Gaps in Diagnosis, Treatment, and Outcomes Among Patients with Brain Tumors in the United States: A State-of-the-Art Review
Simple Summary
Abstract
1. Introduction
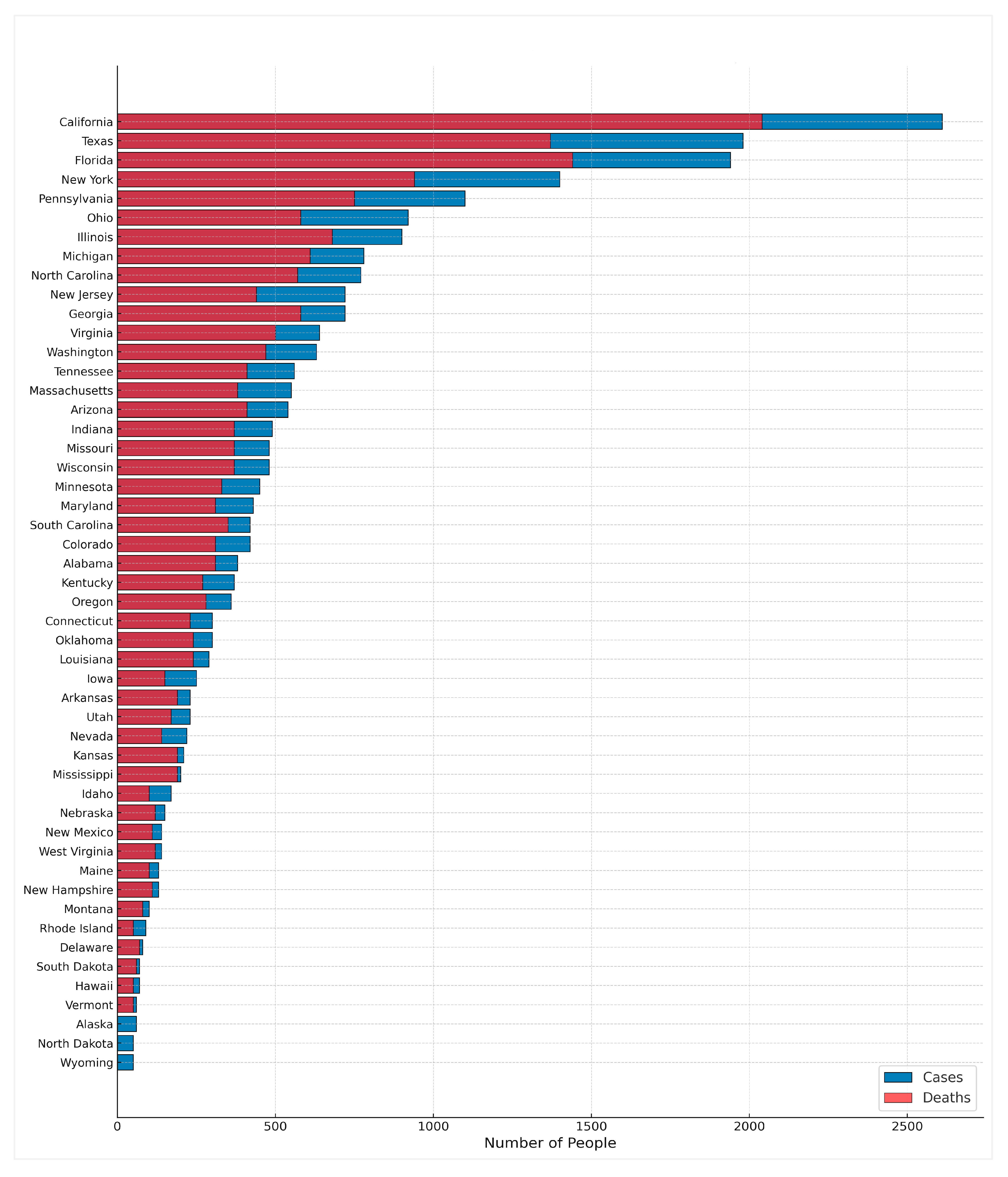
2. Gaps in Diagnosis
3. Gaps in Treatment
| Determinant | Mechanism | Impact on Treatment | References |
|---|---|---|---|
| Socioeconomic Status | Income, education, neighborhood disadvantage; financial toxicity and employment loss | Reduced access to surgery, RT, chemotherapy; omission of systemic therapy; delays in care | [38,39,40,56] |
| Insurance Coverage | Type and adequacy of insurance | Influences multimodal therapy and timeliness of RT | [63,64] |
| Geographic Location | Rurality; distance to specialty centers; uneven distribution of trial-supporting infrastructure | Limited neurosurgical access; higher mortality | [6,44,45] |
| Race/Ethnicity | Structural inequities; referral patterns | Lower likelihood of aggressive or timely treatment; higher perioperative morbidity; lower rates of molecular testing | [49,50,51,55,62] |
| Health Literacy | Ability to navigate diagnosis and treatment | Delayed presentation; reduced adherence | [20,21,22,25,26,27] |
| Referral Pathways | Variation in provider referrals and facility type | Affects access to surgery, SRS, advanced care | [9,49,50,51] |
| Access to Advanced Modalities | Institutional capability | Unequal access to genomic testing and treatments such as SRS, TTFields | [10,28,29] |
| Clinical Trial Access | Structural and logistic barriers; limited infrastructure; provider bias; inadequate outreach | Low enrollment among minorities, uninsured, and rural patients; limited access to novel therapies | [45,57,58,59,60,61,62,65] |
| Supportive Care Infrastructure | Availability of medications, rehabilitation | Impacts QoL, symptom burden, and treatment tolerance; disparities in access to brain tumor–specific palliative care and rehabilitation | [10,52,65] |
4. Gaps in Outcomes
| Study | Study Type | Population | SES Measure | Key Findings |
|---|---|---|---|---|
| Di Nunno et al., 2024 [68] | Systematic Review/Meta-Analysis | 143,303 GBM patients | Economic income | Lower economic income is associated with poorer survival (pooled HR 1.09, 95% CI: 1.02–1.17) |
| Estevez-Ordoñez et al., 2023 [69] | Retrospective Cohort Study | 995 GBM patients (2008–2019) | Patient household income level (categorized low/middle/high); Insurance type; (also analyzed race) | African Americans had longer overall survival (aHR = 0.37) but significantly worse outcomes in low-SES groups (uninsured Black vs. White patients, HR = 15.6) |
| Ramapriyan et al., 2023 [70] | Retrospective Cohort Study | 29,609 GBM patients | Race and social determinants of health | Non-White patients predominantly composed the lowest SES quartile; higher SES is associated with better access to care and outcomes |
| Dy et al., 2022 [71] | Systematic Review/Meta-Analysis | 2552 GBM patients in low- and middle-income countries | Country income level | Patients in low- and middle-income countries have shorter survival times compared to those in high-income countries |
| Rivera Perla et al., 2022 [72] | Retrospective (multi-center) Study | 434 adult GBM patients (2012–2017) | ADI: neighborhood SES composite (top 66% vs. bottom 33% percentile) | GBM patients from high-ADI areas had lower odds of gross total resection (aOR = 0.43) and reduced use of adjuvant therapies, though survival differences were not significant |
| Bower et al., 2020 [73] | Retrospective (Single center) Study | 312 high-grade glioma patients (1999–2017) | Residential community income level (ZIP code median income above vs. below state median) | Patients from low-income areas had shorter OS (15.6 vs. 19.7 months) and living in high-income communities reduced mortality risk by 25% (HR = 0.75, p < 0.05) after adjustment |
| Liu et al., 2020 [66] | Retrospective Cohort Study | 28,952 GBM patients | Median household income | Higher median household income is associated with better overall survival; racial differences also influence outcomes |
| Ostrom et al., 2018 [74] | Retrospective Cohort Study | 244,808 glioma patients of which 150,631 (61.5%) had GBM | Race/Ethnicity | Non-Hispanic whites have higher glioma incidence, but lower survival rates, compared to other racial/ethnic groups |
| Pollom et al., 2018 [63] | Retrospective Cohort Study | 12,738 adult GBM patients (2010–2013) with chemoradiation | Area-level median household income (by ZIP code; above vs. below $48,000/year); Insurance status | Lower-income and uninsured/Medicaid patients had delays >35 days in starting radiotherapy, which correlated with worse survival. Patients from higher-income areas were more likely to receive timely RT (within 35 days) after surgery, conferring a survival benefit |
| Rong et al., 2016 [64] | Retrospective (SEER analysis) Study | 13,665 adult GBM patients (2007–2012) | Insurance status (Uninsured, Medicaid vs. non-Medicaid insured) | Uninsured (HR = 1.14) and Medicaid (HR = 1.10) patients had shorter survival than privately insured ones, with better survival outcomes over time seen only among the insured |
5. Addressing Existing Gaps and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHRQ | Agency for Healthcare Research and Quality |
| ASCO | American Society of Clinical Oncology |
| CBTRUS | Central Brain Tumor Registry of the United States |
| CNS | Central Nervous System |
| DALY | Disability-Adjusted Life Year |
| GBM | Glioblastoma |
| GLOBOCAN | Global Cancer Observatory |
| MRI | Magnetic Resonance Imaging |
| NCCN | National Comprehensive Cancer Network |
| NGS | Next-Generation Sequencing |
| PSI | Psychological Stress Index |
| SDI | Socio-demographic Index |
| SEER | Surveillance, Epidemiology, and End Results |
| SES | Socioeconomic Status |
References
- Fan, Y.; Zhang, X.; Gao, C.; Jiang, S.; Wu, H.; Liu, Z.; Dou, T. Burden and trends of brain and central nervous system cancer from 1990 to 2019 at the global, regional, and country levels. Arch. Public Health 2022, 80, 209. [Google Scholar] [CrossRef]
- Society, A.C. Key Statistics for Brain and Spinal Cord Tumors. Available online: https://www.cancer.org/cancer/types/brain-spinal-cord-tumors-adults/about/key-statistics.html? (accessed on 12 April 2025).
- Curry, W.T.; Barker, F.G. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J. Neuro-Oncol. 2009, 93, 25–39. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Delavar, A.; Al Jammal, O.M.; Maguire, K.R.; Wali, A.R.; Pham, M.H. The impact of rural residence on adult brain cancer survival in the United States. J. Neuro-Oncol. 2019, 144, 535–543. [Google Scholar] [CrossRef]
- Deorah, S.; Lynch, C.F.; Sibenaller, Z.A.; Ryken, T.C. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg. Focus 2006, 20, E1. [Google Scholar] [CrossRef]
- Delavar, A.; Wali, A.R.; Santiago-Dieppa, D.R.; Al Jammal, O.M.; Kidwell, R.L.; Khalessi, A.A. Racial and ethnic disparities in brain tumour survival by age group and tumour type. Br. J. Neurosurg. 2022, 36, 705–711. [Google Scholar] [CrossRef]
- Puthenpura, V.; Canavan, M.E.; Poynter, J.N.; Roth, M.; Pashankar, F.D.; Jones, B.A.; Marks, A.M. Racial/ethnic, socioeconomic, and geographic survival disparities in adolescents and young adults with primary central nervous system tumors. Pediatr. Blood Cancer 2021, 68, e28970. [Google Scholar] [CrossRef] [PubMed]
- Kehm, R.D.; Spector, L.G.; Poynter, J.N.; Vock, D.M.; Altekruse, S.F.; Osypuk, T.L. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer 2018, 124, 4090–4097. [Google Scholar] [CrossRef] [PubMed]
- Zavala, V.A.; Bracci, P.M.; Carethers, J.M.; Carvajal-Carmona, L.; Coggins, N.B.; Cruz-Correa, M.R.; Davis, M.; de Smith, A.J.; Dutil, J.; Figueiredo, J.C. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 2021, 124, 315–332. [Google Scholar] [PubMed]
- International Agency for Research on Cancer (IARC); World Health Organization. Global Cancer Observatory (GCO); IARC: Lyon, France, 2020. Available online: https://gco.iarc.fr (accessed on 8 December 2025).
- CBTRUS. CBTRUS Fact Sheet. Available online: https://cbtrus.org/cbtrus-fact-sheet/ (accessed on 8 December 2025).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Price, M.; Neff, C.; Nagarajan, N.; Kruchko, C.; Waite, K.A.; Cioffi, G.; Cordeiro, B.B.; Willmarth, N.; Penas-Prado, M.; Gilbert, M.R. CBTRUS Statistical Report: American Brain Tumor Association & NCI Neuro-Oncology Branch Adolescent and Young Adult Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro-Oncol. 2024, 26, iii1–iii53. [Google Scholar]
- Plascak, J.J.; Fisher, J.L. Area-based socioeconomic position and adult glioma: A hierarchical analysis of surveillance epidemiology and end results data. PLoS ONE 2013, 8, e60910. [Google Scholar] [CrossRef]
- Patel, A.P.; Fisher, J.L.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Agius, D.; Alahdab, F.; Alam, T. Global, regional, and national burden of brain and other CNS cancer, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J. Global surveillance of trends in cancer survival 2000–2014 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Girardi, F.; Matz, M.; Stiller, C.; You, H.; Marcos Gragera, R.; Valkov, M.Y.; Bulliard, J.-L.; De, P.; Morrison, D.; Wanner, M. Global survival trends for brain tumors, by histology: Analysis of individual records for 556,237 adults diagnosed in 59 countries during 2000–2014 (CONCORD-3). Neuro-Oncol. 2023, 25, 580–592. [Google Scholar] [PubMed]
- Saadi, A.; Himmelstein, D.U.; Woolhandler, S.; Mejia, N.I. Racial disparities in neurologic health care access and utilization in the United States. Neurology 2017, 88, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Brewster, R.; Deb, S.; Pendharkar, A.V.; Ratliff, J.; Li, G.; Desai, A. The effect of socioeconomic status on age at diagnosis and overall survival in patients with intracranial meningioma. Int. J. Neurosci. 2022, 132, 413–420. [Google Scholar] [CrossRef]
- Bytnar, J.A.; Lin, J.; Shriver, C.D.; Zhu, K. Racial differences in brain cancer characteristics and survival: An analysis of SEER data. Cancer Causes Control 2019, 30, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.; Pfister, S.M.; Reifenberger, G. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Omuro, A.M.; Leite, C.C.; Mokhtari, K.; Delattre, J.-Y. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006, 5, 937–948. [Google Scholar] [CrossRef] [PubMed]
- De la Garza Ramos, R.; Benton, J.A.; Gelfand, Y.; Echt, M.; Rodriguez, J.V.F.; Yanamadala, V.; Yassari, R. Racial disparities in clinical presentation, type of intervention, and in-hospital outcomes of patients with metastatic spine disease: An analysis of 145,809 admissions in the United States. Cancer Epidemiol. 2020, 68, 101792. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.C.; de Souza Carvalho, G.; Chaves, F.; de Marchi, P.; de Castro, G., Jr.; Baldotto, C.; Mascarenhas, E.; Pacheco, P.; Gomes, R.; Werutsky, G. Non–small-cell lung cancer with CNS metastasis: Disparities from a real-world analysis (GBOT-LACOG 0417). JCO Glob. Oncol. 2022, 8, e2100333. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Douglas, M.P.; Wordsworth, S.; Buchanan, J.; Marshall, D.A. Availability and funding of clinical genomic sequencing globally. BMJ Glob. Health 2021, 6, e004415. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Francis, S.S.; Barnholtz-Sloan, J.S. Epidemiology of brain and other CNS tumors. Curr. Neurol. Neurosci. Rep. 2021, 21, 68. [Google Scholar] [CrossRef]
- Sahu, B.; Madani, G. Imaging inequality: Exploring the differences in radiology between high-and low-income countries. Clin. Radiol. 2024, 79, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Leece, R.; Xu, J.; Ostrom, Q.T.; Chen, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro-Oncol. 2017, 19, 1553–1564. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Piñeros, M.; Soerjomataram, I.; Deltour, I.; Bray, F. Cancers of the brain and CNS: Global patterns and trends in incidence. Neuro-Oncol. 2017, 19, 270–280. [Google Scholar] [CrossRef]
- Bell, J.S.; Koffie, R.M.; Rattani, A.; Dewan, M.C.; Baticulon, R.E.; Qureshi, M.M.; Wahjoepramono, E.J.; Rosseau, G.; Park, K.; Nahed, B.V. Global incidence of brain and spinal tumors by geographic region and income level based on cancer registry data. J. Clin. Neurosci. 2019, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-Oncol. 2020, 22, iv1–iv96. [Google Scholar] [CrossRef]
- Shakir, M.; Shariq, S.F.; Tahir, I.; Khowaja, A.H.; Irshad, H.A.; Rae, A.I.; Hamzah, R.; Gupta, S.; Park, K.B.; Enam, S.A. Challenges to early detection of brain tumors in Low-and Middle-Income countries: A systematic review. World Neurosurg. 2024, 191, 68–80. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Egan, K.M.; Nabors, L.B.; Gerke, T.; Thompson, R.C.; Olson, J.J.; LaRocca, R.; Chowdhary, S.; Eckel-Passow, J.E.; Armstrong, G. Glioma risk associated with extent of estimated European genetic ancestry in African Americans and Hispanics. Int. J. Cancer 2020, 146, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L. Brain and other central nervous system tumor statistics, 2021. CA A Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef]
- Dutta, R.; Vallurupalli, M.; McVeigh, Q.; Huang, F.W.; Rebbeck, T.R. Understanding inequities in precision oncology diagnostics. Nat. Cancer 2023, 4, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Li, G.; Bhambhvani, H.; Hayden-Gephart, M. Socioeconomic disparities in brain metastasis survival and treatment: A population-based study. World Neurosurg. 2022, 158, e636–e644. [Google Scholar] [CrossRef] [PubMed]
- Bhambhvani, H.P.; Rodrigues, A.J.; Medress, Z.A.; Hayden Gephart, M. Racial and socioeconomic correlates of treatment and survival among patients with meningioma: A population-based study. J. Neuro-Oncol. 2020, 147, 495–501. [Google Scholar] [CrossRef]
- Carstam, L.; Rydén, I.; Gulati, S.; Rydenhag, B.; Henriksson, R.; Salvesen, Ø.; Smits, A.; Jakola, A.S. Socioeconomic factors affect treatment delivery for patients with low grade glioma: A Swedish population-based study. J. Neuro-Oncol. 2020, 146, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Wrensch, M.; Minn, Y.; Chew, T.; Bondy, M.; Berger, M.S. Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro-Oncol. 2002, 4, 278–299. [Google Scholar] [CrossRef] [PubMed]
- Hartley, D. Rural health disparities, population health, and rural culture. Am. J. Public Health 2004, 94, 1675–1678. [Google Scholar] [CrossRef]
- Reschovsky, J.D.; Staiti, A.B. Access and quality: Does rural America lag behind? Health Aff. 2005, 24, 1128–1139. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Kruchko, C.; Barnholtz-Sloan, J.S. Primary brain and other central nervous system tumors in Appalachia: Regional differences in incidence, mortality, and survival. J. Neuro-Oncol. 2019, 142, 27–38. [Google Scholar] [CrossRef]
- Kim, Y.; Armstrong, T.S.; Gilbert, M.R.; Celiku, O. Disparities in the availability of and access to neuro-oncology trial-supporting infrastructure in the United States. J. Natl. Cancer Inst. 2025, 117, 511–516. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar]
- Mendoza, J.; Pangal, D.J.; Cardinal, T.; Bonney, P.A.; Lechtholz-Zey, E.; Strickland, B.A.; Giannotta, S.; Zada, G. Systematic review of racial, socioeconomic, and insurance status disparities in neurosurgical care for intracranial tumors. World Neurosurg. 2022, 158, 38–64. [Google Scholar] [CrossRef]
- Pham, J.; Sisti, J.; Cote, D.J.; Kang, K.; Briggs, R.G.; Gomez, D.; Shah, I.; Lawler, S.E.; Chen, C.C.; Attenello, F. Trends in glioblastoma treatment, survival, and disparities in access to care in the United States from 2004 to 2019: A National Cancer Database analysis. J. Neurosurg. 2025, 143, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.H.; Bhaskara, M.; Deysher, D.; Sadeh, M.; Souter, J.; Mehta, A.I. Racial disparities in incidence, treatment, and survival in adult brain metastases: A 10-year national database analysis. Neurosurg. Focus. 2023, 55, E6. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.T.; Golzarian, S.; Johnson, R.; Fellows, E.; Dhawan, S.; Chen, C.C.; Marcotte, E.L.; Venteicher, A.S. Racial disparities in recommendations for surgical resection of primary brain tumours: A registry-based cohort analysis. Lancet 2022, 400, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Dressler, E.V.; Liu, M.; Garcia, C.R.; Dolecek, T.A.; Pittman, T.; Huang, B.; Villano, J.L. Patterns and disparities of care in glioblastoma. Neuro-Oncol. Pract. 2019, 6, 37–46. [Google Scholar] [CrossRef]
- Muhlestein, W.E.; Akagi, D.S.; Chotai, S.; Chambless, L.B. The impact of race on discharge disposition and length of hospitalization after craniotomy for brain tumor. World Neurosurg. 2017, 104, 24–38. [Google Scholar] [CrossRef]
- McCray, E.; Waguia, R.; De la Garza Ramos, R.; Price, M.J.; Williamson, T.; Dalton, T.; Sciubba, D.M.; Yassari, R.; Goodwin, A.N.; Fecci, P. Racial disparities in inpatient clinical presentation, treatment, and outcomes in brain metastasis. Neuro-Oncol. Pract. 2023, 10, 62–70. [Google Scholar] [CrossRef]
- Gao, S.; Jin, L.; Moliterno, J.; Corbin, Z.A.; Bindra, R.S.; Contessa, J.N.; James, B.Y.; Park, H.S. Impact of radiotherapy delay following biopsy for patients with unresected glioblastoma. J. Neurosurg. 2022, 138, 610–620. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Krebs, H.L.; Patil, N.; Cioffi, G.; Barnholtz-Sloan, J.S. Racial/ethnic disparities in treatment pattern and time to treatment for adults with glioblastoma in the US. J. Neuro-Oncol. 2021, 152, 603–615. [Google Scholar] [CrossRef]
- Xu, F.; Hua, X.; Wang, M.; Cao, W.; Wang, S.; Xu, C.; Chen, J.; Gao, Y.; Chen, L.; Ni, W. Racial and social-economic inequalities in systemic chemotherapy use among adult glioblastoma patients following surgery and radiotherapy. Sci. Rep. 2024, 14, 19079. [Google Scholar] [CrossRef]
- Lee, E.Q.; Chukwueke, U.N.; Hervey-Jumper, S.L.; De Groot, J.F.; Leone, J.P.; Armstrong, T.S.; Chang, S.M.; Arons, D.; Oliver, K.; Verble, K. Barriers to accrual and enrollment in brain tumor trials. Neuro-Oncol. 2019, 21, 1100–1117. [Google Scholar] [CrossRef]
- Unger, J.M.; Vaidya, R.; Hershman, D.L.; Minasian, L.M.; Fleury, M.E. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J. Natl. Cancer Inst. 2019, 111, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Sullenger, R.D.; Deal, A.M.; Grilley Olson, J.E.; Matson, M.; Swift, C.; Lux, L.; Smitherman, A.B. Health insurance payer type and ethnicity are associated with cancer clinical trial enrollment among adolescents and young adults. J. Adolesc. Young Adult Oncol. 2022, 11, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Colon-Otero, G.; Smallridge, R.C.; Solberg, L.A., Jr.; Keith, T.D.; Woodward, T.A.; Willis, F.B.; Dunn, A.N. Disparities in participation in cancer clinical trials in the United States: A symptom of a healthcare system in crisis. Cancer 2008, 112, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.J.; Couillard, S.A.; Arons, D.F.; Yung, W.K.A.; Vogelbaum, M.; Wen, P.Y.; Kingston, A.E. HOUT-15. Brain tumor patient and caregiver survey on clinical trials: Identifying attitudes and barriers to patient participation. Neuro-Oncol. 2017, 19, vi109. [Google Scholar] [CrossRef]
- Reihl, S.J.; Patil, N.; Morshed, R.A.; Mehari, M.; Aabedi, A.; Chukwueke, U.N.; Porter, A.B.; Fontil, V.; Cioffi, G.; Waite, K. A population study of clinical trial accrual for women and minorities in neuro-oncology following the NIH Revitalization Act. Neuro-Oncol. 2022, 24, 1341–1349. [Google Scholar] [CrossRef]
- Pollom, E.L.; Fujimoto, D.K.; Han, S.S.; Harris, J.P.; Tharin, S.A.; Soltys, S.G. Newly diagnosed glioblastoma: Adverse socioeconomic factors correlate with delay in radiotherapy initiation and worse overall survival. J. Radiat. Res. 2018, 59, i11–i18. [Google Scholar] [CrossRef]
- Rong, X.; Yang, W.; Garzon-Muvdi, T.; Caplan, J.M.; Hui, X.; Lim, M.; Huang, J. Influence of insurance status on survival of adults with glioblastoma multiforme: A population-based study. Cancer 2016, 122, 3157–3165. [Google Scholar] [CrossRef]
- Porter, A.B.; Lachance, D.H.; Johnson, D.R. Socioeconomic status and glioblastoma risk: A population-based analysis. Cancer Causes Control 2015, 26, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.K.; Yu, S.; Sulman, E.P.; Kurz, S.C. Racial and socioeconomic disparities differentially affect overall and cause-specific survival in glioblastoma. J. Neuro-Oncol. 2020, 149, 55–64. [Google Scholar] [CrossRef]
- Foster, H.; Polz, P.; Mair, F.; Gill, J.; O’Donnell, C.A. Understanding the influence of socioeconomic status on the association between combinations of lifestyle factors and adverse health outcomes: A systematic review protocol. BMJ Open 2021, 11, e042212. [Google Scholar] [CrossRef]
- Di Nunno, V.; Gatto, L.; Aprile, M.; Bartolini, S.; Tosoni, A.; Franceschi, E. Economic income and survival in patients affected by glioblastoma: A systematic review and meta-analysis. Neuro-Oncol. Pract. 2024, 11, 546–555. [Google Scholar] [CrossRef]
- Estevez-Ordonez, D.; Abdelrashid, M.; Coffee, E.; Laskay, N.M.; Atchley, T.J.; Chkheidze, R.; Fiveash, J.B.; Markert, J.M.; Lobbous, M.; Maveal, B.M. Racial and socioeconomic disparities in glioblastoma outcomes: A single-center, retrospective cohort study. Cancer 2023, 129, 3010–3022. [Google Scholar] [CrossRef]
- Ramapriyan, R.; Ramesh, T.; Yu, H.; Richardson, L.G.; Nahed, B.V.; Carter, B.S.; Barker, F.G.; Curry, W.T.; Choi, B.D. County-level disparities in care for patients with glioblastoma. Neurosurg. Focus 2023, 55, E12. [Google Scholar] [CrossRef] [PubMed]
- Dy, L.F.; Ong, E.P.; Espiritu, A.I.; Spears, J.; Omar, A.T. Survival times of patients with glioblastoma in low-and middle-income countries: A systematic review and meta-analysis. Neurosurg. Rev. 2022, 45, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Rivera Perla, K.M.; Tang, O.Y.; Durfey, S.N.; Vivas-Buitrago, T.; Sherman, W.J.; Parney, I.; Uhm, J.H.; Porter, A.B.; Elinzano, H.; Toms, S.A. Predicting access to postoperative treatment after glioblastoma resection: An analysis of neighborhood-level disadvantage using the Area Deprivation Index (ADI). J. Neuro-Oncol. 2022, 158, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.; Hsu, F.-C.; Weaver, K.E.; Yelton, C.; Merrill, R.; Wicks, R.; Soike, M.; Hutchinson, A.; McTyre, E.; Laxton, A. Community economic factors influence outcomes for patients with primary malignant glioma. Neuro-Oncol. Pract. 2020, 7, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cote, D.J.; Ascha, M.; Kruchko, C.; Barnholtz-Sloan, J.S. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018, 4, 1254–1262. [Google Scholar] [CrossRef]
- Cote, D.J.; Ostrom, Q.T.; Gittleman, H.; Duncan, K.R.; CreveCoeur, T.S.; Kruchko, C.; Smith, T.R.; Stampfer, M.J.; Barnholtz-Sloan, J.S. Glioma incidence and survival variations by county-level socioeconomic measures. Cancer 2019, 125, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Wanner, M.; Rohrmann, S.; Korol, D.; Shenglia, N.; Gigineishvili, T.; Gigineishvili, D. Geographical variation in malignant and benign/borderline brain and CNS tumor incidence: A comparison between a high-income and a middle-income country. J. Neuro-Oncol. 2020, 149, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, J.; Xu, H.; Qin, Z. Geographic variations in the incidence of glioblastoma and prognostic factors predictive of overall survival in US adults from 2004–2013. Front. Aging Neurosci. 2017, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Zuo, T.; Zeng, H.; Zhang, S.; He, J. National cancer incidence and mortality in China, 2012. Chin. J. Cancer Res. 2016, 28, 1–11. [Google Scholar] [PubMed]
- Choi, K.M. Investigation of cancer mortality inequalities between rural and urban areas in South K orea. Aust. J. Rural Health 2016, 24, 61–66. [Google Scholar] [CrossRef]
- Dikshit, R.; Gupta, P.C.; Ramasundarahettige, C.; Gajalakshmi, V.; Aleksandrowicz, L.; Badwe, R.; Kumar, R.; Roy, S.; Suraweera, W.; Bray, F. Cancer mortality in India: A nationally representative survey. Lancet 2012, 379, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Alsan, M.; Stantcheva, S.; Yang, D.; Cutler, D. Disparities in coronavirus 2019 reported incidence, knowledge, and behavior among US adults. JAMA Netw. Open 2020, 3, e2012403. [Google Scholar] [CrossRef]
- 2022 National Healthcare Quality and Disparities Report; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2022.
- Polite, B.N.; Adams-Campbell, L.L.; Brawley, O.W.; Bickell, N.; Carethers, J.M.; Flowers, C.R.; Foti, M.; Gomez, S.L.; Griggs, J.J.; Lathan, C.S.; et al. Charting the Future of Cancer Health Disparities Research: A Position Statement From the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. J. Clin. Oncol. 2017, 35, 3075–3082. [Google Scholar] [CrossRef]
- Kim, Y.Z.; Kim, C.-Y.; Lim, D.H. The overview of practical guidelines for gliomas by KSNO, NCCN, and EANO. Brain Tumor Res. Treat. 2022, 10, 83–93. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Elevating Cancer Equity: Recommendations to Reduce Racial Disparities in Access to Guideline Adherent Cancer Care. 2021. Available online: https://www.nccn.org/docs/default-source/oncology-policy-program/2021_elevating_cancer_equity_webinar_slides.pdf?sfvrsn=e11f66e4_2 (accessed on 8 December 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podder, V.; Sarfraz, Z.; Qidwai, K.A.; Maharaj, A.; Ranjan, T.; Aulakh, S.; Ahluwalia, M.S. Gaps in Diagnosis, Treatment, and Outcomes Among Patients with Brain Tumors in the United States: A State-of-the-Art Review. Cancers 2025, 17, 3982. https://doi.org/10.3390/cancers17243982
Podder V, Sarfraz Z, Qidwai KA, Maharaj A, Ranjan T, Aulakh S, Ahluwalia MS. Gaps in Diagnosis, Treatment, and Outcomes Among Patients with Brain Tumors in the United States: A State-of-the-Art Review. Cancers. 2025; 17(24):3982. https://doi.org/10.3390/cancers17243982
Chicago/Turabian StylePodder, Vivek, Zouina Sarfraz, Khalid Ahmad Qidwai, Arun Maharaj, Tulika Ranjan, Sonikpreet Aulakh, and Manmeet S. Ahluwalia. 2025. "Gaps in Diagnosis, Treatment, and Outcomes Among Patients with Brain Tumors in the United States: A State-of-the-Art Review" Cancers 17, no. 24: 3982. https://doi.org/10.3390/cancers17243982
APA StylePodder, V., Sarfraz, Z., Qidwai, K. A., Maharaj, A., Ranjan, T., Aulakh, S., & Ahluwalia, M. S. (2025). Gaps in Diagnosis, Treatment, and Outcomes Among Patients with Brain Tumors in the United States: A State-of-the-Art Review. Cancers, 17(24), 3982. https://doi.org/10.3390/cancers17243982