Exploring Emerging Therapeutic Targets in Osteosarcoma by Revisiting the Immune and Cancer-Intrinsic Hallmarks of Cancer
Simple Summary
Abstract
1. Introduction
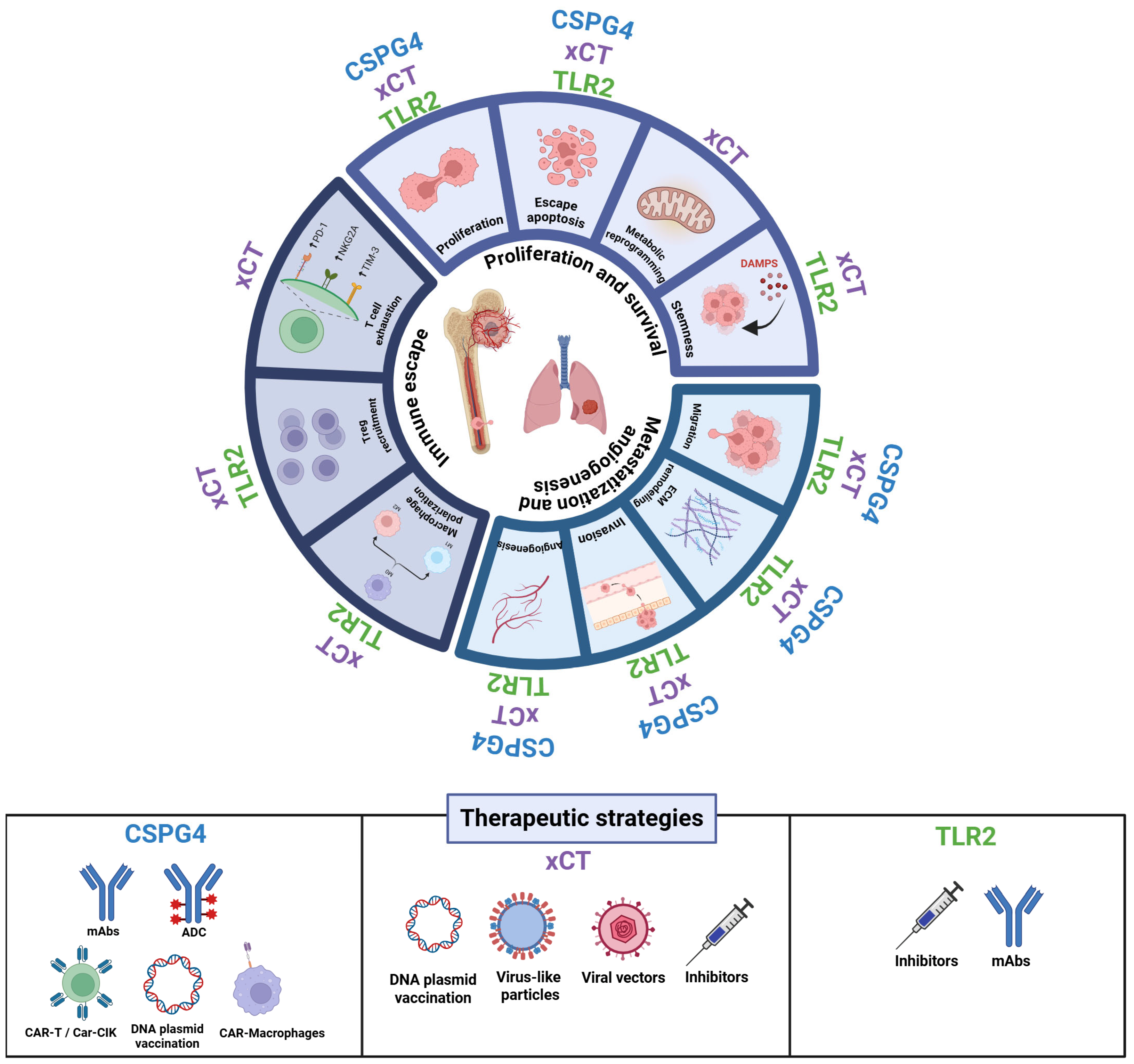
2. Tackling OSA from Multiple Angles
3. CSPG4
4. xCT (SLC7A11)
5. TLR2
6. Investigating Hallmarks of OSA as Therapeutic Vulnerabilities for Novel Treatment Opportunities
6.1. Sustaining Proliferation and Survival
6.2. Metastasization and Angiogenesis
6.3. Immune Evasion
7. Therapeutic Targeting and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCB1 | ATP-Binding Cassette Subfamily B Member 1 (P-glycoprotein) |
| ABCC1 | ATP-Binding Cassette Subfamily C Member 1 (MRP1) |
| ATF4 | Activating Transcription Factor 4 |
| B7-H3 | B7 Homolog 3 |
| Bregs | B Regulatory Cells |
| cAMP | cyclic Adenosine Monophosphate |
| CARs | Chimeric Antigen Receptors |
| CDDP | Cisplatin |
| CEP | Carboxyethylpyrrole |
| CREB | cAMP Response Element-Binding protein |
| CS | Chondroitin-Sulfate |
| CSCs | Cancer stem cells |
| CSPG4 | Chondroitin Sulfate Proteoglycan 4 |
| DAMPs | Damage-Associated Molecular Patterns |
| DCs | Dendritic Cell |
| DOXO | Doxorubicin |
| ECM | Extracellular Matrix |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial-to-Mesenchymal Transition |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1 and 2 |
| ERO1α | Endoplasmic Reticulum Oxidoreductin 1-alpha |
| EV | Extracellular Vesicle |
| FAK | Focal Adhesion Kinase |
| FGF-2 | Fibroblast Growth Factor 2 |
| GSH | Glutathione |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HIF1α | Hypoxia-Inducible Factor 1-alpha |
| HMGB1 | High-Mobility Group Box 1 |
| HSP90 | Heat Shock Protein 90 |
| ICIs | Immune Checkpoint Inhibitors |
| IFN | Interferon |
| IFO | Ifosfamide |
| IGF-1R | Insulin-like Growth Factor 1 Receptor |
| IGFBPs | Insulin-like Growth Factor Binding Proteins |
| IL-10 | Inteleukin-10 |
| IL-21 | Interleukin-21 |
| IL1RAP | IL-1 Receptor Accessory Protein |
| IRFs | Interferon Regulatory Factors |
| mAbs | Monoclonal Antibodies |
| MAPK | Mitogen-Activated Protein Kinase |
| MDSCs | Myeloid Derived Suppressor Cells |
| mGluR | Metabotropic glutamate receptor |
| MLX | MAX-like Protein X |
| MMP | Metalloproteinase |
| mTOR | Mechanistic Target of Rapamycin |
| MTX | Methotrexate |
| MUC1 | Mucin 1 |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| OSA | Osteosarcoma |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PD-1 | Programmed Cell Death Protein-1 |
| PD-L1 | Programmed Death Ligand-1 |
| PDGF | Platelet-Derived Growth Factor |
| PDGFRα | Platelet-Derived Growth Factor Receptor alpha |
| PI3K | Phosphoinositide 3-Kinase |
| PKA | Protein Kinase A |
| PKCα | Protein Kinase C alpha |
| PRRs | Pattern Recognition Receptors |
| RAGE | Receptor for Advanced Glycation End-products |
| ROS | Reactive Oxygen Species |
| SAS | Sulfasalazine |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TAAs | Tumor-Associated Antigens |
| TCR | T-cell receptor |
| TLR2 | Toll-like Receptor 2 |
| TME | Tumor Microenvironment |
| Tregs | T Regulatory Cells |
| VEGF | Vascular Endothelial Growth Factor |
| ZAP70 | Zeta-chain Associated Protein Kinase 70 |
References
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef]
- Kim, C.; Davis, L.E.; Albert, C.M.; Samuels, B.; Roberts, J.L.; Wagner, M.J. Osteosarcoma in Pediatric and Adult Populations: Are Adults Just Big Kids? Cancers 2023, 15, 5044. [Google Scholar] [CrossRef] [PubMed]
- Banjara, R.; Kumar, V.S.; Khan, S.A.; Majeed, A.; Poudel, R.R.; Kanwat, H.; Thapa, S. Relationship between height and osteosarcoma at the time of diagnosis in the Indian population: A retrospective study. J. Clin. Orthop. Trauma 2021, 14, 162–166. [Google Scholar] [CrossRef]
- Sheng, G.; Gao, Y.; Yang, Y.; Wu, H. Osteosarcoma and Metastasis. Front. Oncol. 2021, 11, 780264. [Google Scholar] [CrossRef]
- de Azevedo, J.W.V.; de Medeiros Fernandes, T.A.A.; Fernandes, J.V., Jr.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araujo, J.M.G.; Fernandes, J.V. Biology and pathogenesis of human osteosarcoma. Oncol. Lett. 2020, 19, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Marchandet, L.; Lallier, M.; Charrier, C.; Baud’huin, M.; Ory, B.; Lamoureux, F. Mechanisms of Resistance to Conventional Therapies for Osteosarcoma. Cancers 2021, 13, 683. [Google Scholar] [CrossRef]
- Patel, T.D.; Grimm, S.L.; Kanchi, R.S.; Gandhi, T.; Koirala, A.; Yustein, J.T.; Coarfa, C. Identification of an early survival prognostic gene signature for localized osteosarcoma patients. Sci. Rep. 2024, 14, 7327. [Google Scholar] [CrossRef]
- Fagioli, F.; Biasin, E.; Mereuta, O.M.; Muraro, M.; Luksch, R.; Ferrari, S.; Aglietta, M.; Madon, E. Poor prognosis osteosarcoma: New therapeutic approach. Bone Marrow Transpl. 2008, 41 (Suppl. S2), S131–S134. [Google Scholar] [CrossRef]
- Garcia-Ortega, D.Y.; Cabrera-Nieto, S.A.; Caro-Sanchez, H.S.; Cruz-Ramos, M. An overview of resistance to chemotherapy in osteosarcoma and future perspectives. Cancer Drug Resist. 2022, 5, 762–793. [Google Scholar] [CrossRef]
- Tarone, L.; Giacobino, D.; Camerino, M.; Maniscalco, L.; Iussich, S.; Parisi, L.; Giovannini, G.; Dentini, A.; Bolli, E.; Quaglino, E.; et al. A chimeric human/dog-DNA vaccine against CSPG4 induces immunity with therapeutic potential in comparative preclinical models of osteosarcoma. Mol. Ther. 2023, 31, 2342–2359. [Google Scholar] [CrossRef]
- Riccardo, F.; Tarone, L.; Iussich, S.; Giacobino, D.; Arigoni, M.; Sammartano, F.; Morello, E.; Martano, M.; Gattino, F.; Maria, R.; et al. Identification of CSPG4 as a promising target for translational combinatorial approaches in osteosarcoma. Ther. Adv. Med. Oncol. 2019, 11, 1758835919855491. [Google Scholar] [CrossRef] [PubMed]
- Rolih, V.; Barutello, G.; Iussich, S.; De Maria, R.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. CSPG4: A prototype oncoantigen for translational immunotherapy studies. J. Transl. Med. 2017, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Price, M.A.; Colvin Wanshura, L.E.; Yang, J.; Carlson, J.; Xiang, B.; Li, G.; Ferrone, S.; Dudek, A.Z.; Turley, E.A.; McCarthy, J.B. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell Melanoma Res. 2011, 24, 1148–1157. [Google Scholar] [CrossRef]
- Kurokawa, T.; Imai, K. Chondroitin sulfate proteoglycan 4: An attractive target for antibody-based immunotherapy. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2024, 100, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Koya, Y.; Yoshihara, M.; Iyoshi, S.; Kitami, K.; Sugiyama, M.; Miyamoto, E.; Mogi, K.; Fujimoto, H.; Yamakita, Y.; et al. Chondroitin Sulfate Proteoglycan 4 Provides New Treatment Approach to Preventing Peritoneal Dissemination in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 1626. [Google Scholar] [CrossRef]
- Niibori-Nambu, A.; Yamasaki, Y.; Kobayashi, D.; Angata, K.; Kuno, A.; Panawan, O.; Silsirivanit, A.; Narimatsu, H.; Araki, N. Chondroitin sulfate modification of CSPG4 regulates the maintenance and differentiation of glioma-initiating cells via integrin-associated signaling. J. Biol. Chem. 2024, 300, 105706. [Google Scholar] [CrossRef]
- Chen, X.; Habib, S.; Alexandru, M.; Chauhan, J.; Evan, T.; Troka, J.M.; Rahimi, A.; Esapa, B.; Tull, T.J.; Ng, W.Z.; et al. Chondroitin Sulfate Proteoglycan 4 (CSPG4) as an Emerging Target for Immunotherapy to Treat Melanoma. Cancers 2024, 16, 3260. [Google Scholar] [CrossRef]
- Tarone, L.; Mareschi, K.; Tirtei, E.; Giacobino, D.; Camerino, M.; Buracco, P.; Morello, E.; Cavallo, F.; Riccardo, F. Improving Osteosarcoma Treatment: Comparative Oncology in Action. Life 2022, 12, 2099. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Lu, B.; Yu, J.; Xu, M.; Huang, R.; Cheng, M.; Yang, M.; Zhao, W.; Zou, C. Super-enhancer-driven MLX mediates redox balance maintenance via SLC7A11 in osteosarcoma. Cell Death Dis. 2023, 14, 439. [Google Scholar] [CrossRef]
- Zou, Q.; Zhou, X.; Lai, J.; Zhou, H.; Su, J.; Zhang, Z.; Zhuang, X.; Liu, L.; Yuan, R.; Li, S.; et al. Targeting p62 by sulforaphane promotes autolysosomal degradation of SLC7A11, inducing ferroptosis for osteosarcoma treatment. Redox Biol. 2025, 79, 103460. [Google Scholar] [CrossRef]
- Wen, R.J.; Dong, X.; Zhuang, H.W.; Pang, F.X.; Ding, S.C.; Li, N.; Mai, Y.X.; Zhou, S.T.; Wang, J.Y.; Zhang, J.F. Baicalin induces ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4 regulatory axis. Phytomedicine 2023, 116, 154881. [Google Scholar] [CrossRef]
- Huang, R.; Chu, D.; Shi, J.; Xu, R.; Wang, K. Shikonin suppresses proliferation of osteosarcoma cells by inducing ferroptosis through promoting Nrf2 ubiquitination and inhibiting the xCT/GPX4 regulatory axis. Front. Pharmacol. 2024, 15, 1490759. [Google Scholar] [CrossRef]
- Qin, Q.; Zhang, H.; Lai, M.; Wei, J.; Qian, J.; Chen, X.; Wang, X.; Wang, Y. Sulfasalazine induces ferroptosis in osteosarcomas by regulating Nrf2/SLC7A11/GPX4 signaling axis. Sci. Rep. 2025, 15, 30197. [Google Scholar] [CrossRef]
- Wang, P.; Xiao, J.; Zeng, J.; Yang, F.; Lin, M.; Liang, T.; Liu, H.; Zhan, H. PSAT1 inhibits ferroptosis in osteosarcoma cells by activating the Xct/GPX4 signaling axis. Sci. Rep. 2025, 15, 26425. [Google Scholar] [CrossRef]
- Jing, Y.; Liang, H.; Zhang, Y.; Cleveland, J.; Yan, J.; Zhang, D. Up-regulation of Toll-like Receptor 9 in Osteosarcoma. Anticancer Res. 2015, 35, 5839–5843. [Google Scholar]
- Magri, J.; Gasparetto, A.; Conti, L.; Calautti, E.; Cossu, C.; Ruiu, R.; Barutello, G.; Cavallo, F. Tumor-Associated Antigen xCT and Mutant-p53 as Molecular Targets for New Combinatorial Antitumor Strategies. Cells 2021, 10, 108. [Google Scholar] [CrossRef]
- Landuzzi, L.; Ruzzi, F.; Pellegrini, E.; Lollini, P.L.; Scotlandi, K.; Manara, M.C. IL-1 Family Members in Bone Sarcomas. Cells 2024, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Bolli, E.; Tarone, L.; Cavallo, F.; Conti, L. Toll-Like Receptor 2 at the Crossroad between Cancer Cells, the Immune System, and the Microbiota. Int. J. Mol. Sci. 2020, 21, 9418. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, C.; Fang, Q.; Zhang, W.; Liu, W. Abnormal signal pathways and tumor heterogeneity in osteosarcoma. J. Transl. Med. 2023, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, E.; Dallatomasina, A.; Quartararo, J.; Cortelazzi, B.; Mangieri, D.; Lazzaretti, M.; Perris, R. Structural deciphering of the NG2/CSPG4 proteoglycan multifunctionality. FASEB J. 2019, 33, 3112–3128. [Google Scholar] [CrossRef]
- Ilieva, K.M.; Cheung, A.; Mele, S.; Chiaruttini, G.; Crescioli, S.; Griffin, M.; Nakamura, M.; Spicer, J.F.; Tsoka, S.; Lacy, K.E.; et al. Chondroitin Sulfate Proteoglycan 4 and Its Potential As an Antibody Immunotherapy Target across Different Tumor Types. Front. Immunol. 2017, 8, 1911. [Google Scholar] [CrossRef]
- Liu, X.; Du, S.; Wang, S.; Ye, K. Ferroptosis in osteosarcoma: A promising future. Front. Oncol. 2022, 12, 1031779. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B. The road to Toll. Nat. Rev. Immunol. 2004, 4, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Lyu, J.H.; Kim, J.S.; Chin, H.; Bae, Y.S.; Baek, S.H. Role of JAK2-STAT3 in TLR2-mediated tissue factor expression. J. Cell. Biochem. 2013, 114, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Lanzardo, S.; Arigoni, M.; Antonazzo, R.; Radaelli, E.; Cantarella, D.; Calogero, R.A.; Cavallo, F. The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J. 2013, 27, 4731–4744. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Bolli, E.; Ruiu, R.; Ferrauto, G.; Di Gregorio, E.; Avalle, L.; Savino, A.; Poggio, P.; Merighi, I.F.; Riccardo, F.; et al. Toll-like receptor 2 promotes breast cancer progression and resistance to chemotherapy. Oncoimmunology 2022, 11, 2086752. [Google Scholar] [CrossRef]
- Wang, Y.F.; Feng, J.Y.; Zhao, L.N.; Zhao, M.; Wei, X.F.; Geng, Y.; Yuan, H.F.; Hou, C.Y.; Zhang, H.H.; Wang, G.W.; et al. Aspirin triggers ferroptosis in hepatocellular carcinoma cells through restricting NF-κB p65-activated SLC7A11 transcription. Acta Pharmacol. Sin. 2023, 44, 1712–1724. [Google Scholar] [CrossRef]
- Linher-Melville, K.; Singh, G. The complex roles of STAT3 and STAT5 in maintaining redox balance: Lessons from STAT-mediated xCT expression in cancer cells. Mol. Cell. Endocrinol. 2017, 451, 40–52. [Google Scholar] [CrossRef]
- Lee, S.B.; Sellers, B.N.; DeNicola, G.M. The Regulation of NRF2 by Nutrient-Responsive Signaling and Its Role in Anabolic Cancer Metabolism. Antioxid. Redox Signal. 2018, 29, 1774–1791. [Google Scholar] [CrossRef]
- Groft, S.G.; Nagy, N.; Boom, W.H.; Harding, C.V. Toll-Like Receptor 2-Tpl2-Dependent ERK Signaling Drives Inverse Interleukin 12 Regulation in Dendritic Cells and Macrophages. Infect. Immun. 2020, 89, e00323-20. [Google Scholar] [CrossRef]
- Pathria, G.; Ronai, Z.A. Harnessing the Co-vulnerabilities of Amino Acid-Restricted Cancers. Cell Metab. 2021, 33, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, L.; Yu, Y.; Xiong, Y.; Xiao, P.; Fu, X.; Luo, X. Hydroxysafflor yellow A induced ferroptosis of Osteosarcoma cancer cells by HIF-1α/HK2 and SLC7A11 pathway. Oncol. Res. 2024, 32, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Keleg, S.; Titov, A.; Heller, A.; Giese, T.; Tjaden, C.; Ahmad, S.S.; Gaida, M.M.; Bauer, A.S.; Werner, J.; Giese, N.A. Chondroitin sulfate proteoglycan CSPG4 as a novel hypoxia-sensitive marker in pancreatic tumors. PLoS ONE 2014, 9, e100178. [Google Scholar] [CrossRef] [PubMed]
- Kuhlicke, J.; Frick, J.S.; Morote-Garcia, J.C.; Rosenberger, P.; Eltzschig, H.K. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE 2007, 2, e1364. [Google Scholar] [CrossRef]
- Geldres, C.; Savoldo, B.; Hoyos, V.; Caruana, I.; Zhang, M.; Yvon, E.; Del Vecchio, M.; Creighton, C.J.; Ittmann, M.; Ferrone, S.; et al. T lymphocytes redirected against the chondroitin sulfate proteoglycan-4 control the growth of multiple solid tumors both in vitro and in vivo. Clin. Cancer Res. 2014, 20, 962–971. [Google Scholar] [CrossRef]
- Yang, J.; Liao, Q.; Price, M.; Moriarity, B.; Wolf, N.; Felices, M.; Miller, J.S.; Geller, M.A.; Bendzick, L.; Hopps, R.; et al. Chondroitin sulfate proteoglycan 4, a targetable oncoantigen that promotes ovarian cancer growth, invasion, cisplatin resistance and spheroid formation. Transl. Oncol. 2022, 16, 101318. [Google Scholar] [CrossRef]
- Schiffer, D.; Mellai, M.; Boldorini, R.; Bisogno, I.; Grifoni, S.; Corona, C.; Bertero, L.; Cassoni, P.; Casalone, C.; Annovazzi, L. The Significance of Chondroitin Sulfate Proteoglycan 4 (CSPG4) in Human Gliomas. Int. J. Mol. Sci. 2018, 19, 2724. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yu, L.; Sakakura, K.; Visus, C.; Schwab, J.H.; Ferrone, C.R.; Favoino, E.; Koya, Y.; Campoli, M.R.; et al. CSPG4 in cancer: Multiple roles. Curr. Mol. Med. 2010, 10, 419–429. [Google Scholar] [CrossRef]
- Rivera, Z.; Ferrone, S.; Wang, X.; Jube, S.; Yang, H.; Pass, H.I.; Kanodia, S.; Gaudino, G.; Carbone, M. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin. Cancer Res. 2012, 18, 5352–5363. [Google Scholar] [CrossRef]
- Chen, K.; Yong, J.; Zauner, R.; Wally, V.; Whitelock, J.; Sajinovic, M.; Kopecki, Z.; Liang, K.; Scott, K.F.; Mellick, A.S. Chondroitin Sulfate Proteoglycan 4 as a Marker for Aggressive Squamous Cell Carcinoma. Cancers 2022, 14, 5564. [Google Scholar] [CrossRef]
- Mellai, M.; Casalone, C.; Corona, C.; Crociara, P.; Favole, A.; Cassoni, P.; Schiffer, D.; Boldorini, R. Chondroitin Sulphate Proteoglycans in the Tumour Microenvironment. Adv. Exp. Med. Biol. 2020, 1272, 73–92. [Google Scholar] [CrossRef]
- Nicolosi, P.A.; Dallatomasina, A.; Perris, R. Theranostic impact of NG2/CSPG4 proteoglycan in cancer. Theranostics 2015, 5, 530–544. [Google Scholar] [CrossRef]
- Chekenya, M.; Krakstad, C.; Svendsen, A.; Netland, I.A.; Staalesen, V.; Tysnes, B.B.; Selheim, F.; Wang, J.; Sakariassen, P.O.; Sandal, T.; et al. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene 2008, 27, 5182–5194. [Google Scholar] [CrossRef]
- Wilson, B.S.; Imai, K.; Natali, P.G.; Ferrone, S. Distribution and molecular characterization of a cell-surface and a cytoplasmic antigen detectable in human melanoma cells with monoclonal antibodies. Int. J. Cancer 1981, 28, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, M. SLC7A11: The Achilles heel of tumor? Front. Immunol. 2024, 15, 1438807. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Ganapathy, V. Amino Acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015, 75, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Chen, R.S.; Song, Y.M.; Zhou, Z.Y.; Tong, T.; Li, Y.; Fu, M.; Guo, X.L.; Dong, L.J.; He, X.; Qiao, H.X.; et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/β-catenin pathway. Oncogene 2009, 28, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Dornier, E.; Rabas, N.; Mitchell, L.; Novo, D.; Dhayade, S.; Marco, S.; Mackay, G.; Sumpton, D.; Pallares, M.; Nixon, C.; et al. Glutaminolysis drives membrane trafficking to promote invasiveness of breast cancer cells. Nat. Commun. 2017, 8, 2255. [Google Scholar] [CrossRef]
- Wang, S.F.; Chen, M.S.; Chou, Y.C.; Ueng, Y.F.; Yin, P.H.; Yeh, T.S.; Lee, H.C. Mitochondrial dysfunction enhances cisplatin resistance in human gastric cancer cells via the ROS-activated GCN2-eIF2α-ATF4-xCT pathway. Oncotarget 2016, 7, 74132–74151. [Google Scholar] [CrossRef]
- Lanzardo, S.; Conti, L.; Rooke, R.; Ruiu, R.; Accart, N.; Bolli, E.; Arigoni, M.; Macagno, M.; Barrera, G.; Pizzimenti, S.; et al. Immunotargeting of Antigen xCT Attenuates Stem-like Cell Behavior and Metastatic Progression in Breast Cancer. Cancer Res. 2016, 76, 62–72. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tsuchihashi, K.; Ishimoto, T.; Yae, T.; Motohara, T.; Sugihara, E.; Onishi, N.; Masuko, T.; Yoshizawa, K.; Kawashiri, S.; et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Nagano, O.; Okazaki, S.; Saya, H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene 2013, 32, 5191–5198. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takahashi, H.; Rajabi, H.; Alam, M.; Suzuki, Y.; Yin, L.; Tagde, A.; Maeda, T.; Hiraki, M.; Sukhatme, V.P.; et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget 2016, 7, 11756–11769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, M.; Ghoda, L.Y.; Zhao, D.; Valerio, M.; Nafie, E.; Gonzalez, A.; Ly, K.; Parcutela, B.; Choi, H.; et al. IL1RAP-specific T cell engager depletes acute myeloid leukemia stem cells. J. Hematol. Oncol. 2024, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Carroll, P.A.; Freie, B.W.; Cheng, P.F.; Kasinathan, S.; Gu, H.; Hedrich, T.; Dowdle, J.A.; Venkataramani, V.; Ramani, V.; Wu, X.; et al. The glucose-sensing transcription factor MLX balances metabolism and stress to suppress apoptosis and maintain spermatogenesis. PLoS Biol. 2021, 19, e3001085. [Google Scholar] [CrossRef]
- Bolli, E.; O’Rourke, J.P.; Conti, L.; Lanzardo, S.; Rolih, V.; Christen, J.M.; Barutello, G.; Forni, M.; Pericle, F.; Cavallo, F. A Virus-Like-Particle immunotherapy targeting Epitope-Specific anti-xCT expressed on cancer stem cell inhibits the progression of metastatic cancer in vivo. Oncoimmunology 2018, 7, e1408746. [Google Scholar] [CrossRef]
- Ruiu, R.; Rolih, V.; Bolli, E.; Barutello, G.; Riccardo, F.; Quaglino, E.; Merighi, I.F.; Pericle, F.; Donofrio, G.; Cavallo, F.; et al. Fighting breast cancer stem cells through the immune-targeting of the xCT cystine-glutamate antiporter. Cancer Immunol. Immunother. 2019, 68, 131–141. [Google Scholar] [CrossRef]
- Donofrio, G.; Tebaldi, G.; Lanzardo, S.; Ruiu, R.; Bolli, E.; Ballatore, A.; Rolih, V.; Macchi, F.; Conti, L.; Cavallo, F. Bovine herpesvirus 4-based vector delivering the full length xCT DNA efficiently protects mice from mammary cancer metastases by targeting cancer stem cells. Oncoimmunology 2018, 7, e1494108. [Google Scholar] [CrossRef]
- Conti, L.; Bolli, E.; Di Lorenzo, A.; Franceschi, V.; Macchi, F.; Riccardo, F.; Ruiu, R.; Russo, L.; Quaglino, E.; Donofrio, G.; et al. Immunotargeting of the xCT Cystine/Glutamate Antiporter Potentiates the Efficacy of HER2-Targeted Immunotherapies in Breast Cancer. Cancer Immunol. Res. 2020, 8, 1039–1053. [Google Scholar] [CrossRef]
- Barutello, G.; Di Lorenzo, A.; Gasparetto, A.; Galiazzi, C.; Bolli, E.; Conti, L.; Cavallo, F. Immunotherapy against the Cystine/Glutamate Antiporter xCT Improves the Efficacy of APR-246 in Preclinical Breast Cancer Models. Biomedicines 2022, 10, 2843. [Google Scholar] [CrossRef]
- Cossu, C.; Di Lorenzo, A.; Fiorilla, I.; Todesco, A.M.; Audrito, V.; Conti, L. The Role of the Toll-like Receptor 2 and the cGAS-STING Pathways in Breast Cancer: Friends or Foes? Int. J. Mol. Sci. 2023, 25, 456. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.H.; Scheeren, F.A. Cell-intrinsic TLR2/MyD88 pathway in breast and colon cancer. Cell Cycle 2014, 13, 3785–3786. [Google Scholar] [CrossRef]
- McCoy, M.G.; Nascimento, D.W.; Veleeparambil, M.; Murtazina, R.; Gao, D.; Tkachenko, S.; Podrez, E.; Byzova, T.V. Endothelial TLR2 promotes proangiogenic immune cell recruitment and tumor angiogenesis. Sci. Signal. 2021, 14, eabc5371. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Lee, H.J.; Yoon, C.H.; Choi, Y.R.; Ryu, J.S.; Oh, J.Y. Activation of Toll-like receptor 2 promotes mesenchymal stem/stromal cell-mediated immunoregulation and angiostasis through AKR1C1. Theranostics 2024, 14, 4713–4729. [Google Scholar] [CrossRef]
- Lundy, J.; Gearing, L.J.; Gao, H.; West, A.C.; McLeod, L.; Deswaerte, V.; Yu, L.; Porazinski, S.; Pajic, M.; Hertzog, P.J.; et al. TLR2 activation promotes tumour growth and associates with patient survival and chemotherapy response in pancreatic ductal adenocarcinoma. Oncogene 2021, 40, 6007–6022. [Google Scholar] [CrossRef]
- Tirtei, E.; Campello, A.; Sciannameo, V.; Asaftei, S.D.; Meazza, C.; Sironi, G.; Longhi, A.; Ibrahim, T.; Tamburini, A.; Coccoli, L.; et al. Prolonged 14-day continuous infusion of high-dose ifosfamide for patients with relapsed and refractory high-grade osteosarcoma: A retrospective multicentre cohort study. BMC Cancer 2024, 24, 747. [Google Scholar] [CrossRef]
- Harris, M.A.; Hawkins, C.J. Recent and Ongoing Research into Metastatic Osteosarcoma Treatments. Int. J. Mol. Sci. 2022, 23, 3817. [Google Scholar] [CrossRef]
- Wu, C.C.; Livingston, J.A. Genomics and the Immune Landscape of Osteosarcoma. Adv. Exp. Med. Biol. 2020, 1258, 21–36. [Google Scholar] [CrossRef]
- Meijer, D.M.; Ruano, D.; Briaire-de Bruijn, I.H.; Wijers-Koster, P.M.; van de Sande, M.A.J.; Gelderblom, H.; Cleton-Jansen, A.M.; de Miranda, N.; Kuijjer, M.L.; Bovee, J. The Variable Genomic Landscape During Osteosarcoma Progression: Insights From a Longitudinal WGS Analysis. Genes Chromosomes Cancer 2024, 63, e23253. [Google Scholar] [CrossRef]
- Rajan, S.; Zaccaria, S.; Cannon, M.V.; Cam, M.; Gross, A.C.; Raphael, B.J.; Roberts, R.D. Structurally Complex Osteosarcoma Genomes Exhibit Limited Heterogeneity within Individual Tumors and across Evolutionary Time. Cancer Res. Commun. 2023, 3, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yang, Y.; Guo, W.; Che, D.; Xu, J.; Sun, X.; Liu, K.; Ren, T.; Liu, X.; Yang, Y.; et al. The Clinical Implications of Tumor Mutational Burden in Osteosarcoma. Front. Oncol. 2020, 10, 595527. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Zhang, K.; Guo, Y. TP53 Mutations and Survival in Osteosarcoma Patients: A Meta-Analysis of Published Data. Dis. Markers 2016, 2016, 4639575. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Sun, M.; Zuo, D.; Wang, H.; Shen, J.; Jiang, W.; Mu, H.; Ma, X.; Yin, F.; et al. Multi-omics analysis identifies osteosarcoma subtypes with distinct prognosis indicating stratified treatment. Nat. Commun. 2022, 13, 7207. [Google Scholar] [CrossRef]
- Zoumpoulidou, G.; Alvarez-Mendoza, C.; Mancusi, C.; Ahmed, R.M.; Denman, M.; Steele, C.D.; Tarabichi, M.; Roy, E.; Davies, L.R.; Manji, J.; et al. Therapeutic vulnerability to PARP1,2 inhibition in RB1-mutant osteosarcoma. Nat. Commun. 2021, 12, 7064. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular Biology of Osteosarcoma. Cancers 2020, 12, 2130. [Google Scholar] [CrossRef]
- Zhang, N.; Jing, Z.; Song, J.; Liang, Q.; Xu, Y.; Xu, Z.; Wen, L.; Wei, P. Discovery of Drugs Targeting Mutant p53 and Progress in Nano-Enabled Therapeutic Strategy for p53-Mutated Cancers. Biomolecules 2025, 15, 763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Luo, Y.; Xu, J.; Zhang, B.; Feng, P.; Guo, C.; Wang, Y.; Huang, Z.; Kong, Q.; et al. Reactivating P53 to treat osteosarcoma: A tetrahedral framework nucleic acids-based approach. Int. J. Biol. Macromol. 2025, 304, 140765. [Google Scholar] [CrossRef] [PubMed]
- Linn, P.; Kohno, S.; Sheng, J.; Kulathunga, N.; Yu, H.; Zhang, Z.; Voon, D.; Watanabe, Y.; Takahashi, C. Targeting RB1 Loss in Cancers. Cancers 2021, 13, 3737. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Mao, Y.; Gao, W.; Duan, S. Targeting the p53 signaling pathway in cancers: Molecular mechanisms and clinical studies. MedComm 2023, 4, e288. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shi, Q.; Xu, J.; Ren, T.; Huang, Y.; Guo, W. Current progress and open challenges for applying tyrosine kinase inhibitors in osteosarcoma. Cell Death Discov. 2022, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Niu, X.; Yao, W. Receptor Tyrosine Kinases in Osteosarcoma Treatment: Which Is the Key Target? Front. Oncol. 2020, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Honoki, K.; Tsujiuchi, T.; Kishi, S.; Kuniyasu, H. Revisiting ‘Hallmarks of Cancer’ In Sarcomas. J. Cancer 2024, 15, 1786–1804. [Google Scholar] [CrossRef]
- Assi, A.; Farhat, M.; Hachem, M.C.R.; Zalaquett, Z.; Aoun, M.; Daher, M.; Sebaaly, A.; Kourie, H.R. Tyrosine kinase inhibitors in osteosarcoma: Adapting treatment strategiesa. J. Bone Oncol. 2023, 43, 100511. [Google Scholar] [CrossRef]
- Wang, J.; Svendsen, A.; Kmiecik, J.; Immervoll, H.; Skaftnesmo, K.O.; Planaguma, J.; Reed, R.K.; Bjerkvig, R.; Miletic, H.; Enger, P.O.; et al. Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma. PLoS ONE 2011, 6, e23062. [Google Scholar] [CrossRef]
- Wang, X.; Osada, T.; Wang, Y.; Yu, L.; Sakakura, K.; Katayama, A.; McCarthy, J.B.; Brufsky, A.; Chivukula, M.; Khoury, T.; et al. CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. J. Natl. Cancer Inst. 2010, 102, 1496–1512. [Google Scholar] [CrossRef]
- Hsu, S.C.; Nadesan, P.; Puviindran, V.; Stallcup, W.B.; Kirsch, D.G.; Alman, B.A. Effects of chondroitin sulfate proteoglycan 4 (NG2/CSPG4) on soft-tissue sarcoma growth depend on tumor developmental stage. J. Biol. Chem. 2018, 293, 2466–2475. [Google Scholar] [CrossRef]
- Kuijjer, M.L.; Rydbeck, H.; Kresse, S.H.; Buddingh, E.P.; Lid, A.B.; Roelofs, H.; Burger, H.; Myklebost, O.; Hogendoorn, P.C.; Meza-Zepeda, L.A.; et al. Identification of osteosarcoma driver genes by integrative analysis of copy number and gene expression data. Genes Chromosomes Cancer 2012, 51, 696–706. [Google Scholar] [CrossRef]
- Kuijjer, M.L.; Peterse, E.F.; van den Akker, B.E.; Briaire-de Bruijn, I.H.; Serra, M.; Meza-Zepeda, L.A.; Myklebost, O.; Hassan, A.B.; Hogendoorn, P.C.; Cleton-Jansen, A.M. IR/IGF1R signaling as potential target for treatment of high-grade osteosarcoma. BMC Cancer 2013, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Ammons, D.; Dow, S.W. Role of companion dogs in cancer immunotherapy development. J. Immunol. 2025, 214, 2515–2522. [Google Scholar] [CrossRef]
- Simpson, S.; Rizvanov, A.A.; Jeyapalan, J.N.; de Brot, S.; Rutland, C.S. Canine osteosarcoma in comparative oncology: Molecular mechanisms through to treatment discovery. Front. Vet. Sci. 2022, 9, 965391. [Google Scholar] [CrossRef]
- Mannheimer, J.D.; Tawa, G.; Gerhold, D.; Braisted, J.; Sayers, C.M.; McEachron, T.A.; Meltzer, P.; Mazcko, C.; Beck, J.A.; LeBlanc, A.K. Transcriptional profiling of canine osteosarcoma identifies prognostic gene expression signatures with translational value for humans. Commun. Biol. 2023, 6, 856. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Martano, M.; Buracco, P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef]
- Polton, G.; Borrego, J.F.; Clemente-Vicario, F.; Clifford, C.A.; Jagielski, D.; Kessler, M.; Kobayashi, T.; Lanore, D.; Queiroga, F.L.; Rodrigues, L.; et al. Osteosarcoma of the appendicular skeleton in dogs: Consensus and guidelines. Front. Vet. Sci. 2025, 12, 1633593. [Google Scholar] [CrossRef]
- Liu, Y.D.; Ji, C.B.; Li, S.B.; Yan, F.; Gu, Q.S.; Balic, J.J.; Yu, L.; Li, J.K. Toll-like receptor 2 stimulation promotes colorectal cancer cell growth via PI3K/Akt and NF-κB signaling pathways. Int. Immunopharmacol. 2018, 59, 375–383. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Hu, X. Robinin inhibits pancreatic cancer cell proliferation, EMT and inflammation via regulating TLR2-PI3k-AKT signaling pathway. Cancer Cell Int. 2023, 23, 328. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Jia, Y.; Ma, J.; Wu, S.; Xu, Y.; Shang, M.; Mao, A. TLR2 promotes human intrahepatic cholangiocarcinoma cell migration and invasion by modulating NF-κB pathway-mediated inflammatory responses. FEBS J. 2016, 283, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Zhuang, L.; Olszewski, K.; Gan, B. NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation. Genes Dis. 2021, 8, 731–745. [Google Scholar] [CrossRef]
- Su, Z.; Liu, Y.; Wang, L.; Gu, W. Regulation of SLC7A11 as an unconventional checkpoint in tumorigenesis through ferroptosis. Genes Dis. 2025, 12, 101254. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018, 38, 12. [Google Scholar] [CrossRef]
- Prickett, T.D.; Samuels, Y. Molecular pathways: Dysregulated glutamatergic signaling pathways in cancer. Clin. Cancer Res. 2012, 18, 4240–4246. [Google Scholar] [CrossRef]
- Jyotsana, N.; Ta, K.T.; DelGiorno, K.E. The Role of Cystine/Glutamate Antiporter SLC7A11/xCT in the Pathophysiology of Cancer. Front. Oncol. 2022, 12, 858462. [Google Scholar] [CrossRef]
- Sato, M.; Kusumi, R.; Hamashima, S.; Kobayashi, S.; Sasaki, S.; Komiyama, Y.; Izumikawa, T.; Conrad, M.; Bannai, S.; Sato, H. The ferroptosis inducer erastin irreversibly inhibits system xc− and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci. Rep. 2018, 8, 968. [Google Scholar] [CrossRef]
- Polewski, M.D.; Reveron-Thornton, R.F.; Cherryholmes, G.A.; Marinov, G.K.; Cassady, K.; Aboody, K.S. Increased Expression of System xc- in Glioblastoma Confers an Altered Metabolic State and Temozolomide Resistance. Mol. Cancer Res. 2016, 14, 1229–1242. [Google Scholar] [CrossRef]
- Ge, C.; Cao, B.; Feng, D.; Zhou, F.; Zhang, J.; Yang, N.; Feng, S.; Wang, G.; Aa, J. The down-regulation of SLC7A11 enhances ROS induced P-gp over-expression and drug resistance in MCF-7 breast cancer cells. Sci. Rep. 2017, 7, 3791. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, K.; Huang, X.; Zhao, C.; Mei, Y.; Li, X.; Jiao, L.; Yang, H. lncRNA SLC7A11-AS1 Promotes Chemoresistance by Blocking SCFβ-TRCP-Mediated Degradation of NRF2 in Pancreatic Cancer. Mol. Ther. Nucleic Acids 2020, 19, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Starheim, K.K.; Holien, T.; Misund, K.; Johansson, I.; Baranowska, K.A.; Sponaas, A.M.; Hella, H.; Buene, G.; Waage, A.; Sundan, A.; et al. Intracellular glutathione determines bortezomib cytotoxicity in multiple myeloma cells. Blood Cancer J. 2016, 6, e446. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, X.; Huang, P. xCT: A Critical Molecule That Links Cancer Metabolism to Redox Signaling. Mol. Ther. 2020, 28, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Cesar-Razquin, A.; Girardi, E.; Yang, M.; Brehme, M.; Saez-Rodriguez, J.; Superti-Furga, G. In silico Prioritization of Transporter-Drug Relationships From Drug Sensitivity Screens. Front. Pharmacol. 2018, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Girardi, E.; Cesar-Razquin, A.; Lindinger, S.; Papakostas, K.; Konecka, J.; Hemmerich, J.; Kickinger, S.; Kartnig, F.; Gurtl, B.; Klavins, K.; et al. A widespread role for SLC transmembrane transporters in resistance to cytotoxic drugs. Nat. Chem. Biol. 2020, 16, 469–478. [Google Scholar] [CrossRef]
- Wolf, G.; Craigon, C.; Teoh, S.T.; Essletzbichler, P.; Onstein, S.; Cassidy, D.; Uijttewaal, E.C.H.; Dvorak, V.; Cao, Y.; Bensimon, A.; et al. The efflux pump ABCC1/MRP1 constitutively restricts PROTAC sensitivity in cancer cells. Cell Chem. Biol. 2025, 32, 291–306.e6. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, H.; Chen, H.; Gong, A.; Liu, Y.; Song, L.; Xu, X.; You, T.; Fan, X.; Wang, D.; et al. Dedifferentiation process driven by radiotherapy-induced HMGB1/TLR2/YAP/HIF-1α signaling enhances pancreatic cancer stemness. Cell Death Dis. 2019, 10, 724. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wu, Y.N.; Huang, J.Q.; Wang, W.; Xu, M.; Jia, J.P.; Han, G.; Mao, B.B.; Bi, W.Z. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin. J. Cancer 2016, 35, 47. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y. HMGB1 in inflammation and cancer. J. Hematol. Oncol. 2020, 13, 116. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Yang, Y.; Jiang, J. An analysis of the expression and function of myeloid differentiation factor 88 in human osteosarcoma. Oncol. Lett. 2018, 16, 4929–4936. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X. Association of MMP-2 expression and prognosis in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2015, 8, 14965–14970. [Google Scholar]
- Cui, J.; Dean, D.; Hornicek, F.J.; Chen, Z.; Duan, Z. The role of extracelluar matrix in osteosarcoma progression and metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 178. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, T.; Wang, W. Prognostic significance of matrix metalloproteinase 9 expression in osteosarcoma: A meta-analysis of 16 studies. Medicine 2018, 97, e13051. [Google Scholar] [CrossRef] [PubMed]
- Doppelt-Flikshtain, O.; Asbi, T.; Younis, A.; Ginesin, O.; Cohen, Z.; Tamari, T.; Berg, T.; Yanovich, C.; Aran, D.; Zohar, Y.; et al. Inhibition of osteosarcoma metastasis in vivo by targeted downregulation of MMP1 and MMP9. Matrix Biol. 2024, 134, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ruiu, R.; Cossu, C.; Iacoviello, A.; Conti, L.; Bolli, E.; Ponzone, L.; Magri, J.; Rumandla, A.; Calautti, E.; Cavallo, F. Cystine/glutamate antiporter xCT deficiency reduces metastasis without impairing immune system function in breast cancer mouse models. J. Exp. Clin. Cancer Res. 2023, 42, 254. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Y.; Sun, Y. KDM4A-mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma. Biochem. Biophys. Res. Commun. 2021, 550, 77–83. [Google Scholar] [CrossRef]
- He, P.; Liu, F.; Wang, Z.; Gong, H.; Zhang, M.; Jia, Z.; Zhai, X. CircKIF4A enhances osteosarcoma proliferation and metastasis by sponging MiR-515-5p and upregulating SLC7A11. Mol. Biol. Rep. 2022, 49, 4525–4535. [Google Scholar] [CrossRef]
- Li, M.J.; Li, F.; Xu, J.; Liu, Y.D.; Hu, T.; Chen, J.T. rhHMGB1 drives osteoblast migration in a TLR2/TLR4- and NF-κB-dependent manner. Biosci. Rep. 2016, 36, e00300. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, R.; Zhou, Y.; Fu, H.; Meng, Q. TLR2 Ligand Pam3CSK4 Regulates MMP-2/9 Expression by MAPK/NF-κB Signaling Pathways in Primary Brain Microvascular Endothelial Cells. Neurochem. Res. 2018, 43, 1897–1904. [Google Scholar] [CrossRef]
- Ozerdem, U. Targeting pericytes diminishes neovascularization in orthotopic uveal melanoma in nerve/glial antigen 2 proteoglycan knockout mouse. Ophthalmic Res. 2006, 38, 251–254. [Google Scholar] [CrossRef]
- Sato, S.; Tang, Y.J.; Wei, Q.; Hirata, M.; Weng, A.; Han, I.; Okawa, A.; Takeda, S.; Whetstone, H.; Nadesan, P.; et al. Mesenchymal Tumors Can Derive from Ng2/Cspg4-Expressing Pericytes with β-Catenin Modulating the Neoplastic Phenotype. Cell Rep. 2016, 16, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Li, X.; Liu, W.D.; Liu, X.Z.; Wu, S.J.; Hu, X.H. Inhibition of Growth and Metastasis of Tumor in Nude Mice after Intraperitoneal Injection of Bevacizumab. Orthop. Surg. 2016, 8, 234–240. [Google Scholar] [CrossRef]
- Navid, F.; Santana, V.M.; Neel, M.; McCarville, M.B.; Shulkin, B.L.; Wu, J.; Billups, C.A.; Mao, S.; Daryani, V.M.; Stewart, C.F.; et al. A phase II trial evaluating the feasibility of adding bevacizumab to standard osteosarcoma therapy. Int. J. Cancer 2017, 141, 1469–1477. [Google Scholar] [CrossRef]
- Volz, N.B.; Stintzing, S.; Zhang, W.; Yang, D.; Ning, Y.; Wakatsuki, T.; El-Khoueiry, R.E.; Li, J.E.; Kardosh, A.; Loupakis, F.; et al. Genes involved in pericyte-driven tumor maturation predict treatment benefit of first-line FOLFIRI plus bevacizumab in patients with metastatic colorectal cancer. Pharmacogenom. J. 2015, 15, 69–76. [Google Scholar] [CrossRef]
- Chen, D.; Fan, Z.; Rauh, M.; Buchfelder, M.; Eyupoglu, I.Y.; Savaskan, N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene 2017, 36, 5593–5608. [Google Scholar] [CrossRef]
- Briggs, K.J.; Koivunen, P.; Cao, S.; Backus, K.M.; Olenchock, B.A.; Patel, H.; Zhang, Q.; Signoretti, S.; Gerfen, G.J.; Richardson, A.L.; et al. Paracrine Induction of HIF by Glutamate in Breast Cancer: EglN1 Senses Cysteine. Cell 2016, 166, 126–139. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, H.; Liu, W.; Lin, W.; Sun, A.; Ding, Z.; Chen, X.; Wan, X.; Liu, Y.; Hu, Z.; et al. Augmented ERO1α upon mTORC1 activation induces ferroptosis resistance and tumor progression via upregulation of SLC7A11. J. Exp. Clin. Cancer Res. 2024, 43, 112. [Google Scholar] [CrossRef]
- West, X.Z.; Malinin, N.L.; Merkulova, A.A.; Tischenko, M.; Kerr, B.A.; Borden, E.C.; Podrez, E.A.; Salomon, R.G.; Byzova, T.V. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 2010, 467, 972–976. [Google Scholar] [CrossRef]
- Giri, S.; Lamichhane, G.; Pandey, J.; Khadayat, R.; K, C.S.; Devkota, H.P.; Khadka, D. Immune Modulation and Immunotherapy in Solid Tumors: Mechanisms of Resistance and Potential Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 2923. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, I.; Hendriks, M.; van Ginkel, R.J.; Samplonius, D.F.; Bremer, E.; Helfrich, W. Bispecific Antibody Approach for Improved Melanoma-Selective PD-L1 Immune Checkpoint Blockade. J. Investig. Dermatol. 2019, 139, 2343–2351.e3. [Google Scholar] [CrossRef] [PubMed]
- Boudin, L.; de Nonneville, A.; Finetti, P.; Mescam, L.; Le Cesne, A.; Italiano, A.; Blay, J.Y.; Birnbaum, D.; Mamessier, E.; Bertucci, F. CSPG4 expression in soft tissue sarcomas is associated with poor prognosis and low cytotoxic immune response. J. Transl. Med. 2022, 20, 464. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Chen, W.; Shan, B.; Ding, Y.; Zhang, G.; Cao, N.; Liu, L.; Zhang, Y. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS ONE 2013, 8, e70689. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Liu, G.; Li, D.; Gu, Z.; Zhang, L.; Pan, Y.; Cui, X.; Wang, L.; Liu, G.; et al. B7-H3 targeted CAR-T cells show highly efficient anti-tumor function against osteosarcoma both in vitro and in vivo. BMC Cancer 2022, 22, 1124. [Google Scholar] [CrossRef]
- McEachron, T.A.; Triche, T.J.; Sorenson, L.; Parham, D.M.; Carpten, J.D. Profiling targetable immune checkpoints in osteosarcoma. Oncoimmunology 2018, 7, e1475873. [Google Scholar] [CrossRef]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef]
- Cascini, C.; Ratti, C.; Botti, L.; Parma, B.; Cancila, V.; Salvaggio, A.; Meazza, C.; Tripodo, C.; Colombo, M.P.; Chiodoni, C. Rewiring innate and adaptive immunity with TLR9 agonist to treat osteosarcoma. J. Exp. Clin. Cancer Res. 2023, 42, 154. [Google Scholar] [CrossRef]
- Oyama, R.; Nabeshima, A.; Endo, M.; Novikov, A.; Fujiwara, T.; Phelip, C.; Yokoyama, N.; Oda, Y.; Caroff, M.; Matsumoto, Y.; et al. A detoxified TLR4 agonist inhibits tumour growth and lung metastasis of osteosarcoma by promoting CD8+ cytotoxic lymphocyte infiltration. BJC Rep. 2025, 3, 5. [Google Scholar] [CrossRef]
- Sutmuller, R.P.; den Brok, M.H.; Kramer, M.; Bennink, E.J.; Toonen, L.W.; Kullberg, B.J.; Joosten, L.A.; Akira, S.; Netea, M.G.; Adema, G.J. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Investig. 2006, 116, 485–494. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.; Konowich, J.; Salgame, P. Host defense and recruitment of Foxp3+ T regulatory cells to the lungs in chronic Mycobacterium tuberculosis infection requires toll-like receptor 2. PLoS Pathog. 2013, 9, e1003397. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, Q.; Cheng, Y.; Chen, X.; Wang, G.; Shi, M.; Zhang, T.; Cao, Y.; Pan, H.; Zhang, L.; et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J. Immunother. Cancer 2018, 6, 145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Li, P.C.; Pan, N.; Gao, R.; Wen, Z.F.; Zhang, T.Y.; Huang, F.; Wu, F.Y.; Ou, X.L.; Zhang, J.P.; et al. Tumor-released autophagosomes induces CD4+ T cell-mediated immunosuppression via a TLR2–IL-6 cascade. J. Immunother. Cancer 2019, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Li, L.; Jin, X.; Zhang, Z.; Zheng, H.; Pan, J.; Shi, L.; Jiang, Z.; Su, K.; et al. Tumor-derived HMGB1 induces CD62Ldim neutrophil polarization and promotes lung metastasis in triple-negative breast cancer. Oncogenesis 2020, 9, 82. [Google Scholar] [CrossRef]
- Niu, X.; Yin, L.; Yang, X.; Yang, Y.; Gu, Y.; Sun, Y.; Yang, M.; Wang, Y.; Zhang, Q.; Ji, H. Serum amyloid A 1 induces suppressive neutrophils through the Toll-like receptor 2-mediated signaling pathway to promote progression of breast cancer. Cancer Sci. 2022, 113, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.J.; Kelly, A.J.; Li, Y.; Chen, X.; McGee, C.; Krailo, M.; Barkauskas, D.A.; Hicks, J.; Man, T.K. The prognostic significance of circulating serum amyloid A and CXC chemokine ligand 4 in osteosarcoma. Pediatr. Blood Cancer 2017, 64, e26659. [Google Scholar] [CrossRef]
- Jin, J.; Byun, J.K.; Choi, Y.K.; Park, K.G. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp. Mol. Med. 2023, 55, 706–715. [Google Scholar] [CrossRef]
- Ren, L.; Ruiz-Rodado, V.; Dowdy, T.; Huang, S.; Issaq, S.H.; Beck, J.; Wang, H.; Tran Hoang, C.; Lita, A.; Larion, M.; et al. Glutaminase-1 (GLS1) inhibition limits metastatic progression in osteosarcoma. Cancer Metab. 2020, 8, 4. [Google Scholar] [CrossRef]
- Tang, H.Y.; Guo, J.Q.; Sang, B.T.; Cheng, J.N.; Wu, X.M. PDGFRβ modulates aerobic glycolysis in osteosarcoma HOS cells via the PI3K/AKT/mTOR/c-Myc pathway. Biochem. Cell Biol. 2022, 100, 75–84. [Google Scholar] [CrossRef]
- Sheng, G.; Gao, Y.; Ding, Q.; Zhang, R.; Wang, T.; Jing, S.; Zhao, H.; Ma, T.; Wu, H.; Yang, Y. P2RX7 promotes osteosarcoma progression and glucose metabolism by enhancing c-Myc stabilization. J. Transl. Med. 2023, 21, 132. [Google Scholar] [CrossRef]
- Koppula, P.; Zhang, Y.; Shi, J.; Li, W.; Gan, B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 2017, 292, 14240–14249. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.S.; Mishra, P.; Watrous, J.D.; Carelli, V.; D’Aurelio, M.; Jain, M.; Chan, D.C. The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat. Commun. 2017, 8, 15074. [Google Scholar] [CrossRef]
- Song, Z.; Yao, Q.; Huang, L.; Cui, D.; Xie, J.; Wu, L.; Huang, J.; Zhai, B.; Liu, D.; Xu, X. Glucose Deprivation-Induced Disulfidptosis via the SLC7A11-INF2 Axis: Pan-Cancer Prognostic Exploration and Therapeutic Validation. Adv. Sci. 2025, 12, e08556. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, R.; Liu, Y.W.; Li, S.; Sun, J.; Li, J.; Zhu, J.; Moharil, P.; Zhang, B.; Ren, P.; et al. Targeting metabotropic glutamate receptor 4 for cancer immunotherapy. Sci. Adv. 2021, 7, eabj4226. [Google Scholar] [CrossRef]
- de Aquino, M.T.P.; Hodo, T.W.; Ochoa, S.G.; Uzhachenko, R.V.; Mohammed, M.A.; Goodwin, J.S.; Kanagasabai, T.; Ivanova, A.V.; Shanker, A. Glutamate receptor-T cell receptor signaling potentiates full CD8+ T cell activation and effector function in tumor immunity. iScience 2025, 28, 112772. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010, 70, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ge, M.; Xing, P.; Xia, T.; Zhang, C.; Ma, K.; Ma, Y.; Li, S.; Li, W.; Liu, X.; et al. Cystine deprivation triggers CD36-mediated ferroptosis and dysfunction of tumor infiltrating CD8+ T cells. Cell Death Dis. 2024, 15, 145. [Google Scholar] [CrossRef]
- Long, Y.; Tao, H.; Karachi, A.; Grippin, A.J.; Jin, L.; Chang, Y.E.; Zhang, W.; Dyson, K.A.; Hou, A.Y.; Na, M.; et al. Dysregulation of Glutamate Transport Enhances Treg Function That Promotes VEGF Blockade Resistance in Glioblastoma. Cancer Res. 2020, 80, 499–509. [Google Scholar] [CrossRef]
- He, Q.; Liu, M.; Huang, W.; Chen, X.; Zhang, B.; Zhang, T.; Wang, Y.; Liu, D.; Xie, M.; Ji, X.; et al. IL-1β-Induced Elevation of Solute Carrier Family 7 Member 11 Promotes Hepatocellular Carcinoma Metastasis Through Up-regulating Programmed Death Ligand 1 and Colony-Stimulating Factor 1. Hepatology 2021, 74, 3174–3193. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhu, J.; Wang, Y.; Chen, W.; Fang, S.; Mao, W.; Xu, Z.; Yang, Y.; Weng, Q.; Zhao, Z.; et al. Targeted xCT-mediated Ferroptosis and Protumoral Polarization of Macrophages Is Effective against HCC and Enhances the Efficacy of the Anti-PD-1/L1 Response. Adv. Sci. 2023, 10, e2203973. [Google Scholar] [CrossRef]
- Arensman, M.D.; Yang, X.S.; Leahy, D.M.; Toral-Barza, L.; Mileski, M.; Rosfjord, E.C.; Wang, F.; Deng, S.; Myers, J.S.; Abraham, R.T.; et al. Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 9533–9542. [Google Scholar] [CrossRef]
- Giraudo, L.; Cattaneo, G.; Gammaitoni, L.; Iaia, I.; Donini, C.; Massa, A.; Centomo, M.L.; Basirico, M.; Vigna, E.; Pisacane, A.; et al. CSPG4 CAR-redirected Cytokine Induced Killer lymphocytes (CIK) as effective cellular immunotherapy for HLA class I defective melanoma. J. Exp. Clin. Cancer Res. 2023, 42, 310. [Google Scholar] [CrossRef]
- Xiong, Q.; Yin, B.; Jiang, H.; Qiu, Y.; Shi, G.; Xu, J.; Xu, T.; Deng, H. Targeting CSPG4 enhances the anti-tumor activity of CAR-NK cells for glioblastoma. Cell. Oncol. 2025, 48, 1539–1551. [Google Scholar] [CrossRef]
- Leuci, V.; Donini, C.; Grignani, G.; Rotolo, R.; Mesiano, G.; Fiorino, E.; Gammaitoni, L.; D’Ambrosio, L.; Merlini, A.; Landoni, E.; et al. CSPG4-Specific CAR.CIK Lymphocytes as a Novel Therapy for the Treatment of Multiple Soft-Tissue Sarcoma Histotypes. Clin. Cancer Res. 2020, 26, 6321–6334. [Google Scholar] [CrossRef]
- Chauhan, J.; Grandits, M.; Palhares, L.; Mele, S.; Nakamura, M.; Lopez-Abente, J.; Crescioli, S.; Laddach, R.; Romero-Clavijo, P.; Cheung, A.; et al. Anti-cancer pro-inflammatory effects of an IgE antibody targeting the melanoma-associated antigen chondroitin sulfate proteoglycan 4. Nat. Commun. 2023, 14, 2192. [Google Scholar] [CrossRef]
- Hoffmann, R.M.; Crescioli, S.; Mele, S.; Sachouli, E.; Cheung, A.; Chui, C.K.; Andriollo, P.; Jackson, P.J.M.; Lacy, K.E.; Spicer, J.F.; et al. A Novel Antibody-Drug Conjugate (ADC) Delivering a DNA Mono-Alkylating Payload to Chondroitin Sulfate Proteoglycan (CSPG4)-Expressing Melanoma. Cancers 2020, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn, M.; Rybczynska, A.A.; Wei, Y.; Schwenkert, M.; Fey, G.H.; Dierckx, R.A.; van Waarde, A.; Helfrich, W.; Bremer, E. Melanoma-associated Chondroitin Sulfate Proteoglycan (MCSP)-targeted delivery of soluble TRAIL potently inhibits melanoma outgrowth in vitro and in vivo. Mol. Cancer 2010, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Favoino, E.; Wang, Y.; Ma, Y.; Deng, X.; Wang, X. The CSPG4-specific monoclonal antibody enhances and prolongs the effects of the BRAF inhibitor in melanoma cells. Immunol. Res. 2011, 50, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Greiner, D.; Xue, Q.; Waddell, T.Q.; Kurudza, E.; Chaudhary, P.; Belote, R.L.; Dotti, G.; Judson-Torres, R.L.; Reeves, M.Q.; Cheshier, S.H.; et al. Human CSPG4-targeting CAR-macrophages inhibit melanoma growth. Oncogene 2025, 44, 1665–1677. [Google Scholar] [CrossRef]
- Cattaneo, G.; Ventin, M.; Arya, S.; Bailey, C.; Vantaku, V.R.; Jia, J.; Qi, M.; Maggs, L.; Wang, X.; Parangi, S.; et al. B7-H3 and CSPG4-targeted CAR T cells as potent effectors in anaplastic thyroid cancer. J. Exp. Clin. Cancer Res. 2025, 44, 248. [Google Scholar] [CrossRef]
- Mittelman, A.; Chen, G.Z.; Wong, G.Y.; Liu, C.; Hirai, S.; Ferrone, S. Human high molecular weight-melanoma associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: Modulation of the immunogenicity in patients with malignant melanoma. Clin. Cancer Res. 1995, 1, 705–713. [Google Scholar]
- Vincze, O.; Spada, B.; Bilder, D.; Cagan, A.; DeGregori, J.; Gorbunova, V.; Maley, C.C.; Schiffman, J.D.; Seluanov, A.; Giraudeau, M.; et al. Advancing cancer research via comparative oncology. Nat. Rev. Cancer 2025, 25, 740–748. [Google Scholar] [CrossRef]
- Ruzzi, F.; Riccardo, F.; Conti, L.; Tarone, L.; Semprini, M.S.; Bolli, E.; Barutello, G.; Quaglino, E.; Lollini, P.L.; Cavallo, F. Cancer vaccines: Target antigens, vaccine platforms and preclinical models. Mol. Asp. Med 2025, 101, 101324. [Google Scholar] [CrossRef]
- Roerden, M.; Spranger, S. Cancer immune evasion, immunoediting and intratumour heterogeneity. Nat. Rev. Immunol. 2025, 25, 353–369. [Google Scholar] [CrossRef]
- Thanee, M.; Padthaisong, S.; Suksawat, M.; Dokduang, H.; Phetcharaburanin, J.; Klanrit, P.; Titapun, A.; Namwat, N.; Wangwiwatsin, A.; Sa-Ngiamwibool, P.; et al. Sulfasalazine modifies metabolic profiles and enhances cisplatin chemosensitivity on cholangiocarcinoma cells in in vitro and in vivo models. Cancer Metab. 2021, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 2014, 3, e02523. [Google Scholar] [CrossRef]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, H.; Xu, X.; Liu, H.; Wu, C.; Zhao, L. Erastin/sorafenib induces cisplatin-resistant non-small cell lung cancer cell ferroptosis through inhibition of the Nrf2/xCT pathway. Oncol. Lett. 2020, 19, 323–333. [Google Scholar] [CrossRef]
- Zhang, R.; Thoroe-Boveleth, S.; Chigrin, D.N.; Kiessling, F.; Lammers, T.; Pallares, R.M. Nanoscale engineering of gold nanostars for enhanced photoacoustic imaging. J. Nanobiotechnol. 2024, 22, 115. [Google Scholar] [CrossRef]
- Huang, L.; Ge, X.; Liu, Y.; Li, H.; Zhang, Z. The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy. Pharmaceutics 2022, 14, 1228. [Google Scholar] [CrossRef]
- Quaglino, E.; Conti, L.; Cavallo, F. Breast cancer stem cell antigens as targets for immunotherapy. Semin. Immunol. 2020, 47, 101386. [Google Scholar] [CrossRef]
- Tye, H.; Kennedy, C.L.; Najdovska, M.; McLeod, L.; McCormack, W.; Hughes, N.; Dev, A.; Sievert, W.; Ooi, C.H.; Ishikawa, T.O.; et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell 2012, 22, 466–478. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarone, L.; Iacoviello, A.; Di Lorenzo, A.; Verta, R.; Cossu, C.; Conti, L.; Cavallo, F.; Riccardo, F. Exploring Emerging Therapeutic Targets in Osteosarcoma by Revisiting the Immune and Cancer-Intrinsic Hallmarks of Cancer. Cancers 2025, 17, 3846. https://doi.org/10.3390/cancers17233846
Tarone L, Iacoviello A, Di Lorenzo A, Verta R, Cossu C, Conti L, Cavallo F, Riccardo F. Exploring Emerging Therapeutic Targets in Osteosarcoma by Revisiting the Immune and Cancer-Intrinsic Hallmarks of Cancer. Cancers. 2025; 17(23):3846. https://doi.org/10.3390/cancers17233846
Chicago/Turabian StyleTarone, Lidia, Antonella Iacoviello, Antonino Di Lorenzo, Roberta Verta, Chiara Cossu, Laura Conti, Federica Cavallo, and Federica Riccardo. 2025. "Exploring Emerging Therapeutic Targets in Osteosarcoma by Revisiting the Immune and Cancer-Intrinsic Hallmarks of Cancer" Cancers 17, no. 23: 3846. https://doi.org/10.3390/cancers17233846
APA StyleTarone, L., Iacoviello, A., Di Lorenzo, A., Verta, R., Cossu, C., Conti, L., Cavallo, F., & Riccardo, F. (2025). Exploring Emerging Therapeutic Targets in Osteosarcoma by Revisiting the Immune and Cancer-Intrinsic Hallmarks of Cancer. Cancers, 17(23), 3846. https://doi.org/10.3390/cancers17233846






