Targeting Cancer with Redox Catalysis: Manganese Porphyrins and Ascorbate Synergistically Induce Selective Oxidative Stress and Necrotic Cell Death
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Manganese Porphyrins
2.3. Optical Properties
2.4. Cytotoxicity Assay
2.5. Kinetics of Propidium Iodide Uptake
2.6. Detection of Caspase-3/7 Activation
2.7. Cell Migration Assay
2.8. Fluorescence Microscopy
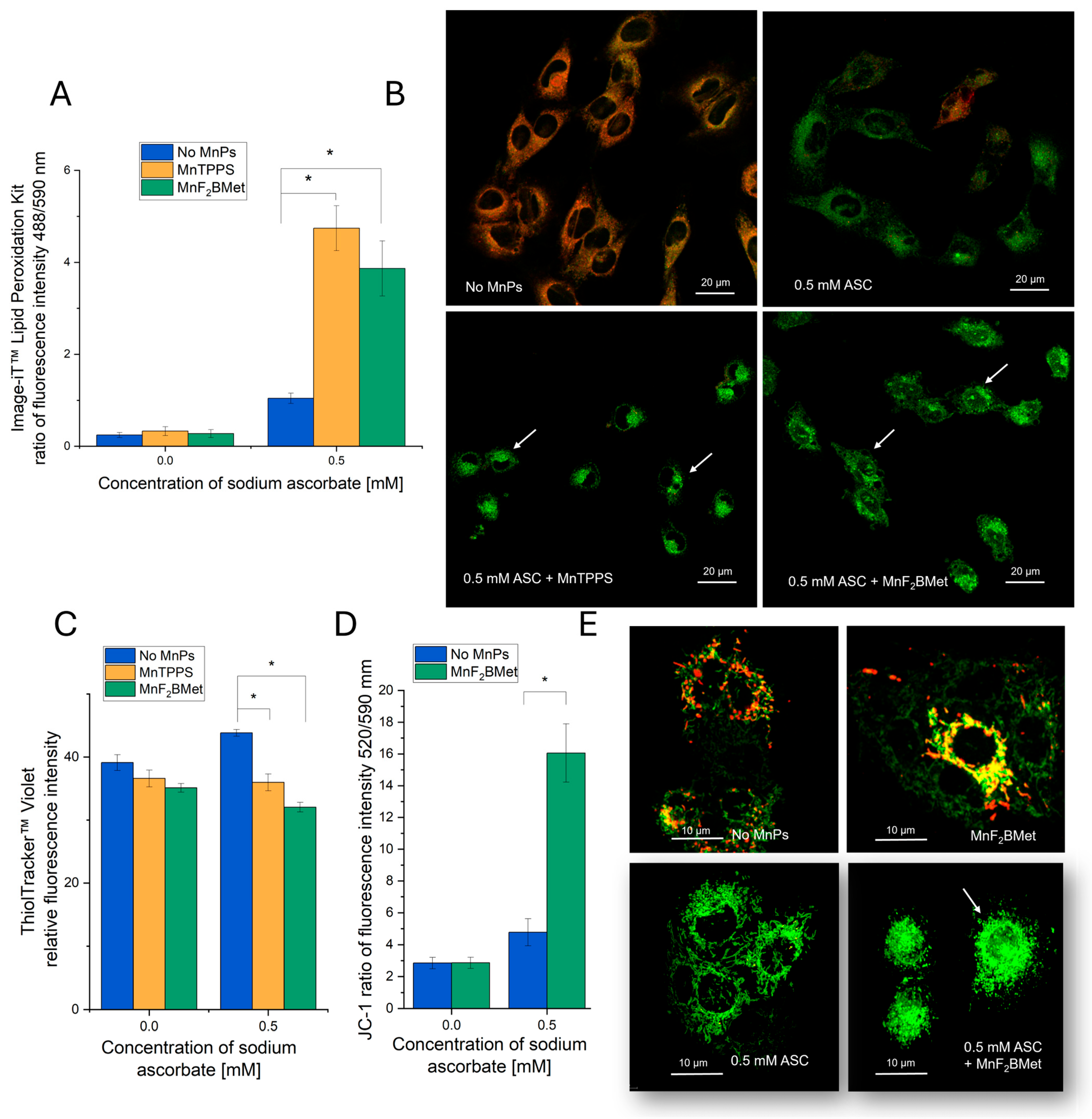
2.9. Quantification of GSH, Lipid Peroxidation, and Mitochondrial Membrane Potential
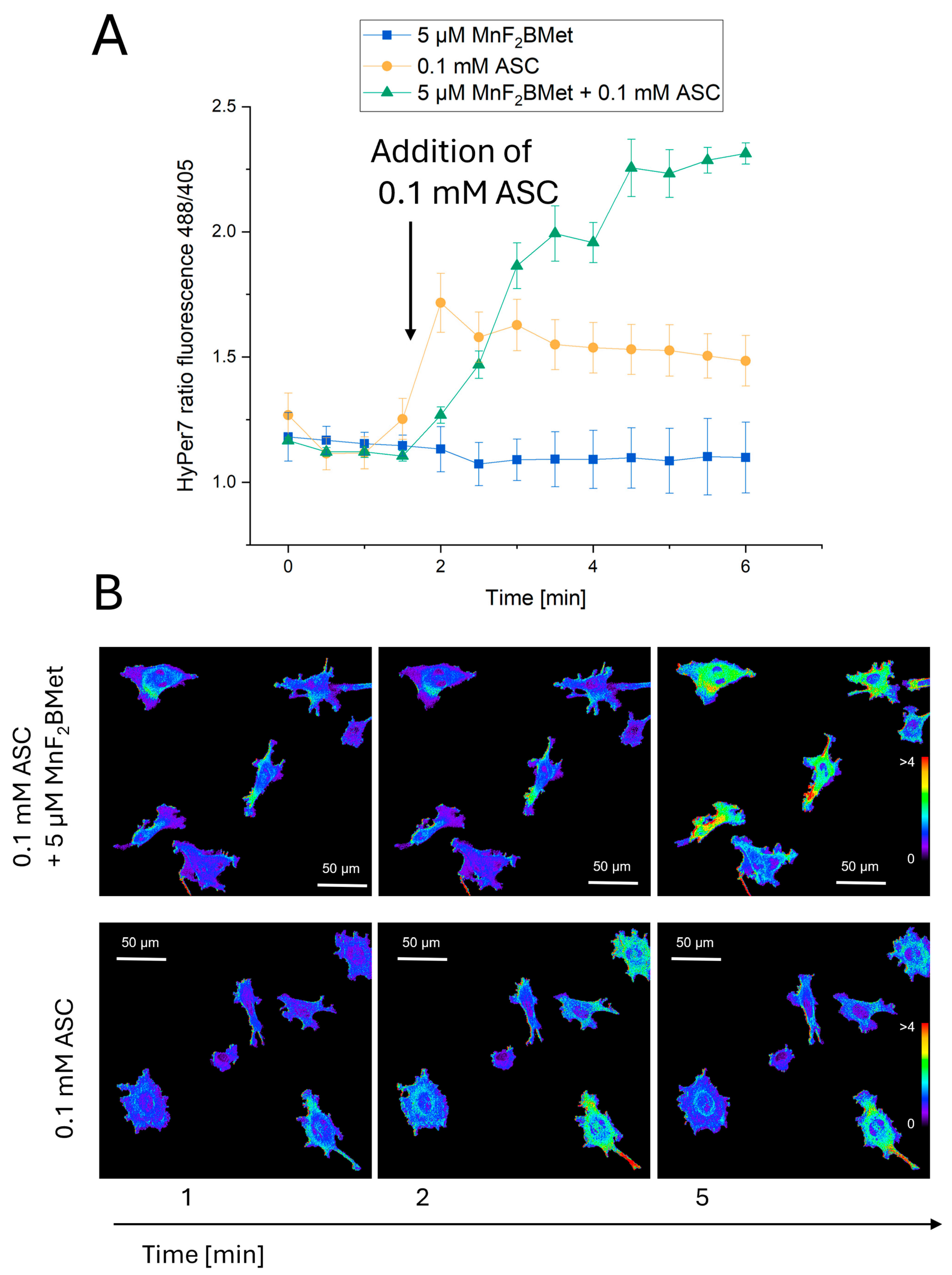
2.10. Quantification of Intracellular H2O2
2.11. Quantification of Intracellular Ascorbate Levels
2.12. Statistical Analysis
3. Results
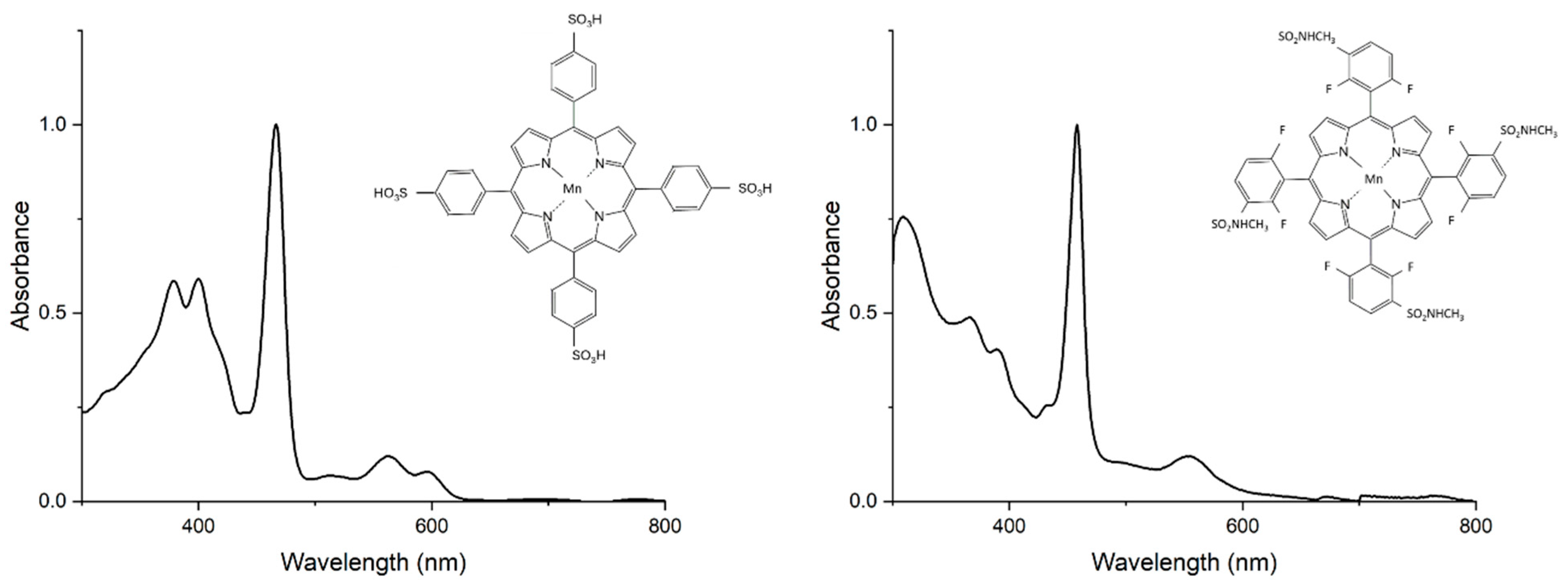
3.1. Optical Properties of Manganese Porphyrins
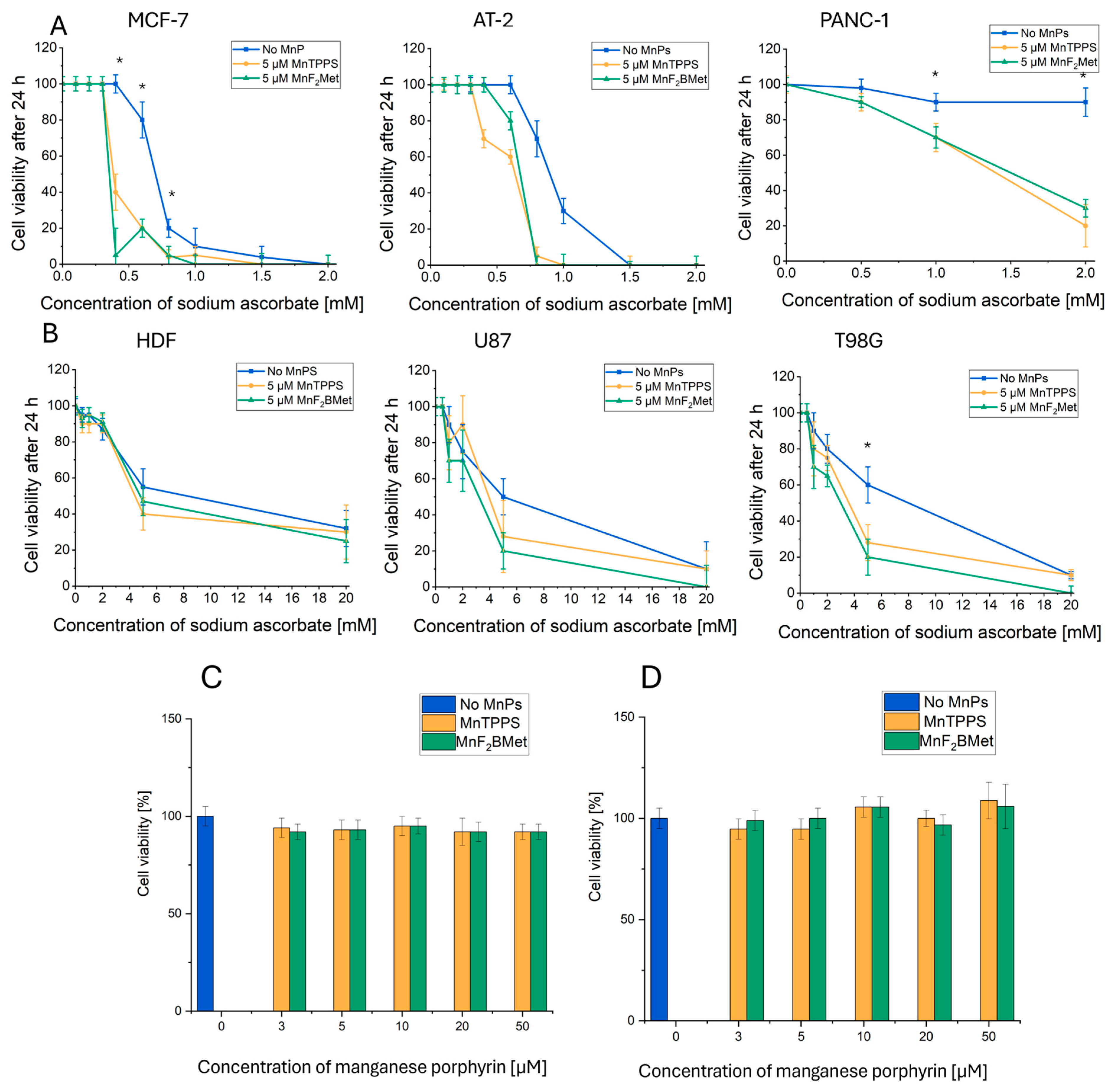
3.2. Selective Cytotoxicity of the MnPs-ASC System Against Cancer Cells: A Screening Approach
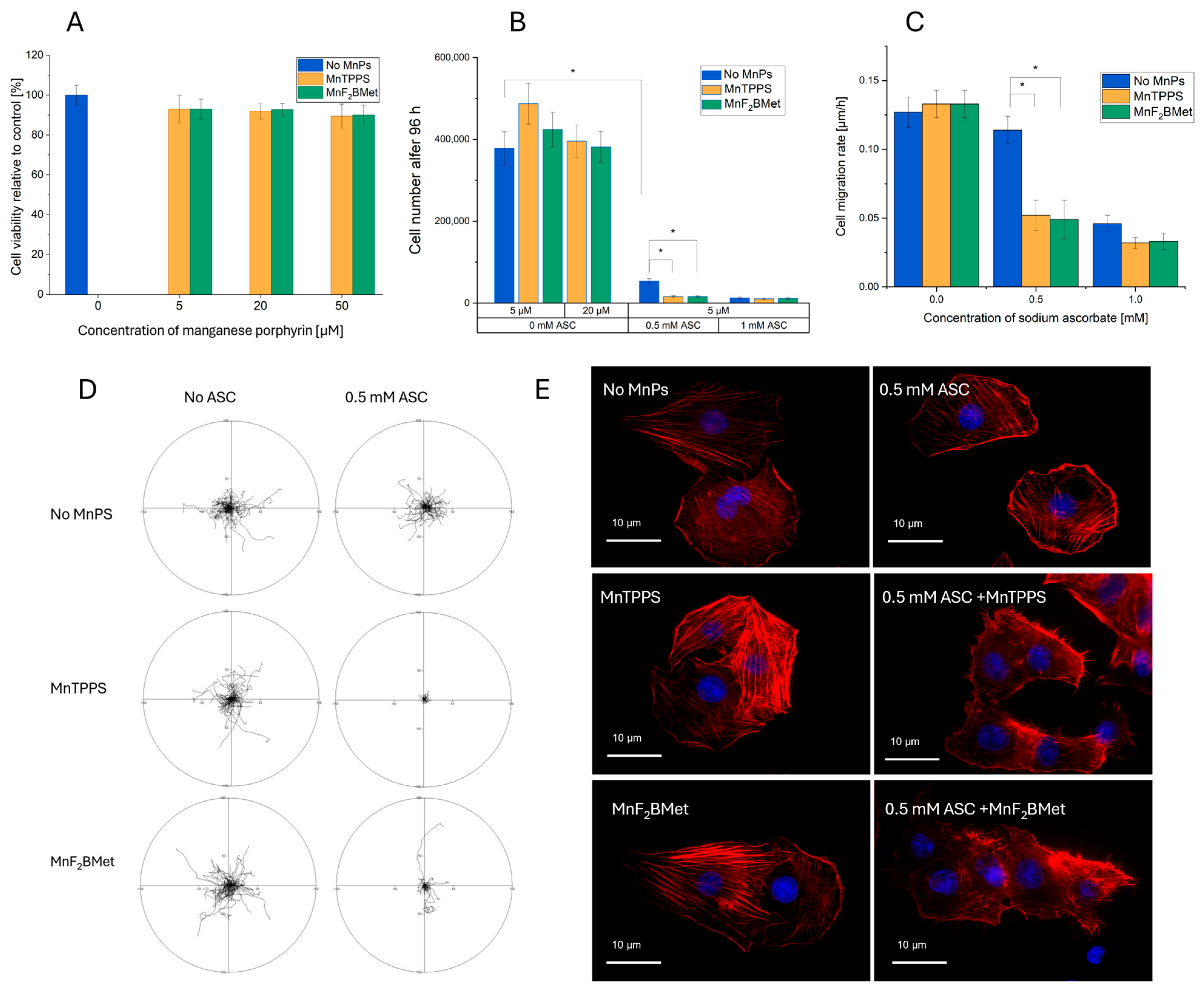
3.3. Effect of MnPs-ASC Treatment on MCF-7 Cell Viability, Proliferation, and Migration
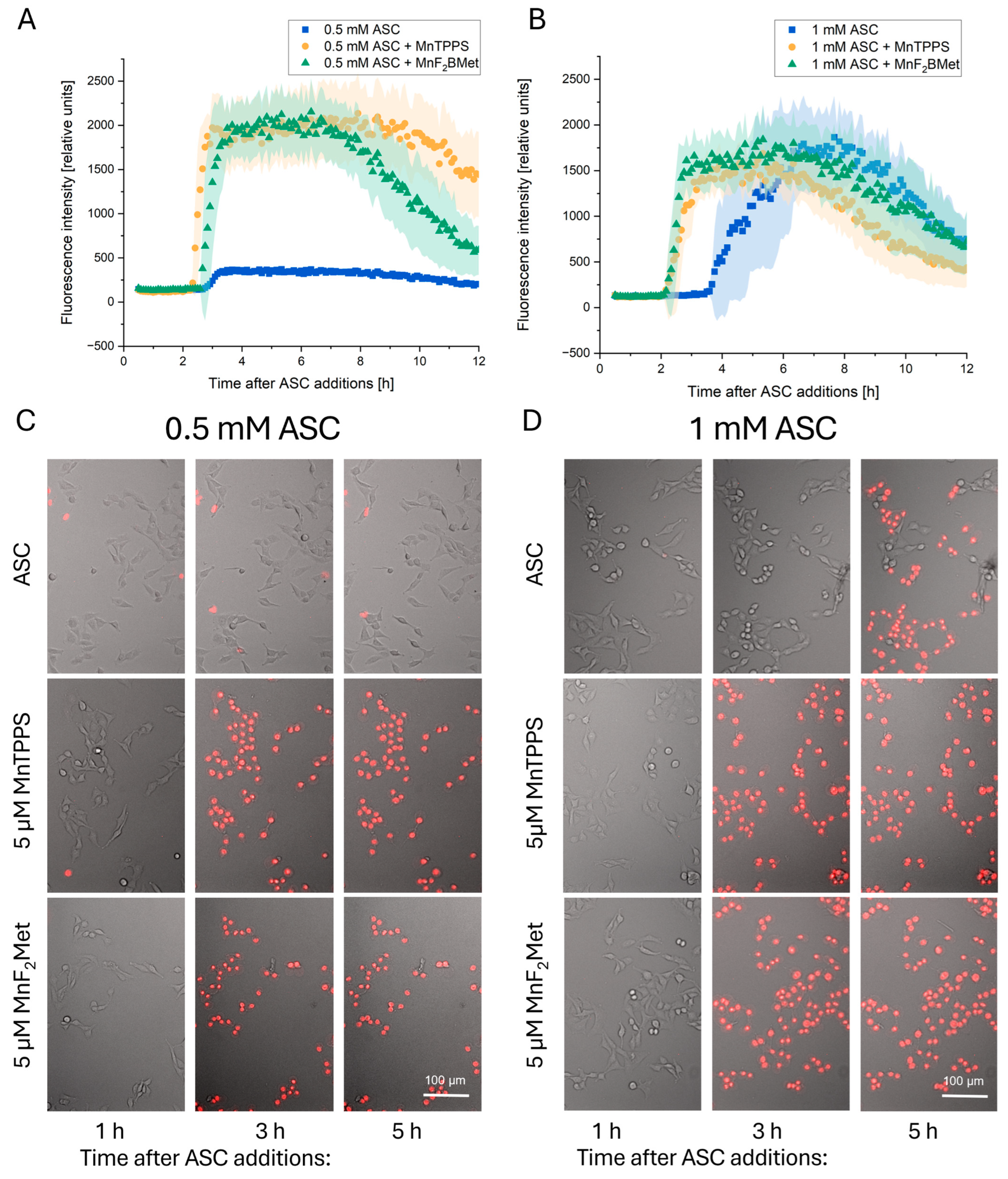
3.4. Kinetics of MCF-7 Cell Death Induced by MnPs-ASC Treatment
3.5. Hallmarks of Oxidative Stress Induced by the MnPs-ASC System
3.6. Combined MnPs/ASC Treatment Increases H2O2 Levels in MCF-7 Cells
3.7. Extracellular Interactions Between ASC and MnPs Mediate Their Combined Cytotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MnPs | Manganese porphyrins |
| ASC | Ascorbate |
| HDF | Human dermal fibroblasts |
| DHA | Dehydroascorbate |
| PDT | Photodynamic therapy |
| ROS | Reactive oxygen species |
| AA | L-ascorbic acid |
| SOD | Superoxide dismutase |
| GSH | Glutathione |
| MnTBAP | Mn(III) tetrakis(4-benzoic acid) porphyrin chloride |
| MnTPPS | Manganese(III) porphyrin: 5,10,15,20-Tetrakis(4-sulfonylphenyl)porphyrin manganese(III) acetate |
| MnF2BMet | 5,10,15,20-Tetrakis [2,6-difluoro-5(N-methylsulfamoyl)phenyl]porphyrin manganese(III) |
| RPMI 1640 | Roswell Park Memorial Institute 1640 medium |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
References
- Král, V.; Králová, J.; Kaplánek, R.; Bríza, T.; Martásek, P. Quo vadis porphyrin chemistry? Physiol. Res. 2006, 55 (Suppl. 2), S3–S26. [Google Scholar] [CrossRef]
- Repetowski, P.; Warszyńska, M.; Dąbrowski, J.M. NIR-activated multifunctional agents for the combined application in cancer imaging and therapy. Adv. Colloid Interface Sci. 2025, 336, 103356. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Warszyńska, M.; Repetowski, P.; Dąbrowski, J.M. Photodynamic therapy combined with immunotherapy: Recent advances and future research directions. Coord. Chem. Rev. 2023, 495, 215350. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Karwicka, M.; Pucelik, B.; Gonet, M.; Elas, M.; Dąbrowski, J.M. Effects of photodynamic therapy with Redaporfin on tumor oxygenation and blood flow in a lung cancer mouse model. Sci. Rep. 2019, 9, 12655. [Google Scholar] [CrossRef]
- Soares, H.T.; Campos, J.R.; Gomes-da-Silva, L.C.; Schaberle, F.A.; Dabrowski, J.M.; Arnaut, L.G. Pro-oxidant and antioxidant effects in photodynamic therapy: Cells recognise that not all exogenous ROS are alike. ChemBioChem 2016, 17, 836–842. [Google Scholar] [CrossRef]
- Faustova, M.; Nikolskaya, E.; Sokol, M.; Zabolotsky, A.; Mollaev, M.; Zhunina, O.; Fomicheva, M.; Lobanov, A.; Severin, E.; Yabbarov, N. High-effective reactive oxygen species inducer based on Mn-tetraphenylporphyrin loaded PLGA nanoparticles in binary catalyst therapy. Free Radic. Biol. Med. 2019, 143, 522–533. [Google Scholar] [CrossRef]
- Houglum, K.P.; Brenner, D.A.; Chojkier, M. Ascorbic acid stimulation of collagen biosynthesis independent of hydroxylation. Am. J. Clin. Nutr. 1991, 54, 1141S. [Google Scholar] [CrossRef]
- Fujii, J.; Osaki, T.; Bo, T. Ascorbate is a primary antioxidant in mammals. Molecules 2022, 27, 6187. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsuda, T.; Miyagantani, Y.; Yukioka, T.; Matsuda, H.; Shimazaki, S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Arch. Surg. 2000, 135, 326–331. [Google Scholar] [CrossRef]
- Lőrincz, T.; Holczer, M.; Kapuy, O.; Szarka, A. The interrelationship of pharmacologic ascorbate-induced cell death and ferroptosis. Pathol. Oncol. Res. 2019, 25, 669–679. [Google Scholar] [CrossRef]
- Calbay, O.; Padia, R.; Akter, M.; Sun, L.; Li, B.; Qian, N.; Guo, J.; Fu, Z.; Jin, L.; Huang, S. ASC/inflammasome-independent pyroptosis in ovarian cancer cells through translational augmentation of caspase-1. iScience 2023, 26, 108408. [Google Scholar] [CrossRef]
- Jankowski, C.S.R.; Rabinowitz, J.D. Selenium Modulates Cancer Cell Response to Pharmacologic Ascorbate. Cancer Res. 2022, 82, 3486–3498. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754. [Google Scholar] [CrossRef]
- Gąbka, M.; Dałek, P.; Przybyło, M.; Gackowski, D.; Oliński, R.; Langner, M. The membrane electrical potential and intracellular pH as factors influencing intracellular ascorbate concentration and their role in cancer treatment. Cells 2021, 10, 2964. [Google Scholar] [CrossRef] [PubMed]
- Pope, A.; O’Leary, B.; Du, J.; Buettner, G.R.; Henry, M.; Cullen, J.J. Pharmacologic ascorbate resistant pancreatic cancer demonstrates enhanced metastatic potential. Redox Biol. 2025, 84, 103694. [Google Scholar] [CrossRef]
- Liang, W.J.; Johnson, D.; Jarvis, S.M. Vitamin C transport systems of mammalian cells. Mol. Membr. Biol. 2001, 18, 87–95. [Google Scholar] [CrossRef]
- Przybyło, M.; Langner, M. On the physiological and cellular homeostasis of ascorbate. Cell. Mol. Biol. Lett. 2020, 25, 32. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Evans, M.K.; Tovmasyan, A.; Batinic-Haberle, I.; Devi, G.R. Mn porphyrin in combination with ascorbate acts as a pro-oxidant and mediates caspase-independent cancer cell death. Free Radic. Biol. Med. 2014, 68, 302–314. [Google Scholar] [CrossRef]
- Ye, X.; Fels, D.; Tovmasyan, A.; Aird, K.M.; Dedeugd, C.; Allensworth, J.L.; Kos, I.; Park, W.; Spasojevic, I.; Devi, G.R.; et al. Cytotoxic effects of Mn(III) N-alkylpyridylporphyrins in the presence of cellular reductant, ascorbate. Free Radic. Res. 2011, 45, 1289–1306. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Rebouças, J.S.; Spasojević, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [PubMed]
- Tovmasyan, A.; Sampaio, R.S.; Boss, M.K.; Bueno-Janice, J.C.; Bader, B.H.; Thomas, M.; Reboucas, J.S.; Orr, M.; Chandler, J.D.; Go, Y.M.; et al. Anticancer therapeutic potential of Mn porphyrin/ascorbate system. Free Radic. Biol. Med. 2015, 89, 1231–1247. [Google Scholar] [CrossRef]
- Clichici, S.; Filip, A.; Daicoviciu, D.; Ion, R.; Mocan, T.; Tatomir, C.; Rogojan, L.; Olteanu, D.; Muresan, A. The dynamics of reactive oxygen species in photodynamic therapy with tetra sulfophenyl-porphyrin. Acta Physiol. Hung. 2010, 97, 41–51. [Google Scholar] [CrossRef]
- Rocha, L.B.; Schaberle, F.; Dąbrowski, J.M.; Simões, S.; Arnaut, L.G. Intravenous single-dose toxicity of Redaporfin-based photodynamic therapy in rodents. Int. J. Mol. Sci. 2015, 16, 29236–29249. [Google Scholar] [CrossRef] [PubMed]
- Frant, M.P.; Trytek, M.; Paduch, R. Assessing the in vitro activity of selected porphyrins in human colorectal cancer cells. Molecules 2022, 27, 2006. [Google Scholar] [CrossRef]
- Isaacs, J.T.; Isaacs, W.B.; Feitz, W.F.J.; Scheres, J. Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate 1986, 9, 261–281. [Google Scholar] [CrossRef]
- Pinto, S.M.; Henriques, C.A.; Tomé, V.A.; Vinagreiro, C.S.; Calvete, M.J.; Dąbrowski, J.M.; Piñeiro, M.; Arnaut, L.G.; Pereira, M.M. Synthesis of meso-substituted porphyrins using sustainable chemical processes. J. Porphyr. Phthalocyanines 2016, 20, 45–60. [Google Scholar] [CrossRef]
- Wybieralska, E.; Koza, M.; Sroka, J.; Czyż, J.; Madeja, Z. Ascorbic acid inhibits the migration of Walker 256 carcinosarcoma cells. Cell. Mol. Biol. Lett. 2008, 13, 103–111. [Google Scholar] [CrossRef]
- Rysiewicz, B.; Błasiak, E.; Dziedzicka-Wasylewska, M.; Polit, A. The polybasic region in Gαi proteins: Relevant or not? Insights from Gαi3 research. Cell. Signal. 2024, 118, 111138. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Chen, Y.; Chen, J.; Zhang, H.; Liao, Y.; Zhou, Y.; Zhou, L.; Qing, C. Anti-tumor effects and mechanism of GA-13315, a novel gibberellin derivative, in human lung adenocarcinoma: An in vitro and in vivo study. Cell. Mol. Biol. Lett. 2019, 24, 6. [Google Scholar] [CrossRef]
- Pak, V.V.; Ezeriņa, D.; Lyublinskaya, O.G.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Gache, S.A.M.; et al. Ultrasensitive genetically encoded indicator for hydrogen peroxide identifies roles for the oxidant in cell migration and mitochondrial function. Cell Metab. 2020, 31, 642–653.e6. [Google Scholar] [CrossRef]
- Szafraniec, M.J.; Szeliga, M.; Urbanska, K.; Fiedor, L. Determinants of the activity and substrate recognition of breast cancer resistance protein (ABCG2). Drug Metab. Rev. 2014, 46, 459–474. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Rawal, M.; Schroeder, S.R.; Wagner, B.A.; Cushing, C.M.; Welsh, J.L.; Button, A.M.; Du, J.; Sibenaller, Z.A.; Buettner, G.R.; Cullen, J.J. Manganoporphyrins increase ascorbate-induced cytotoxicity by enhancing H2O2 generation. Cancer Res. 2013, 73, 5232–5241. [Google Scholar] [CrossRef]
- Tian, J.; Peehl, D.M.; Knox, S.J. Metalloporphyrin synergizes with ascorbic acid to inhibit cancer cell growth through Fenton chemistry. Cancer Biother. Radiopharm. 2010, 25, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Rapta, P.; Moura, N.M.M.; Batinić-Haberle, I.; Šoltés, L. Ortho isomeric Mn(III) N-alkyl- and alkoxyalkylpyridylporphyrins—Enhancers of hyaluronan degradation induced by ascorbate and cupric ions. Int. J. Mol. Sci. 2021, 22, 8608. [Google Scholar] [CrossRef]
- Hasan, B.; Tovmasyan, A.; Batinić-Haberle, I.; Benov, L. Ascorbate-dependent and ascorbate-independent Mn porphyrin cytotoxicity: Anticancer activity of Mn porphyrin-based SOD mimics through ascorbate-dependent and -independent routes. Redox Rep. 2021, 26, 85–93. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Tome, M.E. Thiol regulation by Mn porphyrins, commonly known as SOD mimics. Redox Biol. 2019, 25, 101139. [Google Scholar] [CrossRef]
- Flórido, A.; Saraiva, N.; Cerqueira, S.; Almeida, N.; Parsons, M.; Batinic-Haberle, I.; Miranda, J.P.; Costa, J.G.; Carrara, G.; Castro, M.; et al. The manganese(III) porphyrin MnTnHex-2-PyP5+ modulates intracellular ROS and breast cancer cell migration: Impact on doxorubicin-treated cells. Redox Biol. 2019, 20, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Gilloteaux, J.; Jamison, J.M.; Neal, D.R.; Loukas, M.; Doberzstyn, T.; Summers, J.L. Cell damage and death by autoschizis in human bladder (RT4) carcinoma cells resulting from treatment with ascorbate and menadione. Ultrastruct. Pathol. 2010, 34, 140–160. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwenhuijze, A.E.; van Lopik, T.; Smeenk, R.J.; Aarden, L.A. Time between onset of apoptosis and release of nucleosomes from apoptotic cells: Putative implications for systemic lupus erythematosus. Ann. Rheum. Dis. 2003, 62, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Briehl, M.M.; Crapo, J.D.; Batinic-Haberle, I.; Tome, M.E. Manganese porphyrin, MnTE-2-PyP5+, acts as a pro-oxidant to potentiate glucocorticoid-induced apoptosis in lymphoma cells. Free Radic. Biol. Med. 2012, 52, 1272–1284. [Google Scholar] [CrossRef]
- Piotrowsky, A.; Burkard, M.; Hammerschmidt, K.; Ruple, H.K.; Nonnenmacher, P.; Schumacher, M.; Leischner, C.; Berchtold, S.; Marongiu, L.; Kufer, T.A.; et al. Analysis of High-Dose Ascorbate-Induced Cytotoxicity in Human Glioblastoma Cells and the Role of Dehydroascorbic Acid and Iron. Antioxidants 2024, 13, 1095. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Pucelik, B.; Pereira, M.M.; Arnaut, L.G.; Stochel, G. Towards tuning PDT relevant photosensitizer properties: Comparative study for the free and Zn2+ coordinated meso-tetrakis [2,6-difluoro-5-(N-methylsulfamylo)phenyl] porphyrin. J. Coord. Chem. 2015, 68, 3116–3134. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rąpała, M.; Pudełek, M.; Lasota, S.; Noga, S.; Czyż, J.; Dąbrowski, J.M.; Madeja, Z. Targeting Cancer with Redox Catalysis: Manganese Porphyrins and Ascorbate Synergistically Induce Selective Oxidative Stress and Necrotic Cell Death. Cancers 2025, 17, 3736. https://doi.org/10.3390/cancers17233736
Rąpała M, Pudełek M, Lasota S, Noga S, Czyż J, Dąbrowski JM, Madeja Z. Targeting Cancer with Redox Catalysis: Manganese Porphyrins and Ascorbate Synergistically Induce Selective Oxidative Stress and Necrotic Cell Death. Cancers. 2025; 17(23):3736. https://doi.org/10.3390/cancers17233736
Chicago/Turabian StyleRąpała, Michał, Maciej Pudełek, Sławomir Lasota, Sylwia Noga, Jarosław Czyż, Janusz M. Dąbrowski, and Zbigniew Madeja. 2025. "Targeting Cancer with Redox Catalysis: Manganese Porphyrins and Ascorbate Synergistically Induce Selective Oxidative Stress and Necrotic Cell Death" Cancers 17, no. 23: 3736. https://doi.org/10.3390/cancers17233736
APA StyleRąpała, M., Pudełek, M., Lasota, S., Noga, S., Czyż, J., Dąbrowski, J. M., & Madeja, Z. (2025). Targeting Cancer with Redox Catalysis: Manganese Porphyrins and Ascorbate Synergistically Induce Selective Oxidative Stress and Necrotic Cell Death. Cancers, 17(23), 3736. https://doi.org/10.3390/cancers17233736







