Challenges and Progress for Treatment of Malignant Peripheral Nerve Sheath Tumors in the Context of Recent Successes for Sarcoma Therapy
Simple Summary
Abstract
1. Introduction
2. Sarcoma Treatment
2.1. Surgery
2.2. Radiation Therapy
2.3. Chemotherapy
2.4. Targeted Therapies
2.5. Immunotherapy
3. MPNST Therapy
3.1. Targeted Therapies for MPNST
3.2. Immunotherapy in MPNST
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MPNST | malignant peripheral nerve sheath tumor |
| NF1 | neurofibromatosis type 1 |
| STS | soft tissue sarcoma |
| OS | overall survival |
| RIS | radiation-induced sarcoma |
| ORR | objective response rate |
| PFS | progression free survival |
| TKI | tyrosine kinase inhibitor |
| PKI | protein kinase inhibitor |
| GIST | gastrointestinal stromal tumor |
| IMT | inflammatory myofibroblastic tumor |
| TGCT | tenosynovial giant cell tumor |
| PEComa | perivascular epithelioid cell tumor |
| nab-sirolimus | nanoparticle albumin-bound sirolimus |
| TSC | tuberous sclerosis complex |
| KSHV | Kaposi sarcoma-associated herpesvirus |
| TME | tumor microenvironment |
| ICI | immune checkpoint inhibitor |
| ASPS | alveolar soft part sarcoma |
| ACT | adoptive cell therapy |
| FTI | farnesyl transferase inhibitor |
References
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2020, 113, 70–84. [Google Scholar] [CrossRef]
- Hajdu, S.I. Peripheral nerve sheath tumors histogenesis, classification, and prognosis. Cancer 1993, 72, 3549–3552. [Google Scholar] [CrossRef]
- Ducatman, B.S.; Scheithauer, B.W.; Piepgras, D.G.; Reiman, H.M.; Ilstrup, D.M. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986, 57, 2006–2021. [Google Scholar] [CrossRef]
- Bates, J.E.; Peterson, C.R.; Dhakal, S.; Giampoli, E.J.; Constine, L.S. Malignant peripheral nerve sheath tumors (MPNST): A SEER analysis of incidence across the age spectrum and therapeutic interventions in the pediatric population. Pediatr. Blood Cancer 2014, 61, 1955–1960. [Google Scholar] [CrossRef]
- Lammert, M.; Friedman, J.M.; Kluwe, L.; Mautner, V.F. Prevalence of Neurofibromatosis 1 in German Children at Elementary School Enrollment. Arch. Dermatol. 2005, 141, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Kallionpää, R.A.; Uusitalo, E.; Leppävirta, J.; Pöyhönen, M.; Peltonen, S.; Peltonen, J. Prevalence of neurofibromatosis type 1 in the Finnish population. Genet. Med. Off. J. Am. Coll. Med. Genet. 2018, 20, 1082–1086. [Google Scholar] [CrossRef]
- Evans, D.; Baser, M.; McGaughran, J.; Sharif, S.; Howard, E.; Moran, A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J. Med. Genet. 2002, 39, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Yang, J.; Wang, G. Clinical and molecular prognostic predictors of malignant peripheral nerve sheath tumor. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2014, 16, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Longhi, A.; Errani, C.; Magagnoli, G.; Alberghini, M.; Gambarotti, M.; Mercuri, M.; Ferrari, S. High grade malignant peripheral nerve sheath tumors: Outcome of 62 patients with localized disease and review of the literature. J. Chemother. Florence Italy 2010, 22, 413–418. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, X.; Liu, Z.; Yang, S.; Liao, H.-I.; Jiang, L.; Wei, F. Malignant peripheral nerve sheath tumours of the spine: Clinical manifestations, classification, treatment, and prognostic factors. Eur. Spine J. 2012, 21, 897–904. [Google Scholar] [CrossRef]
- Valentin, T.; Le Cesne, A.; Ray-Coquard, I.; Italiano, A.; Decanter, G.; Bompas, E.; Isambert, N.; Thariat, J.; Linassier, C.; Bertucci, F.; et al. Management and prognosis of malignant peripheral nerve sheath tumors: The experience of the French Sarcoma Group (GSF-GETO). Eur. J. Cancer 2016, 56, 77–84. [Google Scholar] [CrossRef]
- Lim, Z.; Gu, T.Y.; Tai, B.C.; Puhaindran, M.E. Survival outcomes of malignant peripheral nerve sheath tumors (MPNSTs) with and without neurofibromatosis type I (NF1): A meta-analysis. World J. Surg. Oncol. 2024, 22, 14. [Google Scholar] [CrossRef]
- Porter, D.E.; Prasad, V.; Foster, L.; Dall, G.F.; Birch, R.; Grimer, R.J. Survival in Malignant Peripheral Nerve Sheath Tumours: A Comparison between Sporadic and Neurofibromatosis Type 1-Associated Tumours. Sarcoma 2009, 2009, 756395. [Google Scholar] [CrossRef]
- Watson, K.L.; Al Sannaa, G.A.; Kivlin, C.M.; Ingram, D.R.; Landers, S.M.; Roland, C.L.; Cormier, J.N.; Hunt, K.K.; Feig, B.W.; Guadagnolo, B.A.; et al. Patterns of recurrence and survival in sporadic, neurofibromatosis type 1-associated, and radiation-associated malignant peripheral nerve sheath tumors (MPNSTs). J. Neurosurg. 2017, 126, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, M.; Høland, M.; Ågesen, T.H.; Brekke, H.R.; Liestøl, K.; Hall, K.S.; Mertens, F.; Picci, P.; Smeland, S.; Lothe, R.A. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro-oncology 2013, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Al-Mistarehi, A.-H.; Zaitoun, K.J.; Khalifeh, J.; Saint-Germain, M.A.; Horowitz, M.A.; Ghaith, A.K.; Foster, C.H.; Braverman, S.; Albert, A.N.; AlDallal, U.; et al. An Assessment of Surgical Outcomes in Malignant Peripheral Nerve Sheath Tumors: A Systematic Review and Meta-Analysis of Surgical Interventions. Cancers 2025, 17, 1997. [Google Scholar] [CrossRef]
- Sobczuk, P.; Teterycz, P.; Czarnecka, A.M.; Świtaj, T.; Koseła-Paterczyk, H.; Kozak, K.; Falkowski, S.; Rutkowski, P. Systemic Treatment for Advanced and Metastatic Malignant Peripheral Nerve Sheath Tumors—A Sarcoma Reference Center Experience. J. Clin. Med. 2020, 9, 3157. [Google Scholar] [CrossRef] [PubMed]
- Jansma, C.Y.M.N.; Acem, I.; Grünhagen, D.J.; Verhoef, C.; Martin, E.; MONACO Collaborators. Local recurrence in malignant peripheral nerve sheath tumours: Multicentre cohort study. BJS Open 2024, 8, zrae024. [Google Scholar] [CrossRef]
- Akshintala, S.; Mallory, N.C.; Lu, Y.; Ballman, K.V.; Schuetze, S.M.; Chugh, R.; Maki, R.G.; Reinke, D.K.; Widemann, B.C.; Kim, A. Outcome of Patients With Malignant Peripheral Nerve Sheath Tumors Enrolled on Sarcoma Alliance for Research Through Collaboration (SARC) Phase II Trials. Oncologist 2023, 28, 453–459. [Google Scholar] [CrossRef]
- Sobczuk, P.; Teterycz, P.; Czarnecka, A.M.; Świtaj, T.; Koseła-Paterczyk, H.; Kozak, K.; Falkowski, S.; Goryń, T.; Zdzienicki, M.; Morysiński, T.; et al. Malignant peripheral nerve sheath tumors—Outcomes and prognostic factors based on the reference center experience. Surg. Oncol. 2020, 35, 276–284. [Google Scholar] [CrossRef]
- Yuan, J.; Li, X.; Yu, S. Molecular targeted therapy for advanced or metastatic soft tissue sarcoma. Cancer Control 2021, 28, 10732748211038424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Trama, A.; Badalamenti, G.; Baldi, G.G.; Brunello, A.; Caira, M.; Drove, N.; Marrari, A.; Palmerini, E.; Vincenzi, B.; Dei Tos, A.P.; et al. Soft tissue sarcoma in Italy: From epidemiological data to clinical networking to improve patient care and outcomes. Cancer Epidemiol. 2019, 59, 258–264. [Google Scholar] [CrossRef]
- Gatta, G.; Van Der Zwan, J.M.; Casali, P.G.; Siesling, S.; Dei Tos, A.P.; Kunkler, I.; Otter, R.; Licitra, L.; Mallone, S.; Tavilla, A.; et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur. J. Cancer 2011, 47, 2493–2511. [Google Scholar] [CrossRef]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef]
- Gage, M.M.; Nagarajan, N.; Ruck, J.M.; Canner, J.K.; Khan, S.; Giuliano, K.; Gani, F.; Wolfgang, C.; Johnston, F.M.; Ahuja, N. Sarcomas in the United States: Recent trends and a call for improved staging. Oncotarget 2019, 10, 2462–2474. [Google Scholar] [CrossRef]
- Kollár, A.; Rothermundt, C.; Klenke, F.; Bode, B.; Baumhoer, D.; Arndt, V.; Feller, A. Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol. 2019, 63, 101596. [Google Scholar] [CrossRef]
- Florou, V.; Nascimento, A.G.; Gulia, A.; de Lima Lopes, G. Global Health Perspective in Sarcomas and Other Rare Cancers. Am. Soc. Clin. Oncol. Educ. Book. Am. Soc. Clin. Oncol. Annu. Meet. 2018, 38, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, L.M.; Ho, V.K.Y.; Dijkstra, P.D.S.; Schreuder, H.W.B.; Schaap, G.R.; Ploegmakers, J.J.W.; Van Der Geest, I.C.M.; Van De Sande, M.A.J.; Bramer, J.A.; Suurmeijer, A.J.H.; et al. Bone sarcoma incidence in the Netherlands. Cancer Epidemiol. 2019, 60, 31–38. [Google Scholar] [CrossRef]
- Damron, T.A.; Ward, W.G.; Stewart, A. Osteosarcoma, Chondrosarcoma, and Ewing’s Sarcoma: National Cancer Data Base Report. Clin. Orthop. 2007, 459, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Sobczuk, P.; Zdzienicki, M.; Spałek, M.; Rutkowski, P. Malignant peripheral nerve sheath tumour (MPNST). Oncol. Clin. Pract. 2018, 14, 364–376. [Google Scholar] [CrossRef]
- Crago, A.M.; Brennan, M.F. Principles in Management of Soft Tissue Sarcoma. Adv. Surg. 2015, 49, 107–122. [Google Scholar] [CrossRef]
- Aboud, K.; Meissner, M.; Ocen, J.; Jones, R. Cytotoxic chemotherapy: Clinical aspects. Medicine 2023, 51, 23–27. [Google Scholar] [CrossRef]
- Grünewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 2020, 12, e11131. [Google Scholar] [CrossRef]
- Lilley, J.; Murray, L.J. Radiotherapy: Technical aspects. Medicine 2023, 51, 11–16. [Google Scholar] [CrossRef]
- Skliarenko, J.; Barry, A. Clinical and practical applications of radiation therapy: When should radiation therapy be considered for my patient? Medicine 2023, 51, 17–22. [Google Scholar] [CrossRef]
- Gerrand, C.; Athanasou, N.; Brennan, B.; Grimer, R.; Judson, I.; Morland, B.; Peake, D.; Seddon, B.; Whelan, J.; On behalf of the British Sarcoma Group. UK guidelines for the management of bone sarcomas. Clin. Sarcoma Res. 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, A.O.; Yang, G.Q.; Latifi, K.; Gillies, R.; McLeod, H.; Harrison, L.B. The Future of Radiation Oncology in Soft Tissue Sarcoma. Cancer Control 2018, 25, 1073274818815504. [Google Scholar] [CrossRef]
- Snow, A.; Ring, A.; Struycken, L.; Mack, W.; Koç, M.; Lang, J.E. Incidence of radiation induced sarcoma attributable to radiotherapy in adults: A retrospective cohort study in the SEER cancer registries across 17 primary tumor sites. Cancer Epidemiol. 2021, 70, 101857. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Demicco, E.G.; Garcia, R.; Ahn, L.; Merola, P.R.; Cioffi, A.; Maki, R.G. Malignant Peripheral Nerve Sheath Tumors. Oncologist 2014, 19, 193–201. [Google Scholar] [CrossRef]
- Gladdy, R.A.; Qin, L.-X.; Moraco, N.; Edgar, M.A.; Antonescu, C.R.; Alektiar, K.M.; Brennan, M.F.; Singer, S. Do Radiation-Associated Soft Tissue Sarcomas Have the Same Prognosis As Sporadic Soft Tissue Sarcomas? J. Clin. Oncol. 2010, 28, 2064–2069. [Google Scholar] [CrossRef]
- Bjerkehagen, B.; Smeland, S.; Walberg, L.; Skjeldal, S.; Hall, K.S.; Nesland, J.M.; Småstuen, M.C.; Fosså, S.D.; Saeter, G. Radiation-induced sarcoma: 25-year experience from the Norwegian Radium Hospital. Acta Oncol. Stockh. Swed. 2008, 47, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.S.; Gaynor, J.J.; Brennan, M.F. Radiation-associated sarcoma of bone and soft tissue. Arch. Surg. Chic. Ill 1960 1992, 127, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yao, W. Chemotherapeutic drugs for soft tissue sarcomas: A review. Front. Pharmacol. 2023, 14, 1199292. [Google Scholar] [CrossRef]
- Maurel, J.; López-Pousa, A.; de las Peñas, R.; Fra, J.; Martín, J.; Cruz, J.; Casado, A.; Poveda, A.; Martínez-Trufero, J.; Balañá, C.; et al. Efficacy of Sequential High-Dose Doxorubicin and Ifosfamide Compared With Standard-Dose Doxorubicin in Patients With Advanced Soft Tissue Sarcoma: An Open-Label Randomized Phase II Study of the Spanish Group for Research on Sarcomas. J. Clin. Oncol. 2009, 27, 1893–1898. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Wang, B.; Ji, G.; Naeem, H.; Wang, J.; Kantharidis, P.; Powell, D.; Ricardo, S.D. The Use of Targeted Next Generation Sequencing to Explore Candidate Regulators of TGF-β1’s Impact on Kidney Cells. Front. Physiol. 2018, 9, 1755. [Google Scholar] [CrossRef]
- Weiss, A.R.; Chen, Y.-L.; Scharschmidt, T.J.; Chi, Y.-Y.; Tian, J.; Black, J.O.; Davis, J.L.; Fanburg-Smith, J.C.; Zambrano, E.; Anderson, J.; et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): A multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 1110–1122. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.-F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef]
- Ferretti, G.R.; Reymond, E.; Delouche, A.; Sakhri, L.; Jankowski, A.; Moro-Sibilot, D.; Lantuejoul, S.; Toffart, A.C. Personalized chemotherapy of lung cancer: What the radiologist should know. Diagn. Interv. Imaging 2016, 97, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, H.; Kumar, S. Targeted agents in cancer. Medicine 2023, 51, 32–36. [Google Scholar] [CrossRef]
- Definition of targeted therapy—NCI Dictionary of Cancer Terms—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/targeted-therapy (accessed on 7 August 2024).
- Thomson, R.J.; Moshirfar, M.; Ronquillo, Y. Tyrosine Kinase Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK563322/ (accessed on 7 August 2024).
- Blay, J.-Y. A decade of tyrosine kinase inhibitor therapy: Historical and current perspectives on targeted therapy for GIST. Cancer Treat. Rev. 2011, 37, 373–384. [Google Scholar] [CrossRef]
- Demetri, G.D.; Desai, J.; Fletcher, J.A.; Morgan, J.A.; Fletcher, C.D.M.; Kazanovicz, A.; Van Den Abbeele, A.; Baum, C.; Maki, R.; Heinrich, M.C. SU11248, a multi-targeted tyrosine kinase inhibitor, can overcome imatinib (IM) resistance caused by diverse genomic mechanisms in patients (pts) with metastatic gastrointestinal stromal tumor (GIST). J. Clin. Oncol. 2004, 22, 3001. [Google Scholar] [CrossRef]
- Demetri, G.D.; Reichardt, P.; Kang, Y.-K.; Blay, J.-Y.; Rutkowski, P.; Gelderblom, H.; Hohenberger, P.; Leahy, M.; von Mehren, M.; Joensuu, H.; et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib: An international, multicentre, prospective, randomised, placebo-controlled phase 3 trial (GRID). Lancet 2013, 381, 295–302. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; Von Mehren, M.; Schöffski, P.; Serrano, C.; Kang, Y.-K.; Cassier, P.A.; Mir, O.; Eskens, F.; Tap, W.D.; et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncol. 2020, 21, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Suurmeijer, A.J.; Zhang, L.; Sung, Y.-S.; Jungbluth, A.A.; Travis, W.D.; Al-Ahmadie, H.; Fletcher, C.D.; Alaggio, R. Molecular Characterization of Inflammatory Myofibroblastic Tumors with Frequent ALK and ROS1 Fusions and Rare Novel RET Gene Rearrangement. Am. J. Surg. Pathol. 2015, 39, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Mossé, Y.P.; Lim, M.S.; Voss, S.D.; Wilner, K.; Ruffner, K.; Laliberte, J.; Rolland, D.; Balis, F.M.; Maris, J.M.; Weigel, B.J.; et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: A Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013, 14, 472–480. [Google Scholar] [CrossRef]
- Gambacorti-Passerini, C.; Orlov, S.; Zhang, L.; Braiteh, F.; Huang, H.; Esaki, T.; Horibe, K.; Ahn, J.; Beck, J.T.; Edenfield, W.J.; et al. Long-term effects of crizotinib in ALK-positive tumors (excluding NSCLC): A phase 1b open-label study. Am. J. Hematol. 2018, 93, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Dürr, H.R.; Schaefer, I.-M.; Woertler, K.; Haas, R.; Trama, A.; Caraceni, A.; Bajpai, J.; Baldi, G.G.; Bernthal, N.; et al. Best clinical management of tenosynovial giant cell tumour (TGCT): A consensus paper from the community of experts. Cancer Treat. Rev. 2023, 112, 102491. [Google Scholar] [CrossRef]
- Tap, W.D.; Gelderblom, H.; Palmerini, E.; Desai, J.; Bauer, S.; Blay, J.-Y.; Alcindor, T.; Ganjoo, K.; Martín-Broto, J.; Ryan, C.W.; et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): A randomised phase 3 trial. Lancet 2019, 394, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Singh, A.S.; Anthony, S.P.; Sterba, M.; Zhang, C.; Healey, J.H.; Chmielowski, B.; Cohn, A.L.; Shapiro, G.I.; Keedy, V.L.; et al. Results from Phase I Extension Study Assessing Pexidartinib Treatment in Six Cohorts with Solid Tumors including TGCT, and Abnormal CSF1 Transcripts in TGCT. Clin. Cancer Res. 2022, 28, 298–307. [Google Scholar] [CrossRef]
- Iheanacho, I.; Yoo, H.K.; Yang, X.; Dodman, S.; Hughes, R.; Amin, S. Epidemiological and clinical burden associated with plexiform neurofibromas in pediatric neurofibromatosis type-1 (NF-1): A systematic literature review. Neurol. Sci. 2022, 43, 1281–1293. [Google Scholar] [CrossRef]
- Boulanger, J.M.; Larbrisseau, A. Neurofibromatosis Type 1 in a Pediatric Population: Ste-Justine’s Experience. Can. J. Neurol. Sci. 2005, 32, 225–231. [Google Scholar] [CrossRef]
- Prada, C.E.; Rangwala, F.A.; Martin, L.J.; Lovell, A.M.; Saal, H.M.; Schorry, E.K.; Hopkin, R.J. Pediatric Plexiform Neurofibromas: Impact on Morbidity and Mortality in Neurofibromatosis Type 1. J. Pediatr. 2012, 160, 461–467. [Google Scholar] [CrossRef]
- Tucker, T.; Friedman, J.M.; Friedrich, R.E.; Wenzel, R.; Fünsterer, C.; Mautner, V.-F. Longitudinal study of neurofibromatosis 1 associated plexiform neurofibromas. J. Med. Genet. 2009, 46, 81–85. [Google Scholar] [CrossRef]
- Gross, A.M.; Wolters, P.L.; Dombi, E.; Baldwin, A.; Whitcomb, P.; Fisher, M.J.; Weiss, B.; Kim, A.; Bornhorst, M.; Shah, A.C.; et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N. Engl. J. Med. 2020, 382, 1430–1442. [Google Scholar] [CrossRef]
- Gross, A.M.; Dombi, E.; Wolters, P.L.; Baldwin, A.; Dufek, A.; Herrera, K.; Martin, S.; Derdak, J.; Heisey, K.S.; Whitcomb, P.M.; et al. Long-term safety and efficacy of selumetinib in children with neurofibromatosis type 1 on a phase 1/2 trial for inoperable plexiform neurofibromas. Neuro-Oncology 2023, 25, 1883–1894. [Google Scholar] [CrossRef]
- McCaw, T.R.; Inga, E.; Chen, H.; Jaskula-Sztul, R.; Dudeja, V.; Bibb, J.A.; Ren, B.; Rose, J.B. Gamma Secretase Inhibitors in Cancer: A Current Perspective on Clinical Performance. Oncologist 2021, 26, e608–e621. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.; Ratan, R.; Alcindor, T.; Schöffski, P.; van der Graaf, W.T.; Wilky, B.A.; Riedel, R.F.; Lim, A.; Smith, L.M.; Moody, S.; et al. Nirogacestat, a γ-Secretase Inhibitor for Desmoid Tumors. N. Engl. J. Med. 2023, 388, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R.G.; et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013, 369, 341–350. [Google Scholar] [CrossRef]
- Shang, H.; Braggio, D.; Lee, Y.; Al Sannaa, G.A.; Creighton, C.J.; Bolshakov, S.; Lazar, A.J.F.; Lev, D.; Pollock, R.E. Targeting the notch pathway: A potential therapeutic approach for desmoid tumors. Cancer 2015, 121, 4088–4096. [Google Scholar] [CrossRef]
- Wagner, A.J.; Ravi, V.; Riedel, R.F.; Ganjoo, K.; Van Tine, B.A.; Chugh, R.; Cranmer, L.; Gordon, E.M.; Hornick, J.L.; Du, H.; et al. nab-Sirolimus for Patients With Malignant Perivascular Epithelioid Cell Tumors. J. Clin. Oncol. 2021, 39, 3660–3670. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, G.; Pea, M.; Reghellin, D.; Zamboni, G.; Bonetti, F. PEComas: The past, the present and the future. Virchows Arch. 2008, 452, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Manningf, B.D. The TSC1–TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef]
- Kenerson, H.; Folpe, A.L.; Takayama, T.K.; Yeung, R.S. Activation of the mTOR Pathway in Sporadic Angiomyolipomas and Other Perivascular Epithelioid Cell Neoplasms. Hum. Pathol. 2007, 38, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, M.; Woo, D.-H.; Shin, Y.; Shin, J.; Chang, N.; Oh, Y.T.; Kim, H.; Rheey, J.; Nakano, I.; et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell 2013, 23, 839–852. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Lu, X.; Song, B.; Fong, K.-W.; Cao, Q.; Licht, J.D.; Zhao, J.C.; Yu, J. Polycomb- and Methylation-Independent Roles of EZH2 as a Transcription Activator. Cell Rep. 2018, 25, 2808–2820.e4. [Google Scholar] [CrossRef]
- Xu, K.; Wu, Z.J.; Groner, A.C.; He, H.H.; Cai, C.; Lis, R.T.; Wu, X.; Stack, E.C.; Loda, M.; Liu, T.; et al. EZH2 Oncogenic Activity in Castration Resistant Prostate Cancer Cells is Polycomb-Independent. Science 2012, 338, 1465–1469. [Google Scholar] [CrossRef]
- Phelan, M.L.; Sif, S.; Narlikar, G.J.; Kingston, R.E. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol. Cell 1999, 3, 247–253. [Google Scholar] [CrossRef]
- Wilson, B.G.; Wang, X.; Shen, X.; McKenna, E.S.; Lemieux, M.E.; Cho, Y.-J.; Koellhoffer, E.C.; Pomeroy, S.L.; Orkin, S.H.; Roberts, C.W.M. Epigenetic Antagonism between Polycomb and SWI/SNF Complexes during Oncogenic Transformation. Cancer Cell 2010, 18, 316–328. [Google Scholar] [CrossRef]
- Gounder, M.; Schöffski, P.; Jones, R.L.; Agulnik, M.; Cote, G.M.; Villalobos, V.M.; Attia, S.; Chugh, R.; Chen, T.W.-W.; Jahan, T.; et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: An international, open-label, phase 2 basket study. Lancet Oncol. 2020, 21, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of Herpesvirus-Like DNA Sequences in AIDS-Sssociated Kaposi’s Sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef]
- Lacy, M.Q.; McCurdy, A.R. Pomalidomide. Blood 2013, 122, 2305–2309. [Google Scholar] [CrossRef] [PubMed]
- Haslett, P.A.J.; Corral, L.G.; Albert, M.; Kaplan, G. Thalidomide Costimulates Primary Human T Lymphocytes, Preferentially Inducing Proliferation, Cytokine Production, and Cytotoxic Responses in the CD8+ Subset. J. Exp. Med. 1998, 187, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.E.; Raje, N.; Hideshima, T.; Lentzsch, S.; Young, G.; Tai, Y.-T.; Lin, B.; Podar, K.; Gupta, D.; Chauhan, D.; et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 2001, 98, 210–216. [Google Scholar] [CrossRef]
- Bhutani, M.; Polizzotto, M.N.; Uldrick, T.S.; Yarchoan, R. KSHV-associated Malignancies: Epidemiology, Pathogenesis, and Advances in Treatment. Semin. Oncol. 2015, 42, 223–246. [Google Scholar] [CrossRef]
- Polizzotto, M.N.; Uldrick, T.S.; Wyvill, K.M.; Aleman, K.; Peer, C.J.; Bevans, M.; Sereti, I.; Maldarelli, F.; Whitby, D.; Marshall, V.; et al. Pomalidomide for Symptomatic Kaposi’s Sarcoma in People With and Without HIV Infection: A Phase I/II Study. J. Clin. Oncol. 2016, 34, 4125–4131. [Google Scholar] [CrossRef]
- Tap, W.D.; Jones, R.L.; Van Tine, B.A.; Chmielowski, B.; Elias, A.D.; Adkins, D.; Agulnik, M.; Cooney, M.M.; Livingston, M.B.; Pennock, G.; et al. Olaratumab and doxorubicin versus doxorubicin alone in soft tissue sarcoma. Lancet 2016, 388, 488–497. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Bidikian, A.H.; Alameh, I.; Nakib, C.E.; Assi, H.I. Olaratumab’s failure in soft tissue sarcoma. Rare Tumors 2021, 13, 20363613211034115. [Google Scholar] [CrossRef]
- Attia, S.; Villalobos, V.; Hindi, N.; Wagner, A.J.; Chmielowski, B.; Oakley, G.J.; Peterson, P.M.; Ceccarelli, M.; Jones, R.L.; Dickson, M.A. Randomized Phase 2 Clinical Trial of Olaratumab in Combination with Gemcitabine and Docetaxel in Advanced Soft Tissue Sarcomas. Cancers 2023, 15, 4871. [Google Scholar] [CrossRef]
- Schöffski, P.; Bahleda, R.; Wagner, A.J.; Burgess, M.A.; Junker, N.; Chisamore, M.; Peterson, P.; Szpurka, A.M.; Ceccarelli, M.; Tap, W.D. Results of an Open-label, Phase Ia/b Study of Pembrolizumab plus Olaratumab in Patients with Unresectable, Locally Advanced, or Metastatic Soft-Tissue Sarcoma. Clin. Cancer Res. 2023, 29, 3320–3328. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lee, J.X.A. Immunotherapy for cancer. Medicine 2023, 51, 37–41. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Ayodele, O.; Razak, A.R.A. Immunotherapy in Soft-Tissue Sarcoma. Curr. Oncol. 2020, 27, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Movva, S.; Wen, W.; Chen, W.; Millis, S.Z.; Gatalica, Z.; Reddy, S.; von Mehren, M.; Tine, B.A.V. Multi-platform profiling of over 2000 sarcomas: Identification of biomarkers and novel therapeutic targets. Oncotarget 2015, 6, 12234–12247. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Tine, B.A.V.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Lewin, J.; Davidson, S.; Anderson, N.D.; Lau, B.Y.; Kelly, J.; Tabori, U.; Salah, S.; Butler, M.O.; Aung, K.L.; Shlien, A.; et al. Response to Immune Checkpoint Inhibition in Two Patients with Alveolar Soft-Part Sarcoma. Cancer Immunol. Res. 2018, 6, 1001–1007. [Google Scholar] [CrossRef]
- Wilky, B.A.; Trucco, M.M.; Subhawong, T.K.; Florou, V.; Park, W.; Kwon, D.; Wieder, E.D.; Kolonias, D.; Rosenberg, A.E.; Kerr, D.A.; et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 837–848. [Google Scholar] [CrossRef]
- Chen, A.P.; Sharon, E.; O’Sullivan-Coyne, G.; Moore, N.; Foster, J.C.; Hu, J.S.; Tine, B.A.V.; Conley, A.P.; Read, W.L.; Riedel, R.F.; et al. Atezolizumab for Advanced Alveolar Soft Part Sarcoma. N. Engl. J. Med. 2023, 389, 911–921. [Google Scholar] [CrossRef]
- Robbins, P.F.; Kassim, S.H.; Tran, T.L.N.; Crystal, J.S.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Dudley, M.E.; Wunderlich, J.R.; Sherry, R.M.; et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: Long-term follow-up and correlates with response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 1019–1027. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Melchiori, L.; Merchant, M.S.; Bernstein, D.; Glod, J.; Kaplan, R.; Grupp, S.; Tap, W.D.; Chagin, K.; Binder, G.K.; et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 c259T Cells in Synovial Sarcoma. Cancer Discov. 2018, 8, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Van Tine, B.A.; Olszanski, A.J.; Johnson, M.L.; Liebner, D.A.; Trivedi, T.; Lin, Q.; Elefant, E.; Dryer-Minnerly, R.; Navenot, J.-M.; et al. Phase I dose escalation and expansion trial to assess the safety and efficacy of ADP-A2M4 SPEAR T cells in advanced solid tumors. J. Clin. Oncol. 2020, 38, 102. [Google Scholar] [CrossRef]
- Wood, G.E.; Meyer, C.; Petitprez, F.; D’Angelo, S.P. Immunotherapy in Sarcoma: Current Data and Promising Strategies. Am. Soc. Clin. Oncol. Educ. Book. 2024, 44, e432234. [Google Scholar] [CrossRef]
- Baehring, J.M.; Betensky, R.A.; Batchelor, T.T. Malignant peripheral nerve sheath tumor. Neurology 2003, 61, 696–698. [Google Scholar] [CrossRef]
- Angelov, L.; Davis, A.; O’Sullivan, B.; Bell, R.; Guha, A. Neurogenic Sarcomas: Experience at the University of Toronto. Neurosurgery 1998, 43, 56. [Google Scholar] [CrossRef] [PubMed]
- Higham, C.S.; Steinberg, S.M.; Dombi, E.; Perry, A.; Helman, L.J.; Schuetze, S.M.; Ludwig, J.A.; Staddon, A.; Milhem, M.M.; Rushing, D.; et al. SARC006: Phase II Trial of Chemotherapy in Sporadic and Neurofibromatosis Type 1 Associated Chemotherapy-Naive Malignant Peripheral Nerve Sheath Tumors. Sarcoma 2017, 2017, 8685638. [Google Scholar] [CrossRef]
- Gronchi, A.; Stacchiotti, S.; Verderio, P.; Ferrari, S.; Martin Broto, J.; Lopez-Pousa, A.; Llombart-Bosch, A.; Dei Tos, A.P.; Collini, P.; Jurado, J.C.; et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): Long-term follow-up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann. Oncol. 2016, 27, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Palmerini, E.; Quagliuolo, V.; Martin Broto, J.; Lopez Pousa, A.; Grignani, G.; Brunello, A.; Blay, J.-Y.; Tendero, O.; Diaz Beveridge, R.; et al. Neoadjuvant Chemotherapy in High-Risk Soft Tissue Sarcomas: Final Results of a Randomized Trial From Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J. Clin. Oncol. 2020, 38, 2178–2186. [Google Scholar] [CrossRef]
- Hirbe, A.C.; Cosper, P.F.; Dahiya, S.; Van Tine, B.A. Neoadjuvant Ifosfamide and Epirubicin in the Treatment of Malignant Peripheral Nerve Sheath Tumors. Sarcoma 2017, 2017, 3761292. [Google Scholar] [CrossRef]
- Ferrari, A.; Casanova, M.; Bisogno, G.; Mattke, A.; Meazza, C.; Gandola, L.; Sotti, G.; Cecchetto, G.; Harms, D.; Koscielniak, E.; et al. Clear cell sarcoma of tendons and aponeuroses in pediatric patients. Cancer 2002, 94, 3269–3276. [Google Scholar] [CrossRef]
- Frustaci, S.; Gherlinzoni, F.; De Paoli, A.; Bonetti, M.; Azzarelli, A.; Comandone, A.; Olmi, P.; Buonadonna, A.; Pignatti, G.; Barbieri, E.; et al. Adjuvant Chemotherapy for Adult Soft Tissue Sarcomas of the Extremities and Girdles: Results of the Italian Randomized Cooperative Trial. J. Clin. Oncol. 2001, 19, 1238–1247. [Google Scholar] [CrossRef]
- Stucky, C.-C.H.; Johnson, K.N.; Gray, R.J.; Pockaj, B.A.; Ocal, I.T.; Rose, P.S.; Wasif, N. Malignant Peripheral Nerve Sheath Tumors (MPNST): The Mayo Clinic Experience. Ann. Surg. Oncol. 2012, 19, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Spiliopoulos, K.; Plotkin, S.R.; Hornicek, F.J.; Harmon, D.C.; Delaney, T.F.; Williams, Z. Role of resection of malignant peripheral nerve sheath tumors in patients with neurofibromatosis Type 1. J. Neurosurg. 2013, 118, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Dingley, B.; Fiore, M.; Gronchi, A. Personalizing surgical margins in retroperitoneal sarcomas: An update. Expert. Rev. Anticancer. Ther. 2019, 19, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Coert, J.H.; Flucke, U.E.; Slooff, W.-B.M.; Ho, V.K.Y.; Van Der Graaf, W.T.; Van Dalen, T.; Van De Sande, M.A.J.; Van Houdt, W.J.; Grünhagen, D.J.; et al. A nationwide cohort study on treatment and survival in patients with malignant peripheral nerve sheath tumours. Eur. J. Cancer 2020, 124, 77–87. [Google Scholar] [CrossRef]
- Kroep, J.R.; Ouali, M.; Gelderblom, H.; Le Cesne, A.; Dekker, T.J.A.; Van Glabbeke, M.; Hogendoorn, P.C.W.; Hohenberger, P. First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: An EORTC Soft Tissue and Bone Sarcoma Group study. Ann. Oncol. 2011, 22, 207–214. [Google Scholar] [CrossRef]
- van Noesel, M.M.; Orbach, D.; Brennan, B.; Kelsey, A.; Zanetti, I.; de Salvo, G.L.; Gaze, M.N.; Craigie, R.J.; McHugh, K.; Francotte, N.; et al. Outcome and prognostic factors in pediatric malignant peripheral nerve sheath tumors: An analysis of the European Pediatric Soft Tissue Sarcoma Group (EpSSG) NRSTS-2005 prospective study. Pediatr. Blood Cancer 2019, 66, e27833. [Google Scholar] [CrossRef]
- LaFemina, J.; Qin, L.-X.; Moraco, N.H.; Antonescu, C.R.; Fields, R.C.; Crago, A.M.; Brennan, M.F.; Singer, S. Oncologic Outcomes of Sporadic, Neurofibromatosis-Associated, and Radiation-Induced Malignant Peripheral Nerve Sheath Tumors. Ann. Surg. Oncol. 2013, 20, 66–72. [Google Scholar] [CrossRef]
- Knight, S.W.E.; Knight, T.E.; Santiago, T.; Murphy, A.J.; Abdelhafeez, A.H. Malignant Peripheral Nerve Sheath Tumors—A Comprehensive Review of Pathophysiology, Diagnosis, and Multidisciplinary Management. Children 2022, 9, 38. [Google Scholar] [CrossRef]
- Kahn, J.; Gillespie, A.; Tsokos, M.; Ondos, J.; Dombi, E.; Camphausen, K.; Widemann, B.C.; Kaushal, A. Radiation Therapy in Management of Sporadic and Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors. Front. Oncol. 2014, 4, 324. [Google Scholar] [CrossRef]
- Sharif, S.; Ferner, R.; Birch, J.M.; Gillespie, J.E.; Gattamaneni, H.R.; Baser, M.E.; Evans, D.G.R. Second Primary Tumors in Neurofibromatosis 1 Patients Treated for Optic Glioma: Substantial Risks After Radiotherapy. J. Clin. Oncol. 2006, 24, 2570–2575. [Google Scholar] [CrossRef]
- Pellerino, A.; Verdijk, R.M.; Nichelli, L.; Andratschke, N.H.; Idbaih, A.; Goldbrunner, R. Diagnosis and Treatment of Peripheral and Cranial Nerve Tumors with Expert Recommendations: An EUropean Network for RAre CANcers (EURACAN) Initiative. Cancers 2023, 15, 1930. [Google Scholar] [CrossRef] [PubMed]
- Hirbe, A.C.; Dehner, C.A.; Dombi, E.; Eulo, V.; Gross, A.M.; Sundby, T.; Lazar, A.J.; Widemann, B.C. Contemporary Approach to Neurofibromatosis Type 1–Associated Malignant Peripheral Nerve Sheath Tumors. Am. Soc. Clin. Oncol. Educ. Book. 2024, 44, e432242. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, T.; Kim, A.; Ratner, N.; Peinado, H. The Need for New Treatments Targeting MPNST: The Potential of Strategies Combining MEK Inhibitors with Antiangiogenic Agents. Clin. Cancer Res. 2022, 28, 3185–3195. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, N.; Paudyal, B.; Winkler, R.E.; Van Tine, B.A.; Hirbe, A.C. Malignant Peripheral Nerve Sheath Tumor, a Heterogeneous, Aggressive Cancer with Diverse Biomarkers and No Targeted Standard of Care: Review of the Literature and Ongoing Investigational Agents. Target. Oncol. 2024, 19, 665–678. [Google Scholar] [CrossRef]
- Yao, C.; Zhou, H.; Dong, Y.; Alhaskawi, A.; Hasan Abdullah Ezzi, S.; Wang, Z.; Lai, J.; Goutham Kota, V.; Hasan Abdulla Hasan Abdulla, M.; Lu, H. Malignant Peripheral Nerve Sheath Tumors: Latest Concepts in Disease Pathogenesis and Clinical Management. Cancers 2023, 15, 1077. [Google Scholar] [CrossRef]
- Dilworth, J.T.; Kraniak, J.M.; Wojtkowiak, J.W.; Gibbs, R.A.; Borch, R.F.; Tainsky, M.A.; Reiners, J.J.; Mattingly, R.R. Molecular targets for emerging anti-tumor therapies for neurofibromatosis type 1. Biochem. Pharmacol. 2006, 72, 1485–1492. [Google Scholar] [CrossRef]
- Kahen, E.J.; Brohl, A.; Yu, D.; Welch, D.; Cubitt, C.L.; Lee, J.K.; Chen, Y.; Yoder, S.J.; Teer, J.K.; Zhang, Y.O.; et al. Neurofibromin level directs RAS pathway signaling and mediates sensitivity to targeted agents in malignant peripheral nerve sheath tumors. Oncotarget 2018, 9, 22571–22585. [Google Scholar] [CrossRef]
- Furth, M.E.; Davis, L.J.; Fleurdelys, B.; Scolnick, E.M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J. Virol. 1982, 43, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Wong, G.; Arnheim, N.; Nitecki, D.; McCormick, F. Antibodies specific for amino acid 12 of the ras oncogene product inhibit GTP binding. Proc. Natl. Acad. Sci. USA 1985, 82, 5280–5284. [Google Scholar] [CrossRef]
- Mattingly, R.R. Activated Ras as a Therapeutic Target: Constraints on Directly Targeting Ras Isoforms and Wild-Type versus Mutated Proteins. ISRN Oncol. 2013, 2013, 536529. [Google Scholar] [CrossRef]
- Reiss, Y.; Goldstein, J.L.; Seabra, M.C.; Casey, P.J.; Brown, M.S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell 1990, 62, 81–88. [Google Scholar] [CrossRef]
- Whyte, D.B.; Kirschmeier, P.; Hockenberry, T.N.; Nunez-Oliva, I.; James, L.; Catino, J.J.; Bishop, W.R.; Pai, J.-K. K- and N-Ras Are Geranylgeranylated in Cells Treated with Farnesyl Protein Transferase Inhibitors*. J. Biol. Chem. 1997, 272, 14459–14464. [Google Scholar] [CrossRef]
- Brock, E.J.; Ji, K.; Reiners, J.J.; Mattingly, R.R. How to Target Activated Ras Proteins: Direct Inhibition vs. Induced Mislocalization. Mini Rev. Med. Chem. 2016, 16, 358–369. [Google Scholar] [CrossRef]
- Holderfield, M.; Lee, B.J.; Jiang, J.; Tomlinson, A.; Seamon, K.J.; Mira, A.; Patrucco, E.; Goodhart, G.; Dilly, J.; Gindin, Y.; et al. Concurrent inhibition of oncogenic and wild-type RAS-GTP for cancer therapy. Nature 2024, 629, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.-Y.; Xu, M.; Lu, Y.; Liu, J.; Staedtke, V. Pan-RAS inhibitor RMC-7977 is efficacious in treating NF1-related tumors. Neuro-Oncol. Adv. 2025, 7, vdaf065. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.J.; Mathieu, P.A.; Gargano, M.; George, I.; Shen, Y.; Callaghan, J.F.; Borch, R.F.; Mattingly, R.R. Synergistic Suppression of NF1 Malignant Peripheral Nerve Sheath Tumor Cell Growth in Culture and Orthotopic Xenografts by Combinational Treatment with Statin and Prodrug Farnesyltransferase Inhibitor PAMAM G4 Dendrimers. Cancers 2024, 16, 89. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, W.; Li, Y.; Li, H.; Guo, Z.; Wei, C.; Long, M.; Chung, M.; Aimaier, R.; Li, Q.; et al. Preclinical Assessment of MEK Inhibitors for Malignant Peripheral Nerve Sheath Tumors Reveals Differences in Efficacy and Adaptive Response. Front. Oncol. 2022, 12, 903177. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, R.R.; Kraniak, J.M.; Dilworth, J.T.; Mathieu, P.; Bealmear, B.; Nowak, J.E.; Benjamins, J.A.; Tainsky, M.A.; Reiners, J.J. The Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Kinase Inhibitor PD184352 (CI-1040) Selectively Induces Apoptosis in Malignant Schwannoma Cell Lines. J. Pharmacol. Exp. Ther. 2006, 316, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Nagabushan, S.; Lau, L.M.S.; Barahona, P.; Wong, M.; Sherstyuk, A.; Marshall, G.M.; Tyrrell, V.; Wegner, E.A.; Ekert, P.G.; Cowley, M.J.; et al. Efficacy of MEK inhibition in a recurrent malignant peripheral nerve sheath tumor. npj Precis. Oncol. 2021, 5, 9. [Google Scholar] [CrossRef]
- Hitchen, N.; Cross, M.; Laking, G.; Connor, J.; Korenbaum, C. Sporadic Metastatic Malignant Peripheral Nerve Sheath Tumour with an NF1 Mutation Responding to Trametinib: A Case Report. Case Rep. Oncol. 2023, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pollard, K.; Calizo, A.; Pratilas, C.A. Activation of receptor tyrosine kinases mediates acquired resistance to MEK inhibition in malignant peripheral nerve sheath tumors. Cancer Res. 2021, 81, 747–762. [Google Scholar] [CrossRef]
- Widemann, B.C.; Lu, Y.; Reinke, D.; Okuno, S.H.; Meyer, C.F.; Cote, G.M.; Chugh, R.; Milhem, M.M.; Hirbe, A.C.; Kim, A.; et al. Targeting Sporadic and Neurofibromatosis Type 1 (NF1) Related Refractory Malignant Peripheral Nerve Sheath Tumors (MPNST) in a Phase II Study of Everolimus in Combination with Bevacizumab (SARC016). Sarcoma 2019, 2019, 7656747. [Google Scholar] [CrossRef]
- Kim, A.; Lu, Y.; Okuno, S.H.; Reinke, D.; Maertens, O.; Perentesis, J.; Basu, M.; Wolters, P.L.; De Raedt, T.; Chawla, S.; et al. Targeting Refractory Sarcomas and Malignant Peripheral Nerve Sheath Tumors in a Phase I/II Study of Sirolimus in Combination with Ganetespib (SARC023). Sarcoma 2020, 2020, 5784876. [Google Scholar] [CrossRef]
- Manji, G.A.; Van Tine, B.A.; Lee, S.M.; Raufi, A.G.; Pellicciotta, I.; Hirbe, A.C.; Pradhan, J.; Chen, A.; Rabadan, R.; Schwartz, G.K. A Phase I Study of the Combination of Pexidartinib and Sirolimus to Target Tumor-Associated Macrophages in Unresectable Sarcoma and Malignant Peripheral Nerve Sheath Tumors. Clin. Cancer Res. 2021, 27, 5519–5527. [Google Scholar] [CrossRef]
- Maki, R.G.; D’Adamo, D.R.; Keohan, M.L.; Saulle, M.; Schuetze, S.M.; Undevia, S.D.; Livingston, M.B.; Cooney, M.M.; Hensley, M.L.; Mita, M.M.; et al. Phase II Study of Sorafenib in Patients With Metastatic or Recurrent Sarcomas. J. Clin. Oncol. 2009, 27, 3133–3140. [Google Scholar] [CrossRef]
- Chugh, R.; Wathen, J.K.; Maki, R.G.; Benjamin, R.S.; Patel, S.R.; Myers, P.A.; Priebat, D.A.; Reinke, D.K.; Thomas, D.G.; Keohan, M.L.; et al. Phase II Multicenter Trial of Imatinib in 10 Histologic Subtypes of Sarcoma Using a Bayesian Hierarchical Statistical Model. J. Clin. Oncol. 2009, 27, 3148–3153. [Google Scholar] [CrossRef]
- Nishida, Y.; Urakawa, H.; Nakayama, R.; Kobayashi, E.; Ozaki, T.; Ae, K.; Matsumoto, Y.; Tsuchiya, H.; Goto, T.; Hiraga, H.; et al. Phase II clinical trial of pazopanib for patients with unresectable or metastatic malignant peripheral nerve sheath tumors. Int. J. Cancer 2021, 148, 140–149. [Google Scholar] [CrossRef]
- Yoo, K.H.; Kim, H.S.; Lee, S.J.; Park, S.H.; Kim, S.J.; Kim, S.H.; La Choi, Y.; Shin, K.-H.; Cho, Y.J.; Lee, J.; et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: A retrospective case series. BMC Cancer 2015, 15, 154. [Google Scholar] [CrossRef]
- Wang, J.; Pollard, K.; Allen, A.N.; Tomar, T.; Pijnenburg, D.; Yao, Z.; Rodriguez, F.J.; Pratilas, C.A. Combined Inhibition of SHP2 and MEK Is Effective in Models of NF1-Deficient Malignant Peripheral Nerve Sheath Tumors. Cancer Res. 2020, 80, 5367–5379. [Google Scholar] [CrossRef]
- Wang, J.; Calizo, A.; Zhang, L.; Pino, J.C.; Lyu, Y.; Pollard, K.; Zhang, X.; Larsson, A.T.; Conniff, E.; Llosa, N.; et al. CDK4/6 inhibition enhances SHP2 inhibitor efficacy and is dependent upon restoration of RB function in malignant peripheral nerve sheath tumors. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Hao, J.; Zhou, X.; Yan, L.; Dai, H.; Sun, B.; Yang, D.; An, S.; Lv, L.; Jiao, B.; et al. CDKN2B deletion is essential for pancreatic cancer development instead of unmeaningful co-deletion due to juxtaposition to CDKN2A. Oncogene 2018, 37, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Dean, D.C. The Rb/E2F pathway: Expanding roles and emerging paradigms. Genes Dev. 2000, 14, 2393–2409. [Google Scholar] [CrossRef]
- Kohlmeyer, J.L.; Kaemmer, C.A.; Pulliam, C.; Maharjan, C.K.; Samayoa, A.M.; Major, H.J.; Cornick, K.E.; Knepper-Adrian, V.; Khanna, R.; Sieren, J.C.; et al. RABL6A Is an Essential Driver of MPNSTs that Negatively Regulates the RB1 Pathway and Sensitizes Tumor Cells to CDK4/6 Inhibitors. Clin. Cancer Res. 2020, 26, 2997–3011. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Martinez-Garcia, J.; Moura, D.S.; Redondo, A.; Gutierrez, A.; Lopez-Pousa, A.; Martinez-Trufero, J.; Sevilla, I.; Diaz-Beveridge, R.; Solis-Hernandez, M.P.; et al. Phase II trial of CDK4/6 inhibitor palbociclib in advanced sarcoma based on mRNA expression of CDK4/CDKN2A. Signal Transduct. Target. Ther. 2023, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.-T.; Shereda, R.; Lu, Q.; Weisenberger, D.J.; O’Connell, C.; Machida, K.; An, W.; Lenz, H.-J.; El-Khoueiry, A.; et al. DNMT and EZH2 inhibitors synergize to activate therapeutic targets in hepatocellular carcinoma. Cancer Lett. 2022, 548, 215899. [Google Scholar] [CrossRef]
- Patel, A.J.; Warda, S.; Maag, J.L.V.; Misra, R.; Miranda-Román, M.A.; Pachai, M.R.; Lee, C.J.; Li, D.; Wang, N.; Bayshtok, G.; et al. PRC2-Inactivating Mutations in Cancer Enhance Cytotoxic Response to DNMT1-Targeted Therapy via Enhanced Viral Mimicry. Cancer Discov. 2022, 12, 2120–2139. [Google Scholar] [CrossRef]
- Maksimova, V.; Makus, J.; Popova, V.; Prus, A.; Usalka, O.; Trapeznikova, E.; Zhidkova, E.; Belitsky, G.; Yakubovskaya, M.; Kirsanov, K. Histone Methyltransferases as a New Target for Epigenetic Action of Vorinostat. Biochem. Mosc. 2023, 88, 968–978. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Q. Histone deacetylase inhibitors and cell death. Cell. Mol. Life Sci. 2014, 71, 3885–3901. [Google Scholar] [CrossRef] [PubMed]
- De Raedt, T.; Beert, E.; Pasmant, E.; Luscan, A.; Brems, H.; Ortonne, N.; Helin, K.; Hornick, J.L.; Mautner, V.; Kehrer-Sawatzki, H.; et al. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature 2014, 514, 247–251. [Google Scholar] [CrossRef]
- Cooper, J.M.; Patel, A.J.; Chen, Z.; Liao, C.-P.; Chen, K.; Mo, J.; Wang, Y.; Le, L.Q. Overcoming BET Inhibitor Resistance in Malignant Peripheral Nerve Sheath Tumors. Clin. Cancer Res. 2019, 25, 3404–3416. [Google Scholar] [CrossRef]
- Patel, A.J.; Liao, C.-P.; Chen, Z.; Liu, C.; Wang, Y.; Le, L.Q. BET Bromodomain Inhibition Triggers Apoptosis of NF1-Associated Malignant Peripheral Nerve Sheath Tumors through Bim Induction. Cell Rep. 2014, 6, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Jaganathan, A.; Sun, Y.; Ma, N.; Li, N.; Han, X.; Sun, X.; Yi, H.; Fu, S.; et al. BRD4-PRC2 represses transcription of T-helper 2-specific negative regulators during T-cell differentiation. EMBO J. 2023, 42, e111473. [Google Scholar] [CrossRef]
- Cortes-Ciriano, I.; Steele, C.D.; Piculell, K.; Al-Ibraheemi, A.; Eulo, V.; Bui, M.M.; Chatzipli, A.; Dickson, B.C.; Borcherding, D.C.; Feber, A.; et al. Genomic Patterns of Malignant Peripheral Nerve Sheath Tumor (MPNST) Evolution Correlate with Clinical Outcome and Are Detectable in Cell-Free DNA. Cancer Discov. 2023, 13, 654–671. [Google Scholar] [CrossRef]
- Verdijk, R.M.; den Bakker, M.A.; Dubbink, H.J.; Hop, W.C.J.; Dinjens, W.N.M.; Kros, J.M. TP53 Mutation Analysis of Malignant Peripheral Nerve Sheath Tumors. J. Neuropathol. Exp. Neurol. 2010, 69, 16–26. [Google Scholar] [CrossRef]
- Konopleva, M.; Martinelli, G.; Daver, N.; Papayannidis, C.; Wei, A.; Higgins, B.; Ott, M.; Mascarenhas, J.; Andreeff, M. MDM2 inhibition: An important step forward in cancer therapy. Leukemia 2020, 34, 2858–2874. [Google Scholar] [CrossRef]
- Tian, L.; Li, H.; Cui, H.; Tang, C.; Zhao, P.; Wang, X.; Cheng, Y. Oncogenic KRAS mutations drive immune suppression through immune-related regulatory network and metabolic reprogramming. Cell Death Dis. 2025, 16, 785. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.; Russ, A.; Arif-Tiwari, H.; Mahadevan, D.; Elliott, A.; Bhattacharyya, A.; Babiker, H. Pembrolizumab Achieves a Complete Response in an NF-1 Mutated, PD-L1 Positive Malignant Peripheral Nerve Sheath Tumor: A Case Report and Review of the Benchmarks. J. Immunother. 2022, 45, 222. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Bohanes, P.; Bisig, B.; Missiaglia, E.; Tsantoulis, P.; Coukos, G.; Montemurro, M.; Homicsko, K.; Michielin, O. Deep Response to Anti-PD-1 Therapy of Metastatic Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumor with CD274/PD-L1 Amplification. JCO Precis. Oncol. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeyer, J.L.; Lingo, J.J.; Kaemmer, C.A.; Scherer, A.; Warrier, A.; Voigt, E.; Raygoza Garay, J.A.; McGivney, G.R.; Brockman, Q.R.; Tang, A.; et al. CDK4/6-MEK Inhibition in MPNSTs Causes Plasma Cell Infiltration, Sensitization to PD-L1 Blockade, and Tumor Regression. Clin. Cancer Res. 2023, 29, 3484–3497. [Google Scholar] [CrossRef] [PubMed]
- Lingo, J.J.; Elias, E.C.; Quelle, D.E. Novel Therapeutics and the Path Toward Effective Immunotherapy in Malignant Peripheral Nerve Sheath Tumors. Cancers 2025, 17, 2410. [Google Scholar] [CrossRef] [PubMed]
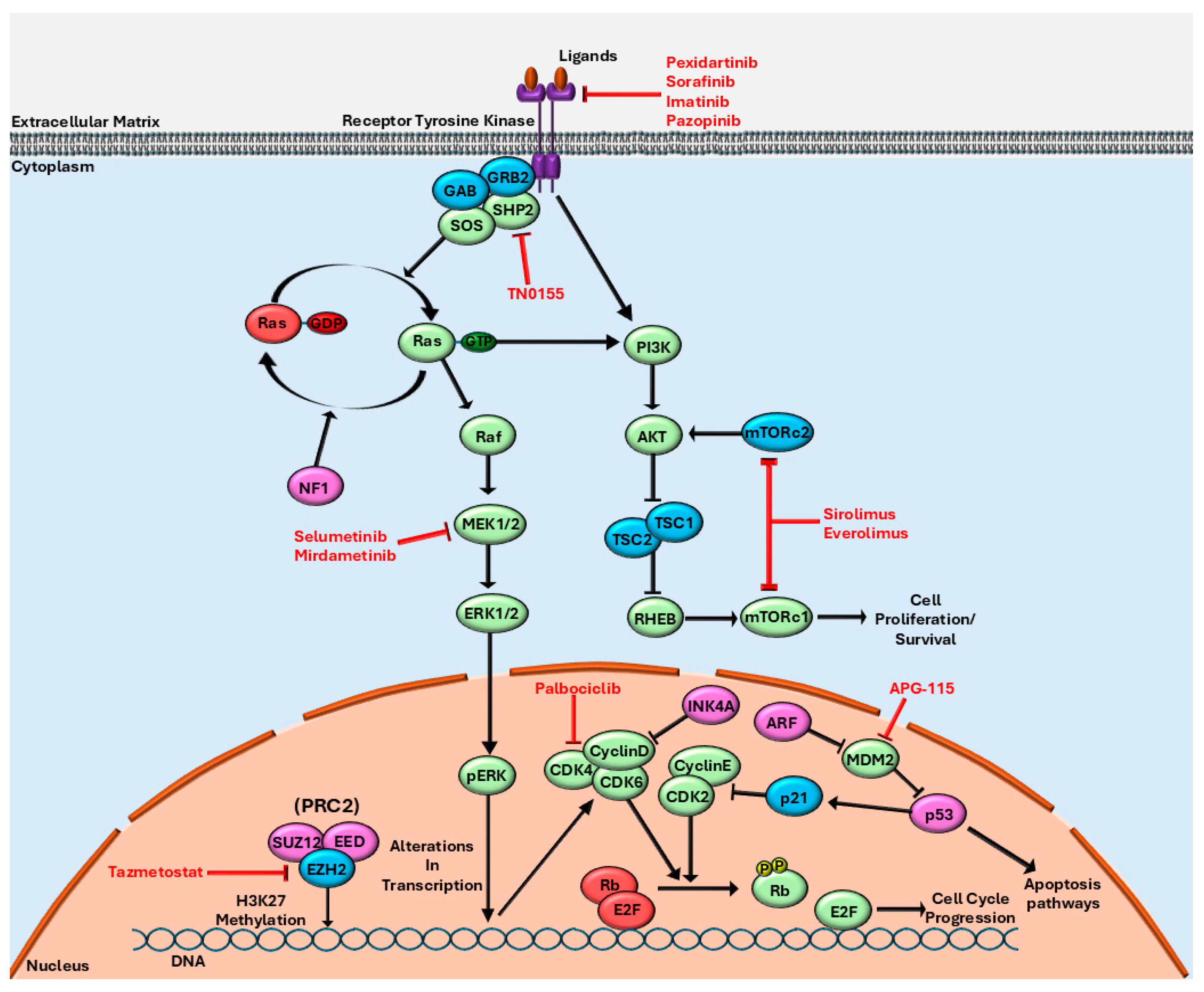
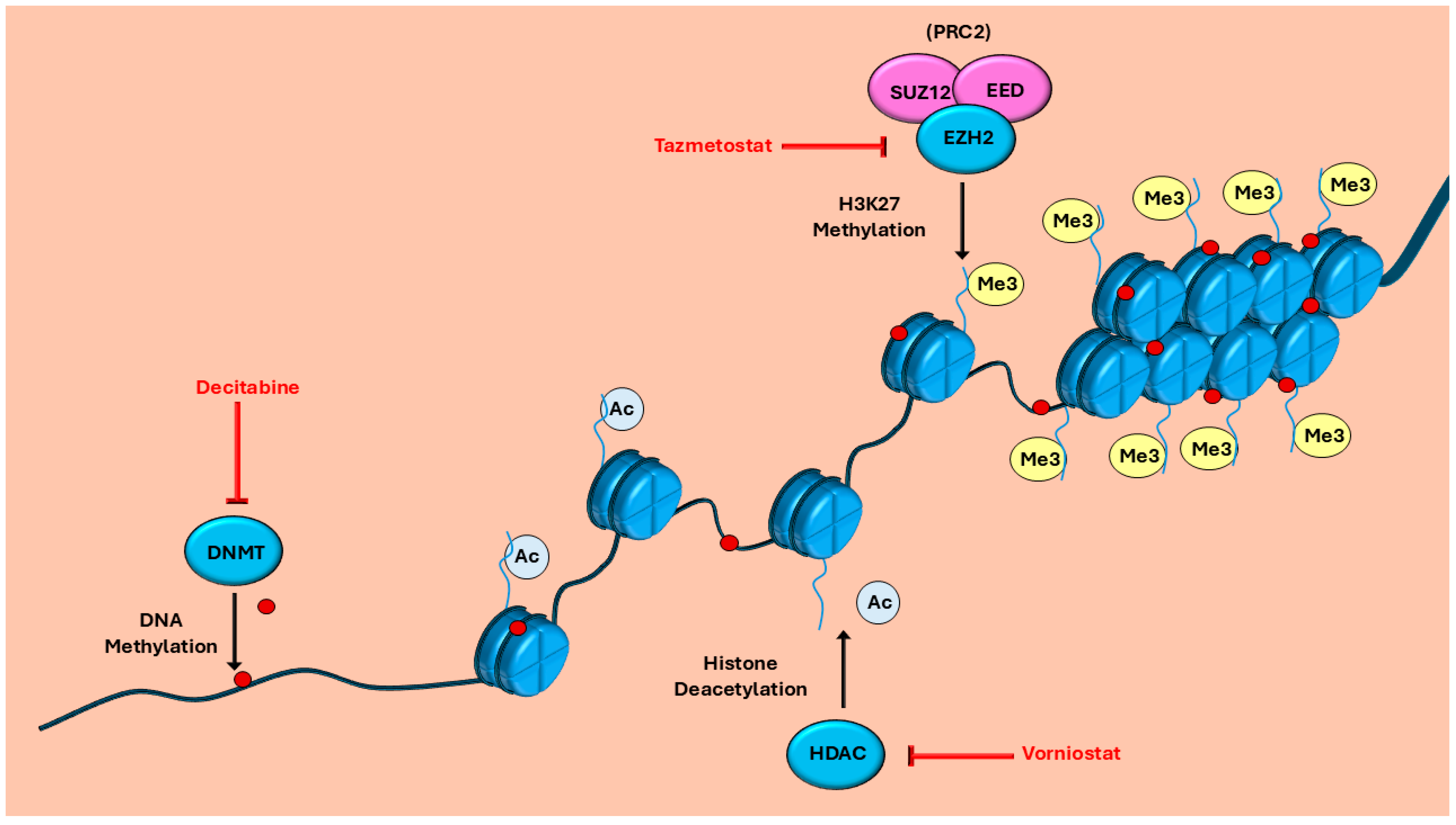
| Drug | Mechanism of Action | Disease Treated |
|---|---|---|
| Imatinib | Multi-kinase inhibitor | Gastrointestinal Stromal Tumors |
| Sunitinib | Multi-kinase inhibitor | Gastrointestinal Stromal Tumors |
| Regorafinib | Multi-kinase inhibitor | Gastrointestinal Stromal Tumors |
| Ripretinib | Multi-kinase inhibitor | Gastrointestinal Stromal Tumors |
| Avapritinib | Multi-kinase inhibitor | Gastrointestinal Stromal Tumors with a PDGFRA D842V Mutation |
| Crizotinib | ALK inhibitor | Inflammatory Myofibroblastic Tumor |
| nab-sirolimus | mTOR inhibitor | Perivascular Epithelioid Tumor |
| Pomalidomide | Angiogenesis inhibitor | Kaposi Sarcoma |
| Tazemetostat | EZH2 inhibitor | Epithelioid Sarcoma |
| Nirogacestat | γ-Secretase Inhibitor | Desmoid Tumors |
| Selumetinib | MEK inhibitor | Inoperable Plexiform Neurofibromas |
| Mirdametinib | MEK inhibitor | Inoperable Symptomatic Plexiform Neurofibromas |
| Pexidartinib | CSF-1R inhibitor | Tenosynovial Giant Cell Tumor |
| Intervention | Mechanism of Action | Phase | Target Tumors | Clinicaltrials.gov ID |
|---|---|---|---|---|
| Pexidartinib and Sirolimus | CSF-1R and mTOR inhibitors | I/II | Unresectable sarcoma and MPNSTs | NCT02584647 |
| Selumetinib and Sirolimus | MEK and mTOR inhibitors | II | Unresectable or metastatic MPNSTs | NCT03433183 |
| Selumetinib and APG-115 | MEK and MDM2 inhibitors | 0/1/2 | Refractory or unresectable MPNST or ANNUBPs | NCT06735820 |
| Mirdametinib and Vorinostat | MEK and histone Deacetylation inhibitors | 0 | Primary MPNST pre-resection. | NCT06693284 |
| Tazemetostat | EZH2 inhibitor | II | Recurrent or metastatic MPNSTs | NCT04917042 |
| ASTX727 | DNA methyl-transferase inhibitor | II | MPNST with a PRC2 mutation | NCT04872543 |
| Nivolumab and Ipilimumab | PD-1 and CTLA-4 inhibitors | I | Pre-malignant and MPNSTs | NCT04465643 |
| MV-NIS | Vaccine therapy | I | Unresectable or recurrent MPNSTs | NCT02700230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callaghan, J.F.; Mattingly, R.R. Challenges and Progress for Treatment of Malignant Peripheral Nerve Sheath Tumors in the Context of Recent Successes for Sarcoma Therapy. Cancers 2025, 17, 3781. https://doi.org/10.3390/cancers17233781
Callaghan JF, Mattingly RR. Challenges and Progress for Treatment of Malignant Peripheral Nerve Sheath Tumors in the Context of Recent Successes for Sarcoma Therapy. Cancers. 2025; 17(23):3781. https://doi.org/10.3390/cancers17233781
Chicago/Turabian StyleCallaghan, John F., and Raymond R. Mattingly. 2025. "Challenges and Progress for Treatment of Malignant Peripheral Nerve Sheath Tumors in the Context of Recent Successes for Sarcoma Therapy" Cancers 17, no. 23: 3781. https://doi.org/10.3390/cancers17233781
APA StyleCallaghan, J. F., & Mattingly, R. R. (2025). Challenges and Progress for Treatment of Malignant Peripheral Nerve Sheath Tumors in the Context of Recent Successes for Sarcoma Therapy. Cancers, 17(23), 3781. https://doi.org/10.3390/cancers17233781







