Development of Facile and Simple Processes for the Heterogeneous Pd-Catalyzed Ligand-Free Continuous-Flow Suzuki–Miyaura Coupling
Abstract
1. Introduction
2. Results and Discussions
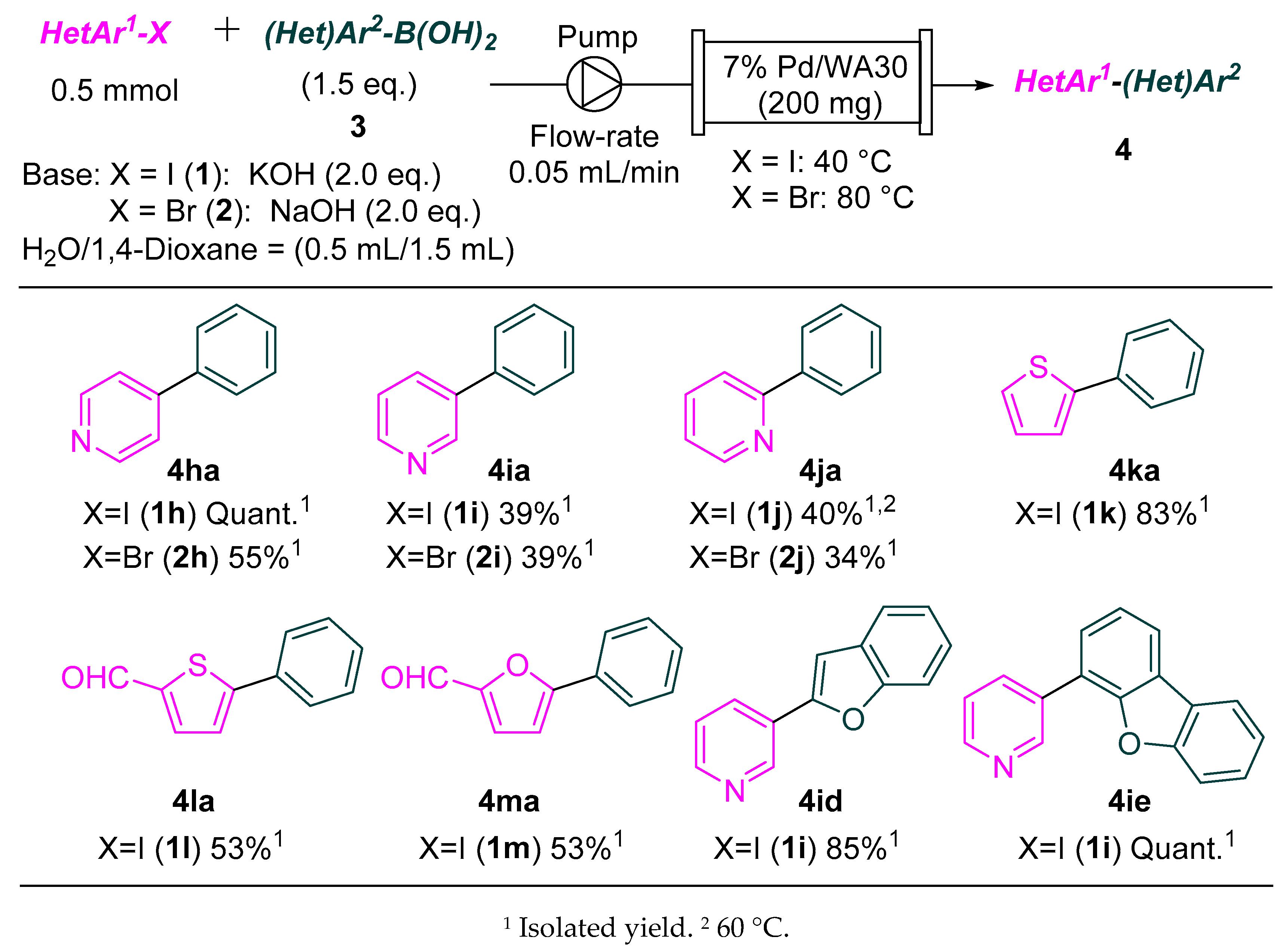
2.1. Continuous-Flow Suzuki–Miyaura Coupling of Aryl Iodides and Bromides with Aryl Boronic Acids
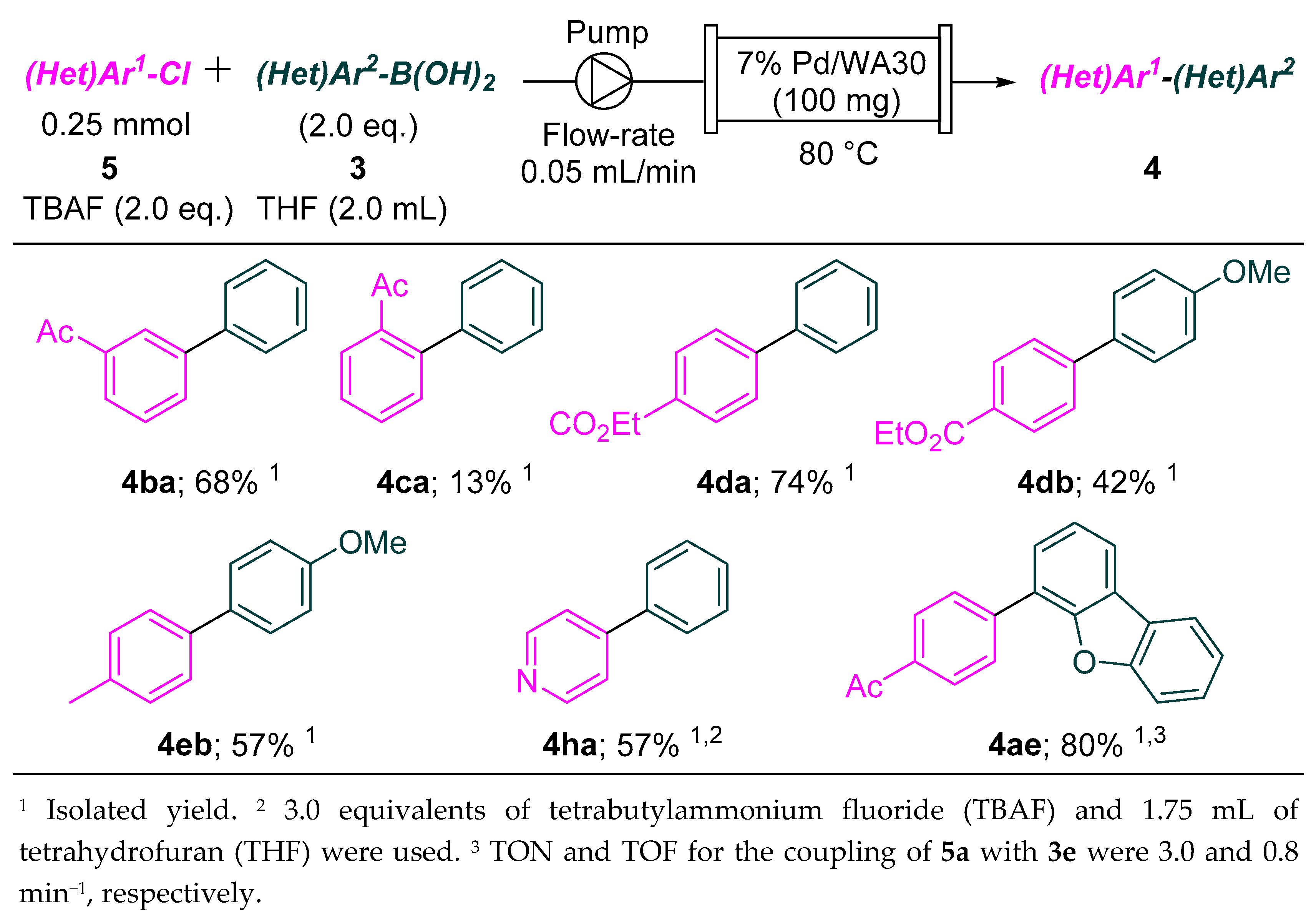
2.2. Continuous-Flow Suzuki–Miyaura Coupling of Aryl Chlorides with Aryl Boronic Acids
2.3. Application of the Continuous-Flow Suzuki–Miyaura Coupling to Gram-Scale Synthesis
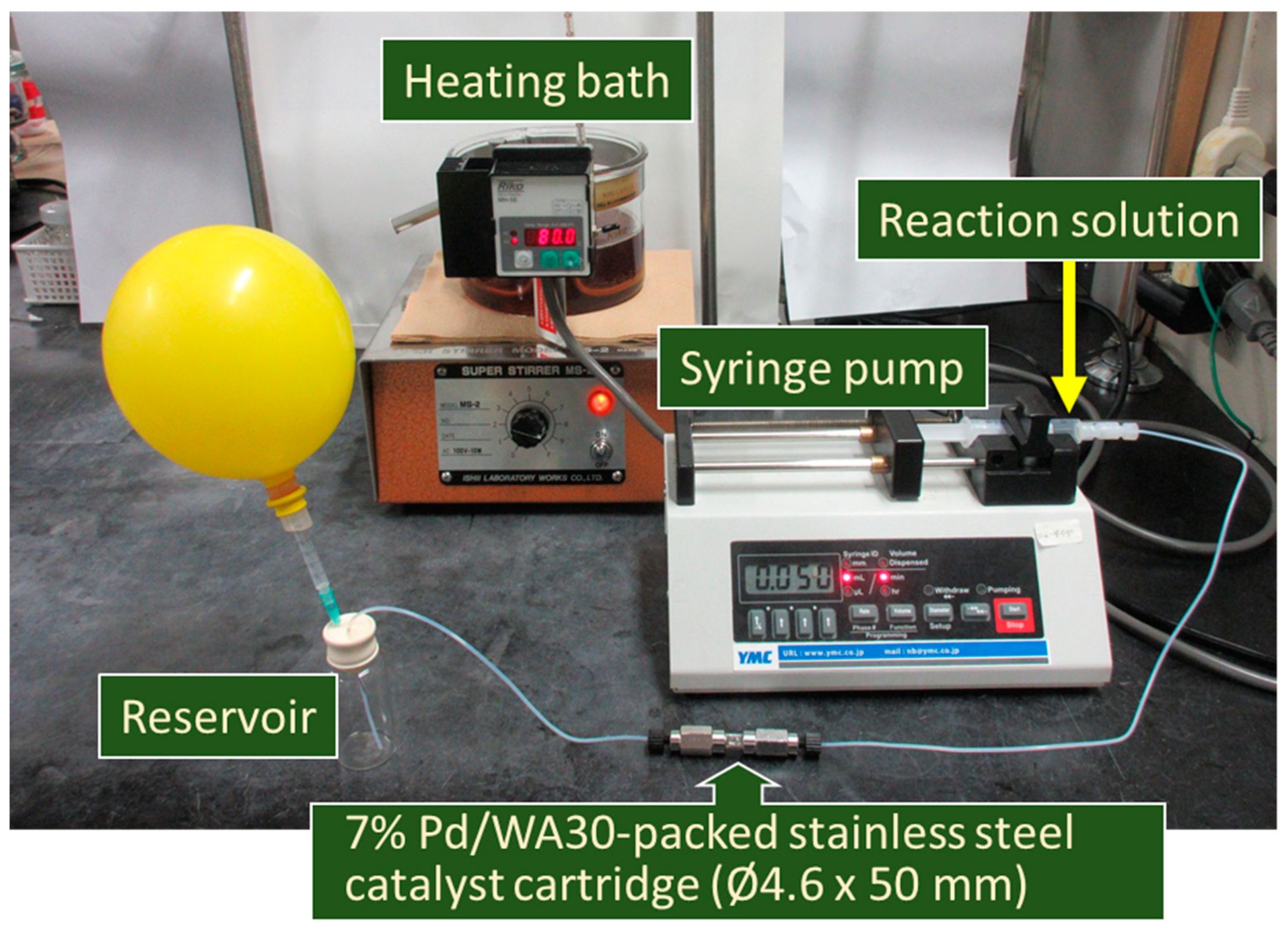
3. Materials and Methods
3.1. General Information
3.2. Preparation of 7% Pd/WA30
3.3. Experimental Procedures
3.3.1. Procedure for the Continuous-Flow Suzuki–Miyaura Coupling of Aryl Iodides and Bromides with Aryl Boronic Acids
3.3.2. Procedure for the Continuous-Flow Suzuki–Miyaura Coupling of Aryl Chlorides with Aryl Boronic Acids
3.3.3. Procedure for the Continuous-Flow Suzuki–Miyaura Coupling Reaction over an Extended Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Hassan, J.; Sèvignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Wu, J.-S.; Cheng, S.-W.; Cheng, Y.-J.; Hsu, C.-S. Donor–acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 2015, 44, 1113–1154. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon-Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef]
- A Smith, D.; Krishnananthan, S.; Meanwell, N.A.; Mathur, A.; Li, J. Multigram Synthesis of BMS-929075, an Allosteric, Palm Site Inhibitor of HCV NS5B Replicase, Involving the Synthesis of a Highly Functionalized Benzofuran through a Telescoped Process. Org. Process Res. Dev. 2020, 24, 1157–1163. [Google Scholar] [CrossRef]
- Konda, V.; Rydfjord, J.; Sämarker, J.; Larhed, M. Safe Palladium-Catalyzed Cross-Couplings with Microwave Heating. Using Continuous-Flow Silicon Carbide Reactors. Org. Process Res. Dev. 2014, 18, 1413–1418. [Google Scholar] [CrossRef]
- Filipponi, P.; Ostacolo, C.; Novellino, E.; Pellicciari, R.; Gioiello, A. Continuous Flow Synthesis of Thieno[2,3-c]isoquinolin-5(4H)-one Scaffold: A Valuable Source of PARP-1 Inhibitors. Org. Process Res. Dev. 2014, 18, 1345–1353. [Google Scholar] [CrossRef]
- Cole, K.P.; Reizman, B.J.; Hess, M.; Groh, J.M.; Laurila, M.E.; Cope, R.F.; Campbell, B.M.; Forst, M.B.; Burt, J.L.; Maloney, T.D.; et al. Small-Volume Continuous Manufacturing of Merestinib. Part 1. Process Development and Demonstration. Org. Process Res. Dev. 2019, 23, 858–869. [Google Scholar] [CrossRef]
- Cole, K.P.; Campbell, B.M.; Forst, M.B.; Groh, J.M.; Hess, M.; Johnson, M.D.; Miller, R.D.; Mitchell, D.; Polster, C.S.; Reizman, B.J.; et al. An Automated Intermittent Flow Approach to Continuous Suzuki Coupling. Org. Process Res. Dev. 2016, 20, 820–830. [Google Scholar] [CrossRef]
- Flick, A.C.; Ding, H.X.; Leverett, C.A.; Fink, S.J.; O’Donnell, C.J. Synthetic Approaches to New Drugs Approved During 2016. J. Med. Chem. 2018, 61, 7004–7031. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Tsuyumine, S.; Kodama, T. Direct Synthesis of a PDE4 Inhibitor by Using Pd–Cu-Catalyzed C–H/C–Br Coupling of Benzoxazole with a Heteroaryl Bromide. Org. Process Res. Dev. 2016, 20, 1053–1058. [Google Scholar] [CrossRef]
- Young, I.S.; Simmons, E.M.; Fenster, M.D.B.; Zhu, J.J.; Katipally, K.R. Palladium-Catalyzed C–O Coupling of a Sterically Hindered Secondary Alcohol with an Aryl Bromide and Significant Purity Upgrade in the API Step. Org. Process Res. Dev. 2018, 22, 585–594. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-Catalyzed Cross-Coupling Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Davis, D.C.; Dai, M. Natural Product Synthesis via Palladium-Catalyzed Carbonylation. J. Org. Chem. 2017, 82, 2319–2328. [Google Scholar] [CrossRef]
- Yamada, T.; Matsuo, T.; Ogawa, Y.; Ichikawa, T.; Kobayashi, Y.; Masuda, H.; Miyamoto, R.; Bai, H.; Meguro, K.; Sawama, Y.; et al. Application of Thiol-Modified Dual-Pore Silica Beads as Practical Scavenger of Leached Palladium Catalyst in C-C Coupling Reaction. Org. Process Res. Dev. 2019, 23, 462–469. [Google Scholar] [CrossRef]
- Yamada, T.; Masuda, H.; Park, K.; Tachikawa, T.; Ito, N.; Ichikawa, T.; Yoshimura, M.; Takagi, Y.; Sawama, Y.; Ohya, Y.; et al. Development of Titanium Dioxide-Supported Pd Catalysts for Ligand-Free Suzuki-Miyaura Coupling of Aryl Chlorides. Catalysts 2019, 9, 461. [Google Scholar] [CrossRef]
- Monguchi, Y.; Sakai, K.; Endo, K.; Fujita, Y.; Niimura, M.; Yoshimura, M.; Mizusaki, T.; Sawama, Y.; Sajiki, H. Carbon–Carbon Bond Formation by Ligand-free Cross-Coupling Reaction Using Palladium Catalyst Supported on Synthetic Adsorbent. ChemCatChem 2012, 4, 546–558. [Google Scholar] [CrossRef]
- Monguchi, Y.; Wakayama, F.; Ueda, S.; Ito, R.; Takada, H.; Inoue, H.; Nakamura, A.; Sawama, Y.; Sajiki, H. Amphipathic monolith-supported palladium catalysts for chemoselective hydrogenation and cross-coupling reactions. RSC Adv. 2017, 7, 1833–1840. [Google Scholar] [CrossRef]
- Maegawa, T.; Kitamura, Y.; Sako, S.; Udzu, T.; Sakurai, A.; Tanaka, A.; Kobayashi, Y.; Endo, K.; Bora, U.; Kurita, T.; et al. Heterogeneous Pd/C-Catalyzed Ligand-Free, Room-Temperature Suzuki–Miyaura Coupling Reactions in Aqueous Media. Chem. Eur. J. 2007, 13, 5937–5943. [Google Scholar] [CrossRef]
- Monguchi, Y.; Ichikawa, T.; Yamada, T.; Sawama, Y.; Sajiki, H. Continuous-flow Suzuki–Miyaura and Mizoroki–Heck Reactions under Microwave Heating Conditions. Chem. Rec. 2019, 19, 3–14. [Google Scholar] [CrossRef]
- Kobayashi, S. Flow “Fine” Synthesis: High Yielding and Selective Organic Synthesis by Flow Methods. Chem. Asian J. 2016, 11, 425–436. [Google Scholar] [CrossRef]
- Masuda, K.; Ichitsuka, T.; Koumura, N.; Sato, K.; Kobayashi, S. Flow fine synthesis with heterogeneous catalysts. Tetrahedron 2018, 74, 1705–1730. [Google Scholar] [CrossRef]
- Cantillo, D.; Kappe, C.O. Immobilized Transition Metals as Catalysts for Cross-Couplings in Continuous Flow—A Critical Assessment of the Reaction Mechanism and Metal Leaching. ChemCatChem 2014, 6, 3286–3305. [Google Scholar] [CrossRef]
- Munirathinam, R.; Huskens, J.; Verboom, W. Supported Catalysis in Continuous-Flow Microreactors. Adv. Synth. Catal. 2015, 357, 1093–1123. [Google Scholar] [CrossRef]
- Len, S.; Bruniaux, S.; Delbecq, F.; Parmar, V.S. Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling in Continuous Flow. Catalysts 2017, 7, 146. [Google Scholar] [CrossRef]
- Ichitsuka, T.; Suzuki, N.; Sairenji, M.; Koumura, N.; Onozawa, S.; Sato, K.; Kobayashi, S. Readily Available Immobilized Pd Catalysts for Suzuki-Miyaura Coupling under Continuous-flow Conditions. ChemCatChem 2019, 11, 2427–2431. [Google Scholar] [CrossRef]
- De Muñouz, J.M.; Alcázar, J.; de la Hoz, A.; Díaz-Ortiz, A. Cross-Coupling in Flow Using Supported Catalysts: Mild, Clean, Efficient and Sustainable Suzuki-Miyaura Coupling in a Single Pass. Adv. Synth. Catal. 2012, 354, 3456–3460. [Google Scholar] [CrossRef]
- Martin, A.D.; Siamaki, A.R.; Belecki, K.; Gupton, B.F. A Flow-Based Synthesis of Telmisartan. J. Flow Chem. 2015, 5, 145–147. [Google Scholar] [CrossRef]
- Greenway, G.M.; Haswell, S.J.; Morgan, D.O.; Skelton, V.; Styring, P. The use of a novel microreactor for high throughput continuous flow organic synthesis. Sens. Actuators B Chem. 2000, 63, 153–158. [Google Scholar] [CrossRef]
- Pandarus, V.; Gingras, G.; Béland, F.; Ciriminna, R.; Pagliaro, M. Process Intensification of the Suzuki−Miyaura Reaction over Sol−Gel Entrapped Catalyst SiliaCat DPP-Pd Under Conditions of Continuous Flow. Org. Process Res. Dev. 2014, 18, 1550–1555. [Google Scholar] [CrossRef]
- He, P.; Haswell, S.J.; Fletcher, P.D.I. Microwave-assisted Suzuki reactions in a continuous flow capillary reactor. Appl. Catal. A Gen. 2004, 274, 111–114. [Google Scholar] [CrossRef]
- He, P.; Haswell, S.J.; Fletcher, P.D.I.; Kelly, S.M.; Mansfield, A. Scaling up of continuous-flow, microwave-assisted, organic reactions by varying the size of Pd-functionalized catalytic monoliths. Beilstein J. Org. Chem. 2011, 7, 1150–1157. [Google Scholar] [CrossRef]
- Mennecke, K.; Kirschning, A. Polyionic Polymers-Heterogeneous Media for Metal Nanoparticles as Catalyst in Suzuki-Miyaura and Heck-Mizoroki Reactions under Flow Conditions. Beilstein J. Org. Chem. 2009, 5. [Google Scholar] [CrossRef]
- Pascanu, V.; Hansen, P.R.; Bermejo-Gomez, A.; Ayats, C.; Platero-Prats, A.E.; Johansson, M.J.; Pericas, M.A.; Martin-Matute, B. Highly functionalized biaryls via Suzuki-Miyaura cross coupling catalyzed by Pd@MOF under batch and continuous flow regimes. ChemSusChem 2015, 8, 123–130. [Google Scholar] [CrossRef]
- Greco, R.; Goessler, W.; Cantillo, D.; Kappe, C.O. Benchmarking immobilized Di- and Triaryphosphine palladium catalysts for continuous-flow cross-coupling reactions: Efficiency, durability, and metal leaching studies. ACS Catal. 2015, 5, 1303–1312. [Google Scholar] [CrossRef]
- Mateos, C.; Rincón, J.A.; Martín-Hidalgo, B.; Villanueva, J. Green and scalable procedure for extremely fast ligandless Suzuki–Miyaura cross-coupling reactions in aqueous IPA using solid-supported Pd in continuous flow. Tetrahedron Lett. 2014, 55, 3701–3705. [Google Scholar] [CrossRef]
- Brinkley, K.W.; Burkholder, M.; Siamaki, A.R.; Belecki, K.; Gupton, B.F. The continuous synthesis and application of graphene supported palladium nanoparticles: A highly effective catalyst for Suzuki-Miyaura cross-coupling reactions. Green Process Synth. 2015, 4, 241–246. [Google Scholar] [CrossRef]
- Trinh, T.N.; Hizartzidis, L.; Lin, A.J.S.; Harman, D.G.; McCluskey, A.; Gordon, C.P. An efficient continuous flow approach to furnish furan-based biaryls. Org. Biomol. Chem. 2014, 12, 9562–9571. [Google Scholar] [CrossRef]
- Hattori, T.; Tsubone, A.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Palladium on carbon-catalyzed Suzuki-Miyaura coupling reaction using an efficient and continuous flow system. Catalysts 2015, 5, 18–25. [Google Scholar] [CrossRef]
- Synthetic Adsorbent Based on a Polystyrene/Divinylbenzene Copolymer HP20 Is Commercially Available. Available online: https://www.diaion.com/en/products/synthetic_adsorbents/ (accessed on 10 September 2020).
- Amphipathic and Monolithic Polystyrene/Divinylbenzene Copolymer Bearing Sulfonic Acid Moieties CM and Bearing Quaternary Ammonium Moieties AM Are Commercially Available. Available online: https://www.organo.co.jp/english/ (accessed on 10 September 2020).
- Tertiary Amine-Functionalized Weakly Basic Anion Exchange Resin WA30 Is Commercially. Available online: https://www.diaion.com/en/products/ion_exchange_resins/weakly_basic_anion/index.html (accessed on 10 September 2020).
- Yamada, T.; Kuwata, M.; Takakura, R.; Monguchi, Y.; Sajiki, H.; Sawama, Y. Organocatalytic Nitroaldol Reaction Associated with Deuterium-Labeling. Adv. Synth. Catal. 2018, 360, 637–641. [Google Scholar] [CrossRef]
- Yamada, T.; Park, K.; Ito, N.; Masuda, H.; Teranishi, W.; Cui, S.; Sajiki, H. Robust Continuous-Flow Synthesis of Deuterium-Labeled β-Nitroalcohols Catalyzed by Basic Anion Exchange Resin. Bull. Chem. Soc. Jpn. 2020, 93, 1000–1006. [Google Scholar] [CrossRef]
- Monguchi, Y.; Ichikawa, T.; Netsu, M.; Hattori, T.; Mizusaki, T.; Sawama, Y.; Sajiki, H. Tertiary-Amino-Functionalized Resin-Supported Palladium Catalyst for the Heterogeneous Suzuki-Miyaura Reaction of Aryl Chlorides. Synlett 2015, 26, 2014–2018. [Google Scholar] [CrossRef]
- Ichikawa, T.; Netsu, M.; Mizuno, M.; Mizusaki, T.; Takagi, Y.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Development of a unique heterogeneous palladium catalyst for Suzuki-Miyaura reaction using (hetero)aryl chlorides and chemoselective hydrogenation. Adv. Synth. Catal. 2017, 359, 2269–2279. [Google Scholar] [CrossRef]
- Ichikawa, T.; Mizuno, M.; Ueda, S.; Ohneda, N.; Odajima, H.; Sawama, Y.; Monguchi, Y.; Sajiki, H. A practical method for heterogeneously-catalyzed Mizoroki–Heck reaction: Flow system with adjustment of microwave resonance as an energy source. Tetrahedron 2018, 74, 1810–1816. [Google Scholar] [CrossRef]
- Wu, X.-M.; Gu, Y.-B. TBAF-Assisted Palladium-Catalyzed Suzuki Reaction in Water Under the Ligand and Base-Free Conditions. Lett. Org. Chem. 2012, 9, 396–400. [Google Scholar] [CrossRef]
- Miyashita, K.; Sakai, T.; Imanishi, T. Total Synthesis of (±)-Spiroxin C. Org. Lett. 2003, 5, 2683–2686. [Google Scholar] [CrossRef] [PubMed]
- Negishi, E.; Tobrman, T.; Rao, H.; Xu, S.; Lee, C.-T. Highly (≥98%) Selective Trisubstituted Alkene Synthesis of Wide Applicability via Fluoride-Promoted Pd-Catalyzed Cross-Coupling of Alkenylboranes. Isr. J. Chem. 2010, 50, 696–701. [Google Scholar] [CrossRef]
- Schmidt, B.; Riemer, M.; Karras, M. 2,2-Biphenols via Protecting Group-Free Thermal or Microwave-Accelerated Suzuki−Miyaura Coupling in Water. J. Org. Chem. 2013, 78, 8680–8688. [Google Scholar] [CrossRef] [PubMed]
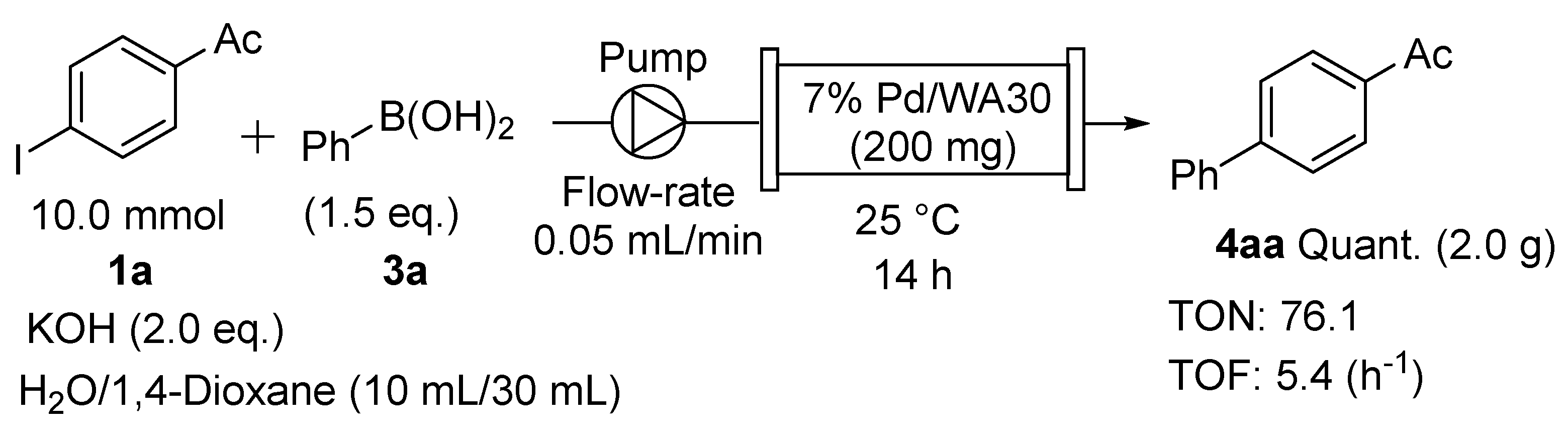
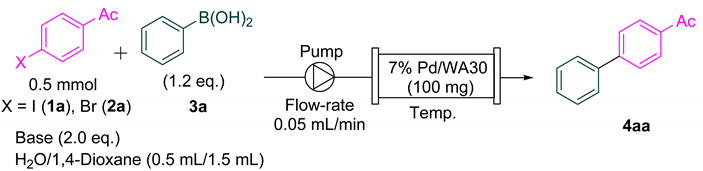
| Entry | X | Base | Temp. | 1H NMR Ratio (1a/2a:4aa) | Yield 1 |
|---|---|---|---|---|---|
| 1 | I | NaOH | 25 °C | 23:77 | 77% |
| 2 | I | KOH | 25 °C | 5:95 | 95% |
| 3 2 | I | KOH | 25 °C | 0:100 | Quant. 3,4 |
| 4 | Br | NaOH | 25 °C | 35:65 | 68% |
| 5 | Br | KOH | 25 °C | 57:43 | 43% |
| 6 | Br | NaOH | 60 °C | 26:76 | 74% |
| 7 2 | Br | NaOH | 60 °C | 0:100 | 95% 3,5 |
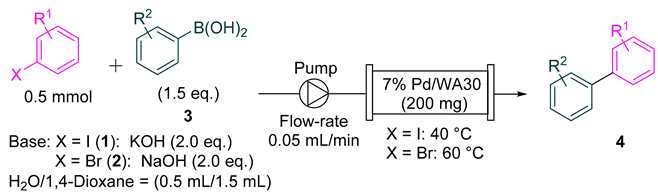
| Entry | X | R1 | R2 | Product | Yield 1 |
|---|---|---|---|---|---|
| 1 2 | I | 3-Ac (1b) | H (3a) | 4ba | Quant. (99) |
| 2 2 | 2-Ac (1c) | H (3a) | 4ca | 83% (83) | |
| 3 | 4-EtO2C (1d) | H (3a) | 4da | 89% (89) | |
| 4 | 4-Me (1e) | H (3a) | 4ea | 85% (84) | |
| 5 | 4-MeO (1f) | H (3a) | 4fa | 88% (88) | |
| 6 | H (1g) | 4-MeO (3b) | 4fa | 72% (72) | |
| 7 3 | H (1g) | 4-Ac (3c) | 4aa | Quant. (96) | |
| 8 | Br | 3-Ac (2b) | H (3a) | 4ba | 84% (81) |
| 9 | 2-Ac (2c) | H (3a) | 4ca | 57% (57) | |
| 10 | 4-EtO2C (2d) | H (3a) | 4da | 50% (50) | |
| 11 | 4-Me (2e) | H (3a) | 4ea | 70% (80) | |
| 12 | 4-MeO (2f) | H (3a) | 4fa | 48% (43) | |
| 13 4 | H (2g) | 4-MeO (3b) | 4fa | 88% (75) |
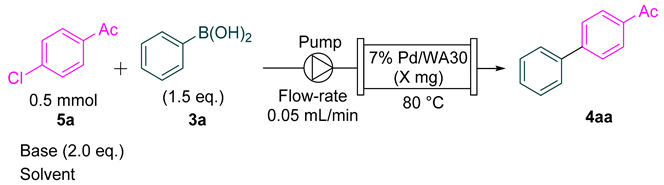
| Entry. | Base | Y (mg) | Solvent | 1H NMR Ratio (5a:4aa) | Yield 1 |
|---|---|---|---|---|---|
| 1 | NaOH | 100 | H2O/1,4-dioxane (0.5/1.5 mL) | 74:26 | 23% |
| 2 | KOH | 100 | H2O/1,4-dioxane (0.5/1.5 mL) | 81:19 | 19% |
| 3 | NatOBu | 100 | H2O/1,4-dioxane (0.5/1.5 mL) | 82:18 | 17% |
| 4 | NaOH | 500 | H2O/1,4-dioxane (0.5/1.5 mL) | 13:87 | 45% |
| 5 2 | NaOH | 500 | H2O/1,4-dioxane (0.5/1.5 mL) | 57:43 | 30% |
| 6 3 | TBAF | 100 | THF (2.0 mL) | 0:100 | 68% |
| 7 3 | TBAF | 300 | THF (2.0 mL) | 0:100 | 68% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, T.; Jiang, J.; Ito, N.; Park, K.; Masuda, H.; Furugen, C.; Ishida, M.; Ōtori, S.; Sajiki, H. Development of Facile and Simple Processes for the Heterogeneous Pd-Catalyzed Ligand-Free Continuous-Flow Suzuki–Miyaura Coupling. Catalysts 2020, 10, 1209. https://doi.org/10.3390/catal10101209
Yamada T, Jiang J, Ito N, Park K, Masuda H, Furugen C, Ishida M, Ōtori S, Sajiki H. Development of Facile and Simple Processes for the Heterogeneous Pd-Catalyzed Ligand-Free Continuous-Flow Suzuki–Miyaura Coupling. Catalysts. 2020; 10(10):1209. https://doi.org/10.3390/catal10101209
Chicago/Turabian StyleYamada, Tsuyoshi, Jing Jiang, Naoya Ito, Kwihwan Park, Hayato Masuda, Chikara Furugen, Moeka Ishida, Seiya Ōtori, and Hironao Sajiki. 2020. "Development of Facile and Simple Processes for the Heterogeneous Pd-Catalyzed Ligand-Free Continuous-Flow Suzuki–Miyaura Coupling" Catalysts 10, no. 10: 1209. https://doi.org/10.3390/catal10101209
APA StyleYamada, T., Jiang, J., Ito, N., Park, K., Masuda, H., Furugen, C., Ishida, M., Ōtori, S., & Sajiki, H. (2020). Development of Facile and Simple Processes for the Heterogeneous Pd-Catalyzed Ligand-Free Continuous-Flow Suzuki–Miyaura Coupling. Catalysts, 10(10), 1209. https://doi.org/10.3390/catal10101209