Abstract
The electrochemical reduction of CO2 is a promising way to recycle it to produce value-added chemicals and fuels. However, the requirement of high overpotential and the low solubility of CO2 in water severely limit their efficient conversion. To overcome these problems, in this work, a new type of electrolyte solution constituted by ionic liquids and propylene carbonate was used as the cathodic solution, to study the conversion of CO2 on an Ag electrode. The linear sweep voltammetry (LSV), Tafel characterization and electrochemical impedance spectroscopy (EIS) were used to study the catalytic effect and the mechanism of ionic liquids in electrochemical reduction of CO2. The LSV and Tafel characterization indicated that the chain length of 1-alkyl-3-methyl imidazolium cation had strong influences on the catalytic performance for CO2 conversion. The EIS analysis showed that the imidazolium cation that absorbed on the Ag electrode surface could stabilize the anion radical (CO2•−), leading to the enhanced efficiency of CO2 conversion. At last, the catalytic performance was also evaluated, and the results showed that Faradaic efficiency for CO as high as 98.5% and current density of 8.2 mA/cm2 could be achieved at −1.9 V (vs. Fc/Fc+).
1. Introduction
The unprecedented increase of CO2 concentration in the atmosphere has led to many concerns about global warming, and even predictable environmental disasters, which make us feel urged to limit the CO2 emission and effectively utilize them [,,,]. The catalytic transformation of CO2 into value-added chemicals and fuels has been regarded as one of the most promising ways to realize the valorization of CO2 []. In this context, several strategic options, including electrochemical [,,,,,,], thermochemical [,,], photocatalytic [,,] and photoelectrochemical [,,,] approaches, have been developed to undertake the CO2 conversion. Among them, the electrochemical reduction of CO2 is regarded as the most prospective way, because it allows one to combine with carbon capture and storage technology, and to utilize renewable energy (such as solar energy and wind energy), as inputting energy and water as a reductant to reduce CO2 into various carbon-based fuels and chemicals (e.g., CO, HCOOH, CH4, C2H4, and CH3OH) in a modular electrochemical reactor under ambient temperature and pressure [,,,,]. However, the linear CO2 molecule is thermodynamically stable and kinetically inert to be reduced, due to its low electron affinity and large energy gap between its highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) []. It has been reported that the main hurdle in CO2 electroreduction lay in the first-step one-electron reduction of CO2 to form an anion radical (CO2•−), because this activation step requires a much high reduction potential of −1.9 V (vs. NHE) [,]. Therefore, to accelerate this process, considerable efforts have been devoted to study the electrocatalysts, because the structure of electrocatalysts provides active sites to activate the reactant of CO2, and great progress has been witnessed in recent years [].
While the electrocatalysts are important in research efforts, the electrolyte on the other side plays an equally pivotal role in catalysis, by interacting with the reactant and intermediate species, ultimately influencing the overall reduction reaction [,]. In this aspect, ionic liquids (ILs), which are composed of relatively large organic cations and small inorganic anions, have shown their advantages in the reduction of CO2, and have been extensively studied in recent years [,,,,,,,,]. The first consideration is that ILs have a high capacity for CO2 capture [,] and unique electrochemical properties [,,,], such as wide electrochemical windows, high conductivity, and high stability. More importantly, ILs could interact with CO2 or the reaction intermediate species, and eventually improve the catalytic activity and influence the product selectivity [,,,,,,,,]. For example, by using the 18 mol% 1-ethyl-3-methylimidazolium tetrafluoroborate [Emim][BF4] aqueous solution as the cathode electrolyte, Rosen et al. [] reported that Faradaic efficiency (FE) greater than 96% for CO from CO2 could be achieved at very low overpotential. In the subsequent work [], they proposed that the formation of an adsorbed CO2–[Emim]+ complex provided a low-energy pathway for CO2 conversion to CO, accounting for the enhanced performance at the presence of ILs. In another ILs assistant CO2 conversion work, Sun et al. [] reported that the cation of 1-ethyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide [Emim][NTf2] played the role of stabilizing effect to prevent the close approach and dimerization between two CO2•− to form oxalate, and thus switched the C-derived product to CO. Furthermore, they proposed that the formation of an imidazolium carboxylate through the coordination of [Emim]+ with CO2•− appeared as a feasible pathway for the CO2 conversion to CO []. However, due to the vast number of ILs through different combinations of various cations and anions, an understanding of the interactions between ILs electrolyte and CO2 molecule or intermediate species, as well as the behind decisive role of ILs is necessary for properly selecting the ILs to enhance the efficiency for CO2 electroreduction.
Because of the high cost and high viscosity, ILs are often mixed with different molecular solvents of water or organic solvent, and then used as supporting electrolytes, as well as active co-catalysts [,,,,]. For water solution, the hurdles for efficient electrochemical conversion of CO2 stem from the low solubility of CO2 in aqueous solution, the complicated CO2 species in water, and the competitive H2 evolution from H2O reduction []. Compared with water, organic solvents have high solubility of CO2 and have been alternatively investigated as solvents of ILs for the conversion of CO2 [,,]. However, these widely investigated organic solvents, such as acetonitrile (AN), N,N-dimethylformamide (DMF), and dimethyl sulfoxide (DMSO), have some severe shortages in practical use. AN is unsuitable for practical use because of its high toxicity and volatility. DMF is also not an appropriate candidate, since it is prone to hydrolysis in water. As for DMSO, owing to its low melting point (18.4 °C), its utilization is highly restricted at ambient conditions. Therefore, the seeking of suitable liquid solvents that can be utilized in CO2 conversion is still a challenging and urgent task. In recent years, propylene carbonate (PC), as a polar aprotic liquid, has attracted much attention and has been regarded as a promising and “green” sustainable alternative solvent in various chemical and electrochemical transformations fields [,,]. This is mainly due to its wide electrochemical window and unique physicochemical properties, such as the low toxicity and vapor pressure, as well as the non-corrosive and biodegradable nature of PC. More importantly, PC also has a high capacity of CO2, which makes PC a promising alternative solvent to overcome the afore-mentioned problem faced in CO2 conversion. Despite these advantages, PC has only been seldom used as a solvent in the electrocatalytic conversion of CO2 [,].
Inspired by these signs of progress, in this study, the electrocatalytic conversion of CO2 was performed in imidazolium-based ILs/PC solution, in which the ILs act as active component and electrolyte, and PC as the solvent. First, the onset potential and the main kinetic parameters of CO2 reduction in PC solution of 1-alkyl-3-methyl imidazolium tetrafluoroborate with different chain length were measured using linear voltammetry (LSV) and Tafel characterization, respectively. Then, the electrochemical impedance spectroscopy (EIS) and equivalent circuit analysis were carried out to investigate the catalytic role of ILs in the course of CO2 reduction. At last, the catalytic performance in the presence of ILs and tetrabutylammonium tetrafluoroborate salt was evaluated and compared.
2. Results and Discussion
2.1. Reference Electrode Calibration in Non-Aqueous Solution
In this work, we used Pt/(I−/I3−) as the reference electrode. For a facile comparison of electrochemical potentials measured in the different non-aqueous electrolyte solution, the electrode potentials versus I−/I3− were first calibrated using ferrocene/ferrocenium (Fc/Fc+) redox couple as an internal standard [], as recommended by International Union of Pure and Applied Chemistry. This calibration method based on the measurement of cyclic voltammograms (CV) at the presence of Fc/Fc+ redox couple in the corresponding catholyte (0.1 M ILs in PC in this work). The obtained CV curves are shown in Figure 1.
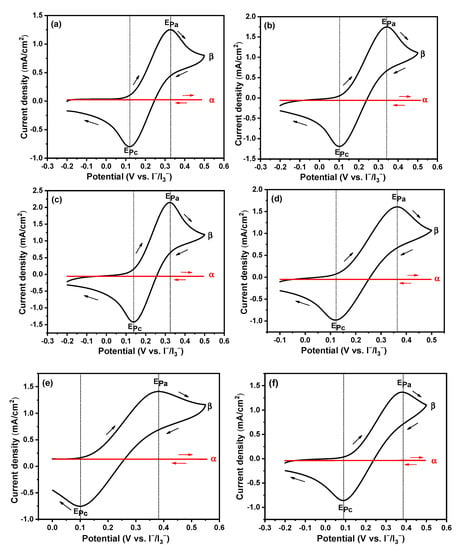
Figure 1.
Cyclic voltammetry measured in [Emim]BF4/PC (a), [Bmim]BF4/PC (b) [Hmim]BF4/PC (c), [Omim]BF4/PC (d), [Dmim]BF4/PC (e), [Bu4N]BF4/PC (f); α: blank, β: 10 mM ferrocene; [Emim]BF4: 1-ethyl-3-methylimidazolium tetrafluoroborate; [Bmim]BF4: 1-butyl-3-methylimidazolium tetrafluoroborate; [Hmim]BF4: 1-hexyl-3-methylimidazolium tetrafluoroborate; [Omim]BF4: 1-octyl-3-methylimidazolium tetrafluoroborate; [Dmim]BF4: 1-decyl-3-methylimidazolium tetrafluoroborate; and [Bu4N]BF4: tetrabutylammonium tetrafluoroborate.
For comparison, the CV curve in the commonly used tetrabutylammonium salt [Bu4N]BF4 catholyte solution is also included. As shown in Figure 1, in all cases, the redox peaks are not observed across the CV curves (the red ones) in the absence of ferrocene. Otherwise, typical redox peaks (the black curves) are observed at the presence of 10 mM ferrocene. The obtained half-wave potentials (E1/2 = (EPc + EPa)/2) are 0.242 V, 0.231 V, 0.223V, 0.222 V, 0.241 V and 0.242 V in 0.1 M [Emim]BF4/PC, [Bmim]BF4/PC, [Hmim]BF4/PC, [Omim]BF4/PC, [Dmim]BF4/PC and [Bu4N]BF4/PC, respectively. Then, all the potentials in different catholyte solutions are calibrated versus the internal standard if Fc/Fc+ according to the equation: E (vs. Fc/Fc+) = E (vs. I−/I3−) − E1/2 [].
2.2. Linear Sweep Voltammetry Measurements
Linear sweep voltammograms (LSV) curves are recorded to study the effect of the alkyl chain length of the imidazolium cations on the electroreduction of CO2, and the results are shown in Figure 2. For comparison, the LSV curve of [Bu4N]BF4 with the same anion BF4− as catholyte is also recorded. In agreement with previous reports [], the onset potential for CO2 reduction was defined as the potential that results in a current density of 0.6 mA/cm2. As shown in Figure 2, though all CV curves show similar profiles, the onset potentials shift anodically when ILs replace [Bu4N]BF4 as supporting electrolytes, indicating that ILs play a promoting role in the process of CO2 electrochemical reduction. Furthermore, it can be observed that the onset potentials change with the alkyl chain length of imidazolium cations. Among these five studied ILs, the [Bmim]BF4 gives the most positive onset potential of −1.702 V (vs. Fc/Fc+), which shifted positively by 133 mV compared with that in [Bu4N]BF4/PC solution (−1.835 V (vs. Fc/Fc+)).
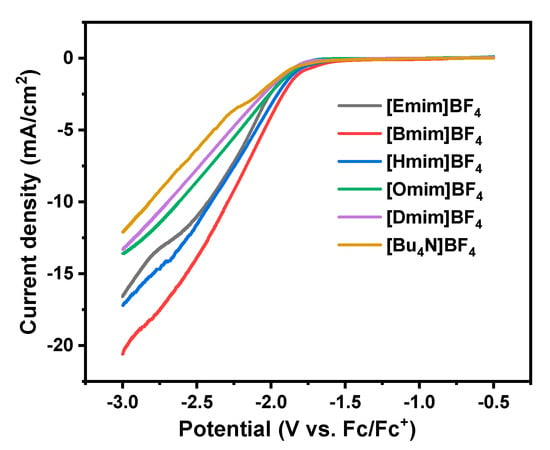
Figure 2.
Linear sweep voltammograms (LSV) curves in CO2-saturated propylene carbonate (PC) solution of ionic liquids (ILs) and [Bu4N]BF4.
2.3. Tafel Analysis
Tafel plots of CO2 reduction in PC solution with different ILs and [Bu4N]BF4 as electrolytes are further conducted, and the results are shown in Figure 3. It can be determined from Figure 3 that the equilibrium potential of CO2 reduction in [Emim]BF4/PC, [Bmim]BF4/PC, [Hmim]BF4/PC, [Omim]BF4/PC, [Dmim]BF4/PC and [Bu4N]BF4/PC solution is −1.512 V, −1.275 V, −1.561 V, −1.633 V, −1.672 V, −1.751 V (vs. Fc/Fc+), respectively. In consistency with the results of LSV characterization, the [Bmim]BF4 IL has the higher catalytic promotion effect than other ILs and the [Bu4N]BF4 salt. The equilibrium potential of CO2 in [Bmim]BF4/PC solution shifted positively 476 mV by comparison with that in [Bu4N]BF4/PC solution. The kinetic parameters of CO2 reduction in different electrolytes were further calculated according to Equations (1) and (2) [], and the results are listed in Table 1.
where η is the overpotential, a is the Tafel constant, b is the Tafel slope, γ is the charge transfer coefficient to indicate the symmetry of the energy barrier, i0 is the exchange current density, n is the number of electrons transferred, F is the Faradaic constant, T is the absolute temperature, and R is the gas constant.
η = a+blog|i|,
a = −2.303RTlogi0/(nγF), b = 2.303RT/(nγF),
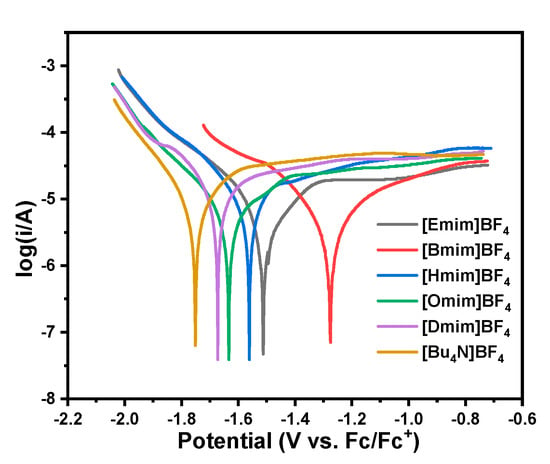
Figure 3.
Tafel plots of CO2 reduction in CO2-saturated PC solution of ILs and [Bu4N]BF4.

Table 1.
Electrochemical kinetic parameters of CO2 reduction in [Emim]BF4/PC, [Bmim]BF4/PC, [Hmim]BF4/PC, [Omim]BF4/PC, [Dmim]BF4/PC, and [Bu4N]BF4/PC electrolyte solution.
It can be seen from Table 1 that, for Tafel constant a and Tafel slope b, these two parameters in all ILs/PC solution are smaller than that in [Bu4N]BF4/PC solution. From Equation (2), the smaller value of a and b means that the activation over potential η will also be lower at the same current density. Moreover, the charge transfer coefficient γ and the exchange current density i0 in all ILs/PC solution are higher than that in [Bu4N]BF4/PC solution. Therefore, these kinetic parameters derived from Tafel characterization confirm that the electrolytes of imidazolium-based ILs can lower the activation energy, and then is expected to enhance the electrochemical conversion efficiency of CO2. Furthermore, [Bmim]BF4 electrolyte gives the smallest value of a (0.791 V) and b (0.160 V/dec), and on the other side, has the largest value of γ (0.185) and i0 (1.14 × 10−5 A/cm2), which means that [Bmim]BF4 would have the highest catalytic activity among all the studied ILs.
2.4. Electrochemical Impedance Analysis
Based on LSV and Tafel characterization, it has been confirmed that ILs, especially [Bmim]BF4, showed an enhanced ability for activating CO2 in the electrochemical conversion process. To further study the underlying role of ILs in CO2 reduction on the Ag electrode, we further performed electrochemical impedance spectroscopy (EIS) analysis. The EIS results in different ILs/PC and [Bu4N]BF4/PC solutions are shown in Figure 4. As shown in Figure 4a, Nyquist plot in [Bu4N]BF4/PC electrolyte shows a semicircle in the region of high frequency, which can be ascribed to the Faradaic electron transfer process, and a nearly straight line can be observed in the low-frequency region, which resulted from the diffusion-control process. The deviation of the line from the typical 45° may be due to the rough surface of the Ag electrode. However, different from that in the [Bu4N]BF4/PC electrolyte, the Nyquist plots in all ILs/PC electrolytes (Figure 4b) show an additional big semicircle. The apparent differences of the Nyquist plot between ILs/PC systems and [Bu4N]BF4/PC electrolyte mean that the reduction mechanism of CO2 is changed when IL is used as the electrolyte.
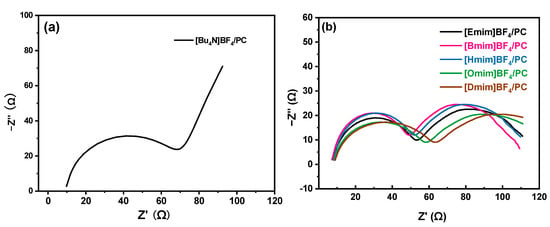
Figure 4.
Nyquist plots of electrochemical impedance spectroscopy (EIS) in 0.1 M CO2-saturated [Bu4N]BF4/PC (a) and different ILs/PC (b) electrolyte solutions.
The equivalent circuits of EIS results were further derived and were shown in Figure 5. Figure 5a is a typical Randles circuit that can well feature the Nyquist plot in [Bu4N]BF4/PC electrolyte (see Figure 4a), in which Rs is the solution resistance, Rct is the charge transfer resistance, C is the double-layer capacitance, and W is the Warburg impedance. The diagram in Figure 5b represented the equivalent circuit of the Nyquist plot in ILs/PC solution. The presence of two semicircles in the Nyquist plot (see Figure 4b) means that there exist two different time constants. Since each time constant is related to an RC component [], an additional RC component should be added in the equivalent circuit. When ILs/PC are used as the electrolyte in CO2 reduction, CO2 molecules have to pass through the absorbed ILs film layer to reach electrode surface, and then anticipate into an electroreduction reaction. Therefore, the additional RC circuit should be connected in parallel with another RC circuit []. So, in the equivalent circuit diagram of Figure 5b, the R1 represents the migration resistance of CO2, through the adsorbed layer of IL. In addition, the second semicircle in Figure 4b is an arc and deviated obviously from the semicircular trajectory. This deviation is due to a dispersion behavior of the real capacitive component. Therefore, a constant phase element (CPE) has been commonly introduced and defined as in Equation (3) [,]:
where Y0 is the constant phase coefficient, n is the dispersion coefficient (if n = 0, it is equivalent to a resistance; if n = 1, it is equivalent to a capacitance), j is the imaginary unit, and ω is angular frequency.
Z(CPE) = (jω)−n/Y0
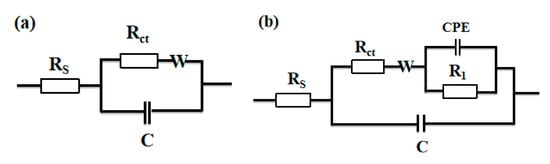
Figure 5.
The equivalent circuit diagram of CO2 in [Bu4N]BF4/PC (a), [Bmim]BF4/PC (b) electrolyte. Rs is the solution resistance, Rct is the charge transfer resistance, C is the double layer capacitance, W is the Warburg impedance, R1 is the migration resistance of CO2 through the ionic liquid adsorption layer, and constant phase element (CPE) is the constant phase element.
According to the equivalent circuit shown in Figure 5, the impedance parameters are further obtained, and the results are shown in Table 2. For example, in [Bmim]BF4/PC solution, the value of the dispersion coefficient n of the CPE is 0.8325. This means its deviation from a pure capacitance, indicating that [Bmim]BF4 formed a film layer on the surface of the Ag electrode and then caused a dispersion behavior [].

Table 2.
Parameter values of equivalent circuit components.
It can be seen from Table 2 that the Rct values of all studied ILs/PC systems are also smaller than that of [Bu4N]BF4/PC solution, confirming the promotion effect of ILs on the electrochemical reduction of CO2. This promotion effect of ILs/PC system is expected because of the catalytic role of the absorbed IL for the activation of CO2 through the complexation and stabilization of the CO2•− radical anion [,,]. In addition, the Rct values of ILs/PC systems have the following trend: [Bmim]BF4/PC (43.07 Ω) < [Hmim]BF4/PC (47.32 Ω) < [Emim]BF4/PC (49.07 Ω) < [Omim]BF4/PC (53.38 Ω) < [Dmim]BF4/PC (55.09 Ω). Among all the studied ILs with different chain lengths, [Bmim]BF4 has the smallest Rct value, indicating its highest catalytic effect for CO2 reduction. This result of EIS characterization is consistent with that obtained by LSV and Tafel analyses.
Furthermore, from the above electrochemical characterizations, it was found that, although the exact promotion effect of ILs on the electrochemical reduction of CO2 is not very clear, previous reports have generally indicated that the ILs adsorbed on the electrode can complex with CO2•−reactive intermediate [,,,,,,], resulting in the reduction of the overpotential and the ultimate facilitation of the CO2 conversion. From this point of view, the adsorption behavior of ILs on the electrode can influence the interaction between the imidazolium cation and CO2•−, and plays a decisive role in the course of CO2 electrochemical conversion [,,]. However, the adsorption behavior (i.e., the adsorption strength, the spatial structure, and the density) is influenced by many factors, such as the material and the potential of electrode, the solvent, the concentration of ILs, and the type of anion. In our study, when decreasing the chain length at N1-position of imidazolium cation from octyl to butyl, the catalytic activities of ILs increase, which can be ascribed to the higher adsorbed quantity with a lower steric hindrance of shorter chain length. However, further deceasing the chain length from butyl to ethyl, the adsorbed [Emim]+ may further increase, and cause the film layer too dense to let CO2 molecules diffuse across, which, on the contrary, is detrimental to CO2 conversion.
2.5. The Catalytic Performance and the Catalytic Mechanism
After the electrochemical characterizations, we then evaluated the CO2 conversion performances in [Bu4N]BF4/PC and [Bmim]BF4/PC electrolyte solution, and the results of Faradaic efficiency (FE) and current density are shown in Figure 6. It can be seen from Figure 6a that the current density in both [Bmim]BF4/PC and [Bu4N]BF4/PC electrolytes increase as the applied potential decrease, and at each potential, the current density enhanced when ILs of [Bmim]BF4 replace [Bu4N]BF4 as the electrolyte. For example, at −1.90 V (vs. Fc/Fc+), the current density in [Bmim]BF4/PC (8.2 mA/cm2) was about three times that in the traditional [Bu4N]BF4/PC (2.7 mA/cm2). The FE results in Figure 6b show that the FE of CO in [Bmim]BF4/PC is much higher than that in [Bu4N]BF4/PC at each applied potential. Correspondingly, the FE of byproduct H2 is reduced when the [Bmim]BF4/PC is alternatively used as the electrolyte solution. More importantly, it can be seen that the FE as high as 98.5% can be obtained in [Bmim]BF4/PC solution when the applied potential is at −1.90 V (vs. Fc/Fc+).
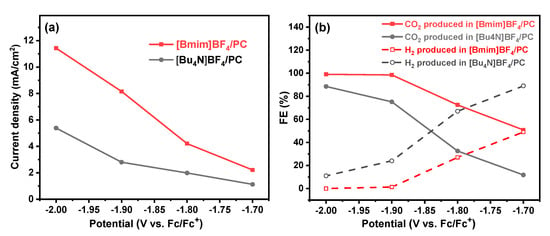
Figure 6.
The current density (a) and Faradaic efficiency (FE) of CO and H2 (b) for electrocatalytic CO2 conversion in [Bu4N]BF4/PC and [Bmim]BF4/PC electrolyte solution.
Based on the above electrochemical characterization, the performance results and previous reports, the reduction mechanism of CO2 at the presence of [Bmim]BF4 is proposed, and the corresponding schematic diagram is shown in Figure 7. Firstly, the imidazolium cation [Bmim]+ adsorbs on the surface of the Ag electrode and forms a film layer of ILs []. Subsequently, the CO2 molecules diffuse through the film layer of ILs and reach the Ag electrode. Then, the CO2 molecule obtains one single electron, resulting in the formation of radical CO2•−, and the generated CO2•− interacts with [Bmim]+ and forms a [Bmim-CO2]ad complex intermediate [,,,,], in which the cation of [Bmim]+ plays the role of stabilizing the radical CO2•−, and in consequence, reduces the required activation energy for the overall reduction of CO2 [,,]. The formed [Bmim-CO2]ad intermediate is further combined with another electron and two protons H+ to ultimately produce CO. It should be noted here that the H+ was supplied from the anodic electrolyte (sulfuric acid) and passed through the Nafion N-117 membrane to reach the cathode cell, to participate into the CO2 reduction reaction. At last, the generated CO diffuses into the solution and overflows the liquid surface.
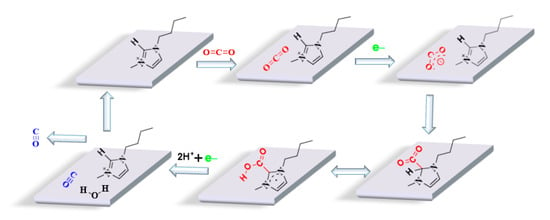
Figure 7.
Schematic diagram of electroreduction of CO2 in [Bmim]BF4/PC solution with Ag as working electrode.
3. Materials and Methods
3.1. Materials
Notably, 1-ethyl-3-methylimidazolium tetrafluoroborate ([Emim]BF4, ≥98%), 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4, ≥99%), 1-hexyl-3-methylimidazolium tetrafluoroborate ([Hmim]BF4, ≥98%), 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim]BF4, ≥98%), 1-decyl-3-methylimidazolium tetrafluoroborate ([Dmim]BF4, ≥98%), and tetrabutylammonium tetrafluoroborate ([Bu4N]BF4, ≥99%) were all supplied by Chengjie Chem. Co., Ltd. (Shanghai, China). Propylene carbonate (PC, ≥99.9%), iodine (I2, ≥99.5%), and tetrabutylammonium iodide (TBAI, ≥99.9%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ferrocene (Fc, ≥99.9%) was purchased from Tianjin Deen Chemical Reagent Co., Ltd. (Tianjin, China). Nafion N-117 membrane (0.180 mm thick, ≥0.90 meg/g exchange capacity) was purchased from Alfa Aesar China Co., Ltd. (Tianjin, China). Ag electrodes (10mm × 10mm, 1mm in thickness, >99.99%) and graphite rod (5 mm diameter, length 15 cm, ≥99.99%) were acquired from Tianjin Aida Hengsheng Technology Development Co., Ltd. (Tianjin, China).
3.2. The Construction of Pt/(I−/I3–) Reference Electrode
In this work, Pt/(I–/I3–) was used as the reference electrode in the ILs/PC non-aqueous system. The Pt/(I–/I3–) reference electrode was prepared by inserting Pt wire into a mixture solution of I2 (0.05 M) and TBAI (0.1 M). In addition, in order to avoid the junction potential and the pollution of the cathodic electrolyte solution from the electrolyte in Pt/(I–/I3–) reference electrode, a salt bridge filled with the same cathodic electrolyte solution is also used. Both bottoms of the Pt/(I–/I3–) reference electrode and the salt bridge were sealed with porous polytetrafluoroethylene.
3.3. Electrochemical Characterization
All electrochemical experiments were carried out on the CHI 660E electrochemical workstation (Shanghai Chenhua, Shanghai, China). The scan rate of linear sweeping voltammetry (LSV) is 20 mV/s and sweeping region from −0.5~−3.0 V (vs. Fc/Fc+). The Tafel scan rate is 10 mV/s. The electrochemical impedance (ESI) is tested at a constant potential, with an amplitude of 10 mV and frequency ranging from 10 kHz to 1 Hz.
3.4. The CO2 Conversion Performance Test
The electrochemical reduction of CO2 was performed in an H-type dual-chamber reactor. The Nafion N-117 ion-exchange membrane was used to separate the cathode chamber and the anode chamber. A silver plate (1cm × 1cm × 0.3mm) and a graphite rod were used as the working electrode and counter electrode, respectively. ILs/PC mixture solution (0.1 M) and H2SO4 aqueous solution (0.1 M) were used as the cathodic and anodic electrolytes, respectively.
In a typical procedure, the reactor was connected to a gas circulation and online sampling system (Labsolar-III AG, Beijing Perfectlight Technology Co., Ltd. (Beijing, China), and the details are stated in our previous work [,]). Subsequently, the air solubilized in the electrolyte solution, and the air in the circulation channel was evacuated for 30 min. Then, CO2 was introduced into the electrolyte solution from the bottom of the cathodic cell for one hour to reach the solubility equilibrium of CO2. Then two hours of the reaction was carried out to make sure that the concentration of products was high enough to exceed the detection limit of the detectors for reducing the analytic errors.
3.5. The Products Analysis and Calculation of Faradaic Efficiency
The products of H2 and CO were online sampled and in-situ quantified by gas chromatography (GC 9790II, Zhejiang Fu Li Analytical Instrument Co. Ltd., Zhejiang, China). The separation of H2, CO, and CO2 feed gas was realized by a TDX-01 column and the separated gas was subsequently brought into two paths. In one path, the products of H2 and CO were guided into a Molsieve5 A column, and the H2 was then measured using a thermal conductivity detector (TCD), while the gas CO was passed through a mechanizer to be transformed into methane by the nickel catalyst at 380 °C, and was ultimately detected by a flame ionization detector (FID). In another path, CO2 and other possible hydrocarbons that have longer retention time than H2 and CO in the TDX-01 column were introduced into a Porapak N column. Finally, the CO2 gas was vented out, and the gas of hydrocarbons were measured by FID.
The FE is calculated from the product analysis by the equation:
where is the mole number of a specific product (mol), is the number of electrons transferred for this product i (for CO and H2), and is the total mole number of electrons passed through the circuit (mol). Furthermore, the (mol) can be determined by the equation:
where Q is the passed charge (C) that can be obtained from the integration of the recorded chronoamperometric (i-t) curve, and F is the Faradaic constant (96500 C/mol).
4. Conclusions
In summary, the ILs/PC mixture solution was constructed and investigated as the electrolyte for electrochemical reduction of CO2 on the Ag plate electrode. The investigation on the alkyl length of imidazole-based ILs indicates that the IL of [Bmim]BF4 gives the lower onset potential and Tafel slope, as well as higher exchange current density, compared to other studied ILs and the traditional [Bu4N]BF4 salt. The EIS characterization further indicated that the imidazolium cation could absorb on the electrode surface and reduce the overpotential through complexing with anion radical (CO2•−) and stabilizing them. The [Bmim]BF4 IL gives the highest performance for the conversion of CO2. The performance tests show that in [Bmim]BF4/PC electrolyte solution, FE for CO as high as 98.5% and current density of 8.2 mA/cm2 can be achieved at −1.9 V (vs. Fc/Fc+).
Author Contributions
Conceptualization, F.J. and W.L.; Funding acquisition, W.L.; Synthesis, characterization, performance test and writing—original draft preparation, F.J. and J.Z.; Formal analysis and writing—review and editing, F.J., W.L. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21673067.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 emissions and climate change from existing energy infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef]
- Lewis, N.S. Research opportunities to advance solar energy utilization. Science 2016, 351, aad1920. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.M.; et al. Net-zero emissions energy systems. Science 2018, 360, eaas9793. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A.; Prakash, G.K.S.; Goeppert, A. Anthropogenic Chemical Carbon Cycle for a Sustainable Future. J. Am. Chem. Soc. 2011, 133, 12881–12898. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Guerra, M.; Albo, J.; Alvarez-Guerra, E.; Irabien, A. Ionic liquids in the electrochemical valorisation of CO2. Energy Environ. Sci. 2015, 8, 2574–2599. [Google Scholar] [CrossRef]
- Faggion, D.; Goncalves, W.D.G.; Dupont, J. CO2 Electroreduction in Ionic Liquids. Front. Chem. 2019, 7, 102. [Google Scholar] [CrossRef]
- Gao, D.; Aran-Ais, R.M.; Jeon, H.S.; Cuenya, B.R. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Sánchez, O.G.; Birdja, Y.Y.; Bulut, M.; Vaes, J.; Breugelmans, T.; Pant, D. Recent advances in industrial CO2 electroreduction. Curr. Opin. Green Sust. Chem. 2019, 16, 47–56. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Chem. 2019, 10, 677. [Google Scholar] [CrossRef]
- Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.-Z. Understanding the Roadmap for Electrochemical Reduction of CO2 to Multi-Carbon Oxygenates and Hydrocarbons on Copper-Based Catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, Y.; Zhang, J.; Xu, P. Reduction of Gas CO2 to CO with High Selectivity by Ag Nanocube-Based Membrane Cathodes in a Photoelectrochemical System. Ind. Eng. Chem. Res. 2020, 59, 5536–5545. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G. Carbon Dioxide Hydrogenation into Higher Hydrocarbons and Oxygenates: Thermodynamic and Kinetic Bounds and Progress with Heterogeneous and Homogeneous Catalysis. ChemSusChem 2017, 10, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, S.; Najari, S.; Fazlollahi, F.; Nikoo, M.K.; Sefidkon, F.; Klemes, J.J.; Baxter, L.L. Mechanisms and kinetics of CO2 hydrogenation to value-added products: A detailed review on current status and future trends. Renew. Sustain. Energy Rev. 2017, 80, 1292–1311. [Google Scholar] [CrossRef]
- Guo, L.-j.; Wang, Y.-j.; He, T. Photocatalytic Reduction of CO2 over Heterostructure Semiconductors into Value-Added Chemicals. Chem. Rec. 2016, 16, 1918–1933. [Google Scholar] [CrossRef]
- Qureshi, M.; Takanabe, K. Insights on Measuring and Reporting Heterogeneous Photocatalysis: Efficiency Definitions and Setup Examples. Chem. Mater. 2017, 29, 158–167. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J.; Ithisuphalap, K.; Wu, G.; Li, Q. Recent advances in Cu-based nanocomposite photocatalysts for CO2 conversion to solar fuels. J. Energy Chem. 2017, 26, 1039–1049. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.-J.; Wang, T.; Gong, J. Nano-designed semiconductors for electro-and photoelectro-catalytic conversion of carbon dioxide. Chem. Soc. Rev. 2018, 47, 5423–5443. [Google Scholar] [CrossRef]
- Zhang, N.; Long, R.; Gao, C.; Xiong, Y. Recent progress on advanced design for photoelectrochemical reduction of CO2 to fuels. Sci. China Mater. 2018, 61, 771–805. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Yang, P.; Zhang, G.; Gong, J. The Development of Cocatalysts for Photoelectrochemical CO2 Reduction. Adv. Mater. 2019, 31, 1804710. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ju, F.; Yao, K.; Wei, X. Photoelectrocatalytic Reduction of CO2 for Efficient Methanol Production: Au Nanoparticles as Electrocatalysts and Light Supports. Ind. Eng. Chem. Res. 2020, 59, 4348–4357. [Google Scholar] [CrossRef]
- Yu, S.; Wilson, A.J.; Kumari, G.; Zhang, X.; Jain, P.K. Opportunities and Challenges of Solar-Energy-Driven Carbon Dioxide to Fuel Conversion with Plasmonic Catalysts. ACS Energy Lett. 2017, 2, 2058–2070. [Google Scholar] [CrossRef]
- Koppenol, W.; Rush, J. Reduction potential of the carbon dioxide/carbon dioxide radical anion: A comparison with other C1 radicals. J. Phys. Chem. 1987, 91, 4429–4430. [Google Scholar] [CrossRef]
- Paik, W.; Andersen, T.N.; Eyring, H. Kinetic studies of electrolytic reduction of carbon dioxide on mercury electrode. Electrochim. Acta 1969, 14, 1217–1232. [Google Scholar] [CrossRef]
- Chen, T.Y.; Shi, J.; Shen, F.X.; Zhen, J.Z.; Li, Y.F.; Shi, F.; Yang, B.; Jia, Y.J.; Dai, Y.N.; Hu, Y.Q. Selection of Low-Cost Ionic Liquid Electrocatalyst for CO2 Reduction in Propylene Carbonate/Tetrabutylammonium Perchlorate. ChemElectroChem 2018, 5, 2295–2300. [Google Scholar] [CrossRef]
- Sharma, P.P.; Zhou, X.D. Electrocatalytic conversion of carbon dioxide to fuels: A review on the interaction between CO2 and the liquid electrolyte. WIRES Energy Environ. 2017, 6, e239. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Jiao, F. Nanostructured Metallic Electrocatalysts for Carbon Dioxide Reduction. ChemCatChem 2015, 7, 38–47. [Google Scholar] [CrossRef]
- Shukia, S.K.; Khokarale, S.G.; Bui, T.Q.; Mikkola, J.P.T. Ionic Liquids: Potential Materials for Carbon Dioxide Capture and Utilization. Front. Mater. 2019, 6, 42. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T.C. Conversion of CO2 to value-added products mediated by ionic liquids. Green Chem. 2019, 21, 2544–2574. [Google Scholar] [CrossRef]
- Lim, H.K.; Kim, H. The Mechanism of Room-Temperature Ionic-Liquid-Based Electrochemical CO2 Reduction: A Review. Molecules 2017, 22, 536. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X. Imidazolium Ionic Liquids, Imidazolylidene Heterocyclic Carbenes, and Zeolitic Imidazolate Frameworks for CO2 Capture and Photochemical Reduction. Angew. Chem. Int. Ed. 2016, 55, 2308–2320. [Google Scholar] [CrossRef] [PubMed]
- Rees, N.V.; Compton, R.G. Electrochemical CO2 sequestration in ionic liquids: A perspective. Energy Environ. Sci. 2011, 4, 403–408. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Sun, J.; Zhang, X.C.; Xin, J.Y.; Miao, Q.Q.; Wang, J.J. Ionic liquid-based green processes for energy production. Chem. Soc. Rev. 2014, 43, 7838–7869. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef] [PubMed]
- Hapiot, P.; Lagrost, C. Electrochemical reactivity in room-temperature ionic liquids. Chem. Rev. 2008, 108, 2238–2264. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Li, J. Ionic liquids in surface electrochemistry. Phys. Chem. Chem. Phys. 2010, 12, 1685–1697. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Pringle, J.M.; Howlett, P.C.; Forsyth, M. Ionic liquids and reactions at the electrochemical interface. Phys. Chem. Chem. Phys. 2010, 12, 1659–1669. [Google Scholar] [CrossRef]
- Zhang, G.R.; Etzold, B.J.M. Ionic liquids in electrocatalysis. J. Energy Chem. 2016, 25, 199–207. [Google Scholar] [CrossRef]
- Rudnev, A.V.; Kiran, K.; Lopez, A.C.; Dutta, A.; Gjuroski, I.; Furrer, J.; Broekmann, P. Enhanced electrocatalytic CO formation from CO2 on nanostructured silver foam electrodes in ionic liquid/water mixtures. Electrochim. Acta 2019, 306, 245–253. [Google Scholar] [CrossRef]
- Wu, H.R.; Song, J.L.; Xie, C.; Hu, Y.; Han, B.X. Highly efficient electrochemical reduction of CO2 into formic acid over lead dioxide in an ionic liquid-catholyte mixture. Green Chem. 2018, 20, 1765–1769. [Google Scholar] [CrossRef]
- Lim, H.K.; Kwon, Y.; Kim, H.S.; Jeon, J.; Kim, Y.H.; Lim, J.A.; Kim, B.S.; Choi, J.; Kim, H. Insight into the Microenvironments of the Metal-Ionic Liquid Interface during Electrochemical CO2 Reduction. ACS Catal. 2018, 8, 2420–2427. [Google Scholar] [CrossRef]
- Medina-Ramos, J.; Lee, S.S.; Fister, T.T.; Hubaud, A.A.; Sacci, R.L.; Mullins, D.R.; DiMeglio, J.L.; Pupillo, R.C.; Velardo, S.M.; Lutterman, D.A.; et al. Structural Dynamics and Evolution of Bismuth Electrodes during Electrochemical Reduction of CO2 in Imidazolium-Based Ionic Liquid Solutions. ACS Catal. 2017, 7, 7285–7295. [Google Scholar] [CrossRef]
- Rosen, B.A.; Salehi-Khojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.A.; Masel, R.I. Ionic Liquid-Mediated Selective Conversion of CO2 to CO at Low Overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef]
- Lu, W.; Jia, B.; Cui, B.; Zhang, Y.; Yao, K.; Zhao, Y.; Wang, J. Efficient photoelectrochemical reduction of carbon dioxide to formic acid: A functionalized ionic liquid as an absorbent and electrolyte. Angew. Chem. Int. Edit. 2017, 56, 11851–11854. [Google Scholar] [CrossRef]
- Feng, J.; Zeng, S.; Feng, J.; Dong, H.; Zhang, X. CO2 Electroreduction in Ionic Liquids: A Review. Chin. J. Chem. 2018, 36, 961–970. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Rivera, S.; Zhou, Y.; Vlachos, D.G.; Jiao, F. Mechanistic Insights into the Electrochemical Reduction of CO2 to CO on Nanostructured Ag Surfaces. ACS Catal. 2015, 5, 4293–4299. [Google Scholar] [CrossRef]
- Sun, L.Y.; Ramesha, G.K.; Kamat, P.V.; Brennecke, J.F. Switching the Reaction Course of Electrochemical CO2 Reduction with Ionic Liquids. Langmuir 2014, 30, 6302–6308. [Google Scholar] [CrossRef]
- Zhu, Q.; Ma, J.; Kang, X.; Sun, X.; Liu, H.; Hu, J.; Liu, Z.; Han, B. Efficient Reduction of CO2 into Formic Acid on a Lead or Tin Electrode using an Ionic Liquid Catholyte Mixture. Angew. Chem. Int. Ed. 2016, 55, 9012–9016. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Q.; Kang, X.; Liu, H.; Qian, Q.; Zhang, Z.; Han, B. Molybdenum-Bismuth Bimetallic Chalcogenide Nanosheets for Highly Efficient Electrocatalytic Reduction of Carbon Dioxide to Methanol. Angew. Chem. Int. Ed. 2016, 55, 6770–6774. [Google Scholar] [CrossRef] [PubMed]
- Huan, T.N.; Simon, P.; Rousse, G.; Genois, I.; Artero, V.; Fontecave, M. Porous dendritic copper: An electrocatalyst for highly selective CO2 reduction to formate in water/ionic liquid electrolyte. Chem. Sci. 2017, 8, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Rudnev, A.V.; Fu, Y.-C.; Gjuroski, I.; Stricker, F.; Furrer, J.; Kovacs, N.; Vesztergom, S.; Broekmann, P. Transport Matters: Boosting CO2 Electroreduction in Mixtures of BMIm BF4/Water by Enhanced Diffusion. ChemPhysChem 2017, 18, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.P.S.; Schreier, M.; Vasilyev, D.; Scopelliti, R.; Gratzel, M.; Dyson, P.J. New Insights Into the Role of Imidazolium-Based Promoters for the Electroreduction of CO2 on a Silver Electrode. J. Am. Chem. Soc. 2016, 138, 7820–7823. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.R.; Clark, E.L.; Bell, A.T. Effects of electrolyte, catalyst, and membrane composition and operating conditions on the performance of solar-driven electrochemical reduction of carbon dioxide. Phys. Chem. Chem. Phys. 2015, 17, 18924–18936. [Google Scholar] [CrossRef] [PubMed]
- Forero, J.S.B.; Munoz, J.A.H.; Jones, J.; da Silva, F.M. Propylene Carbonate in Organic Synthesis: Exploring its Potential as a Green Solvent. Curr. Org. Synth. 2016, 13, 834–846. [Google Scholar] [CrossRef]
- Parker, H.L.; Sherwood, J.; Hunt, A.J.; Clark, J.H. Cyclic Carbonates as Green Alternative Solvents for the Heck Reaction. ACS Sustain. Chem. Eng. 2014, 2, 1739–1742. [Google Scholar] [CrossRef]
- Hfaiedh, A.; Yuan, K.D.; Ben Ammar, H.; Ben Hassine, B.; Soule, J.F.; Doucet, H. Eco-Friendly Solvents for Palladium-Catalyzed Desulfitative C-H Bond Arylation of Heteroarenes. ChemSusChem 2015, 8, 1794–1804. [Google Scholar] [CrossRef]
- Shen, F.X.; Shi, J.; Chen, T.Y.; Shi, F.; Li, Q.Y.; Zhen, J.Z.; Li, Y.F.; Dai, Y.N.; Yang, B.; Qu, T. Electrochemical reduction of CO2 to CO over Zn in propylene carbonate/tetrabutylammonium perchlorate. J. Power Sources 2018, 378, 555–561. [Google Scholar] [CrossRef]
- Shi, J.; Shen, F.-x.; Shi, F.; Song, N.; Jia, Y.-j.; Hu, Y.-Q.; Li, Q.-Y.; Liu, J.-x.; Chen, T.-Y.; Dai, Y.-N. Electrochemical reduction of CO2 into CO in tetrabutylammonium perchlorate/propylene carbonate: Water effects and mechanism. Electrochim. Acta 2017, 240, 114–121. [Google Scholar] [CrossRef]
- Gagne, R.R.; Koval, C.A.; Lisensky, G.C. Ferrocene as an internal standard for electrochemical measurements. Inorg. Chem. 1980, 19, 2854–2855. [Google Scholar] [CrossRef]
- Shi, J.; Li, Q.Y.; Shi, F.; Song, N.; Jia, Y.J.; Hu, Y.Q.; Shen, F.X.; Yang, D.W.; Dai, Y.N. Design of a Two-Compartment Electrolysis Cell for the Reduction of CO2 to CO in Tetrabutylammonium Perchlorate/Propylene Carbonate for Renewable Electrical Energy Storage. J. Electrochem. Soc. 2016, 163, G82–G87. [Google Scholar] [CrossRef]
- Fang, Y.-H.; Liu, Z.-P. Tafel Kinetics of Electrocatalytic Reactions: From Experiment to First-Principles. ACS Catal. 2014, 4, 4364–4376. [Google Scholar] [CrossRef]
- Lates, V.; Falch, A.; Jordaan, A.; Peach, R.; Kriek, R.J. An electrochemical study of carbon dioxide electroreduction on gold-based nanoparticle catalysts. Electrochim. Acta 2014, 128, 75–84. [Google Scholar] [CrossRef]
- González, J.E.G.; Santana, A.F.J.H.; Mirza-rosca, J.C. Effect of bacterial biofilm on 316 SS corrosion in natural seawater by eis. Corros. Sci. 1998, 40, 2141–2154. [Google Scholar] [CrossRef]
- Torresi, R.M.; Lodovico, L.; Benedetti, T.M.; Alcântara, M.R.; Debiemme-Chouvy, C.; Deslouis, C. Convective mass transport in ionic liquids studied by electrochemical and electrohydrodynamic impedance spectroscopy. Electrochim. Acta 2013, 93, 32–43. [Google Scholar] [CrossRef]
- Pajkossy, T. Impedance spectroscopy at interfaces of metals and aqueous solutions—Surface roughness, CPE and related issues. Solid State Ion. 2005, 176, 1997–2003. [Google Scholar] [CrossRef]
- Rosen, B.A.; Haan, J.L.; Mukherjee, P.; Braunschweig, B.; Zhu, W.; Salehi-Khojin, A.; Dlott, D.D.; Masel, R.I. In Situ Spectroscopic Examination of a Low Overpotential Pathway for Carbon Dioxide Conversion to Carbon Monoxide. J. Phys. Chem. C 2012, 116, 15307–15312. [Google Scholar] [CrossRef]
- Braunschweig, B.; Mukherjee, P.; Haan, J.L.; Dlott, D.D. Vibrational sum-frequency generation study of the CO2 electrochemical reduction at Pt/EMIM-BF4 solid/liquid interfaces. J. Electroanal. Chem. 2017, 800, 144–150. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Hatakeyama, M.; Ogata, K.; Wakabayashi, M.; Jin, F.M.; Nakamura, S. Activation of CO2 by ionic liquid EMIM-BF4 in the electrochemical system: A theoretical study. Phys. Chem. Chem. Phys. 2015, 17, 23521–23531. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Kumar, B.; Behranginia, A.; Rosen, B.A.; Baskin, A.; Repnin, N.; Pisasale, D.; Phillips, P.; Zhu, W.; Haasch, R.; et al. Robust carbon dioxide reduction on molybdenum disulphide edges. Nat. Commun. 2014, 5, 4470. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lu, W.; Zhang, J.; Zhang, L. Efficient Hydrolysis of Ammonia Borane for Hydrogen Evolution Catalyzed by Plasmonic Ag@Pd Core-Shell Nanocubes. ACS Sustain. Chem. Eng. 2020, 8, 12366–12377. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).