Abstract
In the palladium-catalyzed C−C coupling reaction, electron-rich phosphine ligands and a catalytic amount of catalyst loading are required in most cases. Herein, a bench-stable, easily modified and less toxic alkynone was utilized in palladium-catalyzed Sonogashira coupling to replace conventional phosphine ligands. With 1-(4-methoxyphenyl)-3-phenyl-2-yn-1-one (L2) as the ligand, catalyst loading was reduced to 5-10 ppm. In this newly developed catalytic system, a variety of (hetero)arene iodines and alkynes could be tolerated, resulting in good yields of the corresponding cross-coupling products.
1. Introduction
The development of palladium-catalyzed C−C cross-coupling reactions has attracted much attention in recent decades [1,2,3]. In conventional homogeneous catalyst systems, the loading of Pd was required at 1–10 mol% level, which significantly increased the cost of catalysts using such noble metal. Furthermore, as far as the toxicity was concerned, the overloaded palladium catalysts might contaminate C−C cross-coupling products in which the residual palladium exceeds the FDA allowable limits. To avoid excess Pd loading, a variety of new ligands were designed and synthesized to construct highly active catalytic systems for trace amounts of Pd-catalyzed coupling reactions. The employment of steric-hindered, electron-rich phosphine ligands is currently recognized as the most effective strategy to maintain the efficiency of Pd at extremely low concentrations (1–500 ppm) (Scheme 1). In 2004, Buchwald et al. used S-Phos (Scheme 1a) to promote a 0.005 mol% Pd-catalyzed Suzuki coupling reaction of brominated and chlorinated aromatic hydrocarbons [4,5]. In the same year, Kwong et al. synthesized a hemilabile phosphine ligand which stabilized the Pd catalyst and promoted oxidative addition. The ligands containing N and P reduced the amount of catalyst down to 1–500 ppm (Scheme 1b) [6,7]. The multidentate phosphine ligand promoted Sonogashira coupling reactions in water under 1–500 ppm Pd (Scheme 1c) [8,9]. Interestingly, N-phenylurea, thiourea, and amide efficiently accelerated the 1–100 ppm, Pd-catalyzed Suzuki reaction (Scheme 1d) [10]. Although these new ligands have a significant role in the trace palladium-catalyzed coupling reactions, most phosphine ligands are expensive, highly toxic, and sensitive to air and water [11,12,13]. More importantly, the strong coordination of these ligands with Pd may completely deactivate the catalyst at ppm levels.

Scheme 1.
PPM Pd catalyst for the Csp2−Csp cross-coupling reaction.
In fact, at low concentrations, Pd showed different behavior in the catalysis system. For instance, the catalytic active Pd specie does not tend to agglomerate and is easily deactivated, compared with conventional catalyst loading (1–10 mol%). Recently, a few P-free, Pd-catalyzed C−C coupling reactions were reported, clearly demonstrating the activity of atomic Pd for the oxidative addition of C−X (Cl, Br, I) bonds [14,15,16,17]. However, to maintain an efficient C−C cross-coupling catalytic cycle, the rate of the reduction elimination needs to be accelerated accordingly [14]. It has been proposed that π acidic additives might accelerate the rate of reduction elimination from the very early stage of development of Pd catalysis methodology [15]. More recently, various small organics such as olefines [16] and 1,3-dicarbonyl compounds [17], even norfloxacin [18], were introduced as additives to enhance the activity of the Pd catalyst. The aromatic and other unsaturated functional groups, such as carbonyl and double bonds, were essential to accelerate the reduction elimination. Therefore, we hypothesized that α, β-alkynone, as highly efficient additives, because their conjugated carbonyl groups and triple bonds supply ideal functional donor groups to interact with atomic Pd, possibly accelerate the reductive elimination. In this work, the activity of alkynone additives in Pd-catalyzed C−C cross-coupling reactions were reported. Comparing with classic P ligands, such as PPh3, X-Phos and XantPhos, alkynone significantly enhanced the activity of palladium at the PPM level. Furthermore, among nine aromatic alkynones, L2 bearing para-methoxy on the ketone side arm showed the strongest accelerating effect on the palladium-catalyzed Sonogashira coupling of aryl iodides and terminal alkynes. The phosphine-free, PPM, Pd-catalyzed Sonogashira coupling reactions were developed for 16 examples with satisfactory yields.
2. Results and Discussion
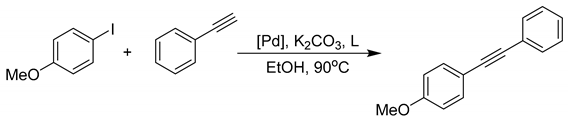
In our initial screening experiments, the Sonogashira coupling of iodobenzene with phenylacetylene was selected as a model reaction to optimize the reaction conditions. The reaction conditions, including the amount of catalyst, the type of base, the type of solvent and the reaction temperature, were investigated. The optimal conditions were obtained: PdCl2 (5 ppm), K2CO3 (2 eq.), EtOH (3 mL) and the reaction was performed at 90 °C for 24 h. To our pleasure, allkynones significantly promoted the cross-coupling of Ar-I and phenylacetylene (Scheme 2). In the control experiment without a ligand, the yield of the product was 37%. In contrast, PPh3 afforded 6% yield, whilst a highly efficient ligand, X-Phos, gave only 3% yield. Multidentate XantPhos deactivated the catalytic atomic Pd, which might be due to its stable coordination preventing the accomplishment of either the oxidative addition or the reduction elimination. These results clearly demonstrated the accelerating effect of α, β-alkynone for Pd catalyzed Csp2−Csp cross-coupling.
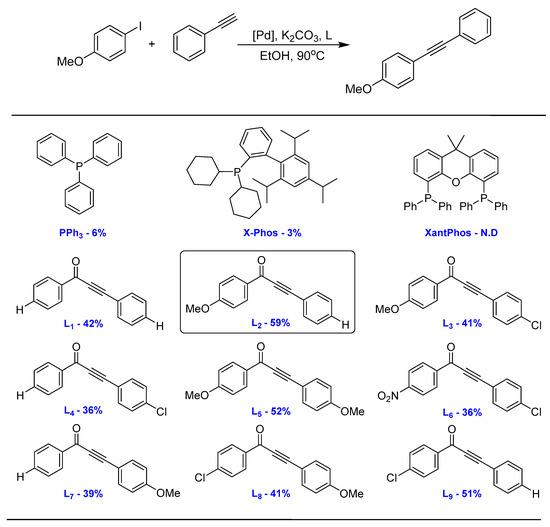
Scheme 2.
Comparison on the accelerating effect of alkynone with classic P ligands. Reaction conditions: 4-Iodoanisole (0.5 mmol), Phenylacetylene (0.6 mmol), PdCl2 (5 ppm), Ligand (5 mol%), EtOH (3 mL), K2CO3 (2 eq.), 90 °C. Determined by 1H NMR.
To understand the electronic factors of the acceleration capabilities of α, β-alkynone, nine para- substituted aromatic allkynones were prepared according to the method outlined in the literature [19] and evaluated in this reaction. The alkynone ligand L1 gave a yield of 42%. Notably, the substituent group on each aromatic ring showed a different impact on the accelerating effect of the PdCl2 pre-catalyst. For instance, the Methoxy group as the electron-donating group enhanced the activity of L2, giving the highest yield of 59%. However, L7 with methoxy on another aromatic ring gave a yield of 39%. A similar trend was observed among alkynone containing Cl as the electron-withdrawing group. L4 afforded 36%, while L9 bearing Cl on the aromatic ketone part gave a 51% yield. As for the dual substituted aromatic alkynones, the electron-donating groups on the carbonyl side showed a stronger acceleration effect than the electron-drawing groups. When alkynone contains both an electron-donating group and an electron-drawing group (L3-41%, L8-41%), the two effects canceled out and the yield was similar to the unsubstituted L1. When the carbonyl-side electron-donating group and the alkynyl-side electron-donating group were both present (L5-52%), the acceleration effect was enhanced accordingly. The alkynone L5 was more active than L1, but less active than L2. L6 showed the same yield as L4 because of the weak acceleration effect with the carbonyl-side electron-donating group. The experimental data unveiled the complicated electronic factor that governed the activity of alkynone, and further confirmed that the alkynone with methoxy group (L2) is the best for the palladium catalyst system.
Through reasonable screening of the amount of alkynone ligands, it was found that the reaction yield increased first and then decreased when increasing the amount of ligand (Table 1). When L2 increased from 0.5 to 1 mol%, the reaction yields increased. The yields did not change much at 1–5 mol% of L2. When we continued to increase L2 to 8 and 10 mol%, the reaction yields decreased. So, we chose the optimal amount of L2 as 1 mol%. Later, the reaction time was doubled to obtain the best yield of 83%.

Table 1.
The amount of ligand and reaction time of a trace-Pd-catalyzed Sonogashira coupling reaction a,b.
The scope of the reaction was subsequently explored, and the influence of the substituted aryl halides and aryl acetylene were evaluated under the optimized reaction conditions (Scheme 3). The catalytic system was suitable for both electron-rich and electron-poor aryl iodides. The electron-donating substituents, such as methyl and ethyl groups, coupled with phenylacetylene in yields of 79% and 73%, respectively. The electron-drawing substituents such as bromo- and trifluoromethyl-groups coupled with phenylacetylene in yields of 45% and 58%–70%. Due to a certain steric effect, ortho- substitution of trifluoromethyl- was more inert than para-substitution. The coupling reaction of 1-iodonaphthalene and 2-iodothiophene can also be achieved with phenylacetylene in yields of 60% and 81%, respectively. The reaction system has relatively good applicability to aryl terminal alkynes including 4-methyl-, 3-methyl-, 4-ethyl-, 4-n-butyl-, 3-chloro-, etc. The aryl terminal alkyne overcame the decrease in activity caused by the different positions of the substituents. For example, 4-methyl- and 3-methyl-phenylacetylene coupled with 4-methoxy-iodobenzene in yields of 80% and 78%, respectively, which had no significant change.
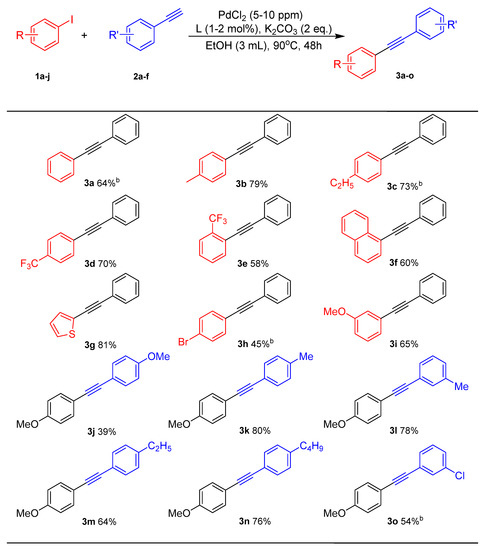
Scheme 3.
The substrate scope of α, β-alkynone accelerates the trace-Pd-catalyzed Sonogashira coupling reaction a,b. a Reaction conditions: Iodobenzene (0.5 mmol), Phenylacetylene (0.6 mmol), PdCl2 (5 ppm), L2 (1 mol%), EtOH (3 mL), K2CO3 (2 eq.), 90 °C, under air. b PdCl2 (10 ppm), L2 (2 mol%).
3. Materials and Methods
3.1. Materials
All reagents were purchased from commercial suppliers and used without further purification. Alkynes and (hetero)arene iodines were purchased from Aladdin (Shanghai, China) The Sonogashira coupling reactions were performed using a reaction vial and heating module under air atmosphere. Column chromatography purifications were performed using silica gel (200–300 mesh) and mixtures of petroleum ether/ethyl acetate as eluents. The names of all the compounds were assigned using ChemBioDraw Ultra 12.0 software. Nuclear magnetic resonance (NMR) spectra were recorded in CDCl3 solutions, unless noted otherwise, on Advance 400 MHz instruments (Madison, America).
3.2. Preparation of PdCl2 Solution
PdCl2 (8.9 mg) was dissolved in 250 mL of an aqueous hydrochloric acid solution to prepare a stock solution. When 1 ppm of PdCl2 was needed, 2.5 μL of the stock solution was added by syringe.
3.3. Synthesis and Spectroscopic Data of α, β-Alkynone Ligands
Under the protection of N2, 3 mL CH3CN was transferred into Schlenk tubes containing Pd(OAc)2 (0.01 mmol) and triazine esters (0.5 mmol). Alkynyl reagent (0.75 mmol) was added by syringe. The reaction mixture was stirred at 50 °C for 10 h. After the reaction was completed, the reaction mixture was concentrated under a vacuum. The crude product was purified by column chromatography on silica gel to afford the corresponding product [19] (see Supplementary Materials).
1,3-Diphenylprop-2-yn-1-one (L1). 1H-NMR spectrum (400 MHz, CDCl3) δ 8.24–8.22 (m, 2H), 7.70–7.67 (m, 2H), 7.65–7.62 (m, 1H), 7.54–7.46 (m, 3H), 7.44–7.40 (m, 2H). 13C-NMR spectrum (400 MHz, CDCl3) δ178.16, 136.94, 134.29, 133.20, 130.96, 129.69, 128.82, 128.76, 120.18, 93.27, 87.00.
1-(4-Methoxyphenyl)-3-phenylprop-2-yn-1-one (L2). 1H-NMR spectrum (400 MHz, CDCl3) δ 8.21–8.19 (d, 2H), 7.69–7.67 (d, 2H), 7.50–7.40 (m, 3H), 7.00–6.98 (d, 2H), 3.91 (s, 3H). 13C-NMR spectrum (400 MHz, CDCl3) δ 176.66, 164.52, 132.97, 131.98, 130.62, 130.31, 128.68, 120.35, 113.92, 92.32, 86.95, 55.61.
3-(4-Chlorophenyl)-1-(4-methoxyphenyl)prop-2-yn-1-one (L3). 1H-NMR spectrum (400 MHz, CDCl3) δ 8.18–8.16 (d, 2H), 7.56–7.33 (m, 4H), 7.00–6.98 (d, 2H), 3.90 (s, 3H). 13C-NMR spectrum (400 MHz, CDCl3) δ 176.32, 164.67, 134.58, 132.56, 132.04, 130.82, 129.94, 122.12, 113.98, 90.14, 87.48, 55.63.
3-(4-Chlorophenyl)-1-phenylprop-2-yn-1-one (L4). 1H-NMR spectrum (400 MHz, CDCl3) δ 8.22–8.20 (d, 2H), 7.66–7.61 (m, 3H), 7.55–7.51(m, 2H), 7.43–7.40 (d, 2H). 13C-NMR spectrum (400 MHz, CDCl3) δ 177.84, 137.23, 136.76, 134.27, 134.26, 129.59, 129.19, 128.69, 118.62, 91.63, 87.59.
1,3-Bis(4-methoxyphenyl)prop-2-yn-1-one (L5). 1H-NMR spectrum (400 MHz, CDCl3) δ 8.18–8.16 (d, 2H), 7.62–7.60 (d, 2H), 6.97–6.89 (dd, 4H), 3.87 (s, 3H), 3.82 (s, 3H). 13C-NMR spectrum (400 MHz, CDCl3) δ 176.74, 164.34, 161.58, 135.00, 131.86, 130.44, 114.40, 113.84, 112.10, 93.46, 86.81, 55.59, 55.44.
3-(4-Chlorophenyl)-1-(4-nitrophenyl)prop-2-yn-1-one (L6). 1H-NMR spectrum (400 MHz, CDCl3) δ 8.39–8.34 (m, 4H), 7.66–7.64 (d, 2H), 7.46–7.44(d, 2H). 13C-NMR spectrum (400 MHz, CDCl3) δ 177.72, 150.95, 140.86, 138.00, 134.45, 129.39, 123.93, 117.85, 93.90, 87.19.
3-(4-Methoxyphenyl)-1-phenylprop-2-yn-1-one (L7). 1H-NMR spectrum (400 MHz, CDCl3) δ 8.22–8.20 (d, 2H), 7.64–7.58 (m, 3H), 7.52–7.48 (m, 2H), 6.92–6.90 (d, 2H), 3.83 (s, 3H). 13C-NMR spectrum (400 MHz, CDCl3) δ 178.02, 161.78, 137.06, 135.17, 133.94, 129.48, 128.59, 114.47, 111.86, 94.38, 86.92, 55.45.
1-(4-Chlorophenyl)-3-(4-methoxyphenyl)prop-2-yn-1-one (L8). 1H-NMR spectrum (400 MHz, CDCl3) δ 8.14–8.12 (d, 2H), 7.63–7.61 (d, 2H), 7.48–7.45 (d, 2H), 6.93–6.91 (d, 2H), 3.84 (s, 3H). 13C-NMR spectrum (400 MHz, CDCl3) δ 176.64, 161.90, 140.44, 135.48, 135.23, 130.78, 128.93, 114.50, 111.62, 94.92, 86.65, 55.47.
1-(4-Chlorophenyl)-3-phenylprop-2-yn-1-one (L9). 1H-NMR spectrum (400 MHz, CDCl3) δ 8.05–8.03 (d, 2H), 7.58–7.56 (m, 2H), 7.38–7.29 (m, 5H). 13C-NMR spectrum (400 MHz, CDCl3) δ 176.65, 140.76, 135.38, 133.17, 131.05, 130.92, 129.06, 128.81, 119.96, 93.69, 86.68.
3.4. General Procedure for a Sonogashira Cross-Coupling Reaction
For a typical Sonogashira cross-coupling reaction, (hetero)arene iodines (0.5 mmol), alkynes (0.6 mmol), PdCl2 (5–10 ppm), L2 (1–2 mol%), K2CO3 (2 eq.), EtOH (3 mL) were added to a reaction tube, and stirred at 90 °C for 48 h under air. After the reaction was completed, the reaction mixture was concentrated under a vacuum. The crude product was purified by column chromatography on silica gel to afford the corresponding product.
4. Conclusions
In summary, α, β-alkynone were evaluated as ligands in the palladium-catalyzed Sonogashira coupling reaction. The best results were obtained by using alkynone (L2) as the ligand, and the PdCl2 loading was dramatically reduced to 5-10 ppm. One of the possibilities about the accelerating effect of the alkynone is that the π-acidicity of the ligand promoted the reductive elimination step. Compared with the conventional phosphine ligands, alkynones are bench stable, easily modified and less toxic. The mild reaction conditions of PPM-Pd catalysts were compatible with many function groups on both aryl iodines and terminal alkynes, such as alkyl, trifluoromethyl, fused aromatic, as well as halogens, flourishing diphenylacetylene derivatives in good yields. Further mechanistic studies and application of the catalytic system to other palladium-catalyzed reactions are under way.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/3/302/s1, more optimization of reaction conditions, the 1H- and 13C-NMR spectra of α, β-alkynone ligands, the analytical and spectroscopic data of Sonogashira cross-coupling products, the 1H- and 13C-NMR spectra of Sonogashira cross-coupling products.
Author Contributions
Conceptualization, Z.X., and W.Z.; methodology, M.G., Z.W. and W.Z.; validation, M.G., Z.W., J.Y. and Z.X.; formal analysis, Z.X., and W.Z.; investigation, M.G. and Z.W.; writing—original draft preparation, M.G.; writing—review and editing, Z.W. and J.Y.; supervision, Z.X. and W.Z.; project administration, W.Z.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the grant from National Natural Science Foundation of China (21771122, 21602129), the 111 Project (B14041), Key Research and Development Project of Shaanxi Science and Technology Department (2017SF-064, 2017GY-124), Fundamental Research Funds for the Central Universities under Grant (GK201903026), Projects of Xi′an Modern Institute of Chemistry (204-J-2018-315-4/6, 204-J-2019-0387-3/6-6).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mañas, M.M.; Pleixats, R. Formation of Carbon-Carbon Bonds under Catalysis by Transition-Metal Nanoparticles. Acc. Chem. Res. 2003, 36, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Árpád, M. Efficient, selective, and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar]
- Bej, A.; Ghosh, K.; Sarkar, A.; Knight, D.W. Palladium nanoparticles in the catalysis of coupling reactions. RSC Adv. 2016, 6, 11446–11453. [Google Scholar] [CrossRef]
- Walker, S.D.; Barder, T.E.; Martinelli, J.R.; Buchwald, S.L. A rationally designed universal catalyst for Suzuki-Miyaura coupling processes. Angew. Chem. Int. Ed. 2004, 43, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Barder, T.E.; Walker, S.D.; Martinelli, J.R.; Buchwald, S.L. Catalysts for Suzuki-Miyaura coupling processes: Scope and studies of the effect of ligand structure. J. Am. Chem. Soc. 2005, 127, 4685–4696. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.M.; So, C.M.; Chung, K.H.; Luk, C.H.; Lau, C.P.; Kwong, F.Y. P,N-Type benzimidazolyl phosphine ligands for the palladium-catalyzed Suzuki coupling of potassium aryltrifluoroborates and aryl chlorides. Tetrahedron Lett. 2012, 53, 3754–3757. [Google Scholar] [CrossRef]
- Wong, S.M.; So, C.M.; Chung, K.H.; Lau, C.P.; Kwong, F.Y. An efficient class of P,N-Type “PhMezole-phos” ligands: Applications in palladium-catalyzed Suzuki coupling of aryl chlorides. Eur. J. Org. Chem. 2012, 2012, 4172–4177. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, W.; Jiang, Z.J.; Fu, H.Y.; Zheng, X.L.; Zhang, C.C.; Chen, H.; Li, R.X. Pd/tetraphosphine catalytic system for Cu-free Sonogashira reaction “on water”. Catal. Sci. Technol. 2014, 4, 746–751. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, W.; Jiang, Z.J.; Wang, K.; Zheng, X.L.; Fu, H.Y.; Chen, H.; Li, R.X. One-pot synthesis of 2-substituted benzo[b]furans via Pd-tetraphosphine catalyzed coupling of 2-halophenols with alkynes. Chem. Commun. 2014, 50, 6023–6026. [Google Scholar] [CrossRef] [PubMed]
- Keesara, S.; Parvathaneni, S.; Mandapati, M.R. N,N’-Mono substituted acyclic thioureas: Efficient ligands for the palladium catalyzed Heck reaction of deactivated aryl bromides. Tetrahedron Lett. 2014, 55, 6769–6772. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W.N.M.; Chadwick, J.C. Homogeneous Catalysts, Activity-Stability-Deactivation; Wiley-VCH: Wein-heim, Germany, 2011; pp. 8–23. [Google Scholar]
- Cai, Y.; Lu, Y.; Liu, Y.; He, M.; Wan, Q. Efficient Heck reactions catalyzed by a palladium/diol-imidazolium salt in aerial atmosphere. Catal. Commun. 2008, 9, 1209–1213. [Google Scholar] [CrossRef]
- Chaudhary, A.R.; Bedekar, A.V. 1-(a-Aminobenzyl)-2-naphthol as phosphinefree ligand for Pd-catalyzed Suzuki and one-pot Wittig-Suzuki reaction. Appl. Organometal. Chem. 2012, 26, 430–437. [Google Scholar] [CrossRef]
- Xu, H.J.; Tang, L.; Zhang, B.; Zheng, F.Y.; Feng, Y.S. Recent progress in palladium catalyzed coupling reactions mediated by phosphine-free ligands. Chin. J. Org. Chem. 2010, 30, 211–219. [Google Scholar]
- Uchino, M.; Yamamoto, A.; Ikeda, S. Preparation of a phenyl-nickel complex, phenyl (dipyridyl) nickel chloride, an olefin dimerization catalyst. J. Org. Chem. 1970, 24, C63–C64. [Google Scholar] [CrossRef]
- Giovannini, R.; Stüdemann, T.; Dussin, G.; Knochel, P. An efficient Nickle-catalyzed cross-coupling between sp3 carbon centers. Angew. Chem. Int. Ed. 1998, 37, 2387–2390. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Liu, L.; Guo, Q.X. 1,3-Dicarbonyl compounds as phosphine-free ligands for Pd-catalyzed Heck and Suzuki reactions. Chin. Chem. Lett. 2007, 18, 625–628. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, L. Fluoroquinolones as effective ligands for palladium catalyzed heck reactions. Chin. J. Org. Chem. 2008, 28, 1655–1659. [Google Scholar]
- Yu, B.; Sun, H.M.; Xie, Z.Y.; Zhang, G.F.; Xu, L.W.; Zhang, W.Q.; Gao, Z.W. Privilege ynone synthesis via palladium-catalyzed alkynylation of “super-active esters”. Org. Lett. 2015, 17, 3298–3301. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).