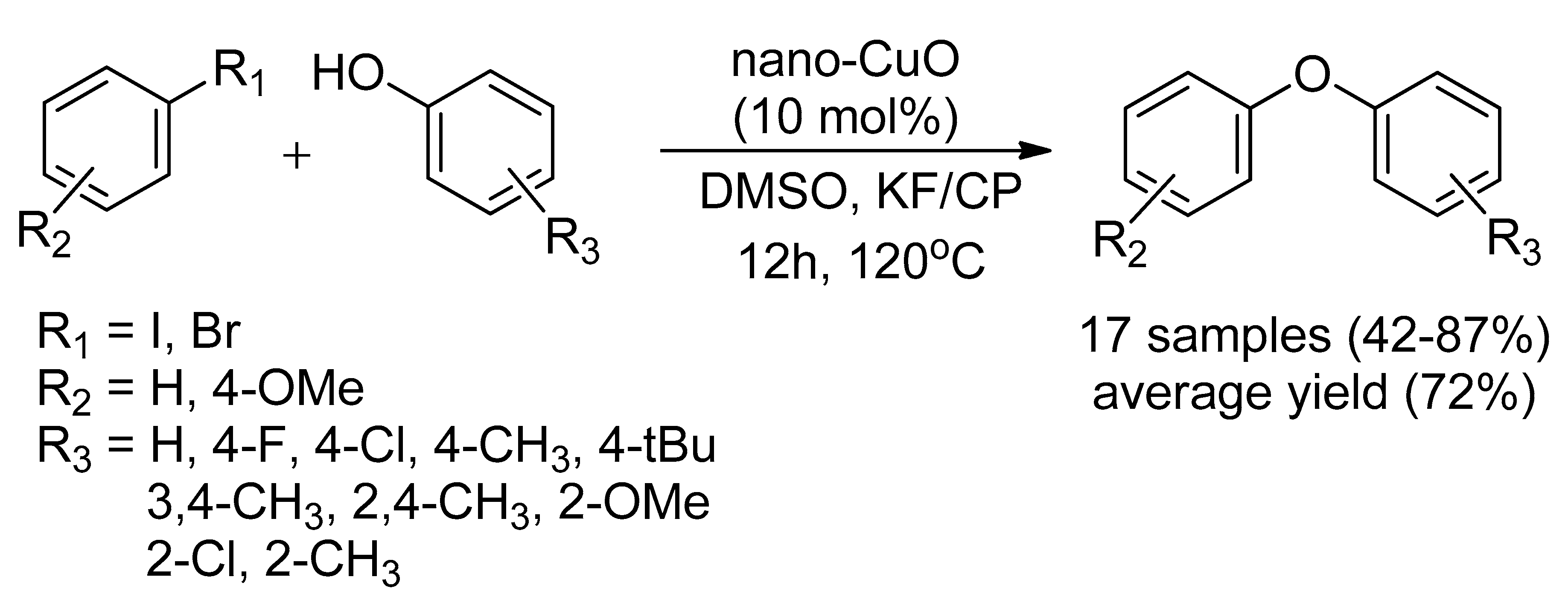
2.1.3. Copper Oxide Nanoparticle
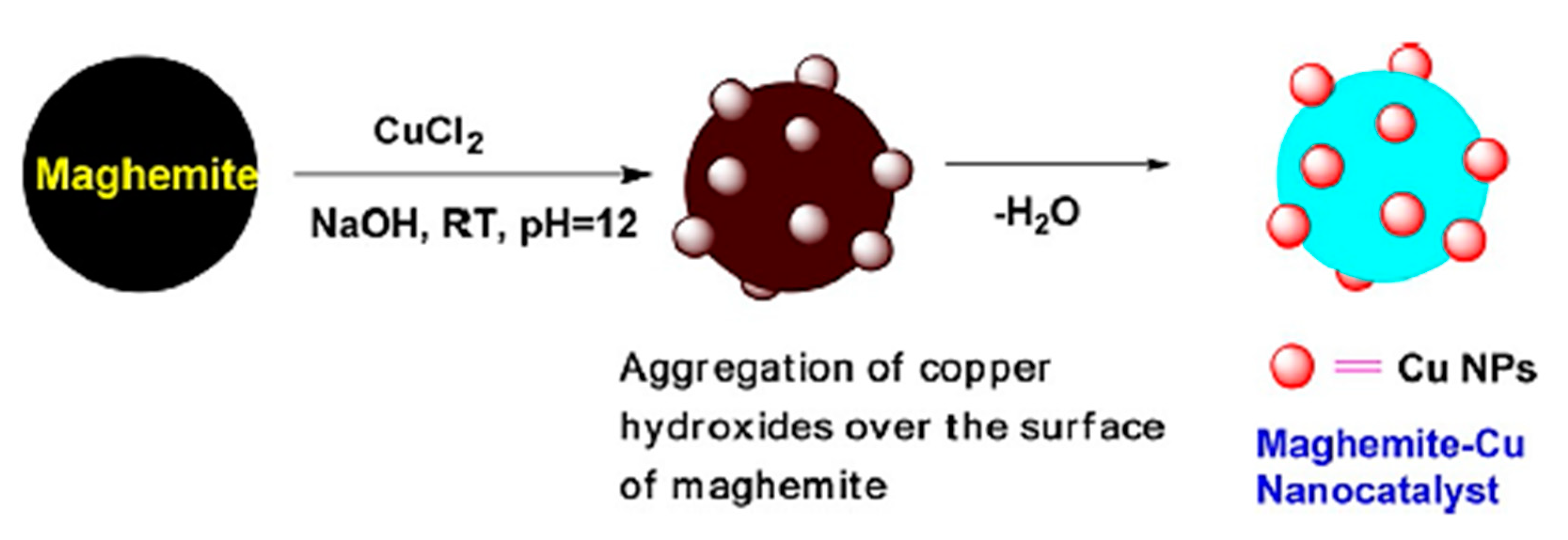
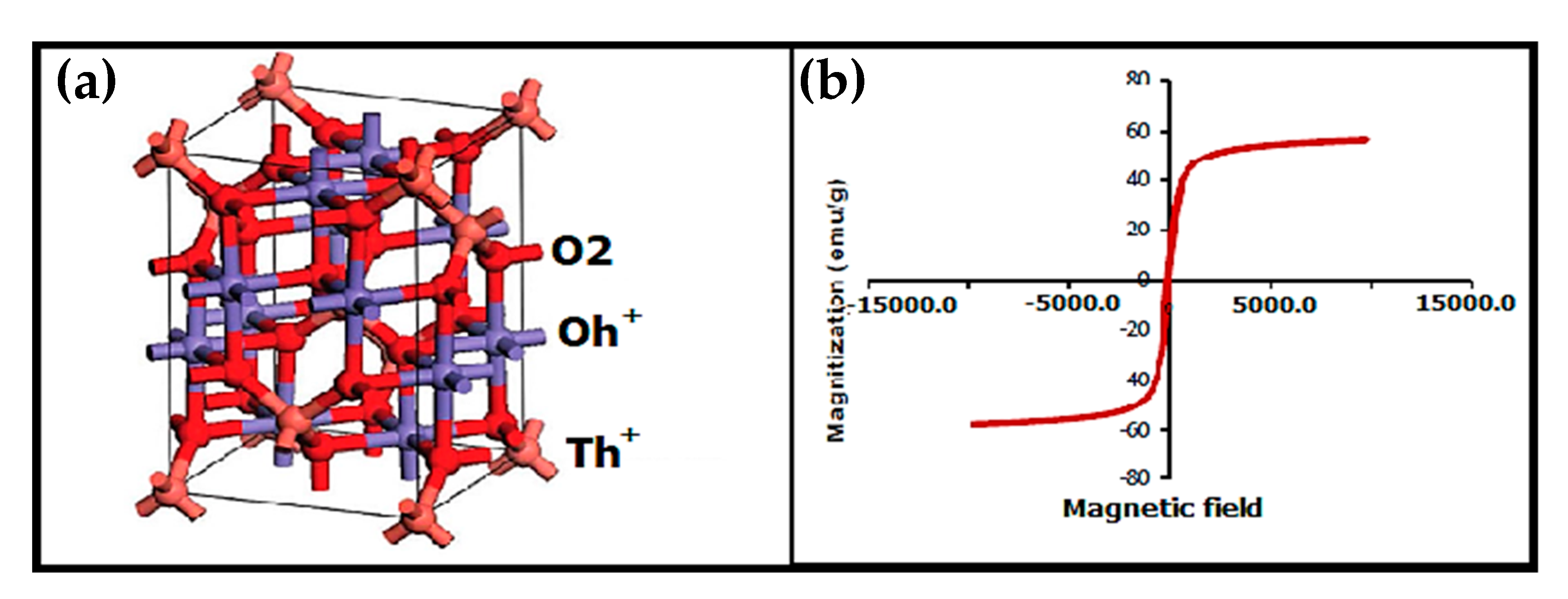
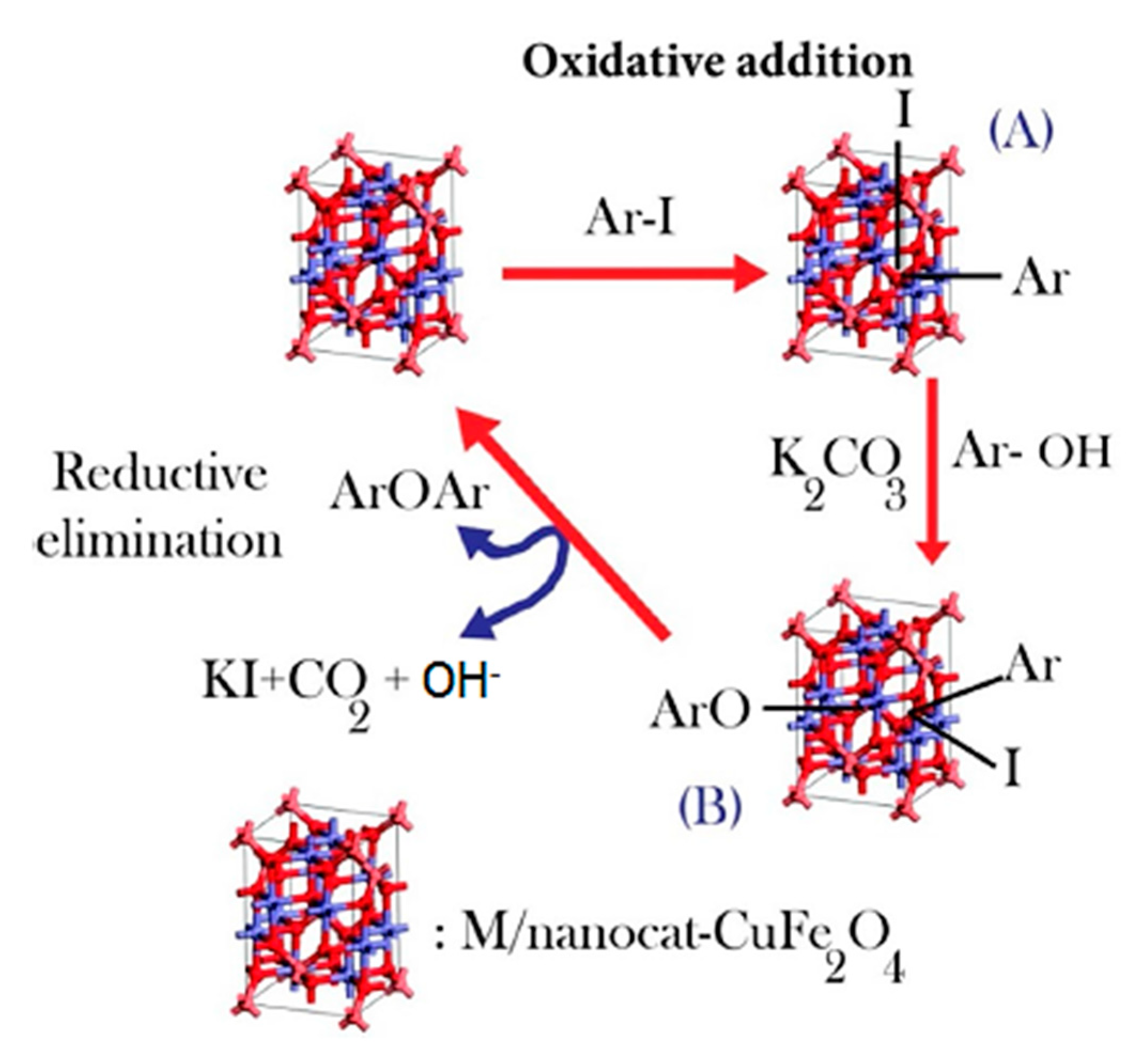
2.1.4. Maghemite-Copper Nanoparticles (CuFe2O4 Nanoparticles)
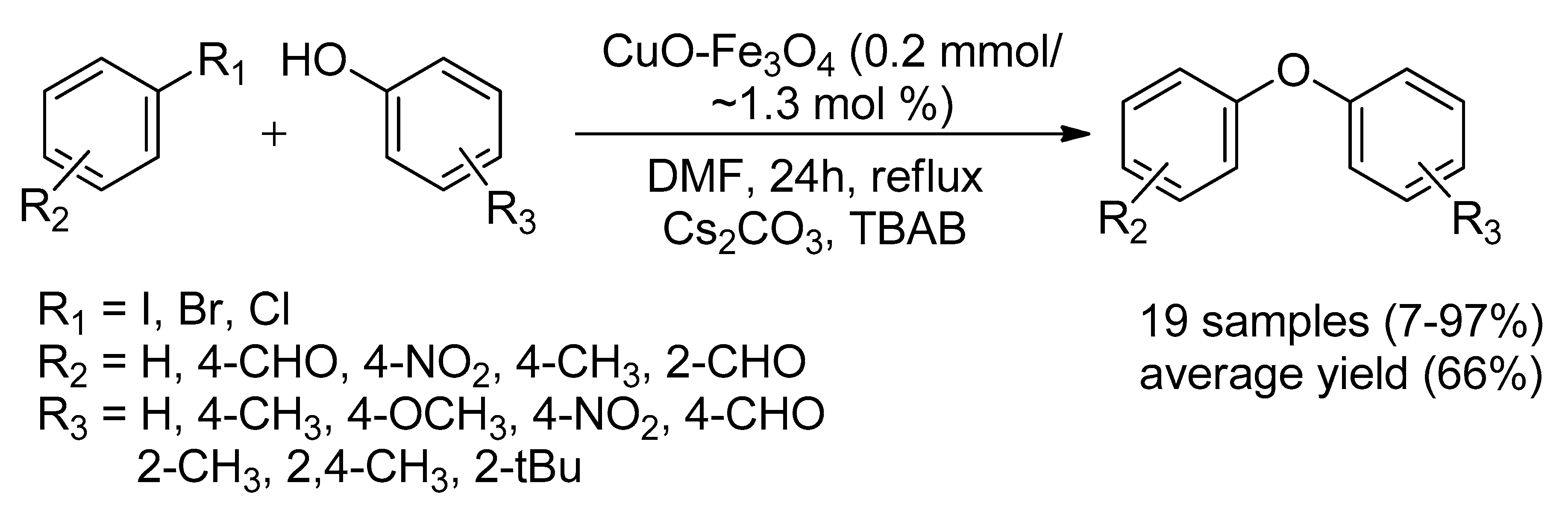
2.1.5. Supported-Copper Nanoparticles Catalyst
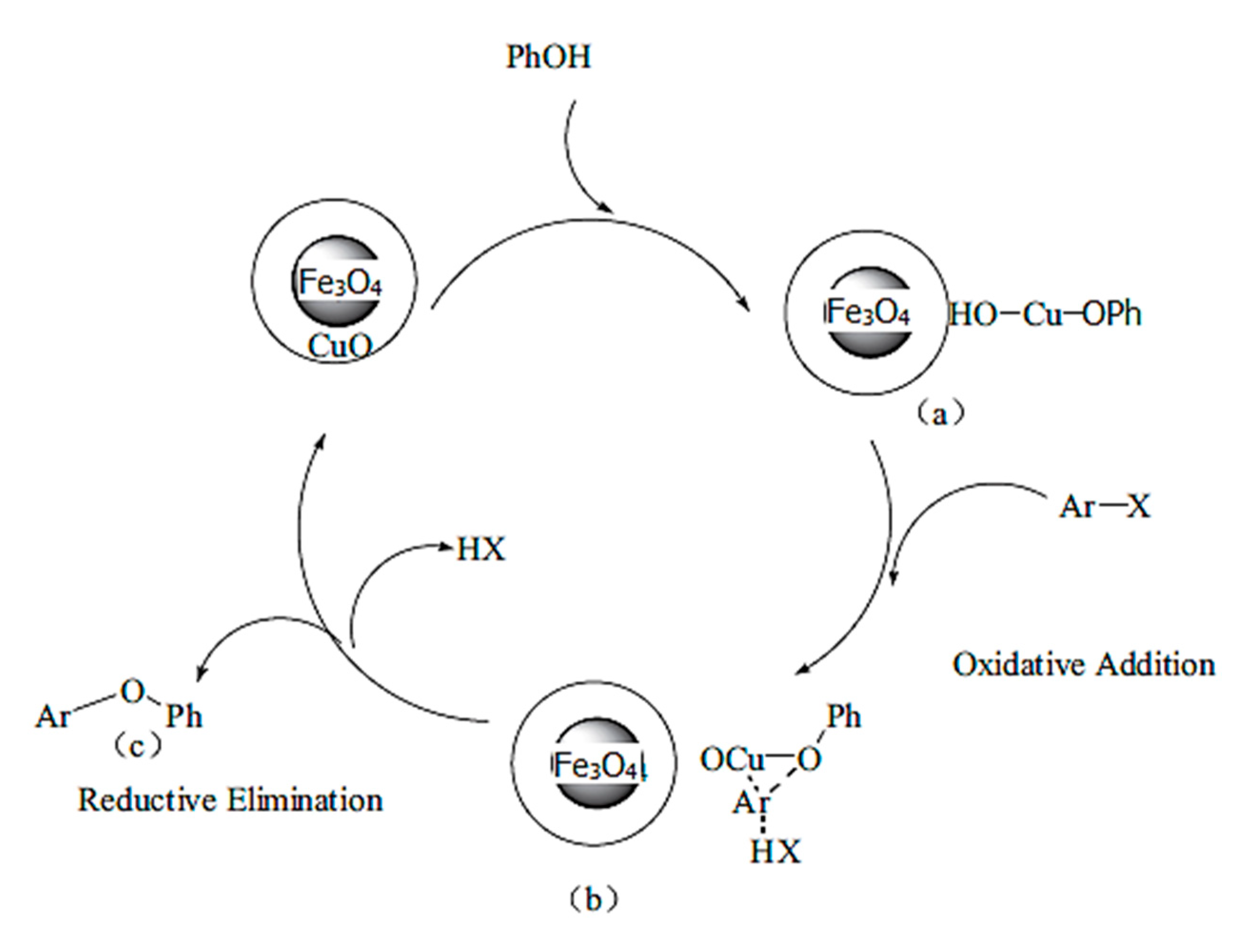
Maghemite-Copper Nanoparticles (CuFe3O4 Nanoparticles)
Carbon-Based Materials Supported
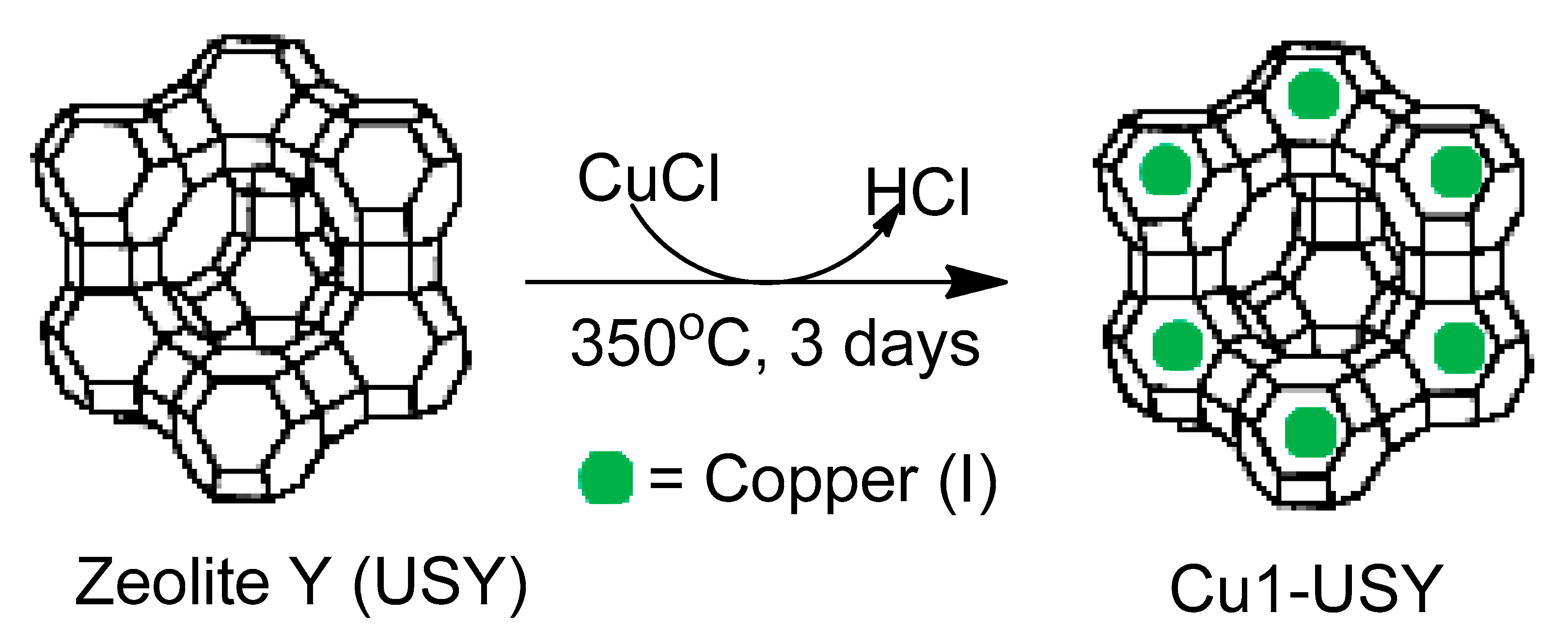
Zeolite Supported
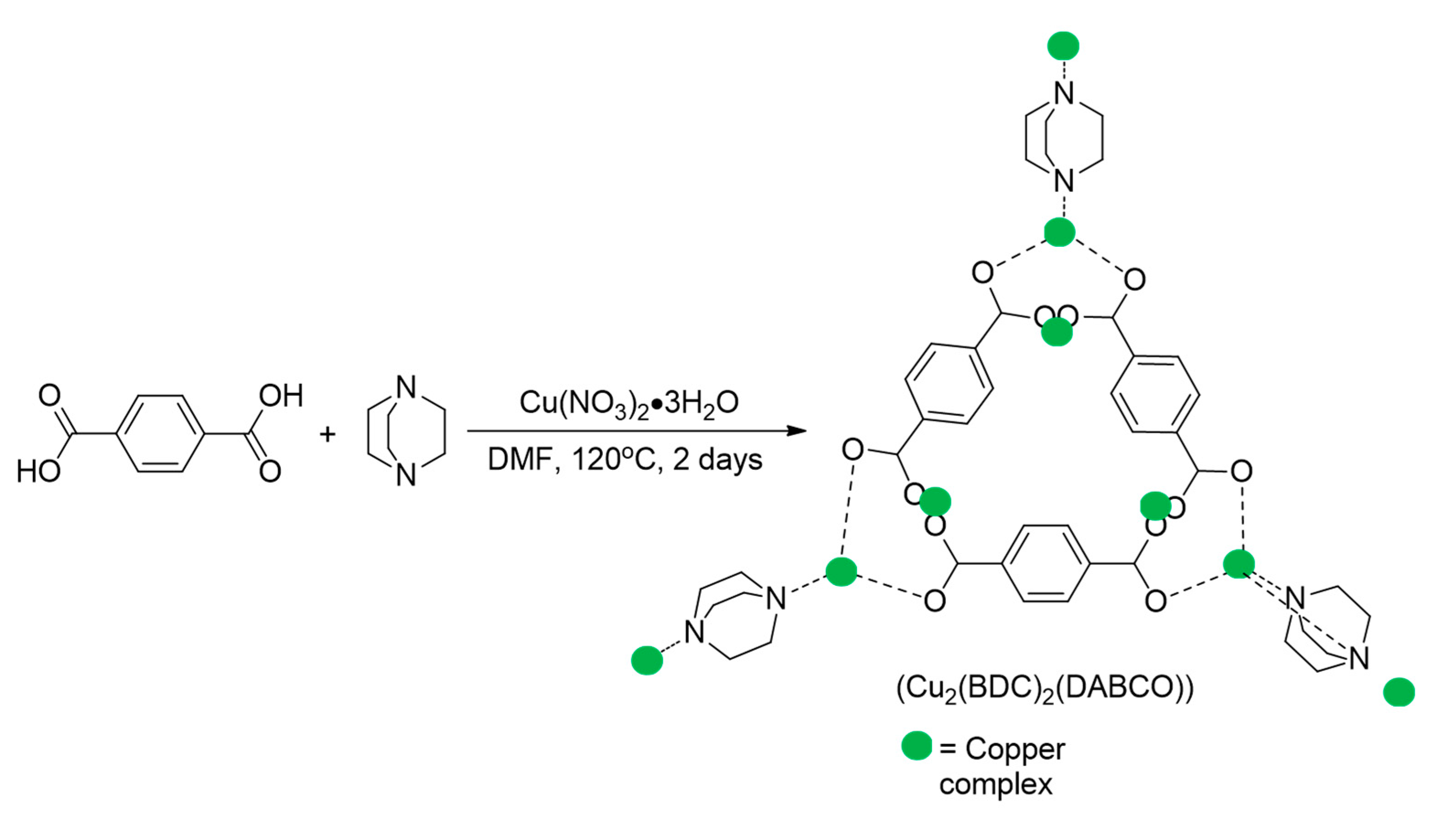
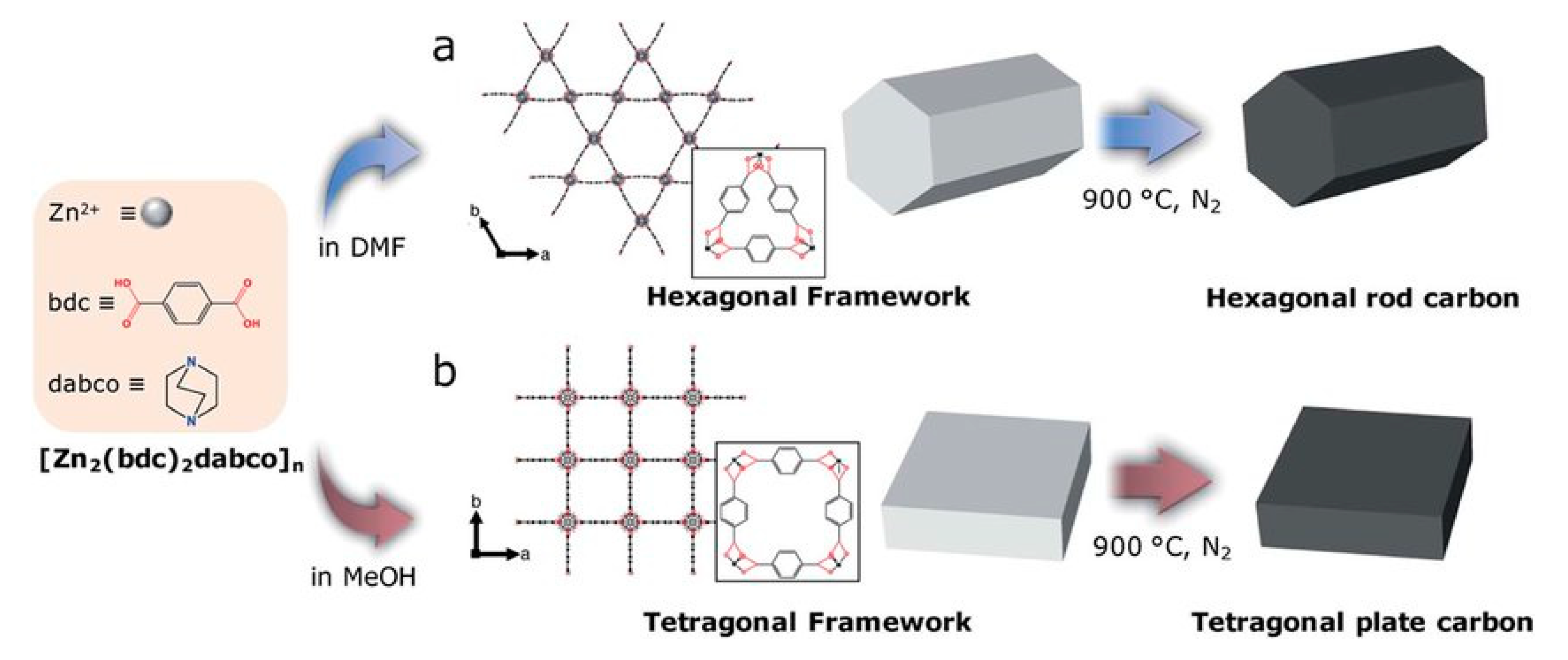
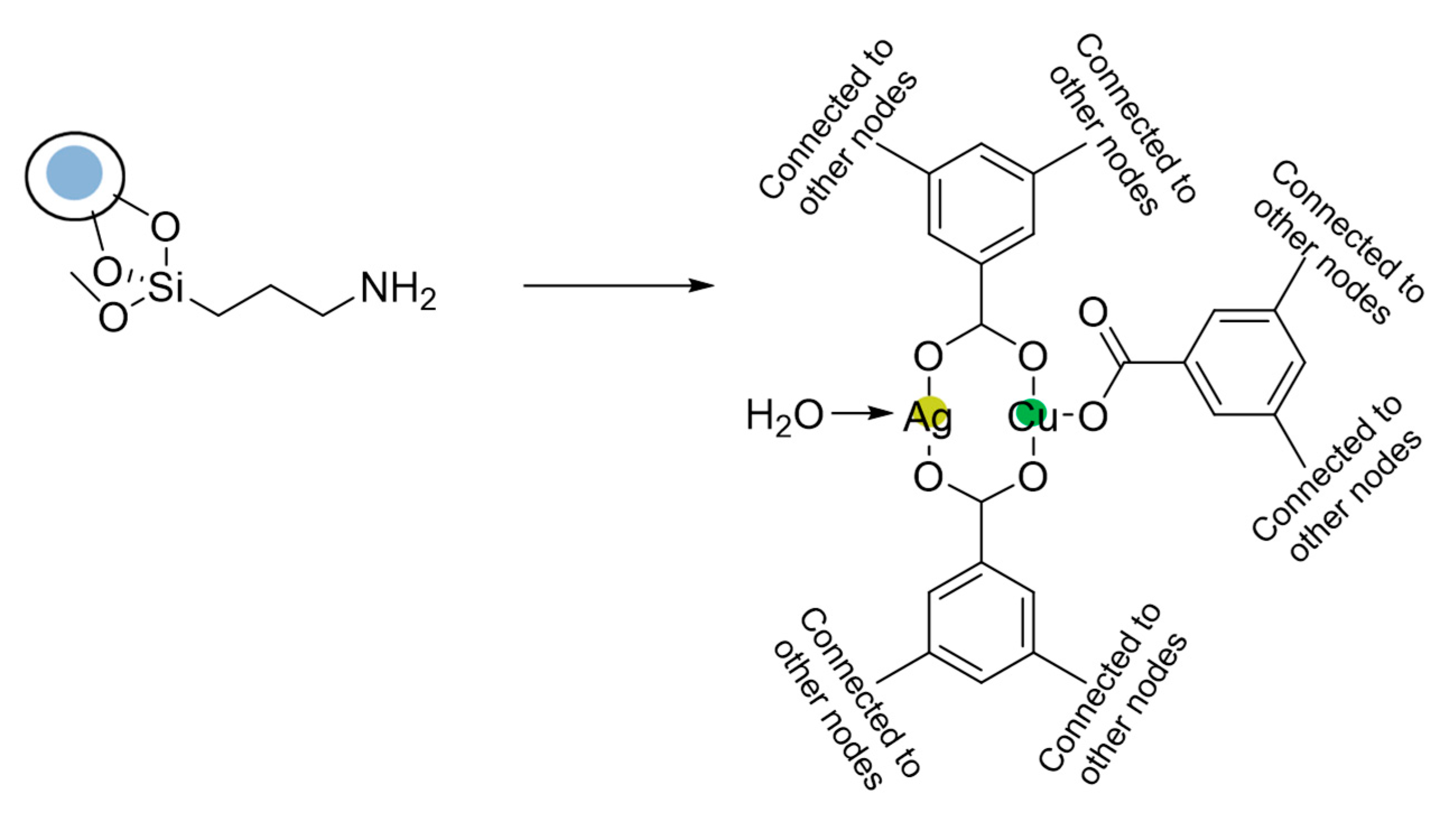
Metal-Organic Framework (MOF) Supported
2.1.6. Cooperative Catalyst (Co-Catalyst)
2.2. Homogeneous Catalyst
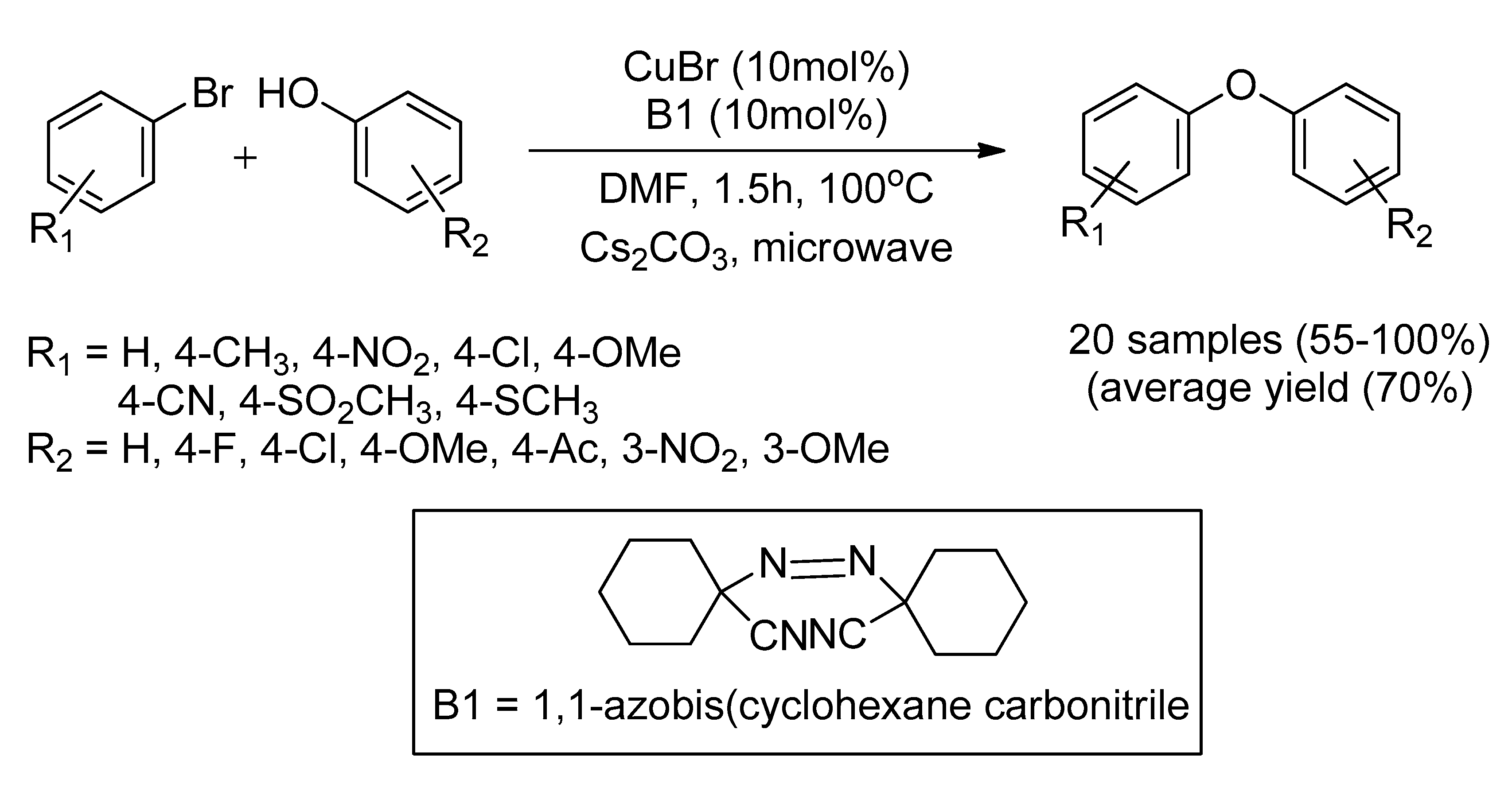
CuI Nanoparticle with the Present of Ligand Precursor (B)
3. Recent Application of Synthetic Ethers in Pharmaceutical and Natural Product
3.1. Medicinal Chemistry
3.2. Natural Product
4. Conclusions
- Ion form of copper: Either metallic copper, Cu(I), Cu(II), or Cu(0) salts and oxides have been applied, but Cu(I) salts commonly provide the extraordinary performance.
- Amount of copper: Commonly in the scope of 5–15 mol% based on the substrate, yet as a typical order higher loaded of copper provides a faster rate of reaction with an excellent outcome.
- Ligand form: Bi-dentate ligands are generally chosen, and the pyridine nucleus, secondary or tertiary amines, carbonyl groups, and imino-groups are generally suitable working ligand moieties; phosphine ligands are generally not very active.
- Ligand loaded: Bi-dentate ligands are used on average in a ratio of (copper: ligand); 1:1 or 1:2, while most of the conditions, a higher ratio leading a better outcome.
- Base: Organic bases ,such as amines, do not work well with C-O Ullmann etherification. On the other hand, inorganic bases, such as potassium phosphate or carbonate and cesium carbonate, give better results in the reaction. The most general loading of the base is 3 equivalents relative to the substrate.
- Solvent: Depending on the reaction and reactant used, polar/non-polar solvents give a better outcome; DMF, DMSO, toluene, or acetonitrile are among the most used; N-Methyl-2-pyrrolidone (NMP) is basically utilized in microwave reactions.
- Temperature: The comment temperature in Ullmann etherification is in the range 70–120 °C, but some cases also conduct at room temperature; a better outcome of the product is generally in higher temperatures.
- Aryl halide: The reactivity of the aryl halide follows the trend: I > Br > Cl; the reactivity of aryl-chlorides can be activated via the strong electron-withdrawing group as substituents, ortho position of substituents/adding a source of I- the reaction (ion exchange reactions are catalyzed by Cu).
- Nucleophile: The better the nucleophile, the better the results, such as amines/thiols> phenol; amides are more active than imides.
- Steric hindrance: A noticeable sensitivity is usually observed, both on the aryl halide and the nucleophile. For example, the presence of the methyl group in the ortho position to the nucleophilic site can significantly reduce the corresponding product.
- Atmosphere: Usually an inert condition; nitrogen/argon atmosphere gives a better organic transformation in Cu-catalyzed ether bond couplings.
Funding
Acknowledgments
Conflicts of Interest
References
- Ayogu, J.I.; Onoabedje, E.A. Recent advances in transition metal-catalysed cross-coupling of (hetero)aryl halides and analogues under ligand-free conditions. Catal. Sci. Technol. 2019, 9, 5233–5255. [Google Scholar] [CrossRef]
- Ciulla, M.G.; Zimmermann, S.; Kumar, K. Cascade reaction based synthetic strategies targeting biologically intriguing indole polycycles. Org. Biomol. Chem. 2019, 17, 413–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boström, J.; Brown, D.G.; Young, R.J.; Keserü, G.M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 2018, 17, 709–727. [Google Scholar] [CrossRef]
- Tahmasbi, B.; Ghorbani-Choghamarani, A.; Moradi, P. Palladium fabricated on boehmite as an organic–inorganic hybrid nanocatalyst for C–C cross coupling and homoselective cycloaddition reactions. New J. Chem. 2020, 44, 3717–3727. [Google Scholar] [CrossRef]
- Mäkelä, E.; González Escobedo, J.L.; Lindblad, M.; Käldström, M.; Meriö-Talvio, H.; Jiang, H.; Puurunen, R.L.; Karinen, R.J.C. Hydrodeoxygenation of levulinic acid dimers on a zirconia-supported ruthenium catalyst. Catalysts 2020, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Hasija, D.C.; Gopalakrishnan, J.; Mishra, A.V.; Ghase, V.D.; Patil, V.R.J.S.A.S. Exploring copper as a catalyst for cost effective synthesis of polyfluorenes: An alternative to platinum and palladium. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Padín, D.; Varela, J.A.; Saá, C. Ruthenium-Catalyzed Tandem Carbene/Alkyne Metathesis/N–H Insertion: Synthesis of Benzofused Six-Membered Azaheterocycles. Org. Lett. 2020. [Google Scholar] [CrossRef]
- Amariucai-Mantu, D.; Mangalagiu, V.; Danac, R.; Mangalagiu, I.I. Microwave Assisted Reactions of Azaheterocycles Formedicinal Chemistry Applications. Molecules 2020, 25, 716. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (US) Chemical Sciences Roundtable. Replacing Critical Materials with Abundant Materials. In The Role of the Chemical Sciences in Finding Alternatives to Critical Resources: A Workshop Summary; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Fan, Q.; Zhu, J.; Gottfried, J.M. On-Surface Ullmann Reaction for the Synthesis of Polymers and Macrocycles. In On-Surface Synthesis II; Springer: Cham, Switzerland, 2018; pp. 83–112. [Google Scholar]
- Abyazisani, M.; Jayalatharachchi, V.; MacLeod, J. 2.14—Directed On-Surface Growth of Covalently-Bonded Molecular Nanostructures. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Oxford, UK, 2019; pp. 299–326. [Google Scholar] [CrossRef]
- Ullmann, F.; Bielecki, J. Ueber Synthesen in der Biphenylreihe. Ber. Dtsch. Chem. Ges. 1901, 34, 2174–2185. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Wu, J.; Meng, Y.; Zhang, Y.; Ye, L.-W.; Zhu, C.J.G.C. Ligand-free palladium catalyzed Ullmann biaryl synthesis:‘household’reagents and mild reaction conditions. Green Chem. 2019, 21, 995–999. [Google Scholar] [CrossRef]
- Jiang, J.; Du, L.; Ding, Y. Aryl-Aryl Bond Formation by Ullmann Reaction: From Mechanistic Aspects to Catalyst. Mini Rev. Org. Chem. 2020, 17, 26–46. [Google Scholar] [CrossRef]
- Gurjar, K.K.; Sharma, R.K.J.H. Synthetic and computational studies on CuI/ligand pair promoted activation of C (Aryl)-Cl bond in C–N coupling reactions. Heliyon 2020, 6, e03233. [Google Scholar] [CrossRef] [PubMed]
- Rovira, M.; Soler, M.; Guell, I.; Wang, M.-Z.; Gomez, L.; Ribas, X. Orthogonal Discrimination among Functional Groups in Ullmann-Type C–O and C–N Couplings. J. Org. Chem. 2016, 81, 7315–7325. [Google Scholar] [CrossRef]
- Cirigottis, K.A.; Ritchie, E.; Taylor, W.C. Studies on the Hurtley reaction. Aust. J. Chem. 1974, 27, 2209–2228. [Google Scholar] [CrossRef]
- Evano, G.; Blanchard, N. Copper-Mediated Cross-Coupling Reactions; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ullmann, F. Ueber eine neue Bildungsweise von Diphenylaminderivaten. Ber. Dtsch. Chem. Ges. 1903, 36, 2382–2384. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, F.; Sponagel, P. Ueber die phenylirung von phenolen. Ber. Dtsch. Chem. Ges. 1905, 38, 2211–2212. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, I. Ueber phenylirungen bei gegenwart von kupfer als katalysator. Ber. Dtsch. Chem. Ges. 1906, 39, 1691–1692. [Google Scholar] [CrossRef] [Green Version]
- Hurtley, W.R.H. CCXLIV—Replacement of halogen in orthobromo-benzoic acid. J. Chem. Soc. 1929, 1870–1873. [Google Scholar] [CrossRef]
- Khan, F.; Dlugosch, M.; Liu, X.; Banwell, M.G. The Palladium-Catalyzed Ullmann Cross-Coupling Reaction: A Modern Variant on a Time-Honored Process. Acc. Chem. Res. 2018, 51, 1784–1795. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Metal Compounds: Knowledge and Myths. Organometallics 2017, 36, 4071–4090. [Google Scholar] [CrossRef] [Green Version]
- Tepale, N.; Fernández-Escamilla, V.V.A.; Carreon-Alvarez, C.; González-Coronel, V.J.; Luna-Flores, A.; Carreon-Alvarez, A.; Aguilar, J. Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications. Crystals 2019, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Prabusankar, G.; Babu, C.N.; Raju, G.; Sampath, N. Silver (I) and copper (II)-imidazolium carboxylates: Efficient catalysts in Ullmann coupling reactions. J. Chem. Sci. 2017, 129, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Rosen, B.M.; Quasdorf, K.W.; Wilson, D.A.; Zhang, N.; Resmerita, A.-M.; Garg, N.K.; Percec, V. Nickel-Catalyzed Cross-Couplings Involving Carbon−Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobisu, M.; Chatani, N. Cross-Couplings Using Aryl Ethers via C–O Bond Activation Enabled by Nickel Catalysts. Acc. Chem. Res. 2015, 48, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Van der Boom, M.E.; Liou, S.-Y.; Ben-David, Y.; Shimon, L.J.W.; Milstein, D. Alkyl− and Aryl−Oxygen Bond Activation in Solution by Rhodium(I), Palladium(II), and Nickel(II). Transition-Metal-Based Selectivity. J. Am. Chem. Soc. 1998, 120, 6531–6541. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Tian, P.; Sheng, Y.; Xu, J.; Han, Y.-F. Oxidative degradation of nitrobenzene by a Fenton-like reaction with Fe-Cu bimetallic catalysts. Appl. Catal. B Environ. 2019, 244, 1–10. [Google Scholar] [CrossRef]
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Bhattacharya, S.K. Size-Dependent Catalytic Activity and Fate of Palladium Nanoparticles in Suzuki-Miyaura Coupling Reactions. ACS Omega 2018, 3, 12905–12913. [Google Scholar] [CrossRef] [Green Version]
- Kidwai, M.; Mishra, N.K.; Bansal, V.; Kumar, A.; Mozumdar, S. Cu-nanoparticle catalyzed O-arylation of phenols with aryl halides via Ullmann coupling. Tetrahedron Lett. 2007, 48, 8883–8887. [Google Scholar] [CrossRef]
- Benaskar, F.; Ben-Abdelmoumen, A.; Patil, N.G.; Rebrov, E.V.; Meuldijk, J.; Hulshof, L.A.; Hessel, V.; Krtschil, U.; Schouten, J.C. Cost Analysis for a Continuously Operated Fine Chemicals Production Plant at 10 Kg/Day Using a Combination of Microprocessing and Microwave Heating. J. Flow Chem. 2011, 1, 74–89. [Google Scholar] [CrossRef]
- Benaskar, F.; Engels, V.; Patil, N.; Rebrov, E.V.; Meuldijk, J.; Hessel, V.; Hulshof, L.A.; Jefferson, D.A.; Schouten, J.C.; Wheatley, A.E.J.T.L. Copper (0) in the Ullmann heterocycle-aryl ether synthesis of 4-phenoxypyridine using multimode microwave heating. Tetrahedron Lett. 2010, 51, 248–251. [Google Scholar] [CrossRef]
- Isomura, Y.; Narushima, T.; Kawasaki, H.; Yonezawa, T.; Obora, Y. Surfactant-free single-nano-sized colloidal Cu nanoparticles for use as an active catalyst in Ullmann-coupling reaction. Chem. Commun. 2012, 48, 3784–3786. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajadi, S.M.; Khalaj, M. Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv. 2014, 4, 47313–47318. [Google Scholar] [CrossRef]
- Garel, C.; Renard, B.-L.; Escande, V.; Galtayries, A.; Hesemann, P.; Grison, C. CC bond formation strategy through ecocatalysis: Insights fromstructural studies and synthetic potential. Appl. Catal. A Gen. 2015, 504. [Google Scholar] [CrossRef]
- Deyris, P.-A.; Grison, C. Nature, ecology and chemistry: An unusual combination for a new green catalysis, ecocatalysis. Curr. Opin. Green Sustain. Chem. 2018, 10, 6–10. [Google Scholar] [CrossRef]
- Clavé, G.; Garel, C.; Poullain, C.; Renard, B.-L.; Olszewski, T.K.; Lange, B.; Shutcha, M.; Faucon, M.-P.; Grison, C. Ullmann reaction through ecocatalysis: Insights from bioresource and synthetic potential. RSC Adv. 2016, 6, 59550–59564. [Google Scholar] [CrossRef]
- Olszewski, T.K.; Adler, P.; Grison, C. Bio-based Catalysts from Biomass Issued after Decontamination of Effluents Rich in Copper—An Innovative Approach towards Greener Copper-based Catalysis. Catalysts 2019, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Damkaci, F.; Sigindere, C.; Sobiech, T.; Vik, E.; Malone, J. N-Picolinamides as ligands in Ullman type C–O coupling reactions. Tetrahedron Lett. 2017, 58, 3559–3564. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Wang, Y.; Zheng, X.; Wang, Z. Nano-CuO-Catalyzed Ullmann Coupling of Phenols with Aryl Halides under Ligand-Free Conditions. Eur. J. Org. Chem. 2008, 2008, 5112–5116. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.C.; Kim, A.; Kim, A.Y.; Lee, H.J.; Song, H.; Park, K. Cu2O Nanocube-Catalyzed Cross-Coupling of Aryl Halides with Phenols via Ullmann Coupling. Eur. J. Org. Chem. 2009, 2009, 4219–4223. [Google Scholar] [CrossRef]
- Babu, S.G.; Karvembu, R. Room temperature Ullmann type C–O and C–S cross coupling of aryl halides with phenol/thiophenol catalyzed by CuO nanoparticles. Tetrahedron Lett. 2013, 54, 1677–1680. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Keipour, H.; Hosseini, A.; Zareyee, D. KF/Clinoptilolite, an effective solid base in Ullmann ether synthesis catalyzed by CuO nanoparticles. New J. Chem. 2014, 38, 42–45. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jafari, S.; Sarvari, S.; Barzegari, E.; Moakedi, F.; Ghorbani, M.; Varnamkhasti, B.S.; Jaymand, M.; Izadi, Z.; Tayebi, L. Biomedical Applications of Zeolitic Nanoparticles, with an Emphasis on Medical Interventions. Int. J. Nanomed. 2020, 15, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabalan, R.; Bertetti, F.P. Cation-Exchange Properties of Natural Zeolites. Rev. Mineral. Geochem. 2001, 45, 453–518. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Shi, A.-H.; Yang, Y.-S.; Li, C.-L. Impregnated copper on magnetite as catalyst for the O-arylation of phenols with aryl halides. Chin. Chem. Lett. 2014, 25, 141–145. [Google Scholar] [CrossRef]
- Gawande, M.B.; Bonifácio, V.D.B.; Varma, R.S.; Nogueira, I.D.; Bundaleski, N.; Ghumman, C.A.A.; Teodoro, O.M.N.D.; Branco, P.S. Magnetically recyclable magnetite–ceria (Nanocat-Fe-Ce) nanocatalyst—Applications in multicomponent reactions under benign conditions. Green Chem. 2013, 15, 1226–1231. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gaur, R.; Yadav, M.; Rathi, A.K.; Pechousek, J.; Petr, M.; Zboril, R.; Gawande, M.B. Maghemite-Copper Nanocomposites: Applications for Ligand-Free Cross-Coupling (C−O, C−S, and C−N) Reactions. ChemCatChem 2015, 7, 3495–3502. [Google Scholar] [CrossRef]
- Kazemi, N.; Mahdavi Shahri, M. Magnetically Separable and Reusable CuFe2O4 Spinel Nanocatalyst for the O-Arylation of Phenol with Aryl Halide Under Ligand-Free Condition. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1264–1273. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Heydari, M. Electrochemical detection of dopamine based on pre-concentration by graphene nanosheets. Analyst 2013, 138, 6044–6051. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Maham, M.; Rostami-Vartooni, A.; Bagherzadeh, M.; Sajadi, S.M. Barberry fruit extract assisted in situ green synthesis of Cu nanoparticles supported on a reduced graphene oxide–Fe3O4 nanocomposite as a magnetically separable and reusable catalyst for the O-arylation of phenols with aryl halides under ligand-free conditions. RSC Adv. 2015, 5, 64769–64780. [Google Scholar]
- Nakhate, A.; Yadav, G. Solvothermal Synthesis of CuFe2O4@rGO: Efficient Catalyst for C-O Cross Coupling and N-arylation Reaction under Ligand-Free Condition. ChemistrySelect 2017, 2, 7150–7159. [Google Scholar] [CrossRef]
- Radfar, I.; Kazemi Miraki, M.; Esfandiary, N.; Ghandi, L.; Heydari, A. Fe3O4@SiO2-copper sucrose xanthate as a green nanocatalyst for N-, O- and S-arylation. Appl. Organomet. Chem. 2019, 33, e4619. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, S.; Pazoki, F.; Radfar, I.; Heydari, A. Copper(I)–creatine complex on magnetic nanoparticles as a green catalyst for N- and O-arylation in deep eutectic solvent. Appl. Organomet. Chem. 2020, 34, e5447. [Google Scholar] [CrossRef]
- Ashraf, M.; Liu, Z.; Peng, W.-X.; Zhou, L. Glycerol Cu(II) Complex Supported on Fe3O4 Magnetic Nanoparticles: A New and Highly Efficient Reusable Catalyst for the Formation of Aryl-Sulfur and Aryl-Oxygen Bonds. Catal. Lett. 2019, 150. [Google Scholar] [CrossRef]
- Khalili, D.; Rezaei, M.; Koohgard, M. Ligand-free copper-catalyzed O-arylation of aryl halides using impregnated copper ferrite on mesoporous graphitic carbon nitride as a robust and magnetic heterogeneous catalyst. Microporous Mesoporous Mater. 2019, 287, 254–263. [Google Scholar] [CrossRef]
- Khodaei, M.M.; Alizadeh, A.; Haghipour, M. Preparation and characterization of isatin complexed with Cu supported on 4-(aminomethyl) benzoic acid-functionalized Fe3O4 nanoparticles as a novel magnetic catalyst for the Ullmann coupling reaction. Res. Chem. Intermed. 2019, 45. [Google Scholar] [CrossRef]
- Mousavi Mashhadi, S.A.; Kassaee, M.Z.; Eidi, E. Magnetically recyclable nano copper/chitosan in O-arylation of phenols with aryl halides. Appl. Organomet. Chem. 2019, 33, e5042. [Google Scholar] [CrossRef]
- Zahedi, R.; Asadi, Z.; Firuzabadi, F.D. Anchored N,O-Cu complex over Fe3O4@SiO2 as a highly efficient and reusable catalyst for CO coupling reaction. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123728. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zhou, J.; Liang, T. Biomass-Derived Porous Carbon Materials for Supercapacitor. Front. Chem. 2019, 7, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Liu, Y.; You, T. Carbon nanofiber based electrochemical biosensors: A review. Anal. Methods 2010, 2, 202–211. [Google Scholar] [CrossRef]
- Dante, R.C. 7—Carbon materials. In Handbook of Friction Materials and Their Applications; Dante, R.C., Ed.; Woodhead Publishing: Boston, MA, USA, 2016; pp. 93–103. [Google Scholar] [CrossRef]
- Luciano, M.A.; Ribeiro, H.; Bruch, G.E.; Silva, G.G. Efficiency of capacitive deionization using carbon materials based electrodes for water desalination. J. Electroanal. Chem. 2020, 859, 113840. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, S.; Han, G.; Li, X.; You, R.; Zhang, Q.; Li, M.; Kaplan, D.L. Facile production of natural silk nanofibers for electronic device applications. Compos. Sci. Technol. 2020, 187, 107950. [Google Scholar] [CrossRef]
- Mergen, Ö.B.; Arda, E.; Evingür, G.A. Electrical, optical, and mechanical percolations of multi-walled carbon nanotube and carbon mesoporous-doped polystyrene composites. J. Compos. Mater. 2020, 54, 31–44. [Google Scholar] [CrossRef]
- Goto, T.; Ito, T.; Mayumi, K.; Maeda, R.; Shimizu, Y.; Hatakeyama, K.; Ito, K.; Hakuta, Y.; Terashima, K. Movable cross-linked elastomer with aligned carbon nanotube/nanofiber as high thermally conductive tough flexible composite. Compos. Sci. Technol. 2020, 190, 108009. [Google Scholar] [CrossRef]
- Wang, C.-C.; Hung, K.-Y.; Ko, T.-E.; Hosseini, S.; Li, Y.-Y. Carbon-nanotube-grafted and nano-Co3O4-doped porous carbon derived from metal-organic framework as an excellent bifunctional catalyst for zinc–air battery. J. Power Sources 2020, 452, 227841. [Google Scholar] [CrossRef]
- Jenie, S.N.A.; Kristiani, A.; Khaerudini, D.S.; Takeishi, K. Magnetic Nanobiochar as Heterogeneous Acid Catalyst for Esterification Reaction. J. Environ. Chem. Eng. 2020, 8, 103912. [Google Scholar] [CrossRef]
- Widiyastuti, W.; Setyawan, H. Sulfonated carbon aerogel derived from coir fiber as high performance solid acid catalyst for esterification. Adv. Powder Technol. 2020, 31, 1412–1419. [Google Scholar]
- Guo, X.; Hao, C.; Jin, G.; Zhu, H.-Y.; Guo, X.-Y. Copper Nanoparticles on Graphene Support: An Efficient Photocatalyst for Coupling of Nitroaromatics in Visible Light. Angew. Chem. Int. Ed. 2014, 53, 1973–1977. [Google Scholar] [CrossRef]
- Zhai, Z.; Guo, X.; Jiao, Z.; Jin, G.; Guo, X.-Y. Graphene-supported Cu2O nanoparticles: An efficient heterogeneous catalyst for C–O cross-coupling of aryl iodides with phenols. Catal. Sci. Technol. 2014, 4, 4196–4199. [Google Scholar] [CrossRef]
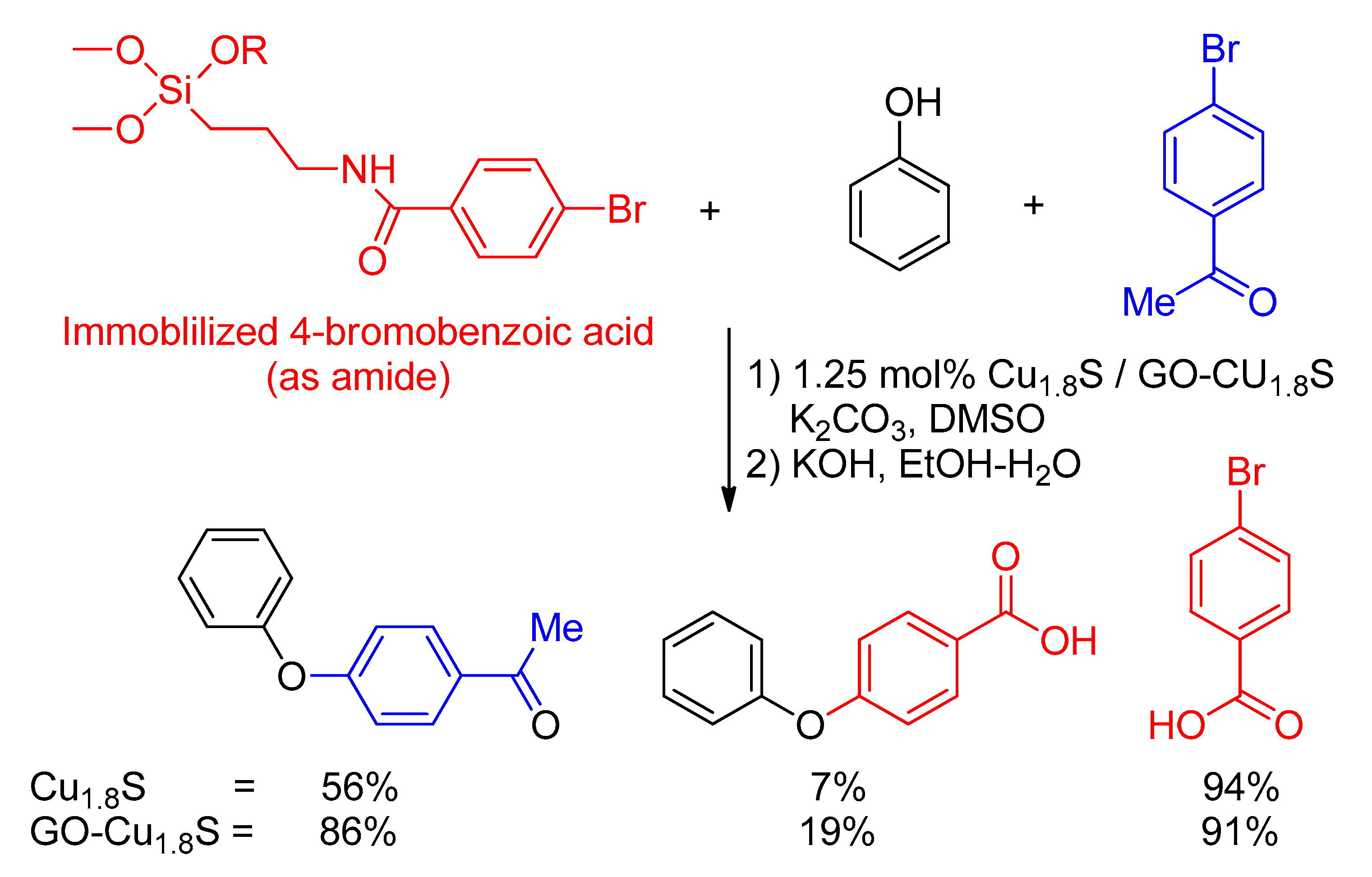
- Singh, V.V.; Singh, A.K. Nanoflowers of Cu1. 8S: Free and Decorated on Graphene Oxide (GO–Cu1. 8S) as Efficient and Recyclable Catalysts for C–O Coupling. ACS Appl. Nano Mater. 2018, 1, 2164–2174. [Google Scholar] [CrossRef]
- Lou, Y.; Chen, X.; Samia, A.C.; Burda, C. Femtosecond Spectroscopic Investigation of the Carrier Lifetimes in Digenite Quantum Dots and Discrimination of the Electron and Hole Dynamics via Ultrafast Interfacial Electron Transfer. J. Phys. Chem. B 2003, 107, 12431–12437. [Google Scholar] [CrossRef]
- Du, X.-S.; Yu, Z.-Z.; Dasari, A.; Ma, J.; Meng, Y.-Z.; Mai, Y.-W. Facile synthesis and assembly of Cu2S nanodisks to corncoblike nanostructures. Chem. Mater. 2006, 18, 5156–5158. [Google Scholar] [CrossRef]
- Yang, W.; Vogler, B.; Lei, Y.; Wu, T. Metallic ion leaching from heterogeneous catalysts: An overlooked effect in the study of catalytic ozonation processes. Environ. Sci. Water Res. Technol. 2017, 3, 1143–1151. [Google Scholar] [CrossRef]
- Vasquez-Cespedes, S.; Chepiga, K.M.; Möller, N.; Schäfer, A.H.; Glorius, F. Direct C–H arylation of heteroarenes with copper impregnated on magnetite as a reusable catalyst: Evidence for CuO nanoparticle catalysis in solution. ACS Catal. 2016, 6, 5954–5961. [Google Scholar] [CrossRef]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef]
- Abdalla, S.; Al-Marzouki, F.; Al-Ghamdi, A.A.; Abdel-Daiem, A. Different Technical Applications of Carbon Nanotubes. Nanoscale Res. Lett. 2015, 10, 358. [Google Scholar] [CrossRef] [Green Version]
- Kharisov, B.I.; Kharissova, O.V.; Dimas, A.V. The dispersion, solubilization and stabilization in “solution” of single-walled carbon nanotubes. RSC Adv. 2016, 6, 68760–68787. [Google Scholar] [CrossRef]
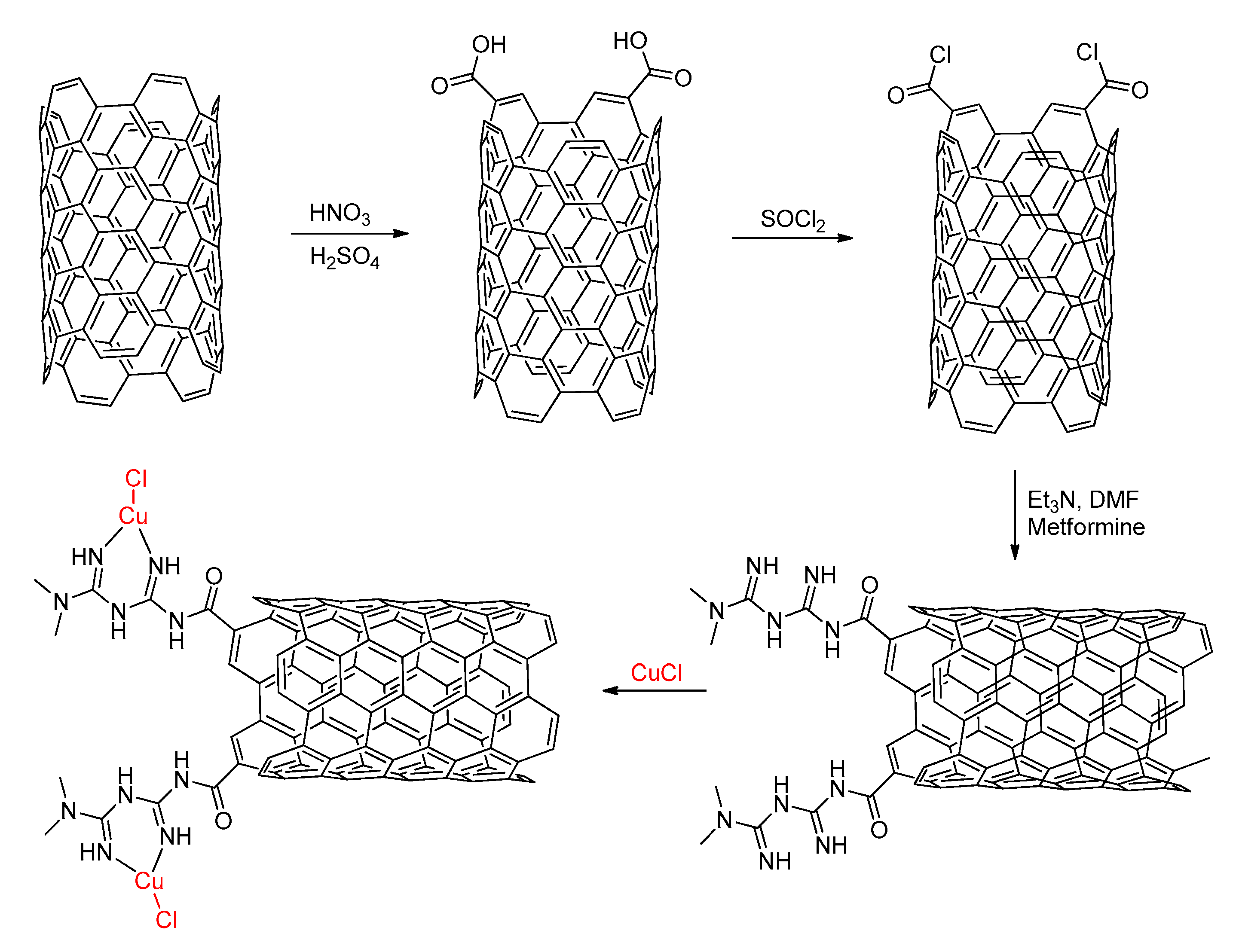
- Akhavan, E.; Hemmati, S.; Hekmati, M.; Veisi, H. CuCl heterogenized on metformine-modified multi walled carbon nanotubes as a recyclable nanocatalyst for Ullmann-type C–O and C–N coupling reactions. New J. Chem. 2018, 42, 2782–2789. [Google Scholar] [CrossRef]
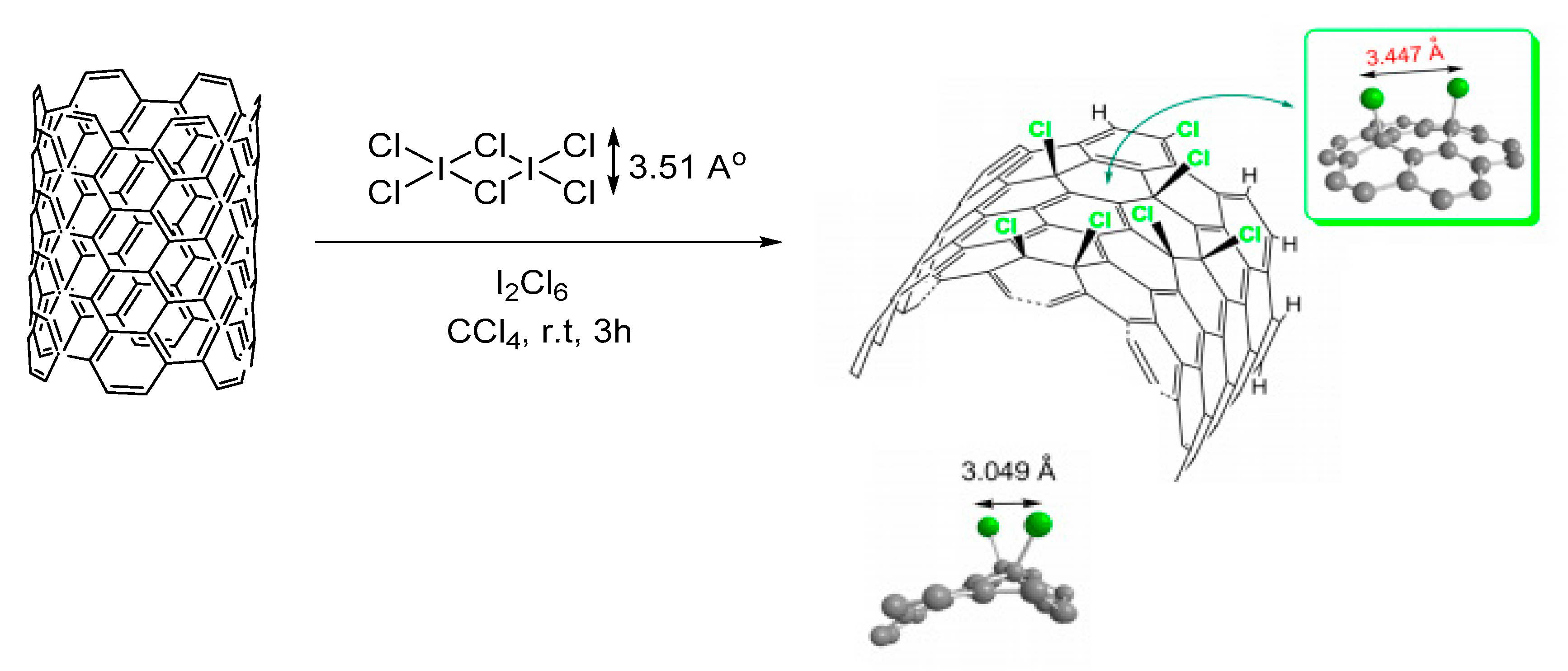
- Kolanowska, A.; Kuziel, A.W.; Jedrysiak, R.G.; Krzywiecki, M.; Korczeniewski, E.; Wisniewski, M.; Terzyk, A.P.; Boncel, S. Ullmann Reactions of Carbon Nanotubes-Advantageous and Unexplored Functionalization toward Tunable Surface Chemistry. Nanomaterials 2019, 9, 1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
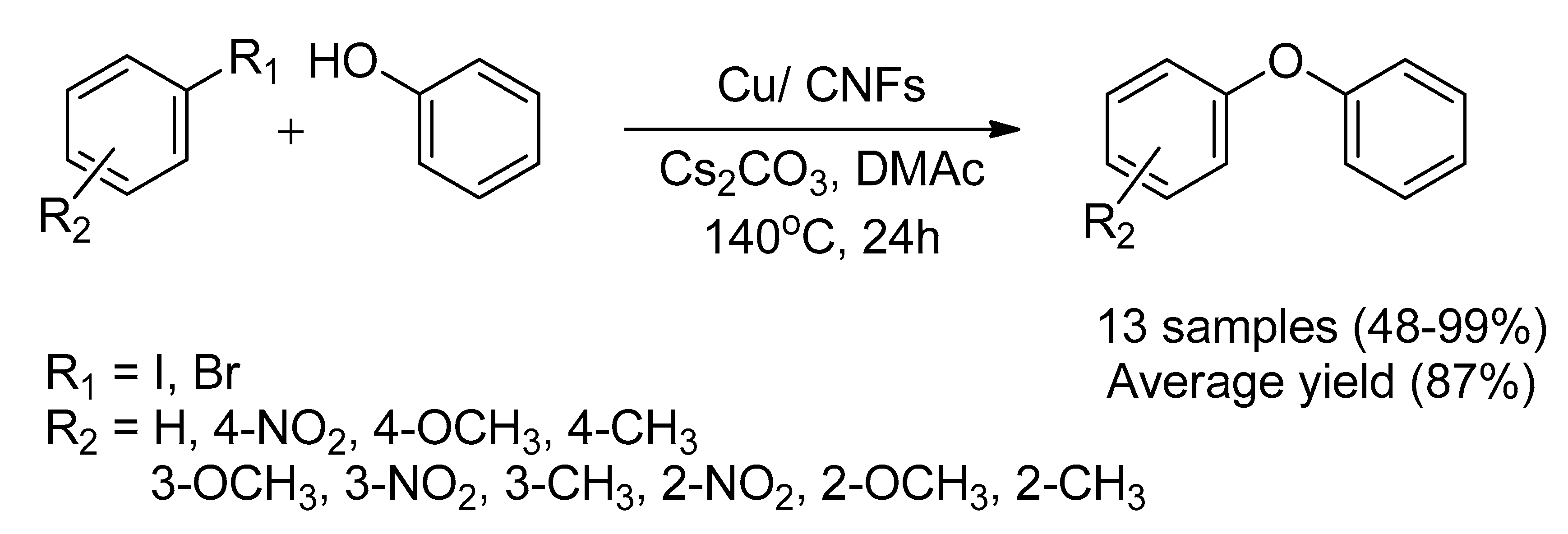
- Mu, H.; Li, C.; Bai, J.; Sun, W. In situ synthesis of Cu/CNFs composite catalyst by electrospun nanofibers wrapped copper chloride and applied for Ullmann coupling reaction. J. Mol. Struct. 2018, 1165, 90–100. [Google Scholar] [CrossRef]
- Chawla, S.; Cai, J.; Naraghi, M. Mechanical tests on individual carbon nanofibers reveals the strong effect of graphitic alignment achieved via precursor hot-drawing. Carbon 2017, 117, 208–219. [Google Scholar] [CrossRef]
- Bin Ali, A.; Renz, F.; Koch, J.; Tegenkamp, C.; Sindelar, R. Graphene Nanoplatelet (GNPs) Doped Carbon Nanofiber (CNF) System: Effect of GNPs on the Graphitic Structure of Creep Stress and Non-Creep Stress Stabilized Polyacrylonitrile (PAN). Nanomaterials 2020, 10, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, L.; Yu, J. Applications of Zeolites in Sustainable Chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef] [Green Version]
- Tahraoui, Z.; Nouali, H.; Marichal, C.; Forler, P.; Klein, J.; Daou, T.J. Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites. Molecules 2020, 25, 944. [Google Scholar] [CrossRef] [Green Version]
- Tuel, A.; Farrusseng, D. Perspectives on Zeolite-Encapsulated Metal Nanoparticles and Their Applications in Catalysis. New J. Chem. 2015, 40. [Google Scholar] [CrossRef]
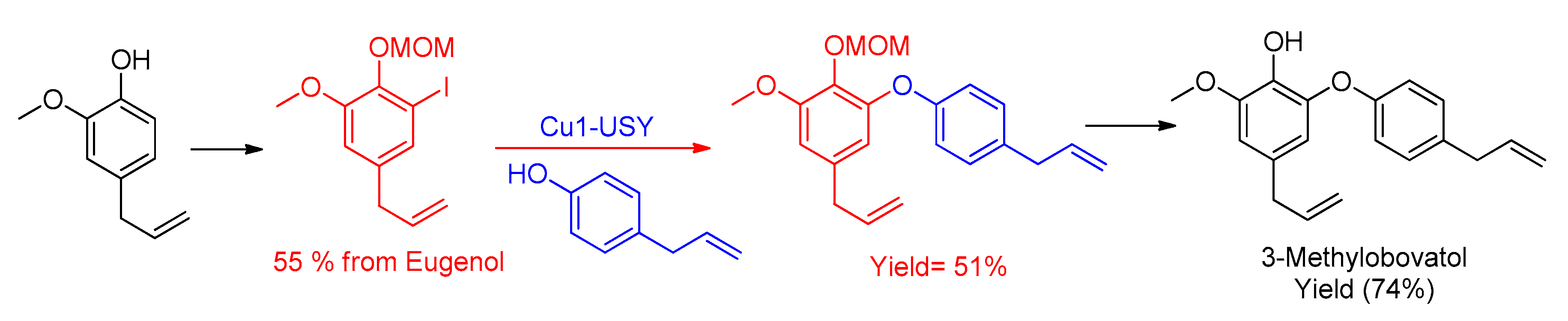
- Magné, V.; Garnier, T.; Danel, M.; Pale, P.; Chassaing, S. CuI–USY as a Ligand-Free and Recyclable Catalytic System for the Ullmann-Type Diaryl Ether Synthesis. Org. Lett. 2015, 17, 4494–4497. [Google Scholar] [CrossRef]
- Garnier, T.; Danel, M.; Magné, V.; Pujol, A.; Bénéteau, V.; Pale, P.; Chassaing, S. Copper(I)–USY as a Ligand-Free and Recyclable Catalyst for Ullmann-Type O-, N-, S-, and C-Arylation Reactions: Scope and Application to Total Synthesis. J. Org. Chem. 2018, 83, 6408–6422. [Google Scholar] [CrossRef]
- Choi, D.-Y.; Lee, J.W.; Peng, J.; Lee, Y.J.; Han, J.-Y.; Lee, Y.H.; Choi, I.S.; Han, S.B.; Jung, J.K.; Lee, W.S.; et al. Obovatol improves cognitive functions in animal models for Alzheimer’s disease. J. Neurochem. 2012, 120, 1048–1059. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Mohamed, M.H.; Banerjee, D.; Thallapally, P.K. Flexibility in Metal–Organic Frameworks: A fundamental understanding. Coord. Chem. Rev. 2018, 358, 125–152. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Lü, J.; Hong, M.; Cao, R. Metal–organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.-N.; Song, X.-Z.; Song, S.-Y.; Zhang, H.-j. Highly efficient heterogeneous catalytic materials derived from metal-organic framework supports/precursors. Coord. Chem. Rev. 2017, 337, 80–96. [Google Scholar] [CrossRef]
- Doonan, C.J.; Sumby, C.J. Metal–organic framework catalysis. CrystEngComm 2017, 19, 4044–4048. [Google Scholar] [CrossRef]
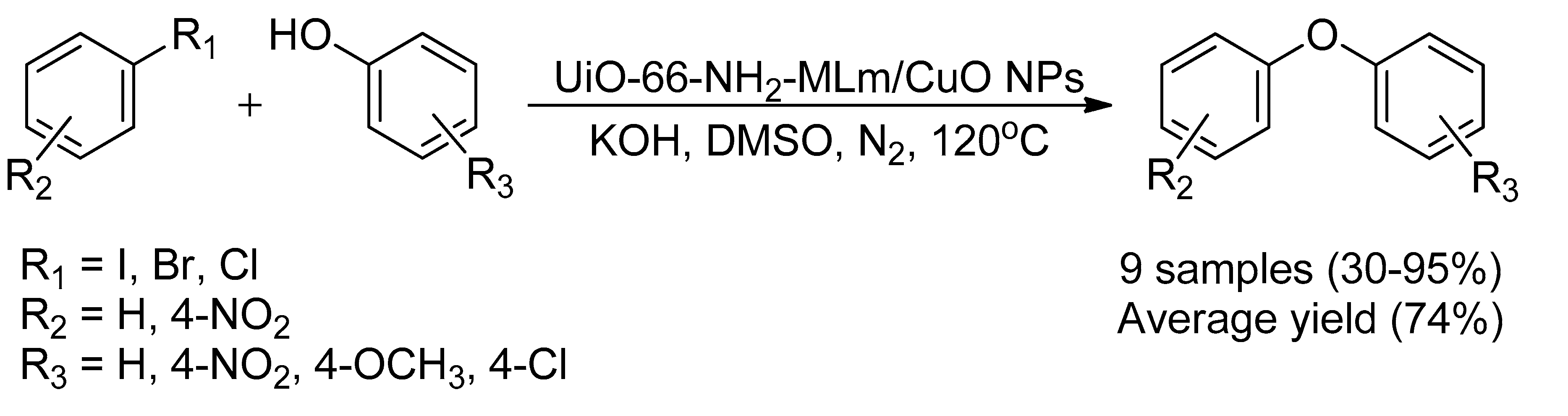
- Sadeghi, S.; Jafarzadeh, M.; Reza Abbasi, A.; Daasbjerg, K. Incorporation of CuO NPs into modified UiO-66-NH2 metal–organic frameworks (MOFs) with melamine for catalytic C–O coupling in the Ullmann condensation. New J. Chem. 2017, 41, 12014–12027. [Google Scholar] [CrossRef]
- Ha, H.Q.; Nguyen, H.T.D.; Pham, T.H.M.; Pham, V.T.; Truong, T. Towards optical application of metal-organic frameworks: Cu-MOFs as sole heterogeneous photocatalyst for arylation of phenols at room temperature. Catal. Commun. 2018, 117, 79–84. [Google Scholar] [CrossRef]
- Hwang, J.; Yan, R.; Oschatz, M.; Schmidt, B. Solvent Mediated Morphology Control of Zn MOFs as Carbon Templates for Application in Supercapacitors. J. Mater. Chem. A 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Allen, A.E.; MacMillan, D.W.C. Synergistic catalysis: A powerful synthetic strategy for new reaction development. Chem. Sci. 2012, 3, 633–658. [Google Scholar] [CrossRef] [PubMed]
- Sammis, G.M.; Danjo, H.; Jacobsen, E.N. Cooperative Dual Catalysis: Application to the Highly Enantioselective Conjugate Cyanation of Unsaturated Imides. J. Am. Chem. Soc. 2004, 126, 9928–9929. [Google Scholar] [CrossRef]
- Trost, B.M.; Luan, X. Contemporaneous Dual Catalysis by Coupling Highly Transient Nucleophilic and Electrophilic Intermediates Generated in Situ. J. Am. Chem. Soc. 2011, 133, 1706–1709. [Google Scholar] [CrossRef]
- Vellayan, K.; González, B.; Trujillano, R.; Vicente, M.A.; Gil, A. Pd supported on Cu-doped Ti-pillared montmorillonite as catalyst for the Ullmann coupling reaction. Appl. Clay Sci. 2018, 160, 126–131. [Google Scholar] [CrossRef]
- Mitsudo, K.; Asada, T.; Inada, T.; Kurimoto, Y.; Mandai, H.; Suga, S. Cu/Fe/O= PPh3-Catalyzed Etherification for the Synthesis of Aryl 3-Benzo [b] thienyl Ethers. Chem. Lett. 2018, 47, 1044–1047. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, L.; Yahyazadeh, A.; Sheykhan, M. The First C–Cl Activation in Ullmann C–O Coupling by MOFs. ChemCatChem 2018, 10, 4636–4651. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Khakyzadeh, V.; Moosavi-Zare, A.R.; Rostami, A.; Zare, A.; Iranpoor, N.; Beyzavi, M.H.; Luque, R. A highly stable and active magnetically separable Pd nanocatalyst in aqueous phase heterogeneously catalyzed couplings. Green Chem. 2013, 15, 2132–2140. [Google Scholar] [CrossRef]
- Baghbanian, S.M.; Yadollahy, H.; Tajbakhsh, M.; Farhang, M.; Biparva, P. Palladium nanoparticles supported on natural nanozeolite clinoptilolite as a catalyst for ligand and copper-free C–C and C–O cross coupling reactions in aqueous medium. RSC Adv. 2014, 4, 62532–62543. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, G.; Li, C.; Xu, S.; Zhang, Z.; Xie, X. The coupling reactions of aryl halides and phenols catalyzed by palladium and MOP-type ligands. Tetrahedron 2015, 71, 4927–4932. [Google Scholar] [CrossRef]
- Joshi, H.; Sharma, K.N.; Sharma, A.K.; Prakash, O.; Singh, A.K. Graphene oxide grafted with Pd 17 Se 15 nano-particles generated from a single source precursor as a recyclable and efficient catalyst for C–O coupling in O-arylation at room temperature. Chem. Commun. 2013, 49, 7483–7485. [Google Scholar] [CrossRef]
- Arundhathi, R.; Damodara, D.; Likhar, P.R.; Kantam, M.L.; Saravanan, P.; Magdaleno, T.; Kwon, S.H. Fe3O4@ mesoporouspolyaniline: A Highly Efficient and Magnetically Separable Catalyst for Cross-Coupling of Aryl Chlorides and Phenols. Adv. Synth. Catal. 2011, 353, 1591–1600. [Google Scholar] [CrossRef]
- Fan, M.; Zhou, W.; Jiang, Y.; Ma, D. CuI/Oxalamide catalyzed couplings of (Hetero) aryl chlorides and phenols for diaryl ether formation. Angew. Chem. Int. Ed. 2016, 55, 6211–6215. [Google Scholar] [CrossRef]
- Xia, N.; Taillefer, M. Copper-or Iron-Catalyzed Arylation of Phenols from respectively Aryl Chlorides and Aryl Iodides. Chem. A Eur. J. 2008, 14, 6037–6039. [Google Scholar] [CrossRef]
- Sreedhar, B.; Arundhathi, R.; Reddy, P.L.; Kantam, M.L. CuI Nanoparticles for C−N and C−O Cross Coupling of Heterocyclic Amines and Phenols with Chlorobenzenes. J. Org. Chem. 2009, 74, 7951–7954. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-W.; Xia, C.-G.; Li, J.-W.; Hu, X.-X. Highly efficient CO cross-coupling reactions with unactivated halides by ligandless, heterogeneous raney Ni-Al alloy/copper (I) salts. Synlett 2003, 2003, 2071–2073. [Google Scholar]
- Truong, T.; Daugulis, O. Divergent reaction pathways for phenol arylation by arynes: Synthesis of helicenes and 2-arylphenols. Chem. Sci. 2013, 4, 531–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, J. Semiconductor Nanocrystals for Environmental Catalysis. In Advanced Nanomaterials for Pollutant Sensing and Environmental Catalysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–163. [Google Scholar]
- Shende, V.S.; Saptal, V.B.; Bhanage, B.M. Recent Advances Utilized in the Recycling of Homogeneous Catalysis. Chem. Rec. 2019, 19, 2022–2043. [Google Scholar] [CrossRef]
- Navarro, L.; Pujol, M.D. Microwave assisted synthesis of selected diaryl ethers under Cu(I)-catalysis. Tetrahedron Lett. 2015, 56, 1812–1815. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Kaneyama, R.; Kodama, K.; Hirose, T. Efficient Pyrazole Moiety-Containing Ligands for Cu-Catalyzed O-Arylation of Phenols. Beilstein Arch. 2019, 1, 94. [Google Scholar]
- Zhao, Y.; Wang, X.; Kodama, K.; Hirose, T. Copper-Catalyzed Coupling Reactions of Aryl Halides and Phenols by 4,4′-Dimethoxy-2,2′-bipyridine and 4,7-Dimethoxy-1,10-phenanthroline. ChemistrySelect 2018, 3, 12620–12624. [Google Scholar] [CrossRef]
- Paul, S.; Joy, B.P.; Rajendran, R.; Gudimetla, V.B. Cost Efficient Synthesis of Diaryl Ethers Catalysed by CuI, Imidazolium Chloride and Cs2CO3. ChemistrySelect 2019, 4, 7181–7186. [Google Scholar] [CrossRef]
- Teng, Q.; Zhu, X.; Guo, Q.; Jiang, W.; Liu, J.; Meng, Q. Synthesis of 9-O-arylated berberines via copper-catalyzed C(Ar)-O coupling reactions. Beilstein J. Org. Chem. 2019, 15, 1575–1580. [Google Scholar] [CrossRef] [Green Version]
- Chan, V.S.; Krabbe, S.W.; Li, C.; Sun, L.; Liu, Y.; Nett, A.J. Identification of an Oxalamide Ligand for Copper-Catalyzed C–O Couplings from a Pharmaceutical Compound Library. ChemCatChem 2019, 11, 5748–5753. [Google Scholar] [CrossRef]
- Queiroz, M.-J.R.P.; Peixoto, D.; Calhelha, R.C.; Soares, P.; dos Santos, T.; Lima, R.T.; Campos, J.F.; Abreu, R.M.V.; Ferreira, I.C.F.R.; Vasconcelos, M.H. New di(hetero)arylethers and di(hetero)arylamines in the thieno[3,2-b]pyridine series: Synthesis, growth inhibitory activity on human tumor cell lines and non-tumor cells, effects on cell cycle and on programmed cell death. Eur. J. Med. Chem. 2013, 69, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-M.; Huang, Z.-N.; Zhou, Z.-S.; Hou, J.; Zheng, M.-Y.; Wang, L.-J.; Jiang, Y.; Zhou, X.-Y.; Chen, Q.-Y.; Li, S.-H.; et al. Structure-based design, structure–activity relationship analysis, and antitumor activity of diaryl ether derivatives. Arch. Pharmacal Res. 2015, 38, 1761–1773. [Google Scholar] [CrossRef]
- Lin, H.; Bruhn, D.F.; Maddox, M.M.; Singh, A.P.; Lee, R.E.; Sun, D. Synthesis and antibacterial evaluation of macrocyclic diarylheptanoid derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 4070–4076. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.S.; Bhat, V.G.; Shenoy, V.P.; Bairy, I.; Shenoy, G.G. Design, synthesis, and evaluation of novel diphenyl ether derivatives against drug-susceptible and drug-resistant strains of Mycobacterium tuberculosis. Chem. Biol. Drug Des. 2019, 93, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current status and future prospects of marine natural products (MNPs) as antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Wang, J.; Nitelet, A. Metal-mediated C–O bond forming reactions in natural product synthesis. Org. Chem. Front. 2017, 4, 2480–2499. [Google Scholar] [CrossRef]
- Tomita, M.; Aoyagi, Y.; Sakatani, Y.; Fujitani, K. Studies on the Alkaloids of Menispermaceous Plants. CCXLI. Synthesis of Cycleanine.(2). Bischler-Napieralski Reaction of N-Acyl-3, 4-dimethoxy-5-(4-substituted-phenoxy)-β-phenethylamines. Chem. Pharm. Bull. 1968, 16, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Tomita, M.; Fujitani, K.; Aoyagi, Y.; Kajita, Y. Studies on the alkaloids of menispermaceous plants. CCXLIV. Synthesis of dl-cepharanthine. Chem. Pharm. Bull. 1968, 16, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Inubushi, Y.; Masaki, Y.; Matsumoto, S.; Takami, F. Studies on the alkaloids of menispermaceous plants. Part CCXLIX. Total synthesis of optically active natural isotetrandrine, phaeanthine, and tetrandrine. J. Chem. Soc. C Org. 1969, 11, 1547–1556. [Google Scholar] [CrossRef]
- Evans, D.A.; Ellman, J.A. The total syntheses of the isodityrosine-derived cyclic tripeptides OF4949-III and K-13. Determination of the absolute configuration of K-13. J. Am. Chem. Soc. 1989, 111, 1063–1072. [Google Scholar] [CrossRef]
- Lygo, B.; Humphreys, L.D. Enantioselective synthesis of renieramide. Synlett 2004, 2004, 2809–2811. [Google Scholar] [CrossRef]
- Toyota, M.; Tori, M.; Takikawa, K.; Shiobara, Y.; Kodama, M.; Asakawa, Y. Perrottetins E, F, and G from Radula perrottetii (liverwort)--isolation, structure determination, and synthesis of perrottetin e. Tetrahedron Lett. 1985, 26, 6097–6100. [Google Scholar] [CrossRef]
- Miao, T.; Wang, L. Immobilization of copper in organic–inorganic hybrid materials: A highly efficient and reusable catalyst for the Ullmann diaryl etherification. Tetrahedron Lett. 2007, 48, 95–99. [Google Scholar] [CrossRef]
- Ingerl, A.; Justus, K.; Hellwig, V.; Steglich, W. Syntheses of retipolide E and ornatipolide, 14-membered biaryl-ether macrolactones from mushrooms. Tetrahedron 2007, 63, 6548–6557. [Google Scholar] [CrossRef]
- Jeong, B.-S.; Wang, Q.; Son, J.-K.; Jahng, Y. A Versatile Synthesis of Cyclic Diphenyl Ether-Type Diarylheptanoids: Acerogenins, (±)-Galeon, and (±)-Pterocarine. Eur. J. Org. Chem. 2007, 2007, 1338–1344. [Google Scholar] [CrossRef]
- Salih, M.Q.; Beaudry, C.M. Chirality in diarylether heptanoids: Synthesis of myricatomentogenin, jugcathanin, and congeners. Org. Lett. 2012, 14, 4026–4029. [Google Scholar] [CrossRef]
- Bryant, V.C.; Kumar, G.D.K.; Nyong, A.M.; Natarajan, A. Synthesis and evaluation of macrocyclic diarylether heptanoid natural products and their analogs. Bioorg. Med. Chem. Lett. 2012, 22, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Sun, D. Total synthesis and structural revision of engelhardione. Tetrahedron Lett. 2011, 52, 4570–4574. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Simmons, C.J.; Sun, D. Microwave-assisted synthesis of macrocycles via intramolecular and/or bimolecular Ullmann coupling. Tetrahedron Lett. 2012, 53, 4173–4178. [Google Scholar] [CrossRef] [Green Version]
- Nicolaou, K.C.; Li, H.; Boddy, C.N.C.; Ramanjulu, J.M.; Yue, T.Y.; Natarajan, S.; Chu, X.J.; Bräse, S.; Rübsam, F. Total synthesis of vancomycin—Part 1: Design and development of methodology. Chem. A Eur. J. 1999, 5, 2584–2601. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Boddy, C.N.C.; Li, H.; Koumbis, A.E.; Hughes, R.; Natarajan, S.; Jain, N.F.; Ramanjulu, J.M.; Bräse, S.; Solomon, M.E. Total synthesis of vancomycin—Part 2: Retrosynthetic analysis, synthesis of amino acid building blocks and strategy evaluations. Chem. A Eur. J. 1999, 5, 2602–2621. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Koumbis, A.E.; Takayanagi, M.; Natarajan, S.; Jain, N.F.; Bando, T.; Li, H.; Hughes, R. Total synthesis of vancomycin—Part 3: Synthesis of the aglycon. Chem. A Eur. J. 1999, 5, 2622–2647. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Mitchell, H.J.; Jain, N.F.; Bando, T.; Hughes, R.; Winssinger, N.; Natarajan, S.; Koumbis, A.E. Total synthesis of vancomycin—Part 4: Attachment of the sugar moieties and completion of the synthesis. Chem. A Eur. J. 1999, 5, 2648–2667. [Google Scholar] [CrossRef]
- Lang, G.; Blunt, J.W.; Cummings, N.J.; Cole, A.L.J.; Munro, M.H.G. Hirsutide, a Cyclic Tetrapeptide from a Spider-Derived Entomopathogenic Fungus, Hirsutella sp. J. Nat. Prod. 2005, 68, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Sarlah, D.; Wu, T.R.; Zhan, W. Total synthesis of hirsutellone B. Angew. Chem. (Int. Ed. Engl.) 2009, 48, 6870–6874. [Google Scholar] [CrossRef]
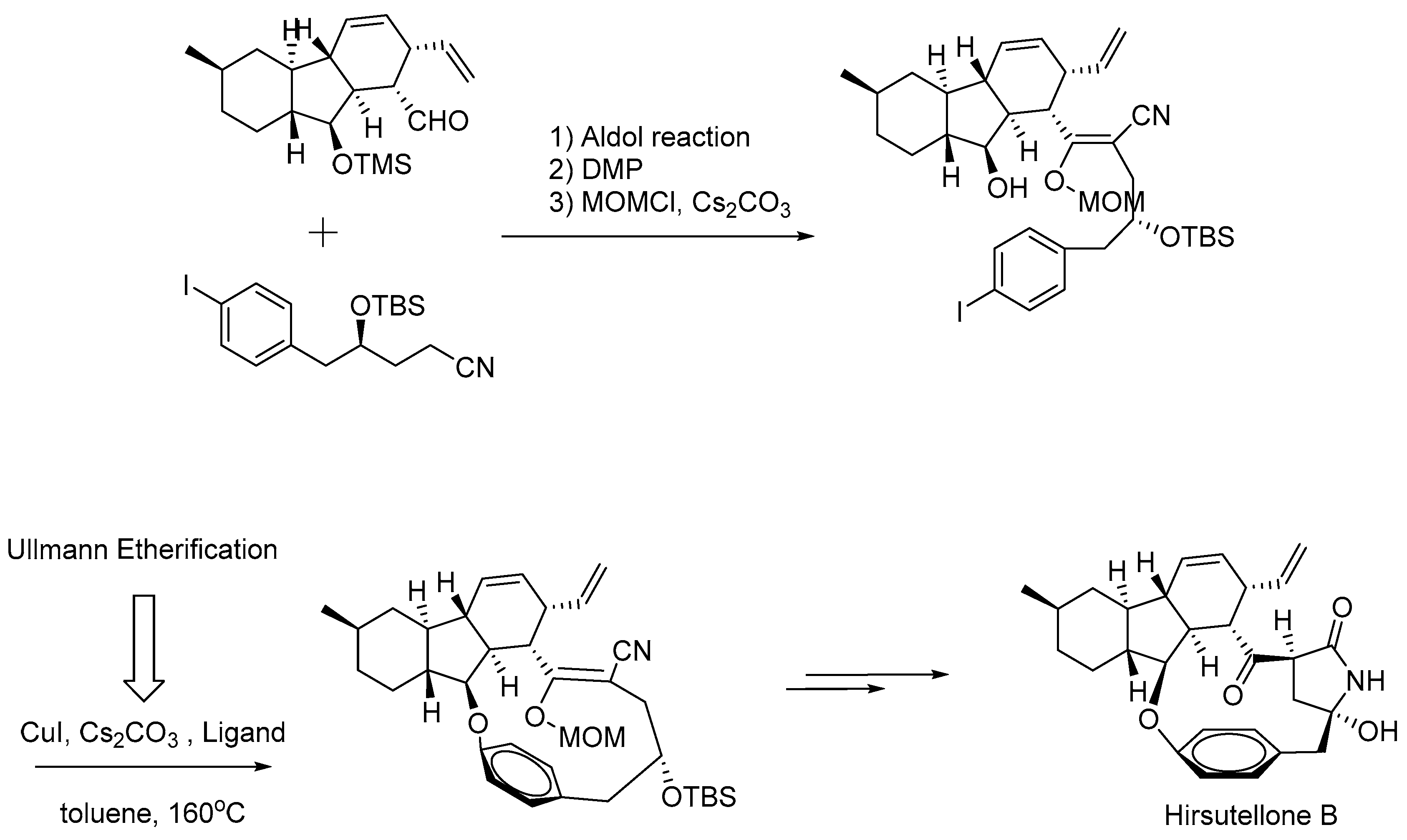
- Uchiro, H.; Kato, R.; Arai, Y.; Hasegawa, M.; Kobayakawa, Y. Total Synthesis of Hirsutellone B via Ullmann-Type Direct 13-Membered Macrocyclization. Org. Lett. 2011, 13, 6268–6271. [Google Scholar] [CrossRef]
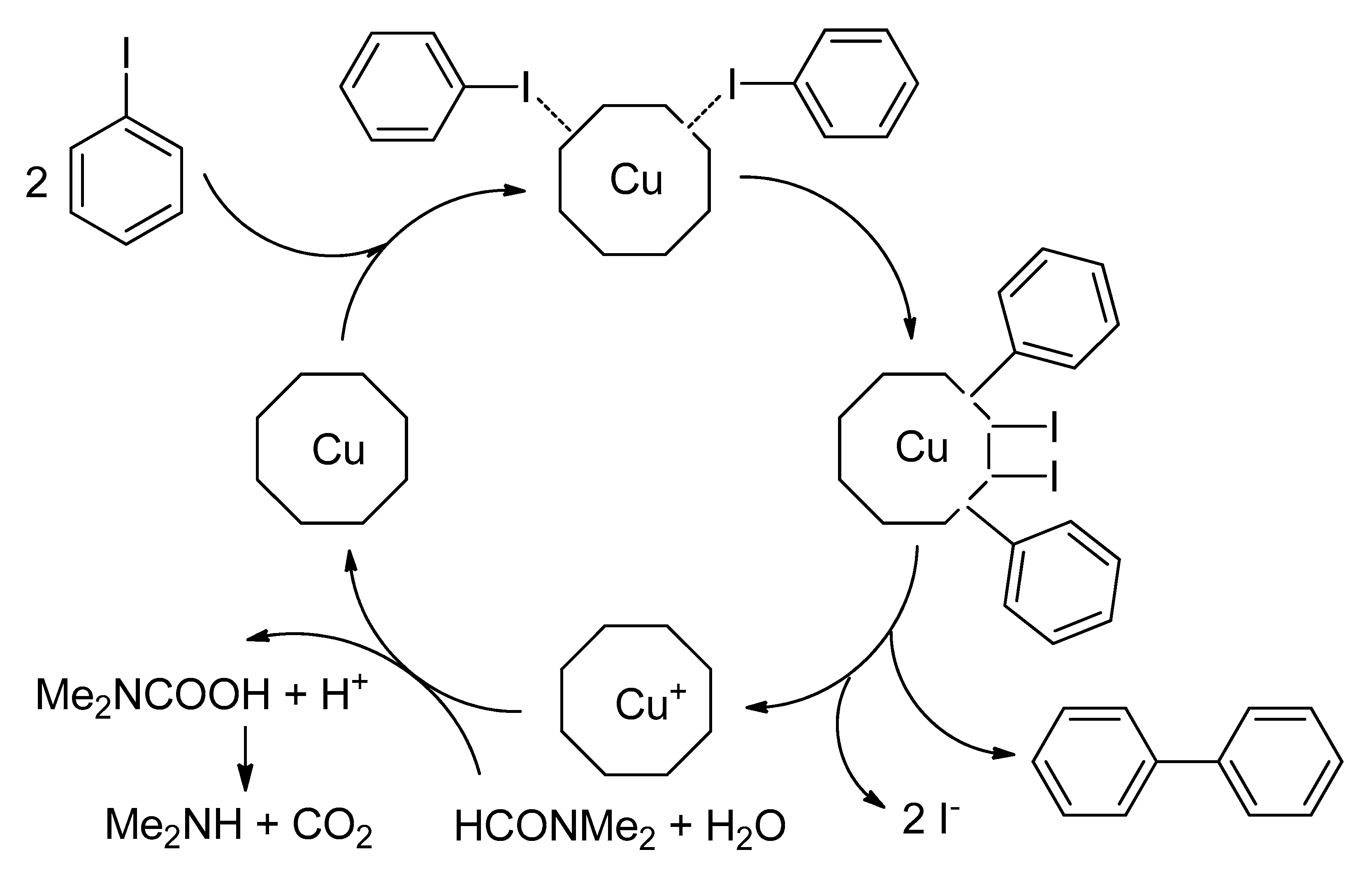
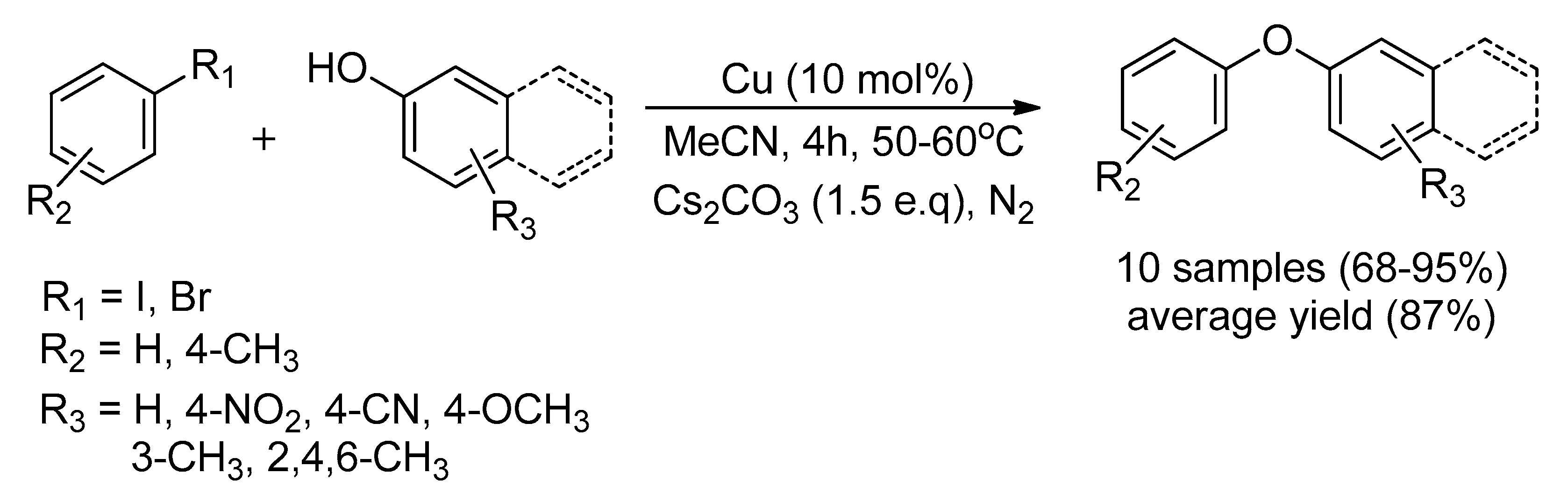
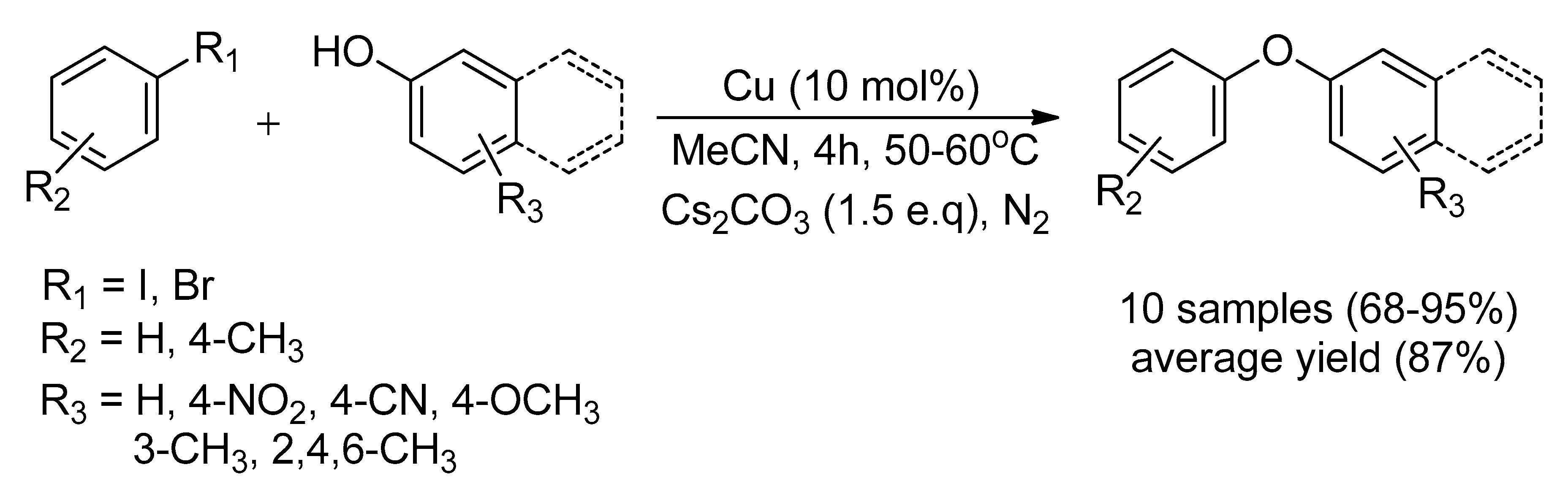


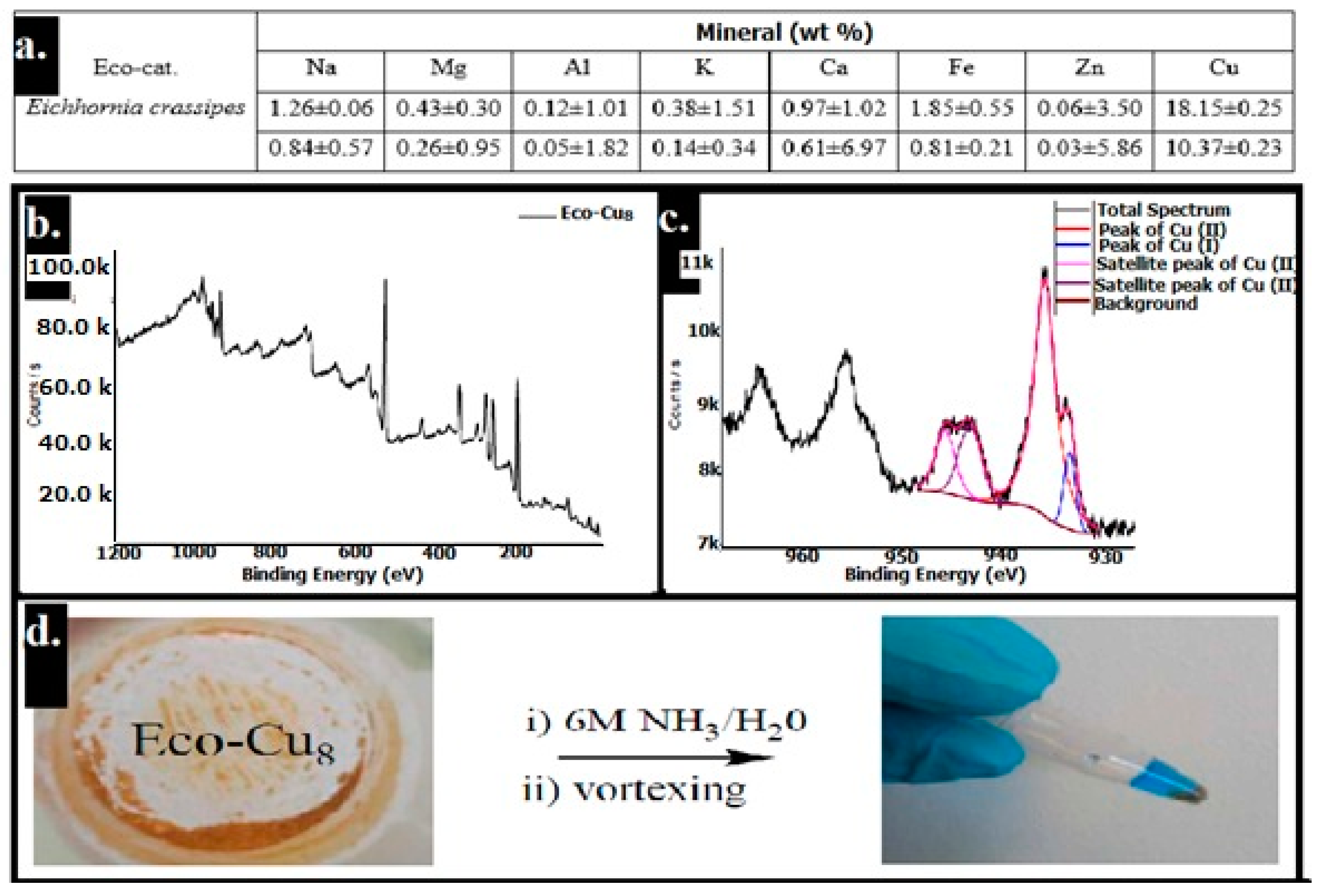

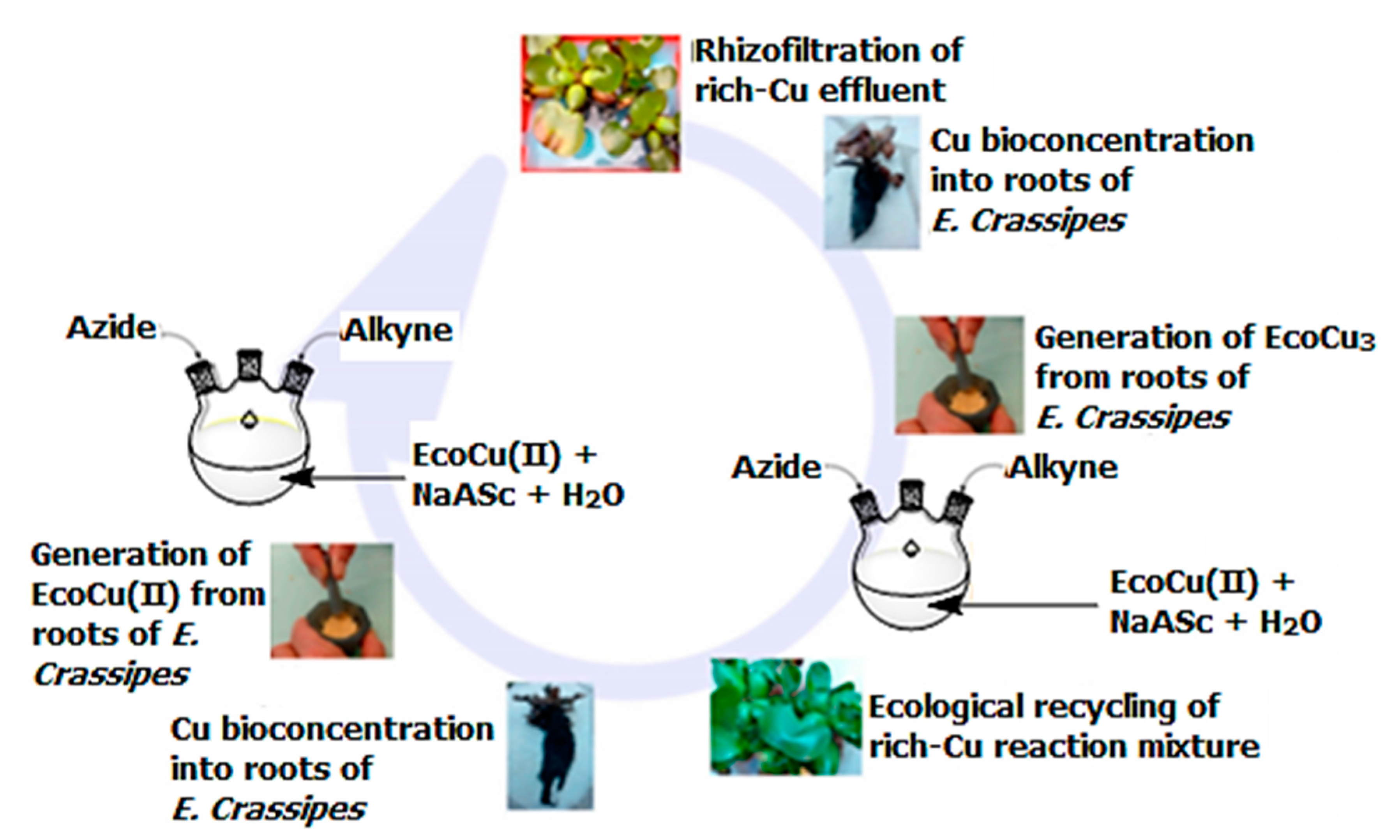
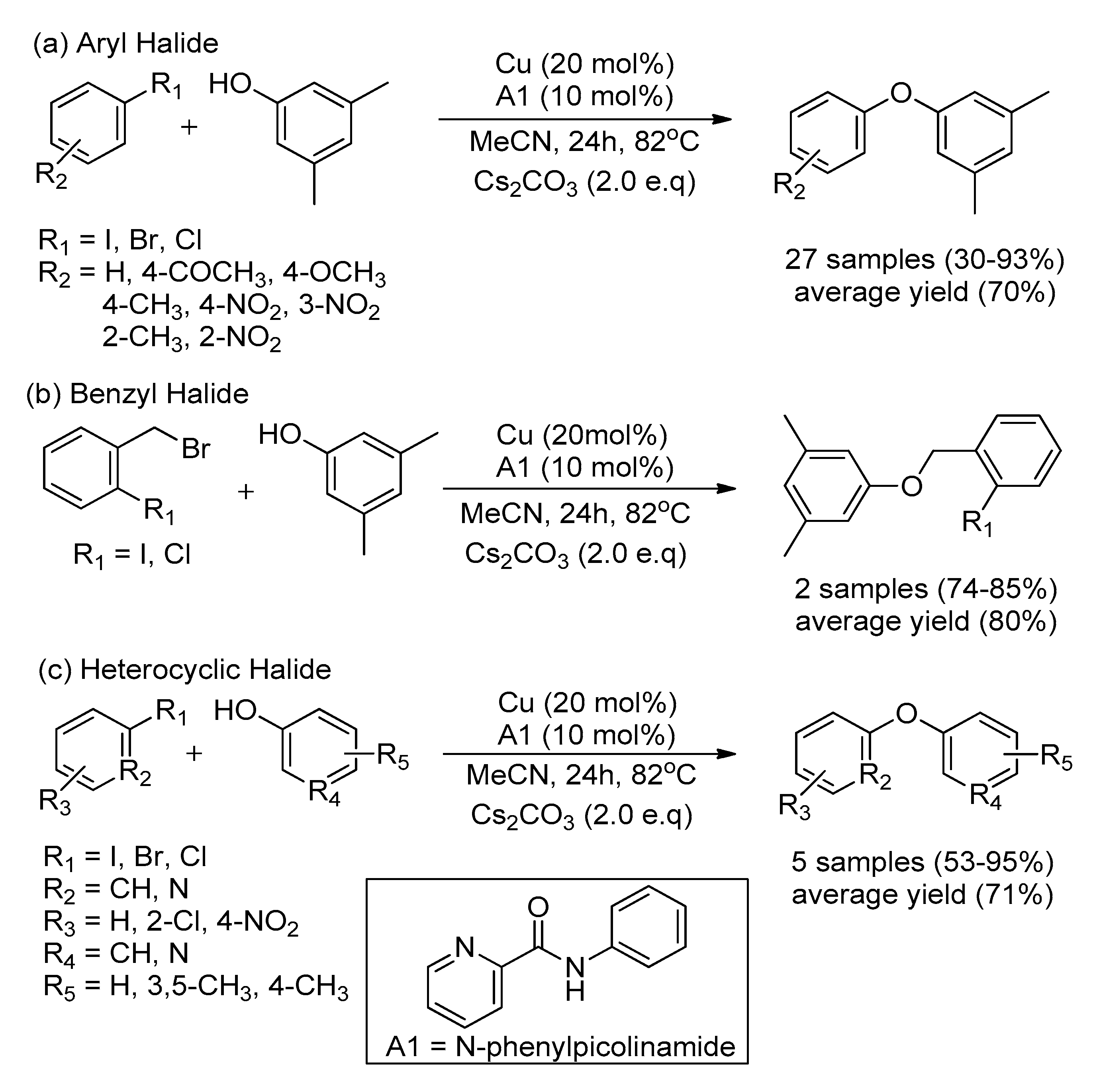
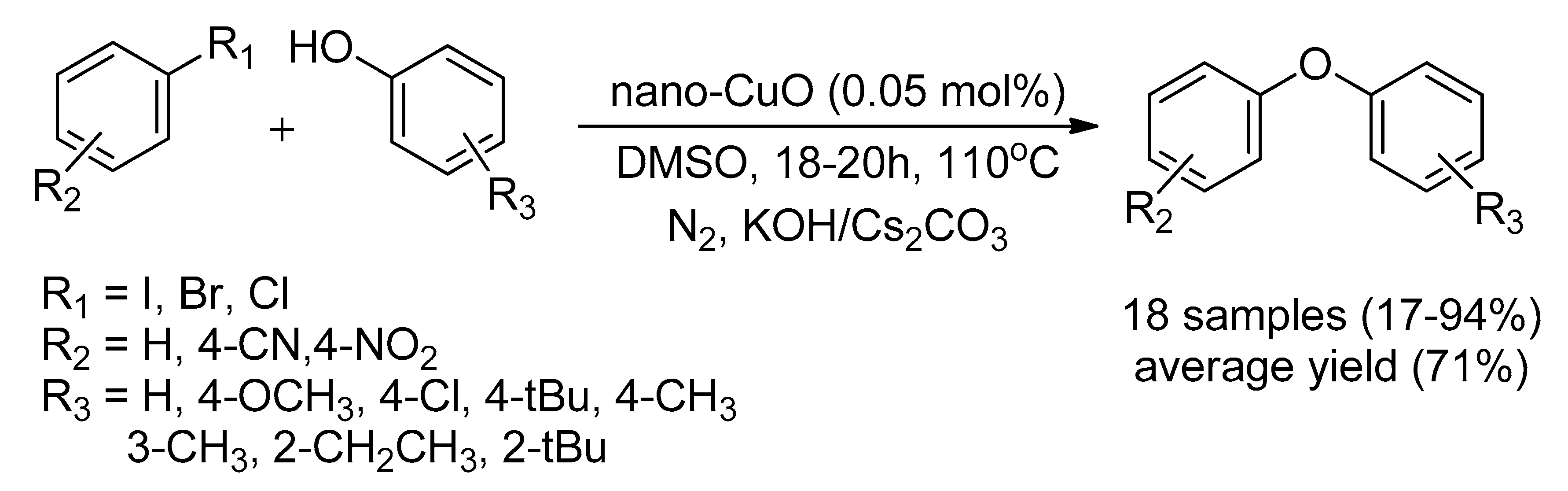


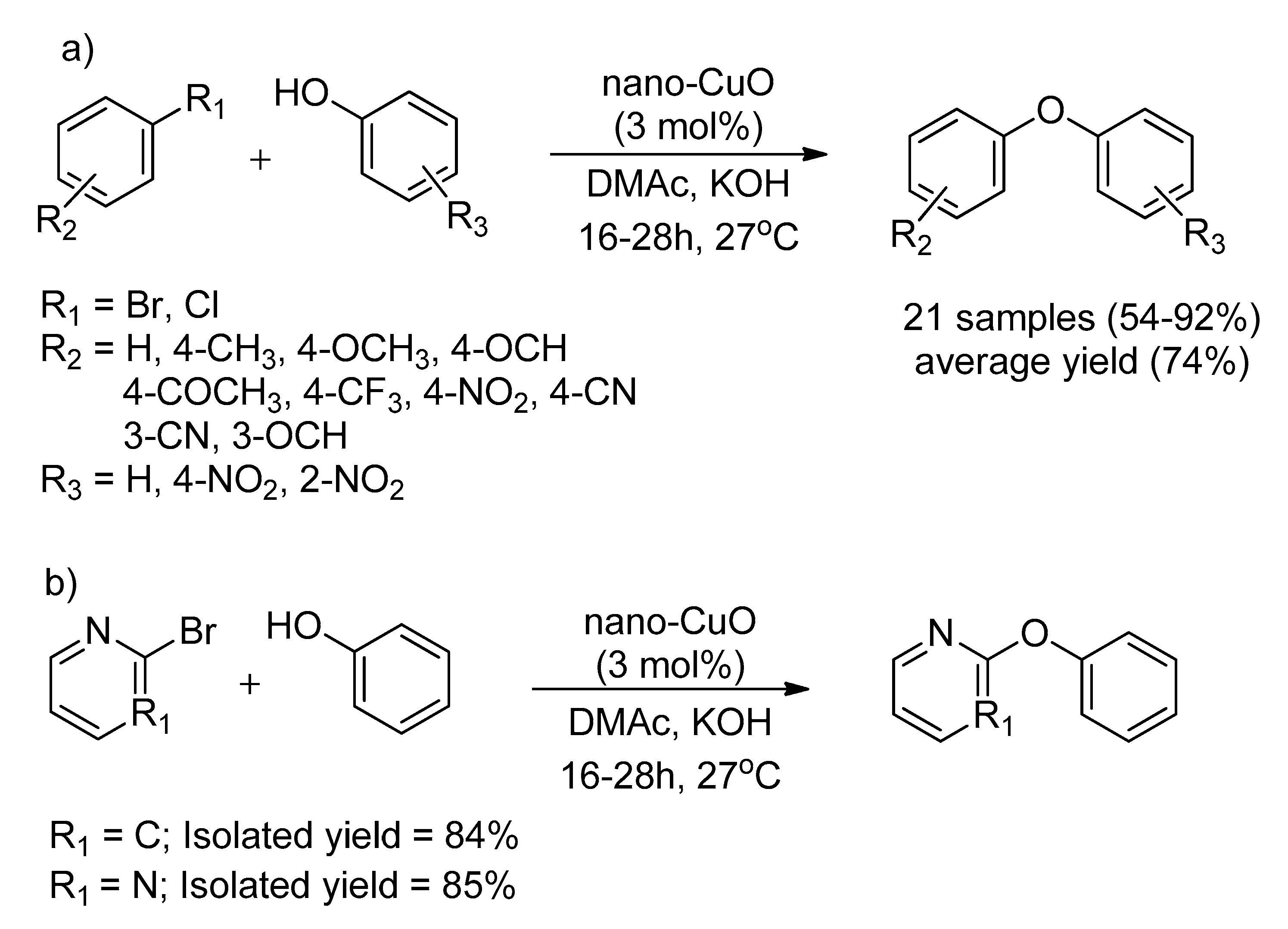

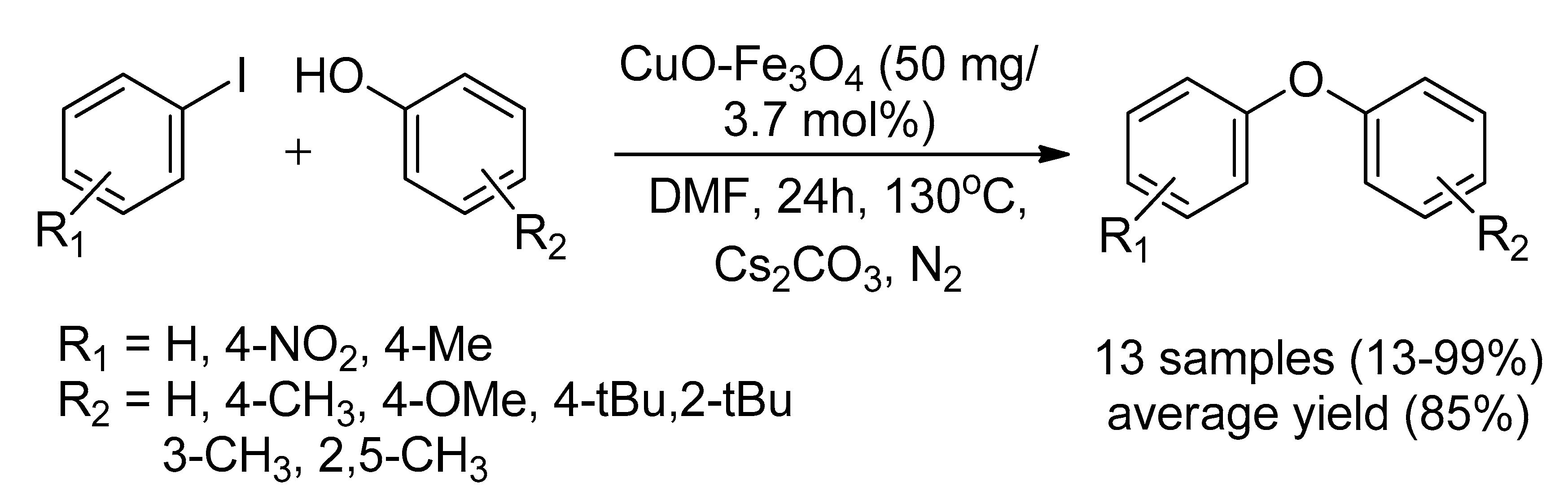
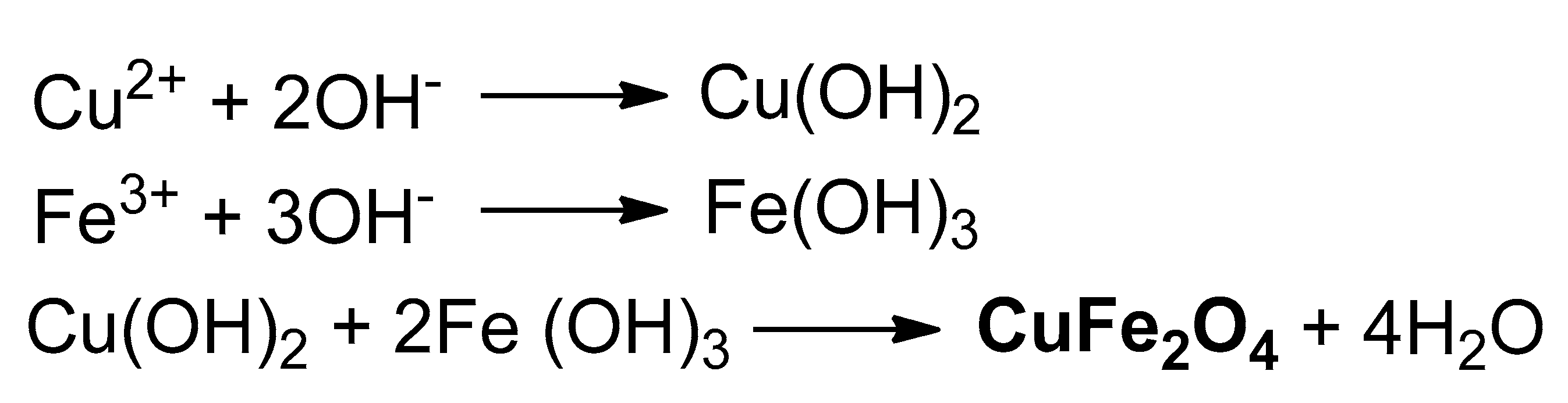
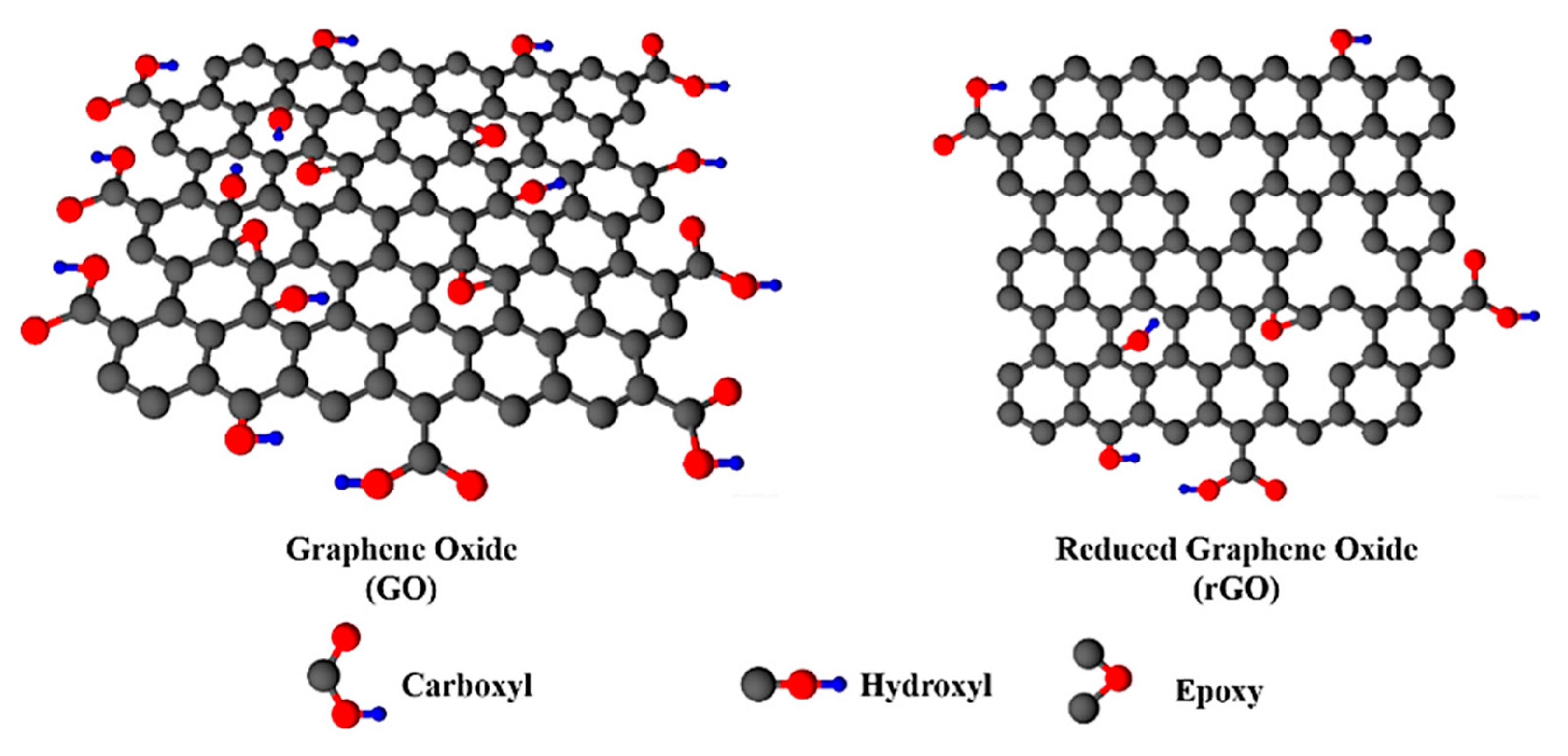

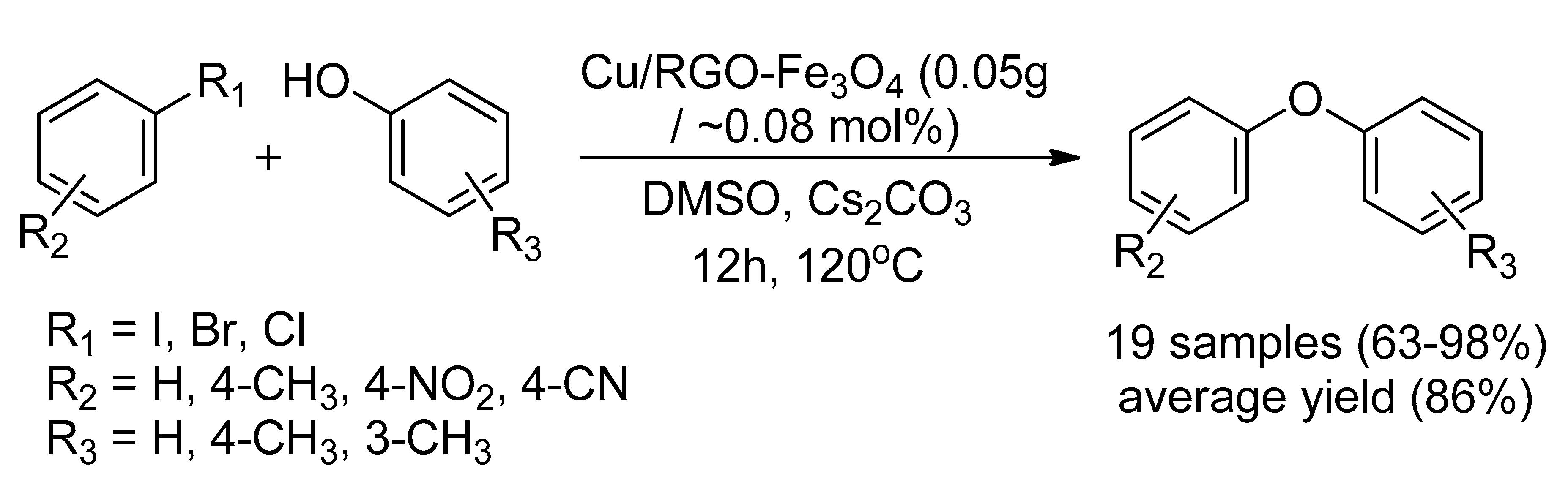
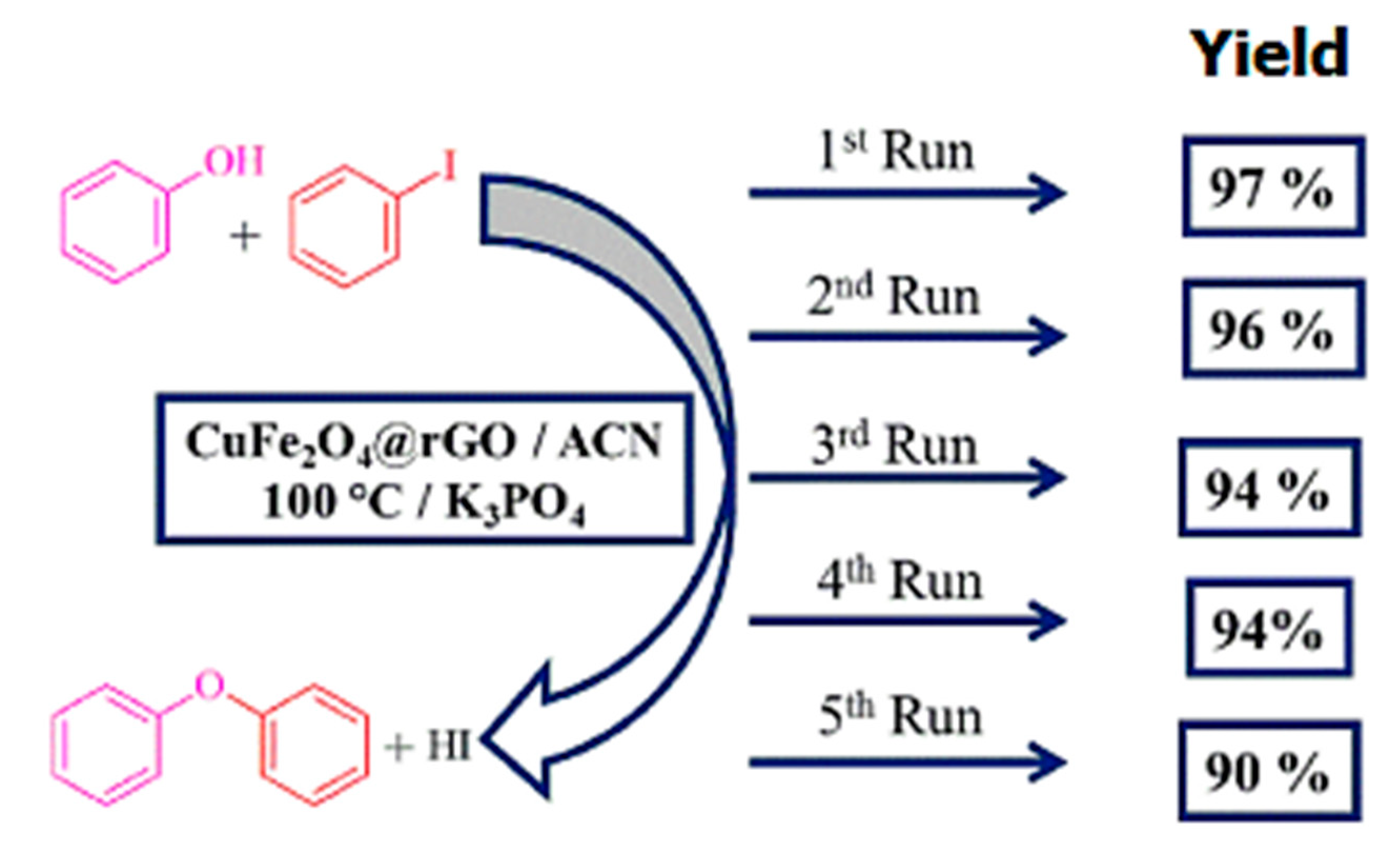
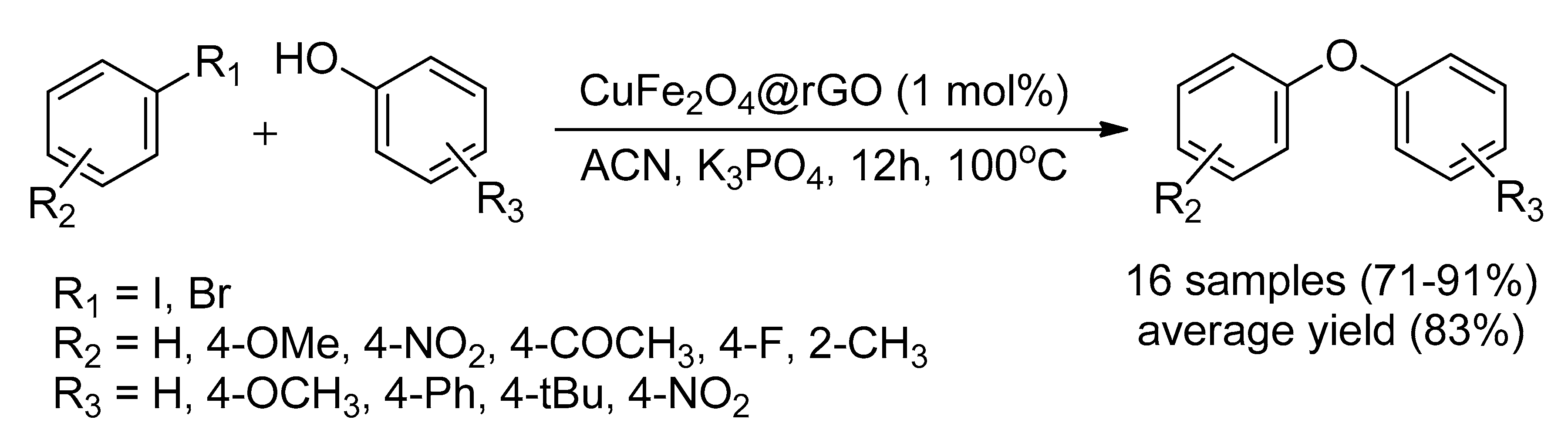
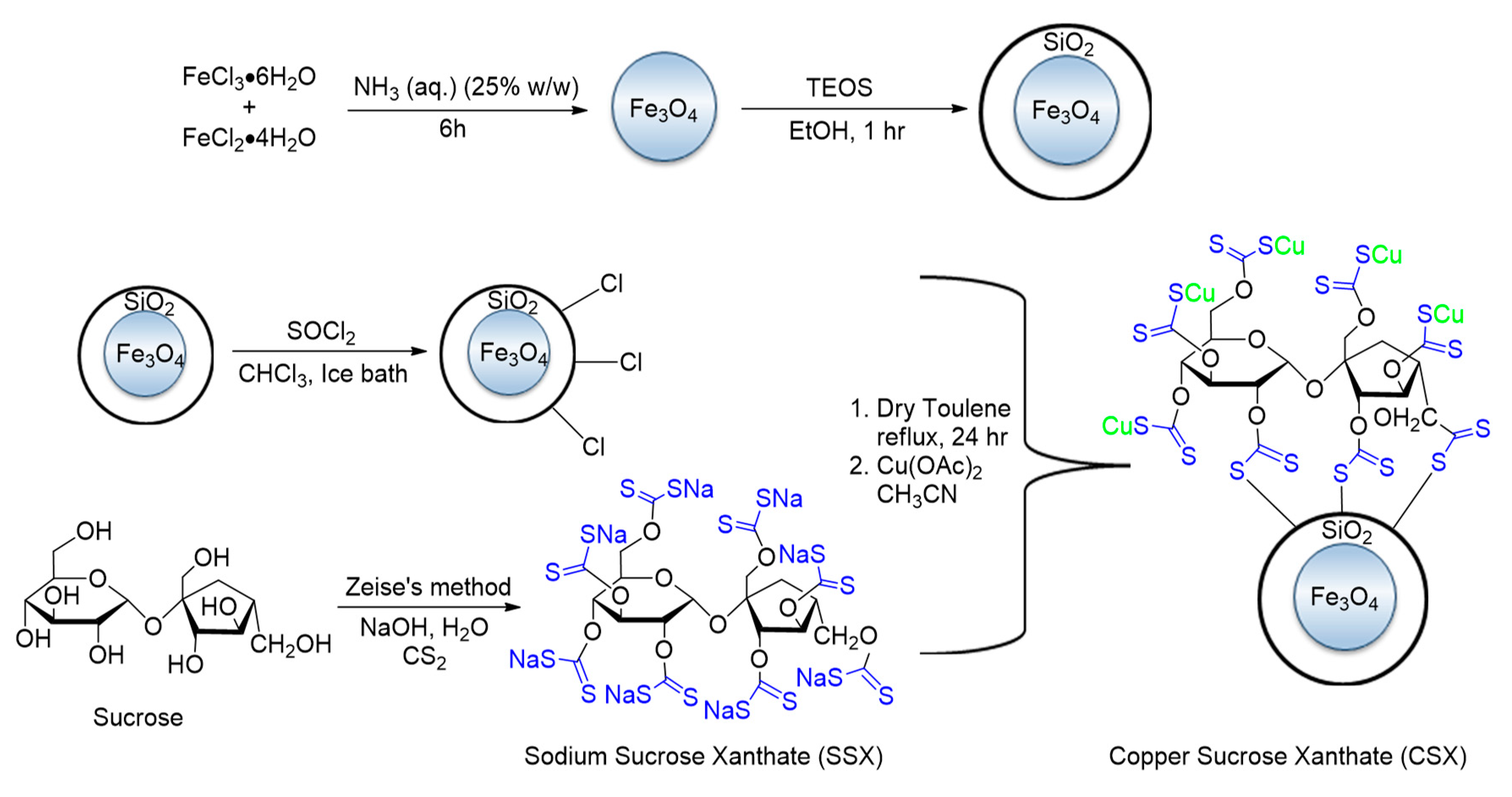

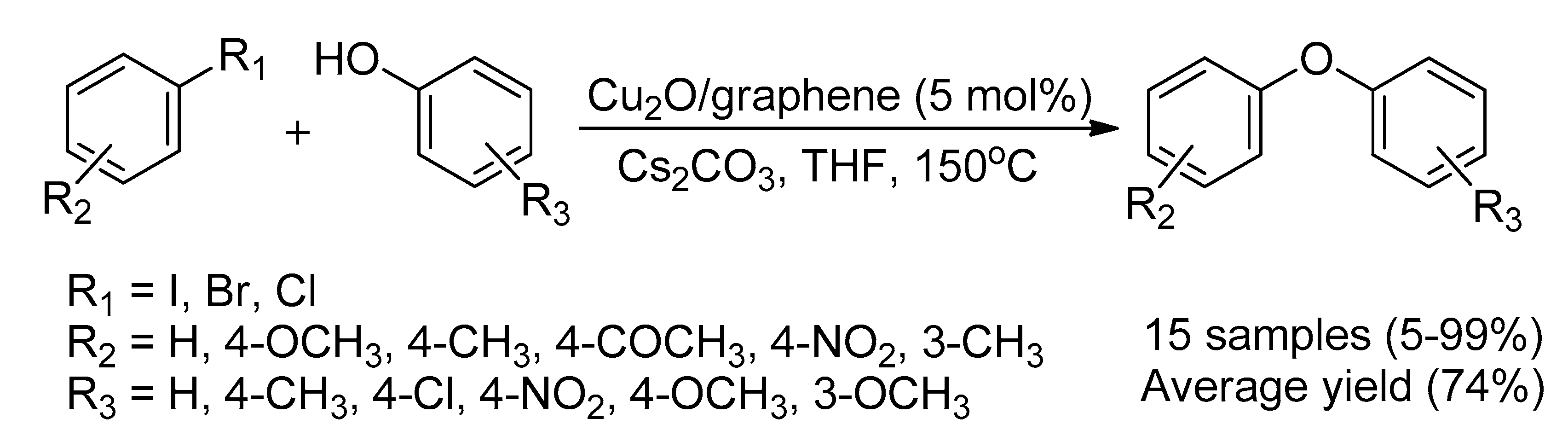
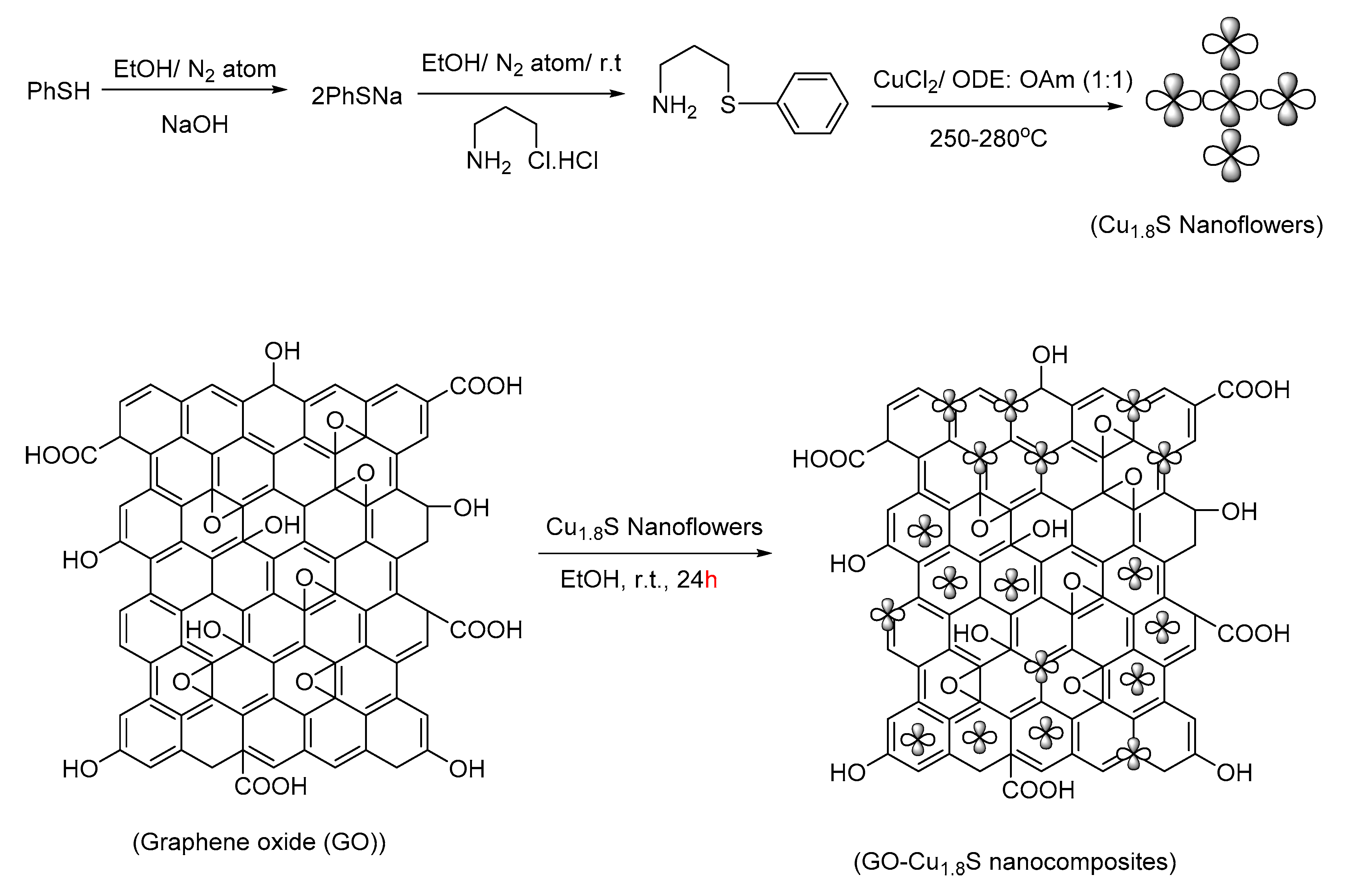
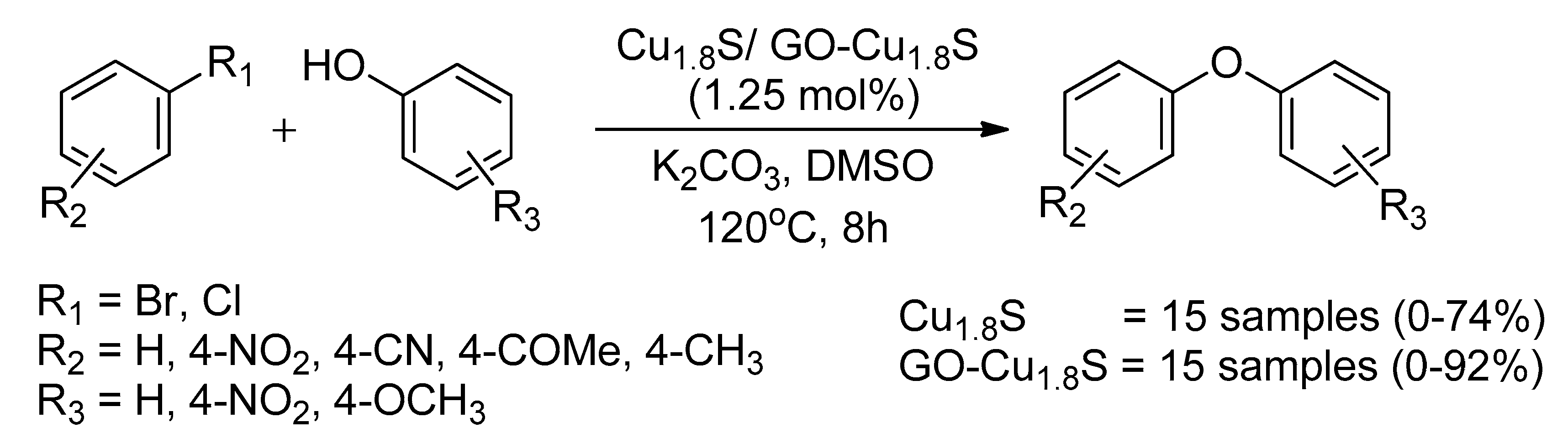






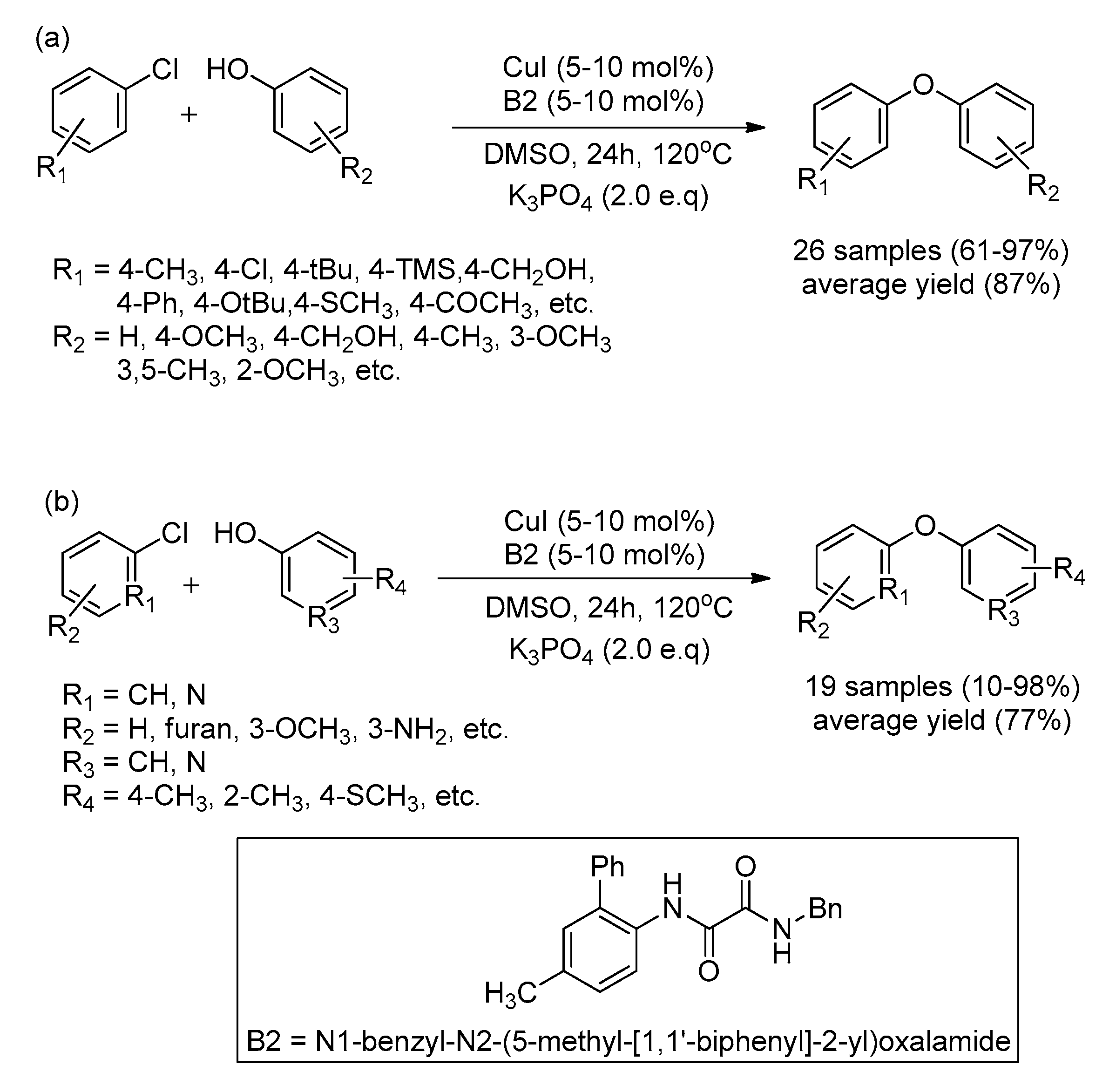
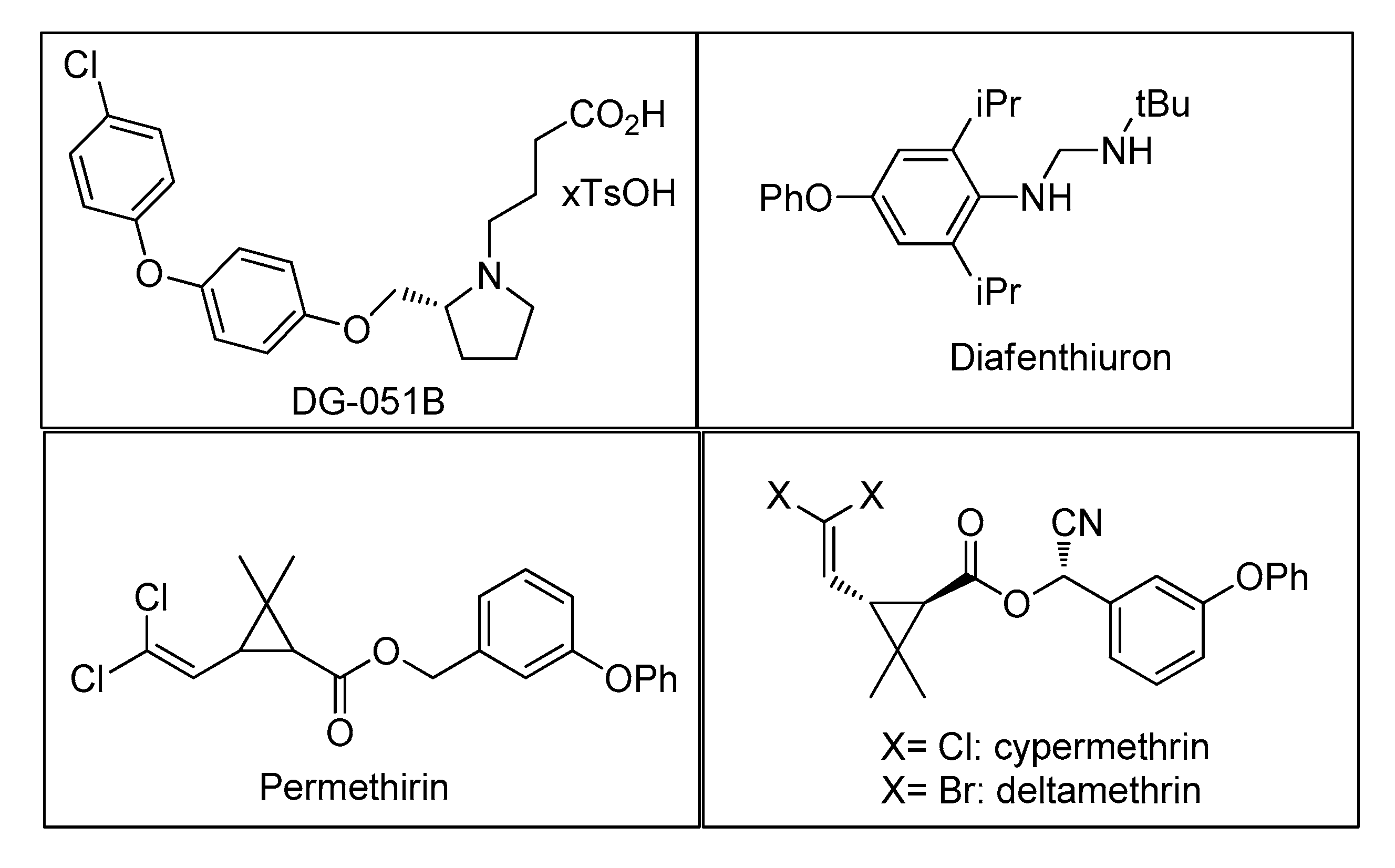
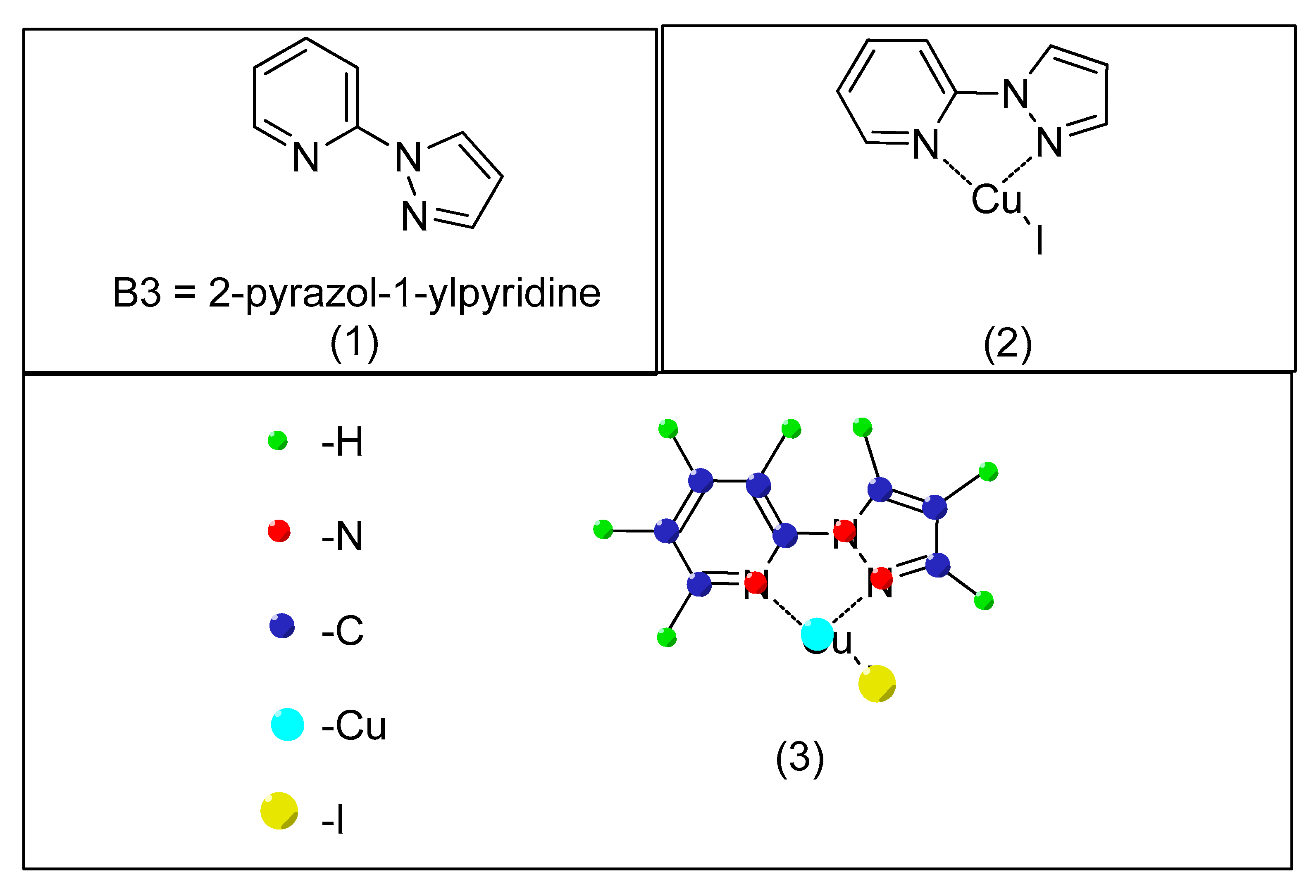
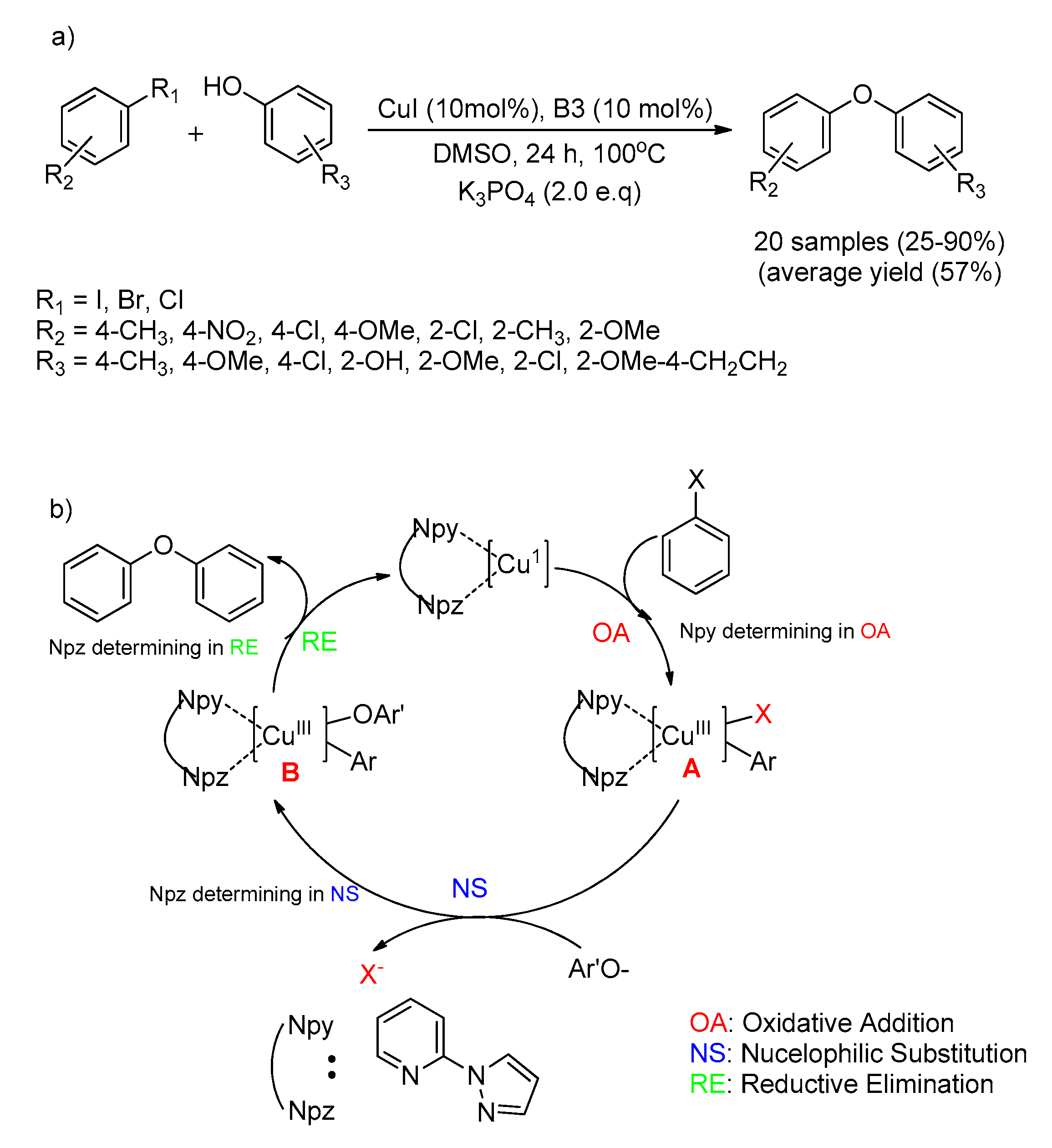
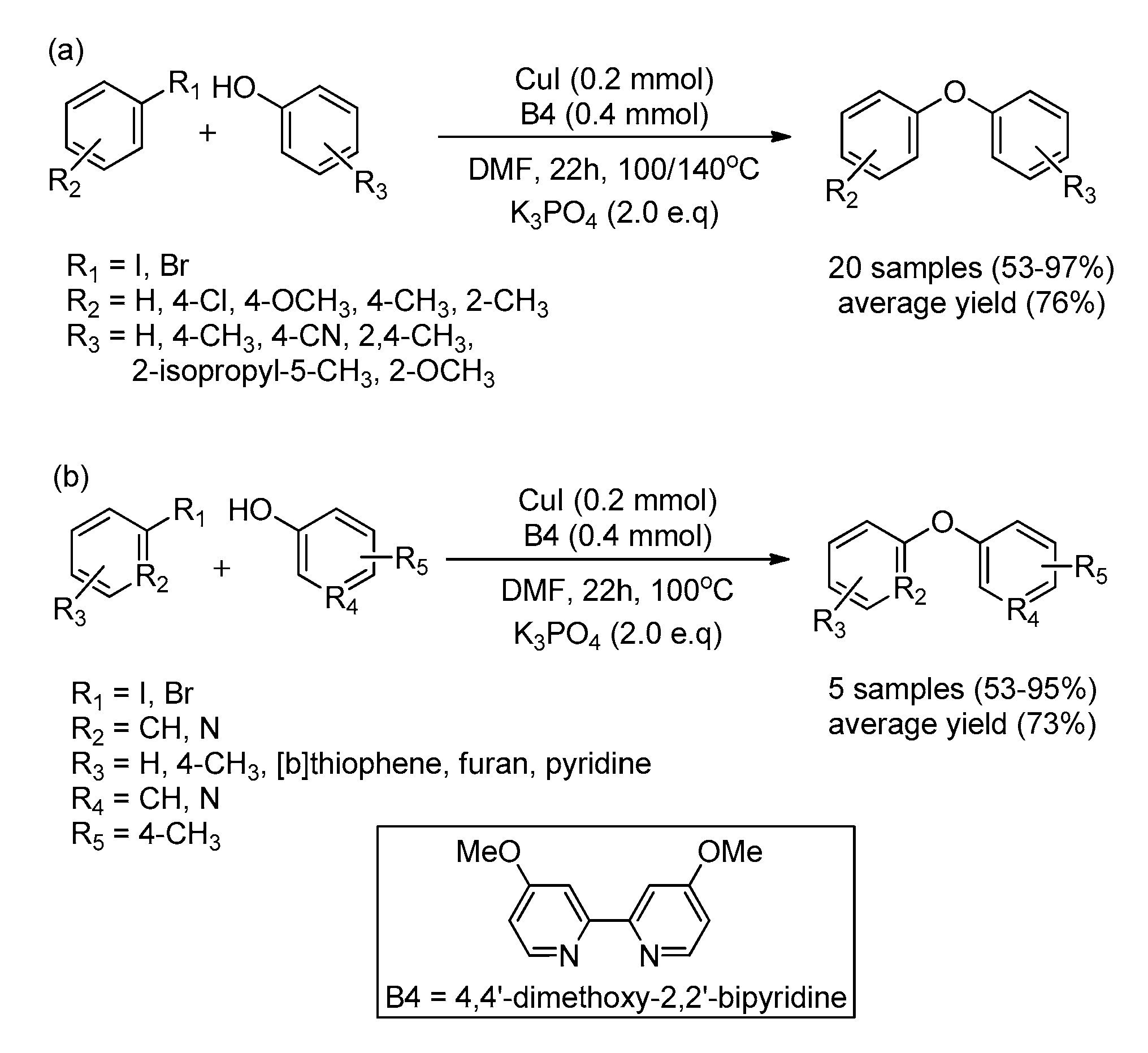
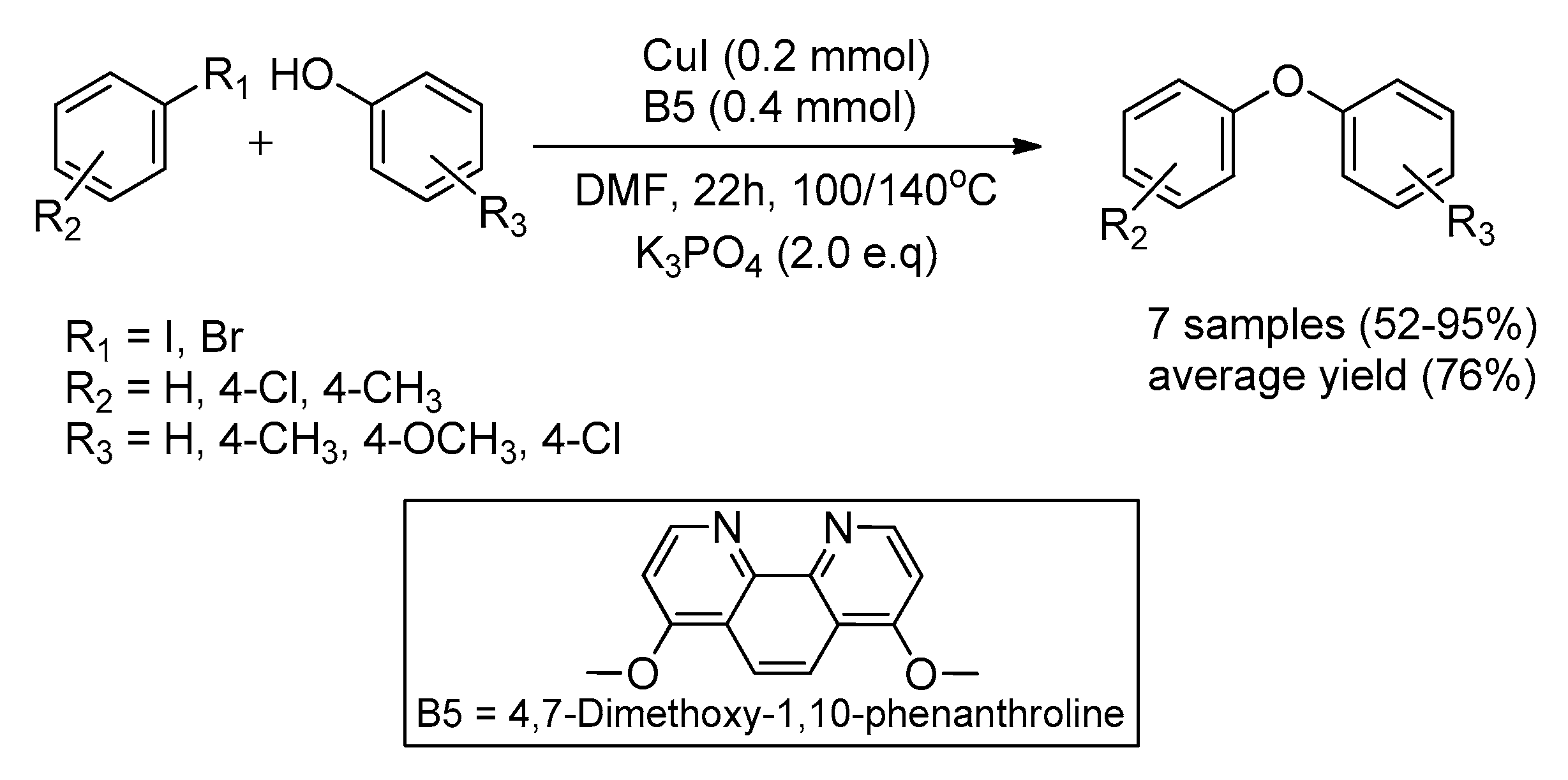
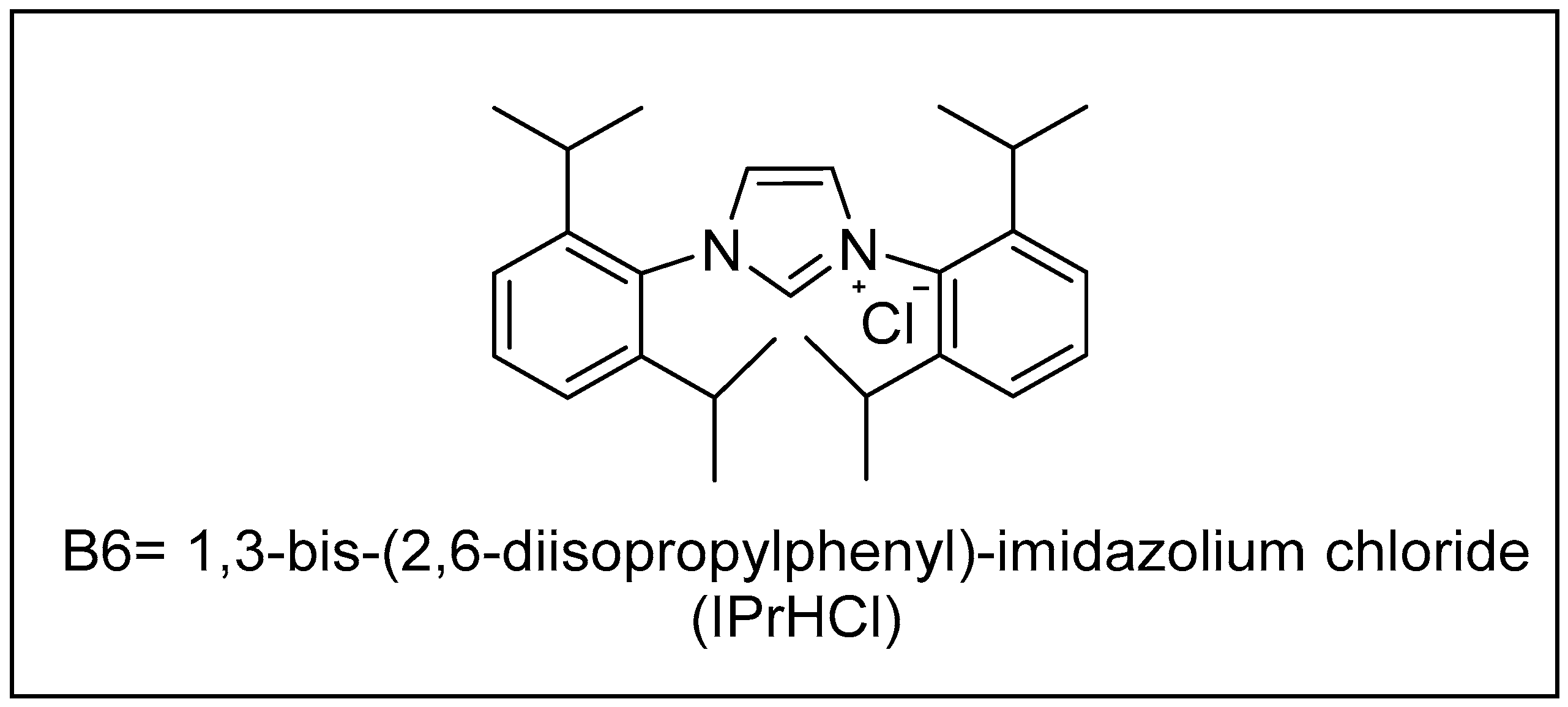
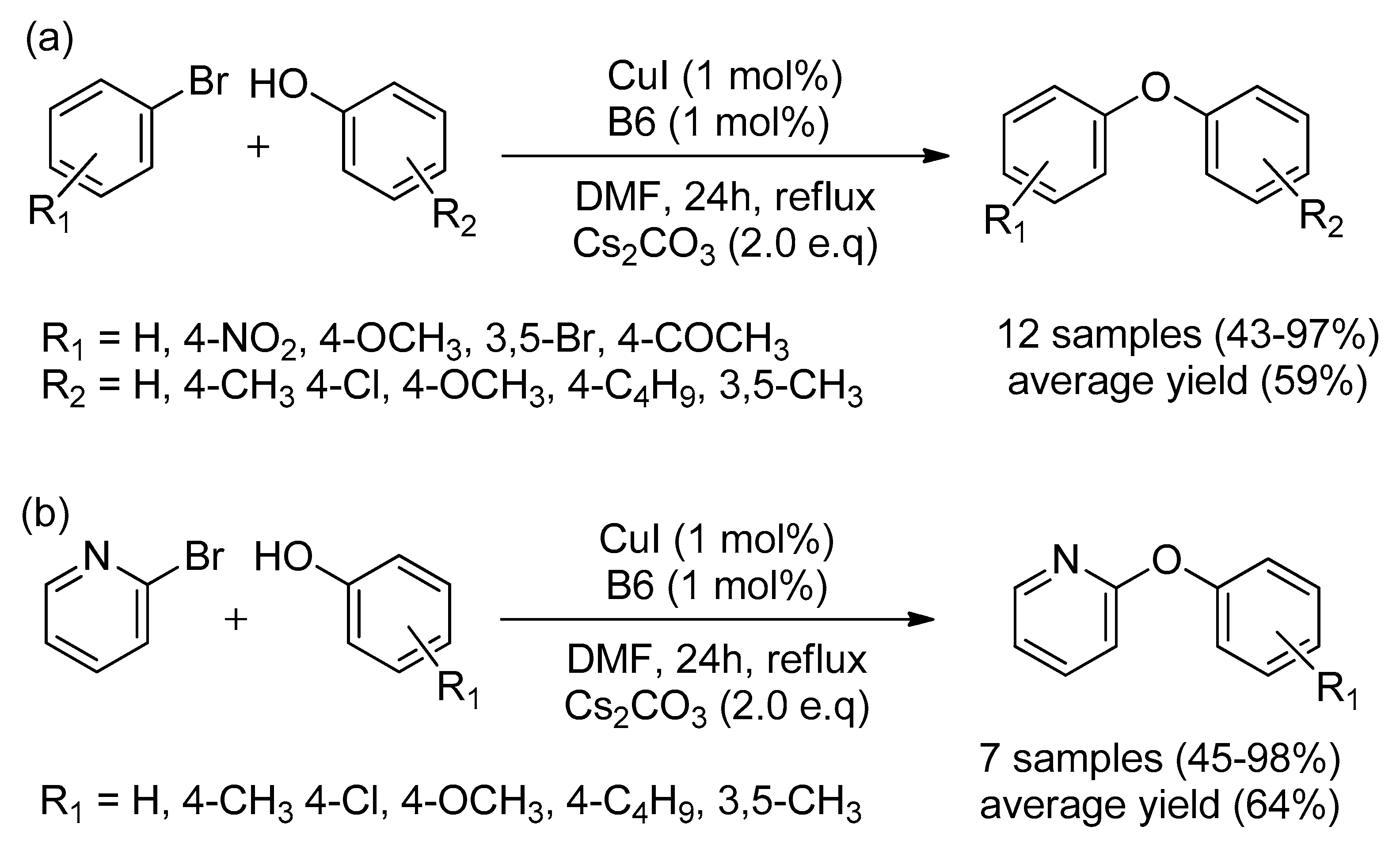


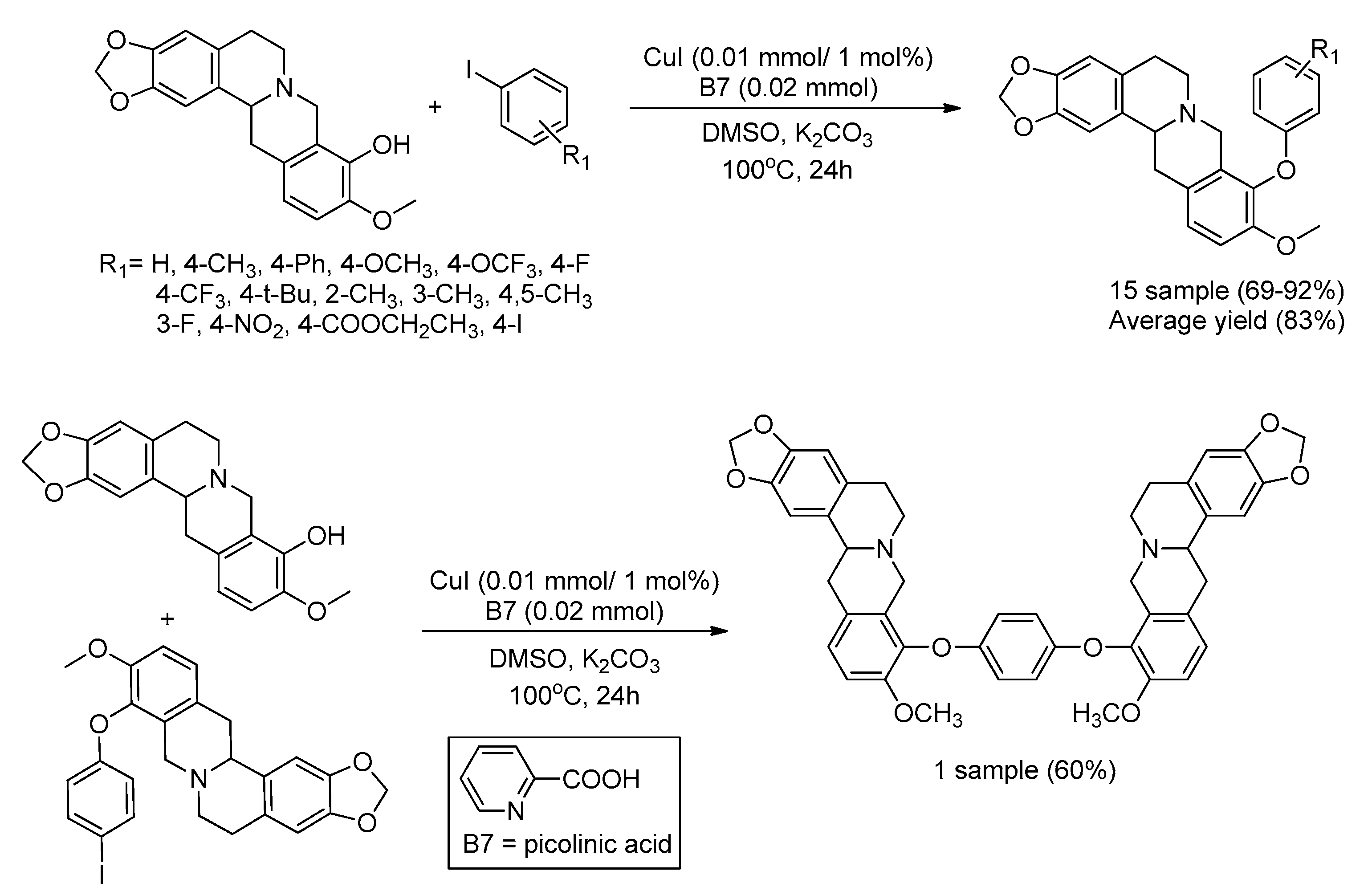

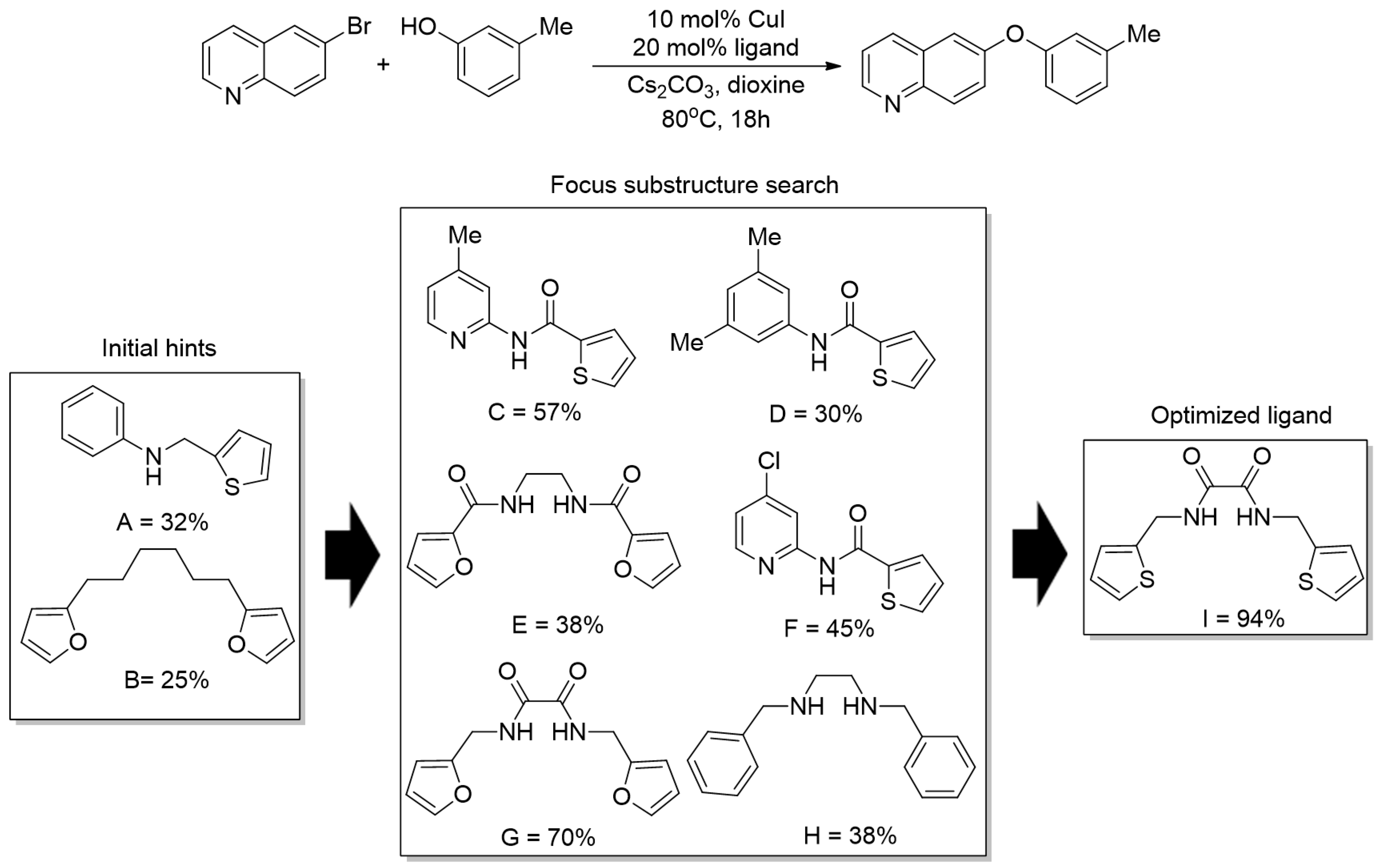
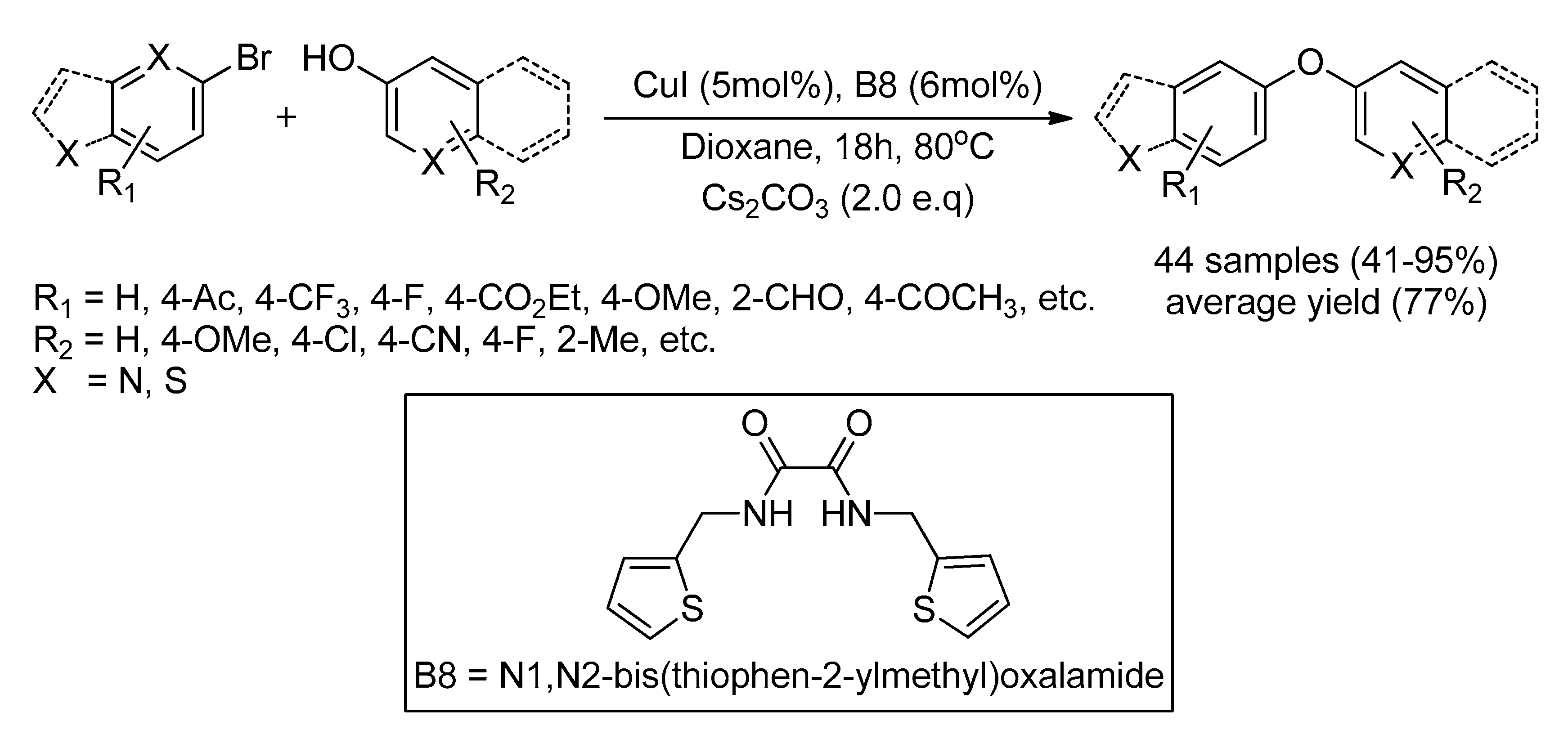
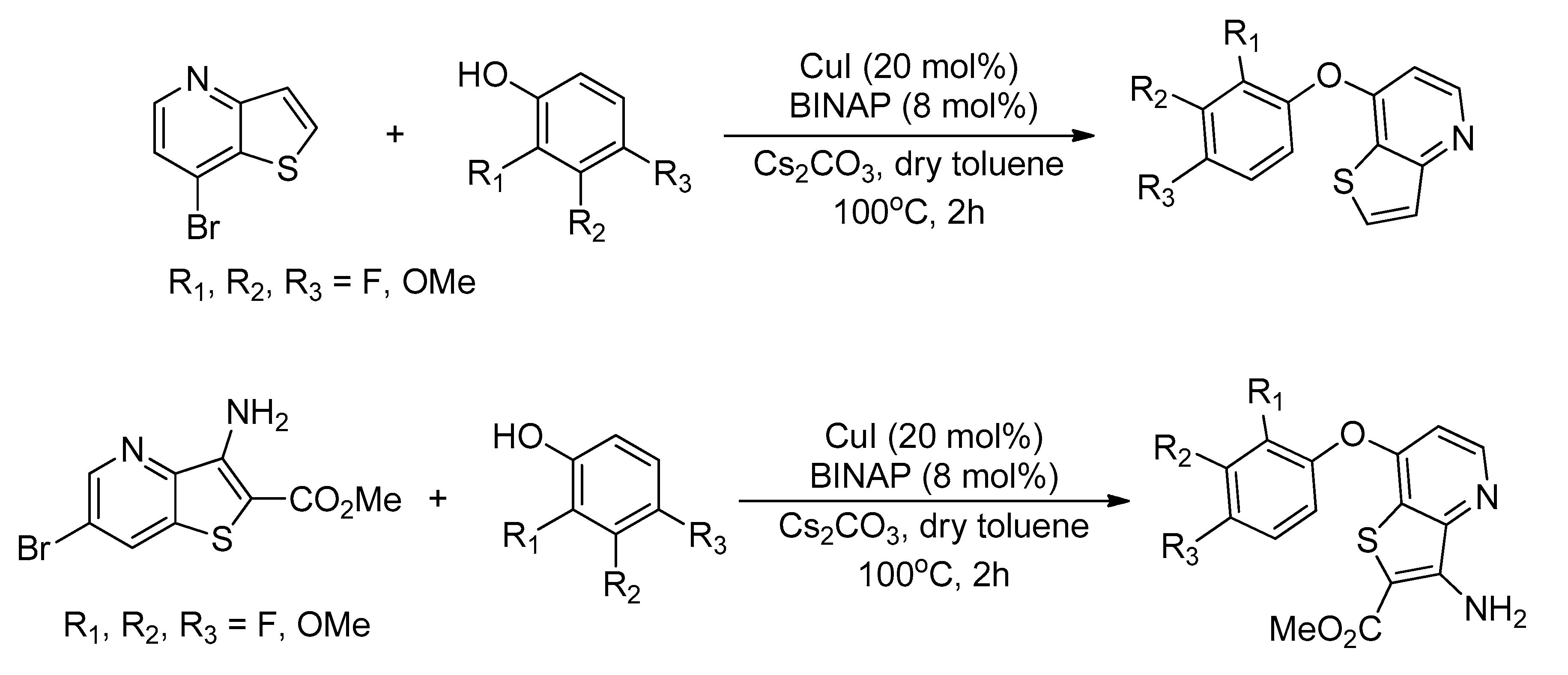
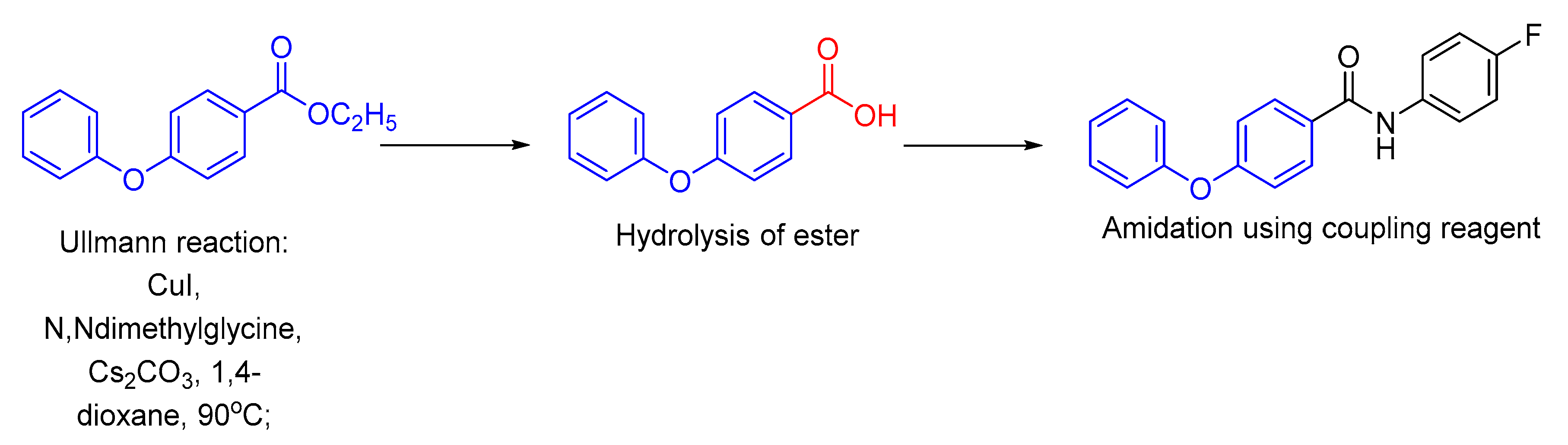
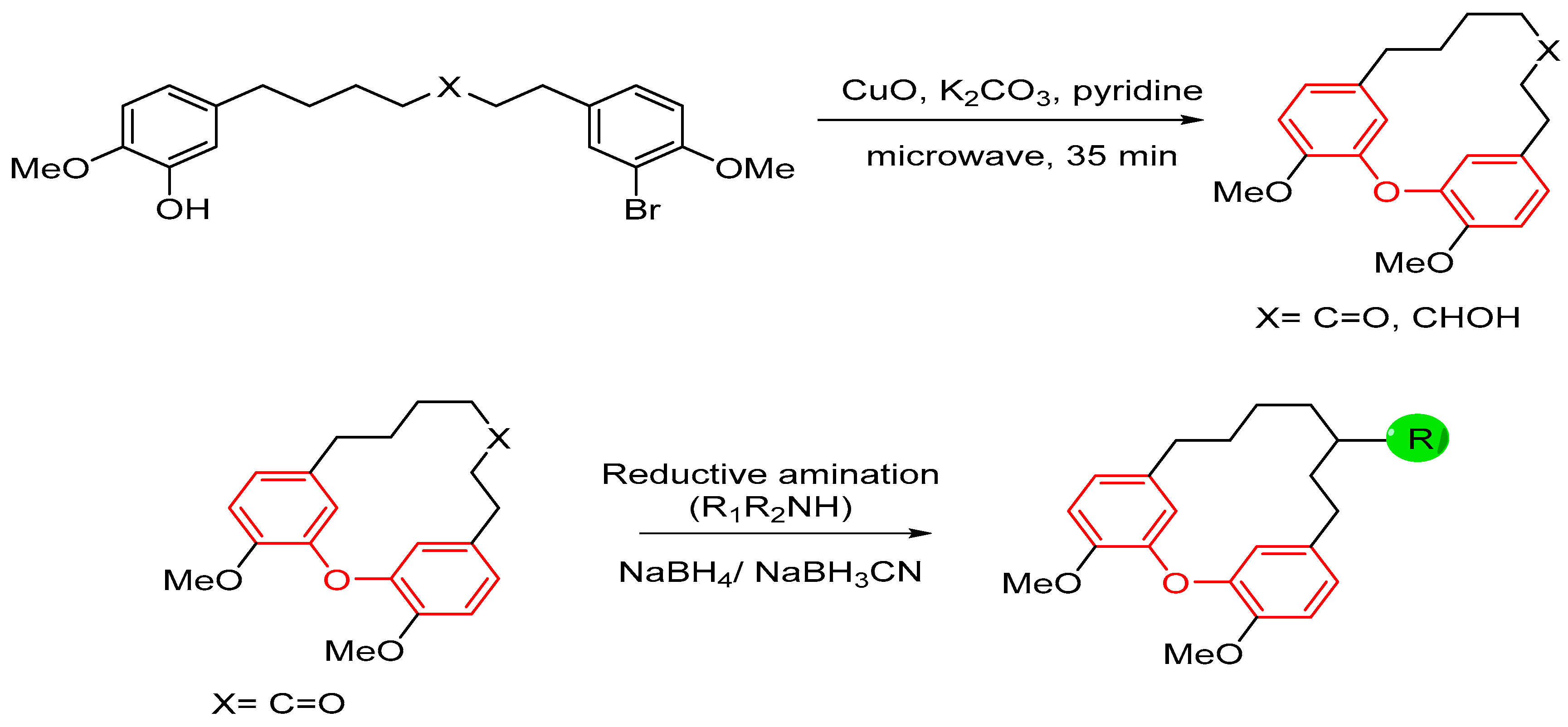
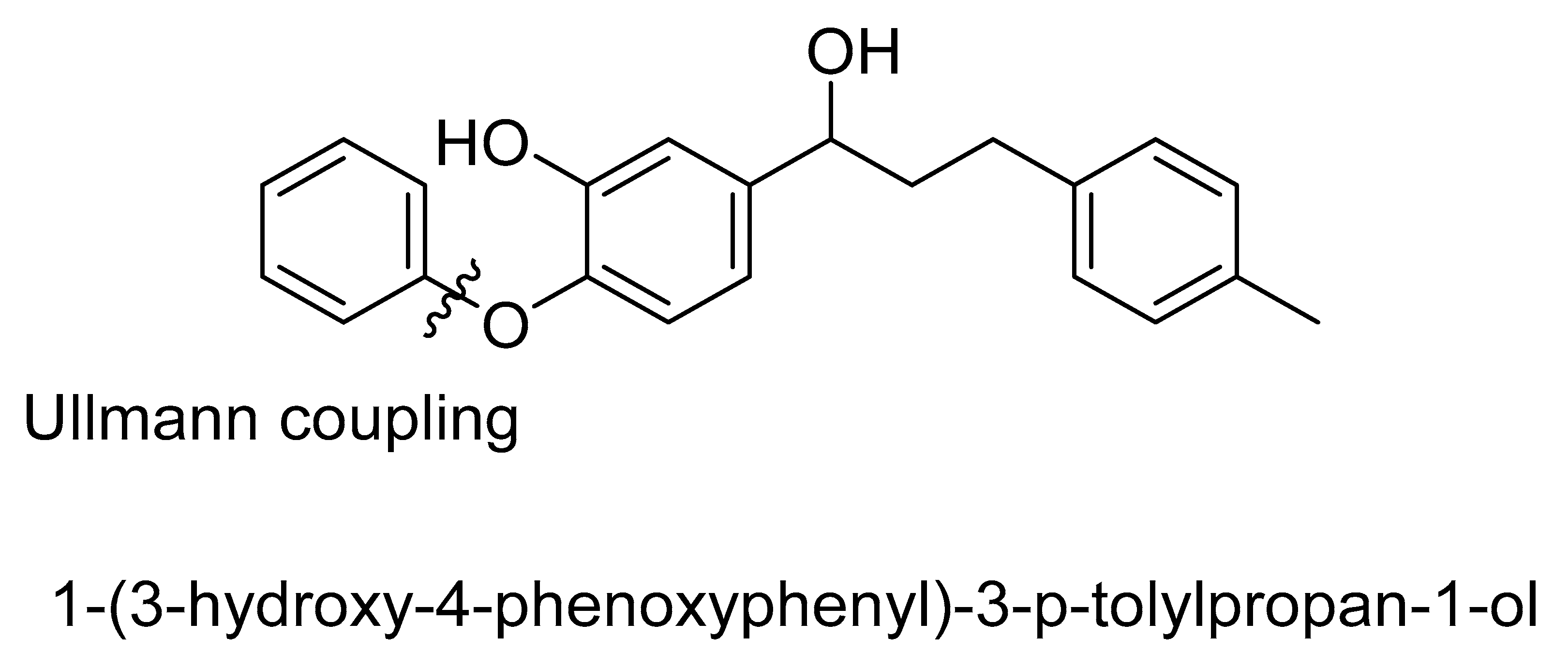
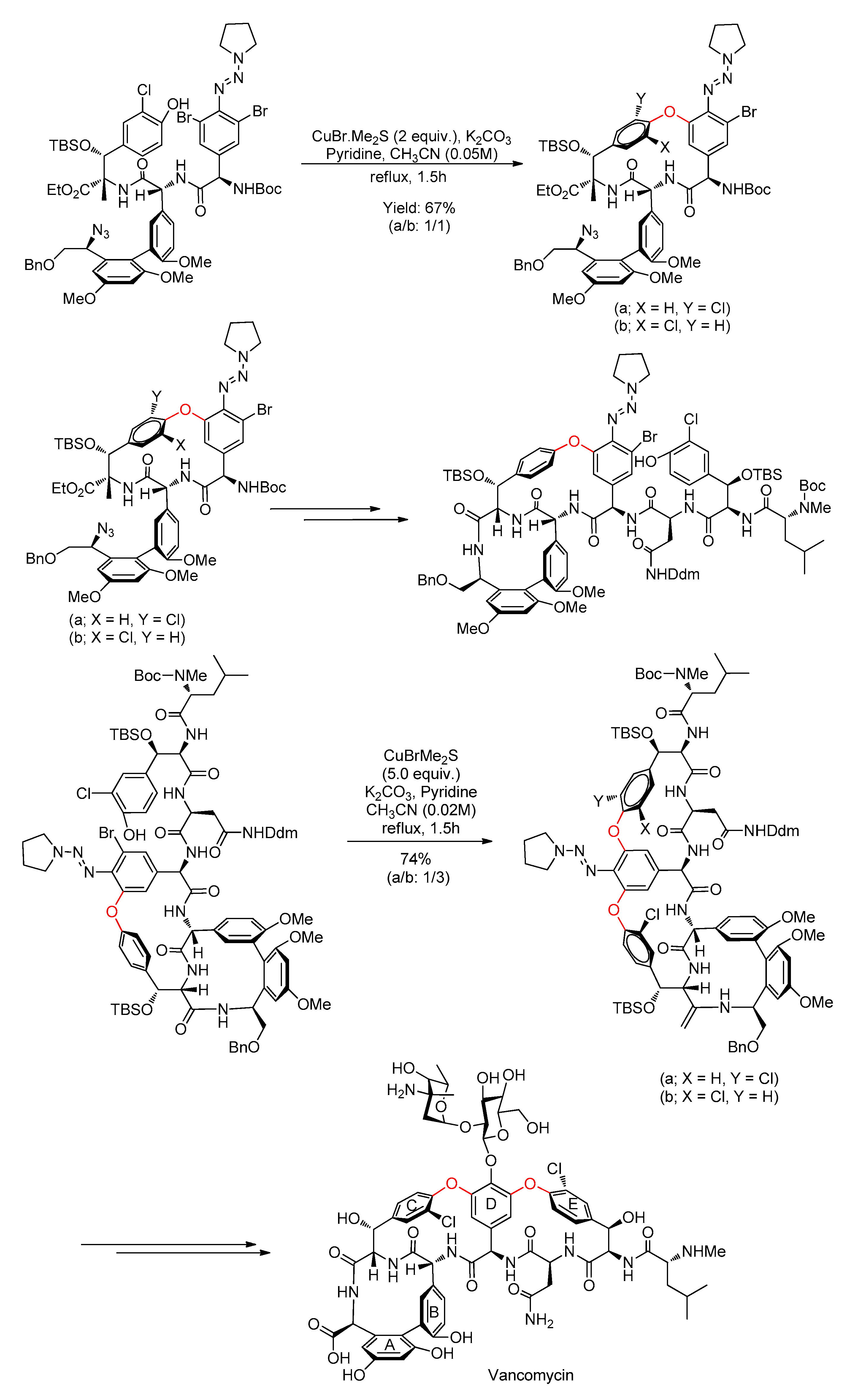
| Bond Type | Author | Year | Catalyst Source | Temperature | Ref. |
|---|---|---|---|---|---|
| C-C | Ullmann | 1901 | Copper powder (stoichiometric) | 200 °C | [12] |
| C-N | Ullmann | 1903 | Copper (stoichiometric) | Reflux | [19] |
| C-O | Ullmann | 1905 | Copper | 200 °C | [20] |
| C-N | Goldberg | 1906 | Copper | Reflux | [21] |
| C-C | Hurtley | 1929 | Copper nanoparticle | Reflux | [22] |
| Entry | Synthesis Route |
|---|---|
| 1 |  |
| 2 | 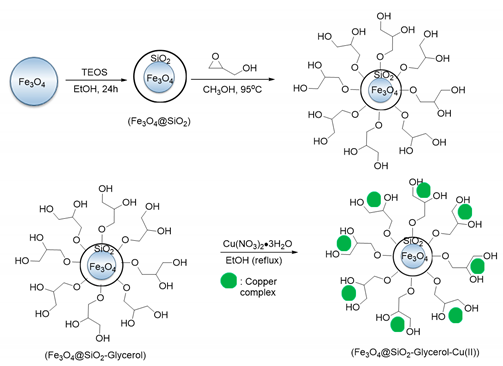 |
| 3 |  |
| 4 | 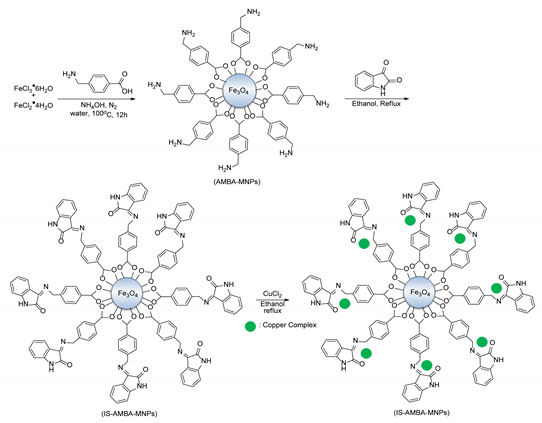 |
| 5 | 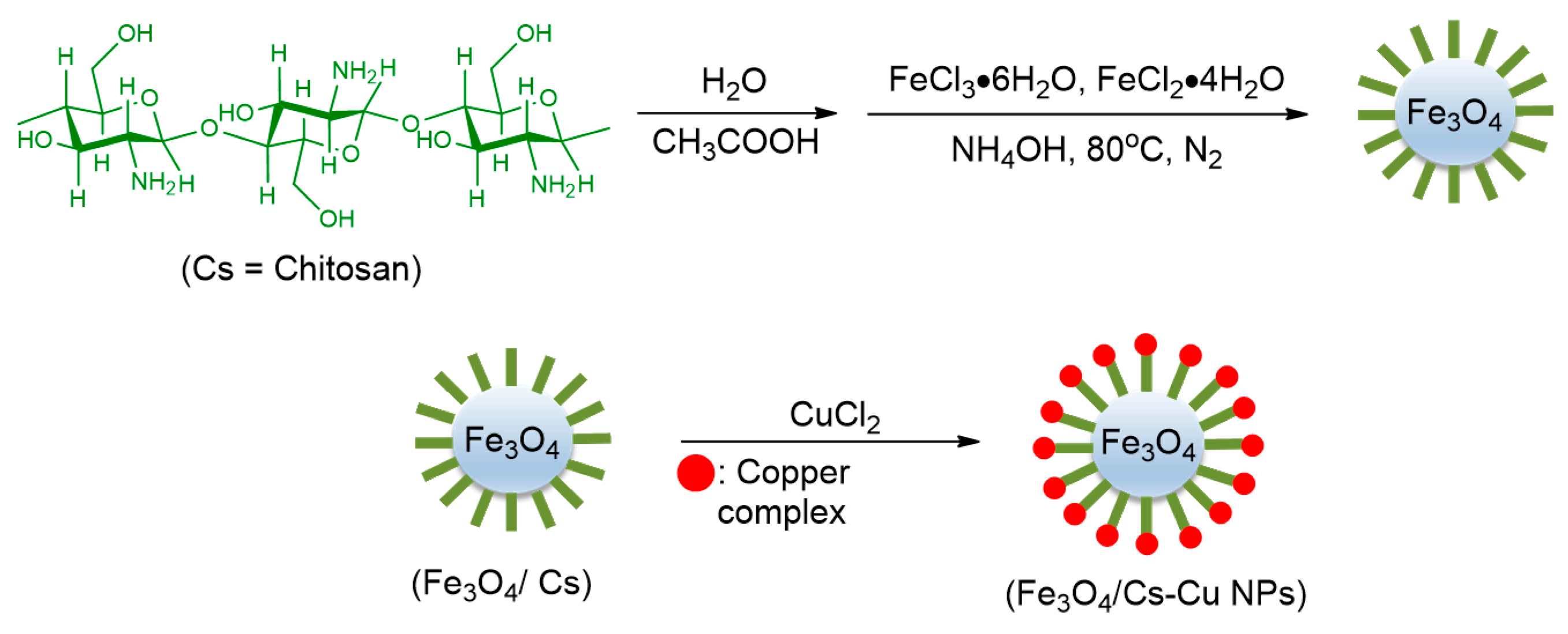 |
| 6 |  |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Type of Supported Catalyst | Substrates | Condition | Yield (%) | Reusability | Ref. | |
| Ar-X | Nucleophile | ||||||
| 1 | Creatine | Aryl-Br | Phenol | Glycerin, 24 h, 80 °C, K2CO3 | 35–80 | Up to 5 times | [59] |
| 2 | Glycerol | Aryl-I, Br, Cl | Phenol | H2O, 5 min-18 h, reflux, KOH | I-84–97 Br-62–91 Cl-38–86 | Up to 5 times | [60] |
| 3 | Mesoporous graphitic carbon nitrile (mpg-C3N4) | Aryl- I, Br, Cl | Phenol | DMF, 5 h, 110 °C, K2CO3 | I-75–90 Br-77–82 Cl-33–40 | Up to 5 times | [61] |
| 4 | Isatin@4-(aminomethyl) benzoic acid- functionalized (IS-AMBA) | Aryl-I, Br | Phenol | DMF, 4 h, 110 °C, K2CO3 | I-21–98 Br-30–63 | Up to 5 times | [62] |
| 5 | Chitosan | Aryl-I | Phenol | DMSO, 15 h, 120 °C, K2CO3 | 55–95 | Up to 5 times | [63] |
| 6 | Covalent anchoring of the ligand (AS) | Aryl-Br | Phenol | DMF, 24 h, reflux, Cs2CO3 | 45–98 | Up to 4 times | [64] |
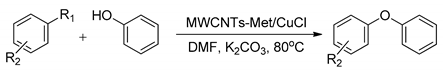 | ||||
|---|---|---|---|---|
| Type of Supported Catalyst | Substrates | Yield (%) | Reusability | |
| Ar-R1, R2 | Nucleophile | |||
| MWCNTs-Met/CuCl | R1 = I, Br, Cl R2 = H, 4-CN, 4-CH3, 4-OCH3, 2-OCH3 | Phenol | 55–96 | Up to 8 times |
| Characteristics | Nanocyl™ NC7000 | In-House MWCNTs | TUBALL™ SWCNTs |
|---|---|---|---|
| Average outer diameter, nm | 9.5 | 60–70 | 1.6 |
| Average length, µm | 1.5 | 200 | >5 |
| Aspect ratio | 150 | 3000 | 3000 |
| Carbon purity, wt.% | 90 | 98 | 85 |
| Fe-base catalyst residue, wt. % | <1 | 5.4 | <1.5 |
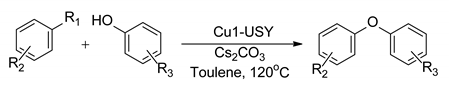 | |||||
|---|---|---|---|---|---|
| Type of Supported Catalyst | Substrates | Yield (%) | Reusability | Ref. | |
| Ar-R1, R2 | Nucleophile | ||||
| Cu1-USY composite catalysts | R1 = I, Br R2 = H, 4-OEt, 4-NEt, 4-CN, 4-NO2, 2-CH3, 2,6-CH3 | R3 = H, 3,5-CH3, 4-CN, 4-NO2, 4-CH3 | 0–85% | Up to 5 times | [93] |
| R1 = I, Br R2 = H, 4-OEt, 4-NEt, 4-CN, 4-NO2, 2-CH3, 2,6-CH3, etc. | R3 = H, 3,5-CH3, 4-CN, 4-NO2, 4-CH3, etc. | 4–85% | [94] | ||
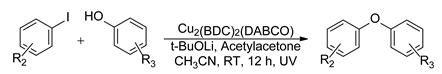 | ||||
|---|---|---|---|---|
| Type of Supported Catalyst | Substrates | Yield (%) | Reusability | |
| Ar-R2 | Nucleophile | |||
| Cu2(BDC)2(DABCO) | R2 = H, 4-Me, 4-OCH3, 4-COCH3, 4-CN, 4-I, 2-Me, 3-F | R3 = H, 4-Br, 3-NO2, 4-COOMe, 2-OMe, 3-OH | 42–81 | Up to 7 times |
| Entry | Catalytic System | Centre | Catalyst Loading | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Fe3O4@SiO2@PPh2@Pd | Pd/Fe | 1.6 × 10−3 mol% | 83 | [109] |
| 2 | AT-Nano CP-Pd | Pd | 6 × 10−5 mol% | 80 | [110] |
| 3 | |||||
| 4 | Pd(dba)2 | Pd | 1 mol% | 90 | [111] |
| 5 | GO-Pd17Se15 | Pd | 1 mol% | 73 | [112] |
| 6 | Fe3O4@mesoporous PANI | Fe | 4.69 mol% | 56 | [113] |
| 7 | CuI/Oxalamide | Cu | 1.5 mol% | 90 | [114] |
| 8 | CuBr | Cu | 10 mol% | 81 | [115] |
| 9 | CuI nanoparticles | Cu | 1.25 mol% | 47 | [116] |
| 10 | CuI/Raney Ni-Al alloy | Cu/Ni | 10 mol% | 32 | [117] |
| 11 | Cu2O/graphene | Cu | 5 mol% | 5 | [76] |
| 12 | Nano CuO | Cu | 5 mol% | 17 | [42] |
| 13 | CuO NPs into UiO-66-NH2 | Cu | 5 × 10−3 mol% | 30 | [43] |
| 14 | AgOAc | Ag | 0.5–2 mol% | 81 | [118] |
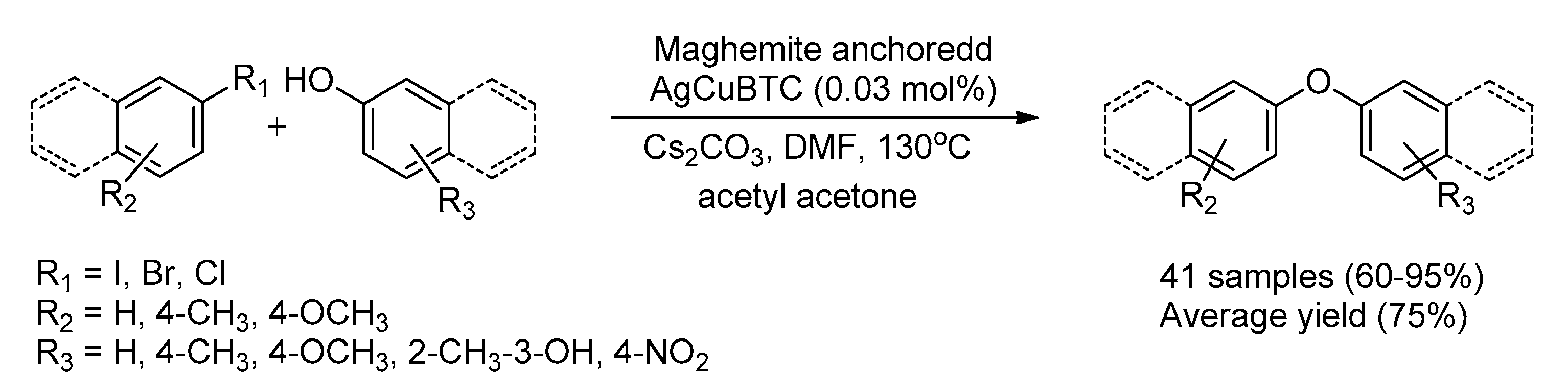
| 15 | Maghemite anchored AgCuBTC | Ag/Cu | 0.03 mol% | 88 | [108] |
| 16 | Cu/Fe/O = PPh3 | Cu/Fe | 5 mol% | 84 | [107] |
| R-X | R-OH | Condition | Diaryl Ether | Natural Product |
|---|---|---|---|---|
 | 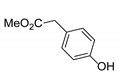 | CuO (28 mol%), K2CO3, Pyridine, 150 °C, 4 h |  |  |
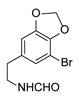 | 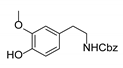 | CuO (26 mol%), K2CO3, Pyridine, 150 °C, 4 h | 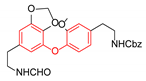 |  |
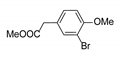 | 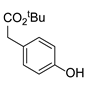 | CuO (15 mol%), K2CO3, Pyridine, 150 °C, 4h |  | |
 | 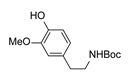 | CuO (2 equiv.), K2CO3, Pyridine, 130 °C, 16 h |  | |
 |  | CuO (2 equiv.), K2CO3, Pyridine, 130 °C, 5 h |  |  |
| Compound | Condition | Structure |
|---|---|---|
| K-13 | CuO (2.0 equiv.), K2CO3, pyridine, 145 °C, 24 h ≫91% (from bromide) |  |
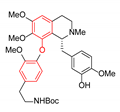
| OF4949-III | CuO (2.0 equiv.), K2CO3, pyridine, 130–145 °C, 12–24 h ≫91–93% (from bromide) | 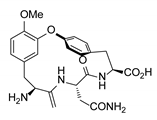 |
| Perrottetin E | CuO, K2CO3, pyridine, reflux, 24 h ≫60% (from bromide) |  |
| Marchantin I | CuO (6.0 equiv.), K2CO3, pyridine, reflux, 24 h ≫60% (from bromide) |  |
| Ornatipolide | CuO (2.5 equiv.), K2CO3, pyridine, 135 °C, 18 h ≫82% (from bromide) |  |
| Retipolide E | CuO (2.5 equiv.), K2CO3, pyridine, 135 °C, 18 h ≫82% (from bromide) | 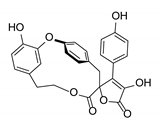 |
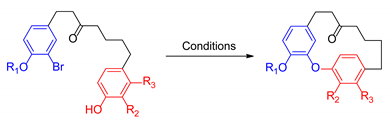 | ||||
|---|---|---|---|---|
| Substituents | Conditions | Yield | Precursor to | Ref. |
| R1 = Me R2 = R3 = H | CuO (2.5 equiv.), K2CO3, pyridine (0.02 M), 90 °C, 48 h | 49% | Acerogenin L (R1 = R2 = R3 = H) | [141] |
| R1 = iPr R2 = R3 = H | CuO (2.5 equiv.), Cs2CO3, pyridine (0.007 M), reflux, 48 h | 81% | Acerogenin L (R1 = R2 = R3 = H) | [142] |
| R1 = Bn R2 = OMe R3 = H | CuO (2.5 equiv.), K2CO3, pyridine (0.02 M), 90 °C, 48 h | 52% | (±)-Galeon (R1 = R3 = H, R2 = OMe) | [141] |
| R1 = iPr R2 = OMe R3 = H | CuO (2.5 equiv.), Cs2CO3, pyridine (0.007 M), reflux, 48 h | 73% | (±)-Pterocarine = (±)-Engelhardione (R1 = R3 = H, R2 = OH) | [142] |
| R1 = H R2 = OMe R3 = H | CuO (10.0 equiv.), K2CO3, pyridine (0.1 M), 200 °C, 30 h | 13% | (±)-Galeon (R1 = R3 = H, R2 = OMe) Direct Obtained | [143] |
| R1 = Me R2 = OMe R3 = H | CuO (2.5 equiv.), K2CO3, pyridine (0.02 M), 175 °C, 4.5 h | 54% | (±)-Pterocarine = (±)-Engelhardione (R1 = R3 = H, R2 = OH) | [144] |
| CuO (2.5 equiv.), K2CO3, pyridine (0.01 M), 220 °C (Microwave), 35 min | 85% | [145] | ||
| CuO (10.0 equiv.), K2CO3, pyridine (0.1 M), 200 °C, 30 h | 12% | (±)-Platycarynol (R1 = Me, R2 = OMe, R3 = H, alcohol instead of ketone) | [143] | |
| R1 = iPr R2 = OMe R3 = OiPr | CuO (2.5 equiv.), Cs2CO3, pyridine (0.007 M), reflux, 69 h | 74% | (±)-Myricatomentogenin (R1 = H, R2 = OMe, R3 = OH) | [142] |
| R1 = Me R2 = OMe R3 = OiPr | CuO (2.5 equiv.), Cs2CO3, pyridine (0.007 M), reflux, 48 h | 63% | (±)-Jugcathanin (R1 = Me, R2 = OMe, R3 = OH) | [142] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fui, C.J.; Sarjadi, M.S.; Sarkar, S.M.; Rahman, M.L. Recent Advancement of Ullmann Condensation Coupling Reaction in the Formation of Aryl-Oxygen (C-O) Bonding by Copper-Mediated Catalyst. Catalysts 2020, 10, 1103. https://doi.org/10.3390/catal10101103
Fui CJ, Sarjadi MS, Sarkar SM, Rahman ML. Recent Advancement of Ullmann Condensation Coupling Reaction in the Formation of Aryl-Oxygen (C-O) Bonding by Copper-Mediated Catalyst. Catalysts. 2020; 10(10):1103. https://doi.org/10.3390/catal10101103
Chicago/Turabian StyleFui, Choong Jian, Mohd Sani Sarjadi, Shaheen M. Sarkar, and Md Lutfor Rahman. 2020. "Recent Advancement of Ullmann Condensation Coupling Reaction in the Formation of Aryl-Oxygen (C-O) Bonding by Copper-Mediated Catalyst" Catalysts 10, no. 10: 1103. https://doi.org/10.3390/catal10101103
APA StyleFui, C. J., Sarjadi, M. S., Sarkar, S. M., & Rahman, M. L. (2020). Recent Advancement of Ullmann Condensation Coupling Reaction in the Formation of Aryl-Oxygen (C-O) Bonding by Copper-Mediated Catalyst. Catalysts, 10(10), 1103. https://doi.org/10.3390/catal10101103





