Targeted Therapies in Oral and Oropharyngeal Cancer: An Overview of Emerging and Repurposed Agents
Simple Summary
Abstract
1. Introduction
2. The Biological Mechanisms of Head and Neck Carcinogenesis
2.1. Cell Signaling Pathways and Oncogenes
2.2. Tumor Suppressor Genes Mutations
2.3. Tumor Microenvironment (TME) and Immune Evasion
2.4. Chronic Inflammation, IL-6/PI3K Signaling, and Pharmacological Impact
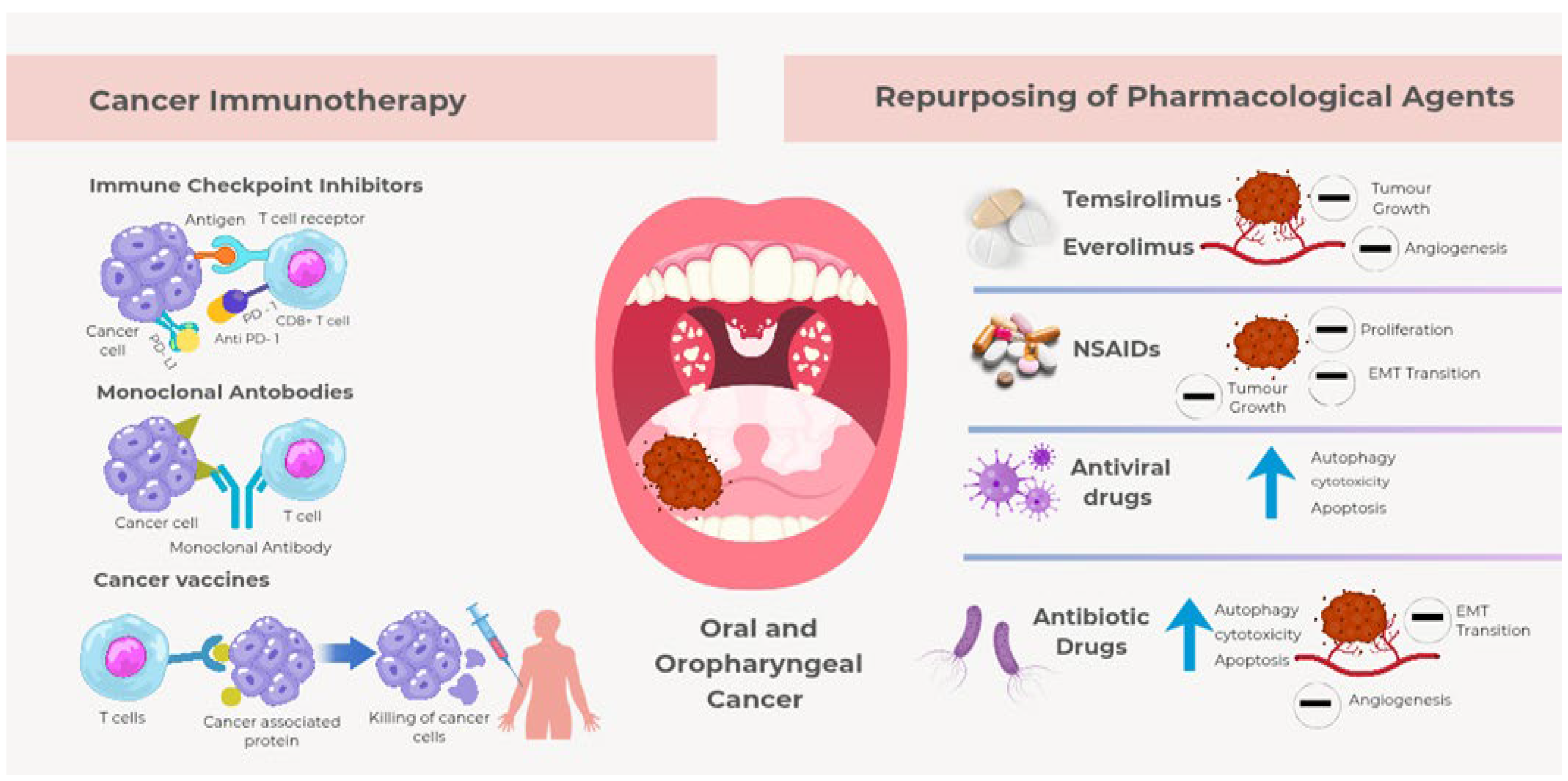
3. The Evolution of Targeted Therapies and Immune Checkpoint
3.1. Drugs That Target the Epidermal Growth Factor Receptor
3.2. Drugs That Target the Programmed Cell Death Receptor
3.3. Drugs That Target Inhibitors of Cyclin-Dependent Kinase (CDK)
3.4. Drugs That Target the Receptor Inhibitors of the Vascular Endothelial Growth Factor
3.5. Drugs That Target the Mammalian Target of Rapamycin Inhibitors
4. Drug Repurposing Opportunities: Targeting Inflammation and PI3K/MTOR to Enhance Immunotherapy
5. Expected Breakthroughs
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAFs | Cancer-Associated Fibroblasts |
| CDK | Cyclin-Dependent Kinase |
| cGvHD | Chronic Graft-Versus-Host Disease |
| CPS | Combined Positive Score |
| ECM | Extracellular Matrix |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial–Mesenchymal Transition |
| EVs | Extracellular Vesicles |
| HCQ | Hydroxychloroquine |
| HDAC | Histone Deacetylase |
| HNCs | Head and Neck Cancers |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| ICIs | Immune Checkpoint Inhibitors |
| IgG1 | Immunoglobulin G1 |
| IL-6 | Interleukin–6 |
| MMPs | Matrix Metalloproteinases |
| mTOR | Mammalian Target of Rapamycin |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| OCT3 | Organic Cation Transporters |
| OLP | Oral Lichen Planus |
| OPSCC | Oropharyngeal Squamous Cell Carcinoma |
| OSCC | Oral Squamous Cell Carcinoma |
| P13K/Akt | Phosphatidylinositol 3-Kinase/Protein Kinase B |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Cell Death Ligand 1 |
| SCFAs | Short-Chain Fatty Acids |
| TAMs | Tumor-Associated Macrophages |
| TGF-α | Transforming Growth Factor–α |
| TLR-9 | Toll-like Receptor-9 |
| TMB | Tumor Mutational Burden |
| TME | Tumor Microenvironment |
| VEGF | Vascular Endothelial Growth Factor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- National Cancer Institute. Head and Neck Cancers Fact Sheet. Available online: https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet (accessed on 30 September 2025).
- El-Bayoumy, K.; Christensen, N.D.; Hu, J.; Viscidi, R.; Stairs, D.B.; Walter, V.; Chen, K.M.; Sun, Y.W.; Muscat, J.E.; Richie, J.P., Jr. An Integrated Approach for Preventing Oral Cavity and Oropharyngeal Cancers: Two Etiologies with Distinct and Shared Mechanisms of Carcinogenesis. Cancer Prev. Res. 2020, 13, 649–660. [Google Scholar] [CrossRef]
- Parke, S.C.; Langelier, D.M.; Cheng, J.T.; Kline-Quiroz, C.; Stubblefield, M.D. State of Rehabilitation Research in the Head and Neck Cancer Population: Functional Impact vs. Impairment-Focused Outcomes. Curr. Oncol. Rep. 2022, 24, 517–532. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering and Medicine; Health and Medicine Division; Board on Health Care Services; National Cancer Policy Forum. Long-Term Survivorship Care After Cancer Treatment: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538366/ (accessed on 30 September 2025).
- Sheikh-Wu, S.F.; Anglade, D.; Downs, C.A. A Cancer Survivorship Model for Holistic Cancer Care and Research. Can. Oncol. Nurs. J. 2023, 33, 4–16. [Google Scholar] [CrossRef]
- Cabral, L.G.S.; Martins, I.M.; Paulo, E.P.A.; Pomini, K.T.; Poyet, J.L.; Maria, D.A. Molecular Mechanisms in the Carcinogenesis of Oral Squamous Cell Carcinoma: A Literature Review. Biomolecules 2025, 15, 621. [Google Scholar] [CrossRef]
- Barroso, L.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Molecular and Genetic Pathogenesis of Oral Cancer: A Basis for Customized Diagnosis and Treatment. Biology 2025, 14, 842. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Chifiriuc, M.C.; Bleotu, C.; Vrancianu, C.O.; Cristian, R.-E.; Bertesteanu, S.V.; Grigore, R.; Bertesteanu, G. Molecular Pathways and Targeted Therapies in Head and Neck Cancers Pathogenesis. Front. Oncol. 2024, 14, 1373821. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Luo, T.; Shi, H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK Pathways for Cancer Therapy. Mol. Biomed. 2022, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral Squamous Cell Carcinomas: State of the Field and Emerging Directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef]
- Baliakas, P.; Soussi, T. The TP53 Tumor Suppressor Gene: From Molecular Biology to Clinical Investigations. J. Intern. Med. 2025, 298, 78–96. [Google Scholar] [CrossRef]
- Capuozzo, M.; Santorsola, M.; Bocchetti, M.; Perri, F.; Cascella, M.; Granata, V.; Celotto, V.; Gualillo, O.; Cossu, A.M.; Nasti, G.; et al. p53: From Fundamental Biology to Clinical Applications in Cancer. Biology 2022, 11, 1325. [Google Scholar] [CrossRef]
- Jiao, Y.; Feng, Y.; Wang, X. Regulation of Tumor Suppressor Gene CDKN2A and Encoded p16-INK4a Protein by Covalent Modifications. Biochemistry 2018, 83, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Wang, X.; Cai, H.; Sheng, Y.; Wu, L.; Huang, K.; Zhu, X. The Cell Senescence Regulator p16 Is a Promising Cancer Prognostic and Immune Check-Point Inhibitor (ICI) Therapy Biomarker. Aging 2023, 15, 2136–2157. [Google Scholar] [CrossRef]
- Gołąbek, K.; Rączka, G.; Gaździcka, J.; Miśkiewicz-Orczyk, K.; Zięba, N.; Krakowczyk, Ł.; Misiołek, M.; Strzelczyk, J.K. Expression Profiles of CDKN2A, MDM2, E2F2 and LTF Genes in Oral Squamous Cell Carcinoma. Biomedicines 2022, 10, 3011. [Google Scholar] [CrossRef]
- Guo, Z.; Li, K.; Ren, X.; Wang, X.; Yang, D.; Ma, S.; Zeng, X.; Zhang, P. The Role of the Tumor Microenvironment in HNSCC Resistance and Targeted Therapy. Front. Immunol. 2025, 16, 1554835. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.H.; Li, N. Immune Evasion in Cancer: Mechanisms and Cutting-Edge Therapeutic Approaches. Signal Transduct. Target. Ther. 2025, 10, 227. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.-J.; Liu, Z. Regulatory Mechanisms of Immune Checkpoints PD-L1 and CTLA-4 in Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Song, Z.; Chen, J.; Tang, Z.; Wang, B. Molecular Basis, Potential Biomarkers, and Future Prospects of OSCC and PD-1/PD-L1 Related Immunotherapy Methods. Heliyon 2024, 10, e25895. [Google Scholar] [CrossRef]
- Arriola Benítez, P.C.; Fusco, M.; Amorin, R.; Picón, C.R.; Piccioni, F.; Victoria, L.; Rizzo, M.M.; Malvicini, M. Unraveling the Role of Tumor-Infiltrating Immune Cells in Head and Neck Squamous Cell Carcinoma: Implications for Antitumor Immune Responses and Immunotherapy. Int. J. Mol. Sci. 2025, 26, 6337. [Google Scholar] [CrossRef] [PubMed]
- Struckmeier, A.-K.; Gosau, M.; Smeets, R. Immunotherapeutic Strategies beyond the PD-1/PD-L1 Pathway in Head and Neck Squamous Cell Carcinoma: A Scoping Review on Current Developments in Agents Targeting TIM-3, TIGIT, LAG-3, and VISTA. Oral Oncol. 2025, 161, 107145. [Google Scholar] [CrossRef]
- Rajneesh; Tiwari, R.; Singh, V.K.; Kumar, A.; Mehrotra, S.; Gautam, V.; Neville, J.F.; Bansal, V.; Pathak, R.; Singh, A.K.; et al. Exploring Metabolic and Immunological Biomarkers for Oral Squamous Cell Carcinoma: Potential Targets for Precision Therapy. Biology 2025, 14, 1109. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, W.; Hu, J.; Tao, L. Editorial: The Tumor Microenvironment and Immunotherapy for Head and Neck Tumors. Front. Oncol. 2025, 15, 1606787. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Fessikh, M.E.; Elhrech, H.; Omari, N.E.; Amanullah, M.; Ming, L.C.; Moshawih, S.; Bouyahya, A. Targeting Inflammation in Cancer Therapy: From Mechanistic Insights to Emerging Therapeutic Approaches. J. Transl. Med. 2025, 23, 588. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Liu, P.; Zeng, X.; Lv, J.; Qiu, S.; Zhang, P. The Role of Inflammation-Associated Factors in Head and Neck Squamous Cell Carcinoma. J. Inflamm. Res. 2023, 16, 4301–4315. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Yang, Y.; Xu, X.; Xiong, X.; Meng, W. Increased Pathogenicity and Pro-Inflammatory Capabilities of Mucosal-Associated Invariant T Cells Involved in Oral Lichen Planus. BMC Oral Health 2024, 24, 829. [Google Scholar] [CrossRef]
- Pugliese, G.; Nitro, L.; Allevi, F.; Biglioli, F.; Coccapani, M.; Felisati, G.; Ferella, F.; Ghilardi, G.; Montavoci, L.; Caretti, A.; et al. Can Serum and Saliva Inflammatory Cytokines Be Considered a Reliable Marker in Chronic Oral Graft-Versus-Host Disease Patients? J. Pers. Med. 2024, 14, 1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Zhang, Z.; Wu, P.; Zhang, Y.; Chen, X. Advances in Targeting Tumor Microenvironment for Immunotherapy. Front. Immunol. 2024, 15, 1472772. [Google Scholar] [CrossRef]
- Yu, J.; Kong, X.; Feng, Y. Tumor Microenvironment-Driven Resistance to Immunotherapy in Non-Small Cell Lung Cancer: Strategies for Cold-to-Hot Tumor Transformation. Cancer Drug Resist. 2025, 8, 21. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, K.; Zhang, Z.; Zeng, X.; Huang, Z.; Qin, P.; Xie, Z.; Cai, X.; Ashrafizadeh, M.; Tian, Y.; et al. PI3K/AKT/mTOR Axis in Cancer: From Pathogenesis to Treatment. MedComm 2025, 6, e70295. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt Signal Transduction for Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Ambati, R.; Gundamaraju, R. Exploring the Crosstalk between Inflammation and Epithelial–Mesenchymal Transition in Cancer. Mediat. Inflamm. 2021, 2021, 9918379. [Google Scholar] [CrossRef]
- Moghbeli, M. PI3K/AKT Pathway as a Pivotal Regulator of Epithelial–Mesenchymal Transition in Lung Tumor Cells. Cancer Cell Int. 2024, 24, 165. [Google Scholar] [CrossRef]
- Poulose, J.V.; Kainickal, C.T. Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinoma: A Systematic Review of Phase-3 Clinical Trials. World J. Clin. Oncol. 2022, 13, 388–411. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, H.; Zhao, G.; Wang, M.; Luo, J.; Liu, J. Immune Checkpoint Inhibitors Serve as the First-Line Treatment for Advanced Head and Neck Cancer. Laryngoscope 2024, 134, 749–761. [Google Scholar] [CrossRef]
- Green, S.E.; McCusker, M.G.; Mehra, R. Emerging Immune Checkpoint Inhibitors for the Treatment of Head and Neck Cancers. Expert Opin. Emerg. Drugs 2020, 25, 501–514. [Google Scholar] [CrossRef]
- Harrington, K.J.; Burtness, B.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Brana, I.; Basté, N.; Neupane, P.; et al. Pembrolizumab with or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. J. Clin. Oncol. 2023, 41, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Lim, D.W.; Keam, B.; Ma, B.; Zhang, L.; Wang, C.; Guo, Y. Management Approaches for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma after Anti-PD-1/PD-L1 Immunotherapy. Cancer Treat. Rev. 2025, 136, 102938. [Google Scholar] [CrossRef]
- Machiels, J.-P.; Tao, Y.; Licitra, L.; Burtness, B.; Tahara, M.; Rischin, D.; Alves, G.; Lima, I.P.F.; Hughes, B.G.M.; Pointreau, Y.; et al. Pembrolizumab plus Concurrent Chemoradiotherapy versus Placebo plus Concurrent Chemoradiotherapy in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-412): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2024, 25, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Aboaid, H.; Khalid, T.; Hussain, A.; Myat, Y.M.; Nanda, R.K.; Srinivasmurthy, R.; Nguyen, K.; Jones, D.T.; Bigcas, J.L.; Thein, K.Z. Advances and Challenges in Immunotherapy in Head and Neck Cancer. Front. Immunol. 2025, 16, 1596583. [Google Scholar] [CrossRef]
- Uppaluri, R.; Lee, N.Y.; Westra, W.; Cohen, E.E.W.; Haddad, R.I.; Temam, S.; Le Tourneau, C.; Chernock, R.; Safina, S.; Klochikhin, A.; et al. KEYNOTE-689: Phase 3 Study of Adjuvant and Neoadjuvant Pembrolizumab Combined with Standard of Care (SOC) in Patients with Resectable, Locally Advanced Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2019, 37 (Suppl. 15), TPS6090. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Vujanovic, L.; Clump, D.A.; Isett, B.P.; Wang, H.; Sica, G.; Bao, R.; Li, H.; Ohr, J.; Skinner, H.D.; et al. Randomized Phase II Study of Concurrent Versus Sequential Pembrolizumab in Combination with Chemoradiation in Locally Advanced Head and Neck Cancer. J. Clin. Oncol. 2025, 43, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Merck. FDA Grants Priority Review to Merck’s Application for KEYTRUDA (Pembrolizumab) Plus Standard of Care as Perioperative Treatment for Resectable Locally Advanced Head and Neck Squamous Cell Carcinoma. News Release, 25 February 2025. Available online: https://tinyurl.com/2kmefvjn (accessed on 30 September 2025).
- Clump, D.A.; Zandberg, D.P.; Skinner, H.D.; Ohr, J.; Fenton, M.J.; Normolle, D.P.; Beitler, J.J.; Bauman, J.E.; Ferris, R.L. A Randomized Phase II Study Evaluating Concurrent or Sequential Fixed-Dose Immune Therapy in Combination with Cisplatin and Intensity-Modulated Radiotherapy in Intermediate- or High-Risk, Previously Untreated, Locally Advanced Head and Neck Cancer (LA SCCHN). J. Clin. Oncol. 2022, 40 (Suppl. 16), 6007. [Google Scholar] [CrossRef]
- Haddad, R.I.; Harrington, K.; Tahara, M.; Ferris, R.L.; Gillison, M.; Fayette, J.; Daste, A.; Koralewski, P.; Zurawski, B.; Taberna, M.; et al. Nivolumab plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J. Clin. Oncol. 2023, 41, 2166–2180. [Google Scholar] [CrossRef]
- Bhateja, P.; Bonomi, M.; Verschraegen, C. Novel Combinations with Programmed Cell Death 1 Inhibitor for Incurable Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (RM HNSCC): Is Cabozantinib a Front Runner? Transl. Cancer Res. 2025, 14, 2548–2552. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Combination Study with Soluble LAG-3 Fusion Protein Eftilagimod Alpha (IMP321) and Pembrolizumab in Patients with Previously Untreated Unresectable or Metastatic NSCLC, or Recurrent PD-X Refractory NSCLC or With Recurrent or Metastatic HNSCC (TACTI-002), ID NCT03625323. Available online: https://clinicaltrials.gov/ct2/show/NCT03625323 (accessed on 30 September 2025).
- Doger de Spéville, B.; Felip, E.; Forster, M.; Majem, M.; Bajaj, P.; Peguero, J.A.; Carcereny, E.; Krebs, M.G.; Mukherjee, U.; Mueller, C.; et al. Final Results from TACTI-002 Part C: A Phase II Study of Eftilagimod Alpha (Soluble LAG-3 Protein) and Pembrolizumab in Patients with Metastatic 2nd Line Head and Neck Squamous Cell Carcinoma Unselected for PD-L1. J. Clin. Oncol. 2023, 41, 6029. [Google Scholar] [CrossRef]
- Ghasemi, K. Tiragolumab and TIGIT: Pioneering the Next Era of Cancer Immunotherapy. Front. Pharmacol. 2025, 16, 1568664. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Wang, Z.; Zhang, J.; Zhou, R. Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials. Mol. Cancer 2023, 22, 93, Erratum in Mol. Cancer 2023, 22, 101. [Google Scholar] [CrossRef]
- Mao, L.; Xiao, Y.; Yang, Q.-C.; Yang, S.-C.; Yang, L.-L.; Zhi, S. TIGIT/CD155 Blockade Enhances Anti-PD-L1 Therapy in Head and Neck Squamous Cell Carcinoma by Targeting Myeloid-Derived Suppressor Cells. Oral Oncol. 2021, 121, 105472. [Google Scholar] [CrossRef]
- Morchón-Araujo, D.; Catani, G.; Mirallas, O.; Pretelli, G.; Sánchez-Pérez, V.; Vieito, M.; Braña, I.; Pujol-Borrell, R.; Garralda, E.; Hernando-Calvo, A. Emerging Immunotherapy Targets in Early Drug Development. Int. J. Mol. Sci. 2025, 26, 5394. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of Pembrolizumab (MK-3475) for First Line Treatment of Recurrent or Metastatic Squamous Cell Cancer of the Head and Neck (MK-3475-048/KEYNOTE-048), ID NCT02358031. Available online: https://clinicaltrials.gov/ct2/show/NCT02358031 (accessed on 30 September 2025).
- Sun, F.; Colevas, A.D. Update: Immunotherapeutic Strategies in HPV-Associated Head and Neck Squamous Cell Carcinoma. Viruses 2025, 17, 712. [Google Scholar] [CrossRef]
- Jiang, B.; Elkashif, A.; Coulter, J.A.; Dunne, N.J.; McCarthy, H.O. Immunotherapy for HPV Negative Head and Neck Squamous Cell Carcinoma. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189138. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Nam, M.; Kwang, C.Y. Tissue-Agnostic Biomarkers in Solid Tumors: Current Approvals and Emerging Candidates. Cancer Metastasis Rev. 2025, 44, 58. [Google Scholar] [CrossRef]
- Haddad, R.I.; Seiwert, T.Y.; Chow, L.Q.M.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; et al. Influence of Tumor Mutational Burden, Inflammatory Gene Expression Profile, and PD-L1 Expression on Response to Pembrolizumab in Head and Neck Squamous Cell Carcinoma. J. Immunother. Cancer 2022, 10, e003026. [Google Scholar] [CrossRef] [PubMed]
- Zadka, Ł.; Grybowski, D.J.; Dzięgiel, P. Modeling of the Immune Response in the Pathogenesis of Solid Tumors and Its Prognostic Significance. Cell Oncol. 2020, 43, 539–575. [Google Scholar] [CrossRef] [PubMed]
- Nicoară, A.; Roi, C.; Roi, A.; Motofelea, A.C.; Rakitovan, M.; Zară, F.; Riviș, M. Systemic Immune-Inflammatory Index and Other Inflammatory Marker Variations in Oral Squamous Cell Carcinoma Management. Medicina 2024, 60, 1840. [Google Scholar] [CrossRef]
- Sutera, S.; Furchì, O.A.; Pentenero, M. Investigating Tumor-Infiltrating Lymphocytes in the Microenvironment of Oral Squamous Cell Carcinoma (OSCC) and Oral Potentially Malignant Disorders (OPMDs): Can They Shift Our Perspective? A Scoping Review. J. Clin. Med. 2025, 14, 606. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and Hot Tumors: From Molecular Mechanisms to Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Kaufman, N.E.M.; Dhingra, S.; Jois, S.D.; Vicente, M.d.G.H. Molecular Targeting of Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor Receptor (VEGFR). Molecules 2021, 26, 1076. [Google Scholar] [CrossRef] [PubMed]
- Purba, E.R.; Saita, E.-i.; Maruyama, I.N. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The “Rotation Model”. Cells 2017, 6, 13. [Google Scholar] [CrossRef]
- Tagliaferro, M.; Ponti, D. The Signaling of Neuregulin-Epidermal Growth Factor Receptors and Its Impact on the Nervous System. Neuroglia 2023, 4, 253–274. [Google Scholar] [CrossRef]
- Abouelkheer, Y.; Bhatia, A. Head and Neck Cancer—Emerging Targeted Therapies. Front. Oncol. 2025, 15, 1640960. [Google Scholar] [CrossRef]
- Hallaji, M.; Allahyari, M.; Teimoori-Toolabi, L.; Yasami-Khiabani, S.; Golkar, M.; Fard-Esfahani, P. Targeted Cancer Treatment Using a Novel EGFR-Specific Fc-Fusion Peptide Based on GE11 Peptide. Sci. Rep. 2025, 15, 5107. [Google Scholar] [CrossRef]
- Pannunzio, S.; Di Bello, A.; Occhipinti, D.; Scala, A.; Messina, G.; Valente, G.; Quirino, M.; Di Salvatore, M.; Tortora, G.; Cassano, A. Multimodality Treatment in Recurrent/Metastatic Squamous Cell Carcinoma of Head and Neck: Current Therapy, Challenges, and Future Perspectives. Front. Oncol. 2024, 13, 1288695. [Google Scholar] [CrossRef]
- Song, X.; Wang, S.; Li, J.; Yan, L.; Chen, F.; Wang, J. Induction Chemotherapy Plus Nimotuzumab Followed by Concurrent Chemoradiotherapy for Advanced Nasopharyngeal Carcinoma. Arch. Med. Sci. 2021, 17, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zhang, D.; Zhao, Y.; Mao, M.; Shen, K.; Wang, X.; Bi, C. Nimotuzumab Combined with Radiotherapy ± Chemotherapy for Definitive Treatment of Locally Advanced Squamous Cell Carcinoma of Head and Neck: A Meta-analysis of Randomized Controlled Trials. Front. Oncol. 2024, 14, 1380428. [Google Scholar] [CrossRef]
- Cui, J.-W.; Li, Y.; Yang, Y.; Yang, H.-K.; Dong, J.-M.; Xiao, Z.-H.; He, X.; Guo, J.-H.; Wang, R.-Q.; Dai, B.; et al. Tumor Immunotherapy Resistance: Revealing the Mechanism of PD-1/PD-L1-Mediated Tumor Immune Escape. Biomed. Pharmacother. 2024, 171, 116203. [Google Scholar] [CrossRef]
- Qiao, X.W.; Jiang, J.; Pang, X.; Huang, M.C.; Tang, Y.J.; Liang, X.H.; Tang, Y.L. The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer. Front. Immunol. 2020, 11, 1721. [Google Scholar] [CrossRef]
- Wahabi, H.; Al Manadili, A. The Expression of PD-L1 and PD-1 in the Microenvironment of Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2024, 25, 3471–3479. [Google Scholar] [CrossRef]
- Zervanos, D.-I.; Galatou, E.; Miliotou, A.N.; Theodoroula, N.F.; Grigoriadis, N.; Vizirianakis, I.S. Assessing the Pharmacological and Pharmacogenomic Data of PD-1/PD-L1 Inhibitors to Enhance Cancer Immunotherapy Outcomes in the Clinical Setting. Future Pharmacol. 2025, 5, 43. [Google Scholar] [CrossRef]
- Pellarin, I.; Dall’Acqua, A.; Favero, A.; Segatto, I.; Rossi, V.; Crestan, N.; Karimbayli, J.; Belletti, B.; Baldassarre, G. Cyclin-Dependent Protein Kinases and Cell Cycle Regulation in Biology and Disease. Signal Transduct. Target. Ther. 2025, 10, 11. [Google Scholar] [CrossRef]
- Chang, J.T.; Wang, H.M.; Chang, K.W.; Chen, W.H.; Wen, M.C.; Hsu, Y.M.; Yung, B.Y.; Chen, I.H.; Liao, C.T.; Hsieh, L.L.; et al. Identification of Differentially Expressed Genes in Oral Squamous Cell Carcinoma (OSCC): Overexpression of NPM, CDK1, NDRG1, and Underexpression of CHES1. Int. J. Cancer 2005, 114, 942–949. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, F.H.; Chen, Q.E.; Wang, Y.Y.; Wang, Y.L.; He, J.C.; Zhou, J. The Clinical Significance of CDK1 Expression in Oral Squamous Cell Carcinoma. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e7–e12. [Google Scholar] [CrossRef]
- Kujan, O.; Huang, G.; Ravindran, A.; Vijayan, M.; Farah, C.S. The Role of Cyclin-Dependent Kinases in Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2019, 48, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yao, Y.; Gao, H.; Hu, X. Mechanisms of Angiogenesis in Tumour. Front. Oncol. 2024, 14, 1359069. [Google Scholar] [CrossRef]
- Pomella, S.; Melaiu, O.; Dri, M.; Martelli, M.; Gargari, M.; Barillari, G. Effects of Angiogenic Factors on the Epithelial-to-Mesenchymal Transition and Their Impact on the Onset and Progression of Oral Squamous Cell Carcinoma: An Overview. Cells 2024, 13, 1294. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Dong, Q.; Li, J.; Niu, W.; Liu, T. Extracellular Vesicle-Bound VEGF in Oral Squamous Cell Carcinoma and Its Role in Resistance to Bevacizumab Therapy. Cancer Cell Int. 2024, 24, 296. [Google Scholar] [CrossRef] [PubMed]
- Laban, S.; Steinmeister, L.; Gleißner, L.; Grob, T.J.; Grénman, R.; Petersen, C.; Gal, A.; Knecht, R.; Dikomey, E.; Kriegs, M. Sorafenib sensitizes head and neck squamous cell carcinoma cells to ionizing radiation. Radiother. Oncol. 2013, 109, 286–292. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, A.; Zhu, M.; Song, L. Dual Inhibition of EGFR and VEGF: An Effective Approach to the Treatment of Advanced Non-Small Cell Lung Cancer with EGFR Mutation (Review). Int. J. Oncol. 2023, 62, 26. [Google Scholar] [CrossRef] [PubMed]
- Yonesi, A.; Tomihara, K.; Takatsuka, D.; Tachinami, H.; Yamazaki, M.; Jadidi, A.R.; Takaichi, M.; Imaue, S.; Fujiwara, K.; Yamada, S.I.; et al. Rapamycin Induces Phenotypic Alterations in Oral Cancer Cells That May Facilitate Antitumor T Cell Responses. Biomedicines 2024, 12, 1078. [Google Scholar] [CrossRef]
- Liao, Y.M.; Kim, C.; Yen, Y. Mammalian Target of Rapamycin and Head and Neck Squamous Cell Carcinoma. Head Neck Oncol. 2011, 3, 22–25. [Google Scholar] [CrossRef]
- Ali, E.S.; Mitra, K.; Akter, S.; Ramproshad, S.; Mondal, B.; Khan, I.N.; Islam, M.T.; Sharifi-Rad, J.; Calina, D.; Cho, W.C. Recent Advances and Limitations of mTOR Inhibitors in the Treatment of Cancer. Cancer Cell Int. 2022, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Advances in mTOR Inhibitors. [Internet]. June 2022. Available online: https://www.bocsci.com/blog/advances-in-mtor-inhibitors (accessed on 30 September 2025).
- Goudarzi, Z.; Mostafavi, M.; Salesi, M.; Jafari, M.; Mirian, I.; Hashemi Meshkini, A.; Keshavarz, K.; Ghasemi, Y. Everolimus and Temsirolimus Are Not the Same Second-Line in Metastatic Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Cost Eff. Resour. Alloc. 2023, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Bauman, J.E.; Arias-Pulido, H.; Lee, S.J.; Fekrazad, M.H.; Ozawa, H.; Fertig, E.; Howard, J.; Bishop, J.; Wang, H.; Olson, G.T.; et al. A Phase II Study of Temsirolimus and Erlotinib in Patients with Recurrent and/or Metastatic, Platinum-Refractory Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2013, 49, 461–467. [Google Scholar] [CrossRef]
- Alam, M.M.; Fermin, J.M.; Knackstedt, M.; Noonan, M.J.; Powell, T.; Goodreau, L.; Daniel, E.K.; Rong, X.; Moore-Medlin, T.; Khandelwal, A.R.; et al. Everolimus Downregulates STAT3/HIF-1α/VEGF Pathway to Inhibit Angiogenesis and Lymphangiogenesis in TP53 Mutant Head and Neck Squamous Cell Carcinoma (HNSCC). Oncotarget 2023, 14, 85–95. [Google Scholar] [CrossRef]
- Li, C.; Dong, X.; Li, B. Tumor Microenvironment in Oral Squamous Cell Carcinoma. Front. Immunol. 2024, 15, 1485174. [Google Scholar] [CrossRef]
- Veerasamy, V.; Veeran, V.; Nagini, S. Dysregulated PI3K/AKT Signaling in Oral Squamous Cell Carcinoma: The Tumor Microenvironment and Epigenetic Modifiers as Key Drivers. Oncol. Res. 2025, 33, 1835–1860. [Google Scholar] [CrossRef]
- Amengual-Cladera, E.; Morla-Barcelo, P.M.; Morán-Costoya, A.; Sastre-Serra, J.; Pons, D.G.; Valle, A.; Roca, P.; Nadal-Serrano, M. Metformin: From Diabetes to Cancer—Unveiling Molecular Mechanisms and Therapeutic Strategies. Biology 2024, 13, 302. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Q.; Ou, Y.; Zhang, Q.; Hu, J. Metformin Induces Cytotoxicity in Oral Squamous Cell Carcinoma Cells by Targeting CCN1/Akt-Axis. Int. J. Pharmacol. 2022, 18, 182–189. [Google Scholar] [CrossRef]
- Li, J.H.; Hsin, P.Y.; Hsiao, Y.C.; Chen, B.J.; Zhuang, Z.Y.; Lee, C.W.; Lee, W.J.; Vo, T.T.T.; Tseng, C.F.; Tseng, S.F.; et al. A Narrative Review: Repurposing Metformin as a Potential Therapeutic Agent for Oral Cancer. Cancers 2024, 16, 3017. [Google Scholar] [CrossRef]
- Ji, M.; Lv, Y.; Chen, C.; Xing, D.; Zhou, C.; Zhao, J.; Qi, Y.; Zhang, J.; Wang, Y.; Ma, X.; et al. Metformin Inhibits Oral Squamous Cell Carcinoma Progression through Regulating RNA Alternative Splicing. Life Sci. 2023, 315, 121274. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xu, W.; Xie, J.; Wang, Y.; Han, S.; Wei, Z.; Ni, Y.; Dong, Y.; Han, W. Metformin Sensitizes the Response of Oral Squamous Cell Carcinoma to Cisplatin Treatment through Inhibition of NF-κB/HIF-1α Signal Axis. Sci. Rep. 2016, 6, 35788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, C.; Zhou, J.; Chen, X.; Cai, K.; Shen, M.; Chen, X.; Jiang, L.; Wang, G. Inhibition of Autophagy Promotes the Anti-Tumor Effect of Metformin in Oral Squamous Cell Carcinoma. Cancers 2022, 14, 4185. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zheng, Y.; Yao, Y.; Jia, R.; Ge, S.; Zhuang, A. Metformin and Cancer Hallmarks: Shedding New Lights on Therapeutic Repurposing. J. Transl. Med. 2023, 21, 403. [Google Scholar] [CrossRef]
- Dongoran, R.; Wang, K.-H.; Lin, T.-J.; Yuan, T.-C.; Liu, C.-H. Anti-Proliferative Effect of Statins Is Mediated by DNMT1 Inhibition and p21 Expression in OSCC Cells. Cancers 2020, 12, 2084. [Google Scholar] [CrossRef]
- Spoerl, S.; Gerken, M.; Fischer, R.; Spoerl, S.; Kirschneck, C.; Wolf, S.; Taxis, J.; Ludwig, N.; Biermann, N.; Reichert, T.E.; et al. Statin Use Ameliorates Survival in Oral Squamous Cell Carcinoma—Data from a Population-Based Cohort Study Applying Propensity Score Matching. Biomedicines 2023, 11, 369. [Google Scholar] [CrossRef]
- Ricco, N.; Kron, S.J. Statins in Cancer Prevention and Therapy. Cancers 2023, 15, 3948. [Google Scholar] [CrossRef]
- Ling, Z.; Li, W.; Hu, J.; Li, Y.; Deng, M.; Zhang, S.; Ren, X.; Wu, T.; Xia, J.; Cheng, B.; et al. Targeting CCL2-CCR4 Axis Suppresses Cell Migration of Head and Neck Squamous Cell Carcinoma. Cell Death Dis. 2022, 13, 158. [Google Scholar] [CrossRef]
- Biselli-Chicote, P.M.; Lotierzo, A.T.; Biselli, J.M.; Paravino, É.C.; Goloni-Bertollo, E.M. Atorvastatin Increases Oxidative Stress and Inhibits Cell Migration of Oral Squamous Cell Carcinoma In Vitro. Oral Oncol. 2019, 90, 109–114. [Google Scholar] [CrossRef]
- Kou, Y.; Zhang, Y.; Rong, X.; Yang, P.; Wang, C.; Zhou, Q.; Liu, H.; Liu, B.; Li, M. Simvastatin Inhibits Proliferation and Promotes Apoptosis of Oral Squamous Cell Carcinoma through KLF2 Signal. J. Oral Biosci. 2023, 65, 347–355. [Google Scholar] [CrossRef]
- Wang, S.; Shi, M.; Wang, H.; Zeng, X.; Zhang, D.; Zhang, Z.; Xu, Z.; Li, Y. Effects on Oral Squamous Carcinoma Cell Lines and Their Mechanisms of Pyrazole N-Aryl Sulfonate: A Novel Class of Selective Cyclooxygenase-2 Inhibitors. Int. J. Mol. Sci. 2025, 26, 8906. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, L.; Zhang, C.; Chen, G.; Mai, H. Blocking β₂-AR and Inhibiting COX-2: A Promising Approach to Suppress OSCC Development. Int. Dent. J. 2025, 75, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Nasry, W.H.S.; Martin, C.K. Intersecting Mechanisms of Hypoxia and Prostaglandin E₂-Mediated Inflammation in the Comparative Biology of Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 539361. [Google Scholar] [CrossRef]
- Benbelkacem, M.; Moulai, N.; Chader, H.; Ouahioune, W.; Bourouba, M. Dichloroacetate and Chloroquine in Combination with Arsenite Suppress ROS-Induced Oral Squamous Cell Carcinoma (OSCC) Development and Improve BALB/c Mice Survival. Free Radic. Biol. Med. 2025, 227, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Corral, D.; García-Valles, P.; Arroyo-Garrapucho, N.; Bueno-Martínez, E.; Ruiz-Robles, J.M.; Ovejero-Sánchez, M.; González-Sarmiento, R.; Herrero, A.B. Chloroquine-Induced DNA Damage Synergizes with DNA Repair Inhibitors Causing Cancer Cell Death. Front. Oncol. 2024, 14, 1390518. [Google Scholar] [CrossRef]
- Faraji-Barhagh, A.; Jahandar-Lashaki, S.; Esfahlan, R.J.; Alizadeh, E. Current Nano Drug Delivery Systems for Targeting Head and Neck Squamous Cell Carcinoma Microenvironment: A Narrative Review. Mol. Biol. Rep. 2025, 52, 369. [Google Scholar] [CrossRef]
- Chou, K.-Y.; Chen, P.-C.; Chang, A.-C.; Tsai, T.F.; Chen, H.E.; Ho, C.Y.; Hwang, T.I. Attenuation of Chloroquine and Hydroxychloroquine on the Invasive Potential of Bladder Cancer through Targeting Matrix Metalloproteinase 2 Expression. Environ. Toxicol. 2021, 36, 2138–2145. [Google Scholar] [CrossRef]
- Rodriguez-Berriguete, G.; Puliyadi, R.; Machado, N.; Barberis, A.; Prevo, R.; McLaughlin, M.; Buffa, F.M.; Harrington, K.J.; Higgins, G.S. Antitumour Effect of the Mitochondrial Complex III Inhibitor Atovaquone in Combination with Anti-PD-L1 Therapy in Mouse Cancer Models. Cell Death Dis. 2024, 15, 32. [Google Scholar] [CrossRef]
- Fiorillo, M.; Lamb, R.; Tanowitz, H.; Mutti, L.; Krstic-Demonacos, M.; Cappello, A.; Martinez-Outschoorn, U.; Sotgia, F.; Lisanti, M. Repurposing Atovaquone: Targeting Mitochondrial Complex III and OXPHOS to Eradicate Cancer Stem Cells. Oncotarget 2016, 7, 34084–34099. [Google Scholar] [CrossRef]
- Kapur, A.; Mehta, P.; Simmons, A.D.; Ericksen, S.S.; Mehta, G.; Palecek, S.P.; Felder, M.; Stenerson, Z.; Nayak, A.; Dominguez, J.M.A.; et al. Atovaquone: An Inhibitor of Oxidative Phosphorylation as Studied in Gynecologic Cancers. Cancers 2022, 14, 2297. [Google Scholar] [CrossRef]
- Wagner, V.P.; Spuldaro, T.R.; Nör, F.; Gaio, E.J.; Castilho, R.M.; Carrard, V.C.; Rösing, C.K. Can Propranolol Act as a Chemopreventive Agent during Oral Carcinogenesis? An Experimental Animal Study. Eur. J. Cancer Prev. 2021, 30, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Chun, K.J.; Kil, H.K.; Jung, N.; Shin, H.; Jang, J.Y.; Choi, H.G.; Oh, K.; Kim, M. β₂ Adrenergic Receptor Expression and the Effects of Norepinephrine and Propranolol on Various Head and Neck Cancer Subtypes. Oncol. Lett. 2021, 22, 804. [Google Scholar] [CrossRef]
- Nakhaei, A.; Marzoughi, S.; Ghoflchi, S.; Hosseini, H.; Afshari, A.R.; Jalili-Nik, M.; Kesharwani, P.; Sahebkar, A. An Exploration of Molecular Signaling in Drug Reprocessing for Oral Squamous Cell Carcinoma. Eur. J. Med. Chem. 2025, 295, 117816. [Google Scholar] [CrossRef]
- Jin, Y.; Zhuang, Y.; Dong, X.; Liu, M. Development of CpG Oligodeoxynucleotide TLR9 Agonists in Anti-Cancer Therapy. Expert Rev. Anticancer Ther. 2021, 21, 841–851. [Google Scholar] [CrossRef]
- Rioja-Blanco, E.; Arroyo-Solera, I.; Álamo, P.; Casanova, I.; Gallardo, A.; Unzueta, U.; Serna, N.; Sánchez-García, L.; Quer, M.; Villaverde, A.; et al. Self-Assembling Protein Nanocarrier for Selective Delivery of Cytotoxic Polypeptides to CXCR4⁺ Head and Neck Squamous Cell Carcinoma Tumors. Acta Pharm. Sin. B 2022, 12, 2578–2591. [Google Scholar] [CrossRef]
- Goldberg, M.; Manzi, A.; Birdi, A.; Laporte, B.; Conway, P.; Cantin, S.; Mishra, V.; Singh, A.; Pearson, A.T.; Goldberg, E.R.; et al. A Nanoengineered Topical Transmucosal Cisplatin Delivery System Induces Anti-Tumor Response in Animal Models and Patients with Oral Cancer. Nat. Commun. 2022, 13, 4829. [Google Scholar] [CrossRef]
- Bagheri, M.; Zandieh, M.A.; Daryab, M.; Samaei, S.S.; Gholami, S.; Rahmanian, P.; Dezfulian, S.; Eary, M.; Rezaee, A.; Rajabi, R.; et al. Nanostructures for Site-Specific Delivery of Oxaliplatin Cancer Therapy: Versatile Nanoplatforms in Synergistic Cancer Therapy. Transl. Oncol. 2024, 39, 101838. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular Vesicles: A Rising Star for Therapeutics and Drug Delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Liu, S.; Yu, L.; Liu, S.; Li, M.; Jin, F. Extracellular Vesicles in Oral Squamous Cell Carcinoma: Current Progress and Future Prospect. Front. Bioeng. Biotechnol. 2023, 11, 1149662. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Meng, Y.; Yao, Z.; Lin, X.; Hu, M.; Cai, S.; Gao, H.; Zhang, H. Extracellular Vesicles as Nature’s Nano Carriers in Cancer Therapy: Insights towards Preclinical Studies and Clinical Applications. Pharmacol. Res. 2025, 217, 107751. [Google Scholar] [CrossRef] [PubMed]
| Target/Pathway | Mechanism of Action | Drugs |
|---|---|---|
| EGFR (Epidermal Growth Factor Receptor) | Tyrosine kinase inhibitors (TKIs) bind to the intracellular ATP-binding site (reversible or irreversible) → block kinase autophosphorylation and downstream signaling Monoclonal antibodies (mAbs) bind to the extracellular ligand-binding domain, preventing ligand binding/receptor dimerization/receptor activation | TKIs: gefitinib, erlotinib, afatinib, dacomitinib, osimertinib, mobocertinib, icotinib, and sunvozertinib mAbs/biologics: cetuximab, necitumumab, panitumumab, nimotuzumab, and amivantamab (bispecific EGFR/MET) |
| PD-1/PD-L1 (Immune Checkpoint) | Monoclonal antibodies block the interaction between PD-1 (on T cells) and PD-L1 (on tumor cells or antigen-presenting cells), preventing the inhibitory signal that dampens T-cell activation/enabling T-cell-mediated tumor killing | Anti–PD-1: pembrolizumab, nivolumab, cemiplimab, and dostarlimab Anti–PD-L1: atezolizumab, durvalumab, and avelumab |
| CDKs (Cyclin-Dependent Kinases) | Small molecule inhibitors of CDKs inhibit kinase activity and block phosphorylation of downstream effectors (e.g., Rb) → cell cycle arrest, especially in G1/S transition | Palbociclib, ribociclib, and abemaciclib (CDK4/6 inhibitors) |
| VEGF/VEGFR (Angiogenesis Pathway) | Monoclonal antibodies bind VEGF ligands → sequester them and receptor tyrosine kinase inhibitors (RTKIs) inhibit VEGFR kinase activity (or broader multikinase inhibition) | Bevacizumab (anti-VEGF) and ziv-aflibercept; RTKIs/multikinase inhibitors: sunitinib, sorafenib, pazopanib, axitinib, lenvatinib, and vandetanib |
| mTOR (Mammalian Target of Rapamycin) | Small-molecule inhibitors of mTOR block the kinase activity (TORC1 or both TORC1/TORC2) → suppress cell growth, protein synthesis, and metabolism | Rapamycin (sirolimus), everolimus, and temsirolimus; second-generation TOR inhibitors (e.g., PP242, Torin1) |
| Interception Mode/Pathway | Repurposed Agent | Mechanism of Action | Evidence (in Cancer with Immunotherapy Context) | Safety/Practical Notes |
|---|---|---|---|---|
| AMPK activation → indirect mTORC1 inhibition/metabolic reprogramming/anti-inflammation | Metformin | Activates AMPK → suppression of mTORC1. Lowers systemic insulin/IGF signaling Reduces NF-κB/IL-6/STAT3 signaling in stroma/CAFs. Down-regulates PD-L1 expression (via IL-6/JAK2/STAT3). Improves metabolic fitness/reduces hypoxia in TME. | Observational/retrospective: Diabetic patients on metformin showed improved responses to ICI in some cohorts. Metformin + anti–PD-1 synergy in murine models. Early prospective/mechanistic: Increasing interest, e.g., metformin co-treatment “boosts immunotherapy” reviews. | Metformin is generally well tolerated, with long use in diabetes. Risk of lactic acidosis in renal impairment or hypoxia. |
| Mevalonate/prenylation/RAS/cholesterol/immunomodulation | Statins (e.g., atorvastatin, simvastatin) | Inhibit HMG-CoA reductase → reduce cholesterol, isoprenoid pathway. Impair prenylation of small GTPases (e.g., RAS/Rho) → downstream PI3K/AKT signaling interference. Anti-inflammatory effects: reduce cytokine secretion, modulate myeloid cell polarization, lower endothelial activation. May modulate PD-L1, myeloid suppression. | Preclinical/mechanistic: Statins shown to enhance chemo/overcome resistance; immunomodulatory signals in tumor models. Observational/retrospective: Some cancer immunotherapy cohorts report improved outcomes in statin users (various cancers, though not robust HNSCC--specific). Early prospective: Few trials explicitly combining statins + ICI | Main risks: myopathy, hepatic effects, drug–drug interactions (notably via CYP3A4). Careful monitoring during CRT (e.g., overlapping toxicity). Need attention to lipophilicity vs. hydrophilicity (some statins penetrate tissues better). |
| COX-2/prostaglandin E2 axis/immunosuppressive inflammation | Celecoxib (selective COX-2 inhibitor), NSAIDs | Inhibit COX-2 → reduce PGE2 production. Lower recruitment/activation of MDSCs, suppress IL-10 macrophages and improve DC and T-cell infiltration. Downstream reduction in immunosuppressive milieu. | Preclinical: Many tumor models show COX-2 inhibition enhances T-cell infiltration and reduces immune suppression. Observational/retrospective: Some ICI cohorts show NSAID/COX-2 use correlates with better outcomes (heterogeneous). Early prospective: Limited, especially in HNSCC. | COX-2 inhibitors carry GI bleeding risk and cardiovascular risk. In peri-operative/CRT settings, bleeding risk must be managed. Dose, timing, and patient selection are critical to mitigate toxicity. |
| Autophagy/lysosome inhibition/microenvironment remodeling | Hydroxychloroquine (HCQ)/Chloroquine (CQ) | Blocking autophagic flux in cancer cells, increasing tumor susceptibility to T-cell killing. Repolarizing macrophages from M2 → M1, reducing MDSCs and regulatory T cells, improving DC function. Modulation of tumor stroma and vasculature, CAFs via lysosomal/TLR/NF-κB mechanisms. | Preclinical: Strong support in many tumor models, HCQ/CQ + immunotherapy synergy in animal models. Clinical/early trials: Multiple cancer trials (various tumor types) with HCQ combinations ongoing. Some early signals in solid tumor trials combining HCQ/CQ + chemotherapy/targeted therapy (phase I) | Retinopathy/ocular toxicity risk with long-term use. QT prolongation risk and GI side effects Dosing needs careful calibration (in many trials, MTD or tolerability is limiting). The degree of autophagy dependence varies by tumor and context. |
| Bioenergetic/mitochondrial metabolism disruptors | Menadione (vitamin K3), pyrvinium pamoate, atovaquone, antimycin A (experimental) | Disrupt oxidative phosphorylation/mitochondrial electron transport → metabolic stress in tumor cells. Collapse of tumor metabolic plasticity → increase immunogenicity, weaken stemness/EMT. | Preclinical: Evidence in cell/animal models of metabolic stress + immunotherapy synergy (largely non-HNSCC). Currently little clinical data in humans for cancer immunotherapy settings. | High risk of off-target toxicities (mitochondrial toxicity). Narrow therapeutic windows; careful monitoring required. Likely restricted to experimental/trial settings. |
| β-adrenergic/stress signaling modulation | Propranolol (and possibly other β-blockers) | Block β-adrenergic signaling → increase PTEN expression and reduce pro-survival signaling. Modulate stress-induced immune suppression and reduce catecholamine-driven immunosuppression. | Preclinical/mechanistic: some tumor and chemo combination models show enhanced sensitivity. Observational/retrospective: In various cancers, β-blocker use has been associated with improved survival/reduced metastasis. Prospective in immunotherapy combinations: Very limited in HNSCC. | β-blockers have known cardiopulmonary side effects (bradycardia, hypotension, and bronchospasm). Must be used with caution in patients with comorbidities. Dose, timing, and cancer specificity are uncertain. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, G.; Sinha, N.; Vale, N.; Mendes, R.A. Targeted Therapies in Oral and Oropharyngeal Cancer: An Overview of Emerging and Repurposed Agents. Cancers 2025, 17, 3761. https://doi.org/10.3390/cancers17233761
Kaur G, Sinha N, Vale N, Mendes RA. Targeted Therapies in Oral and Oropharyngeal Cancer: An Overview of Emerging and Repurposed Agents. Cancers. 2025; 17(23):3761. https://doi.org/10.3390/cancers17233761
Chicago/Turabian StyleKaur, Geetpriya, Neetu Sinha, Nuno Vale, and Rui Amaral Mendes. 2025. "Targeted Therapies in Oral and Oropharyngeal Cancer: An Overview of Emerging and Repurposed Agents" Cancers 17, no. 23: 3761. https://doi.org/10.3390/cancers17233761
APA StyleKaur, G., Sinha, N., Vale, N., & Mendes, R. A. (2025). Targeted Therapies in Oral and Oropharyngeal Cancer: An Overview of Emerging and Repurposed Agents. Cancers, 17(23), 3761. https://doi.org/10.3390/cancers17233761








