The Effect of Tumor Location and Extension on Survival in Patients with Sinonasal Mucosal Melanoma: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reporting Guideline, Registration, and Ethics
2.2. Definitions
2.3. Literature Search Strategy
2.4. Study Selection
2.5. Data Extraction and Quality Assessment
2.6. Statistical Analysis
3. Results
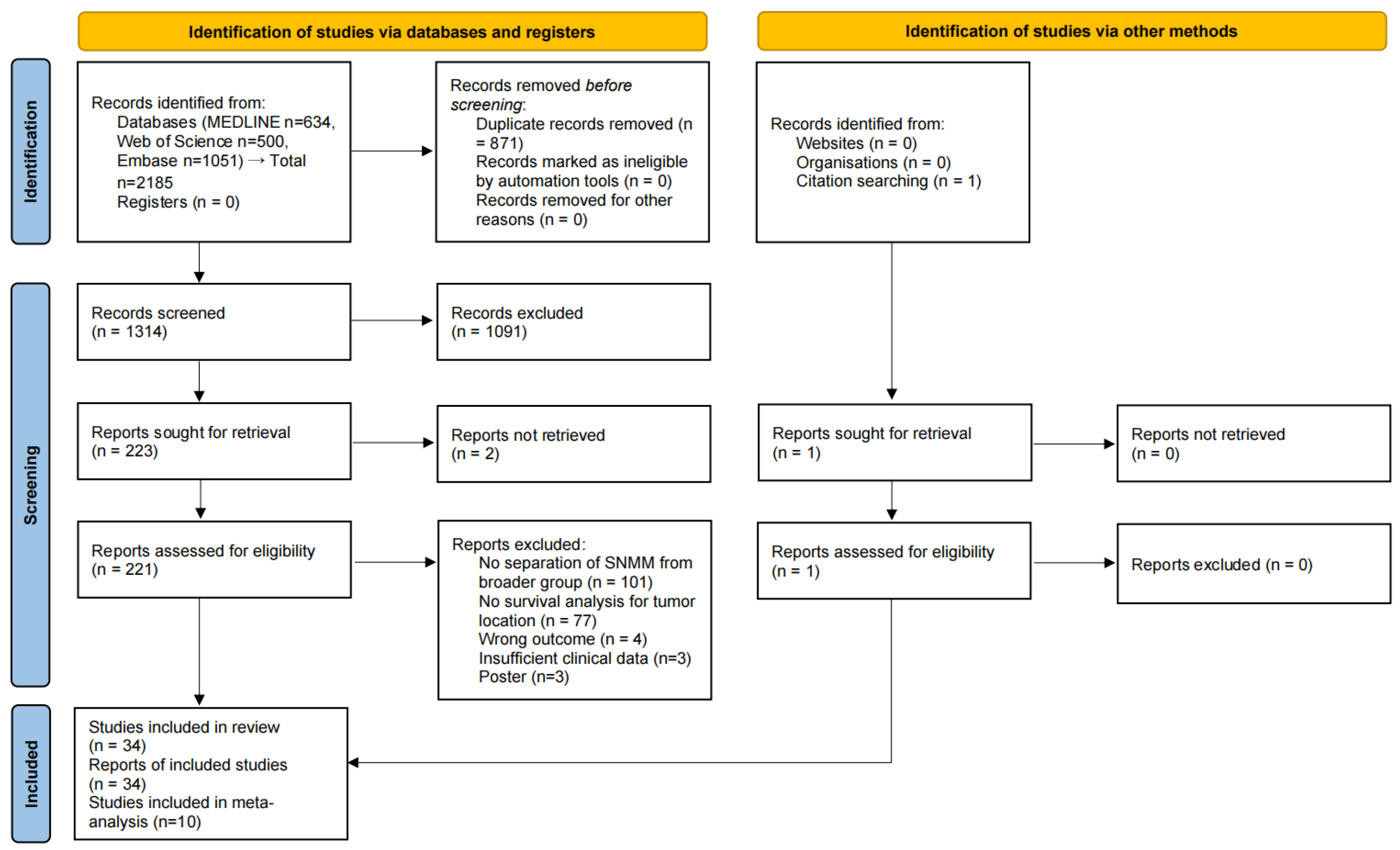
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Systematic Review
3.5. Meta-Analysis Outcomes
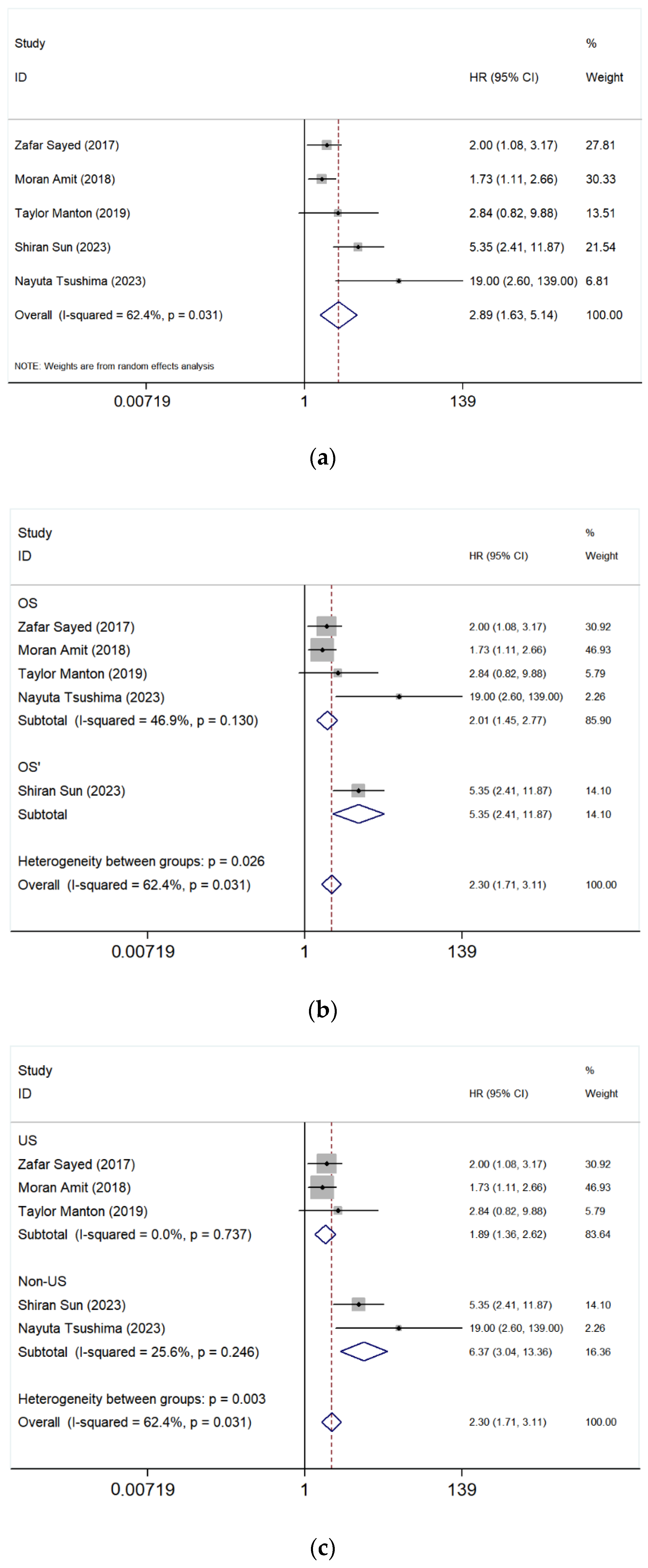
3.5.1. Paranasal Sinus vs. Nasal Cavity
- Overall Analysis
- Subgroup Analysis After Excluding the study of Sun et al. [17]
- Subgroup Analysis by Geographic Region
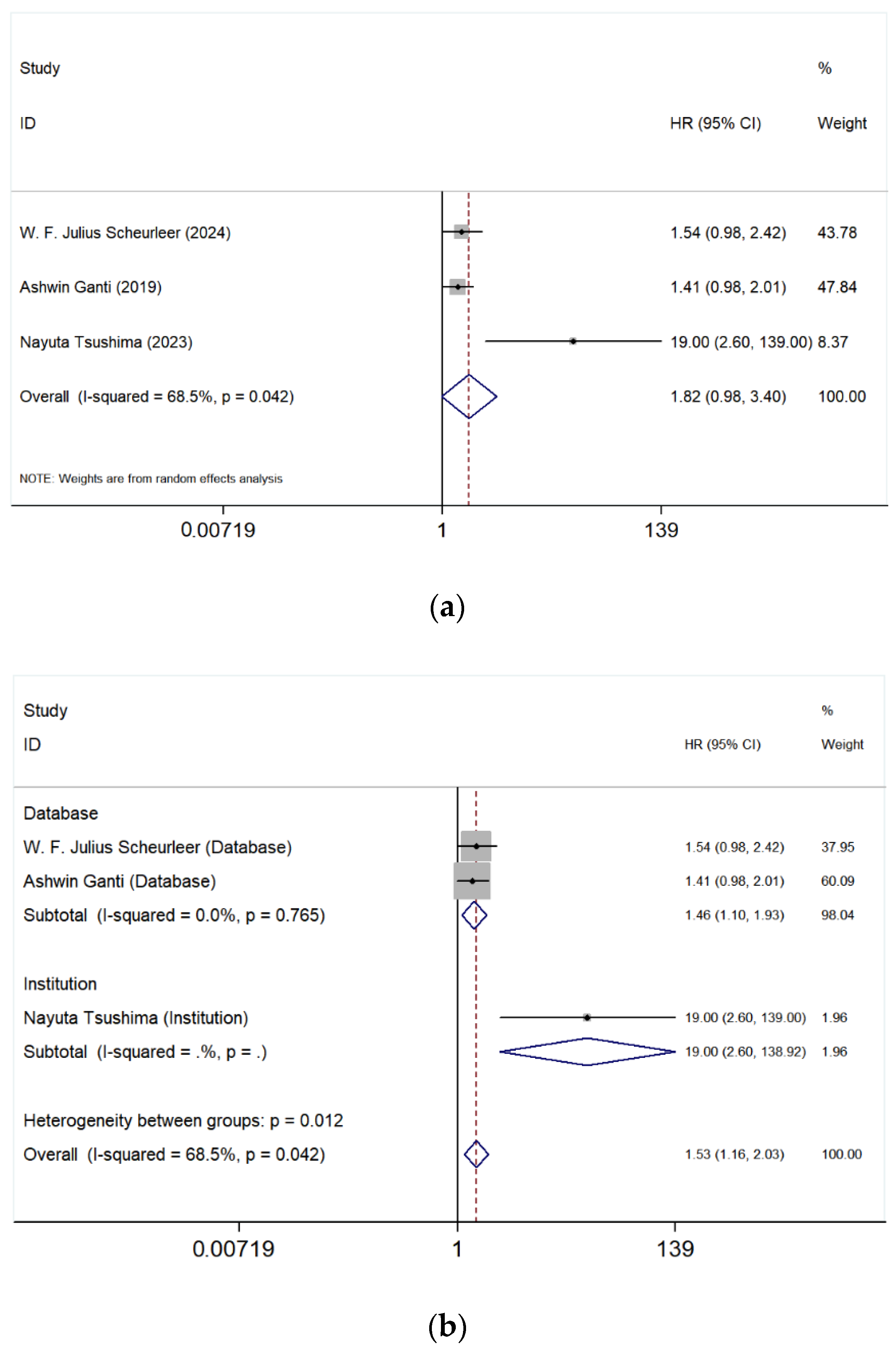
3.5.2. Maxillary Sinus vs. Nasal Cavity
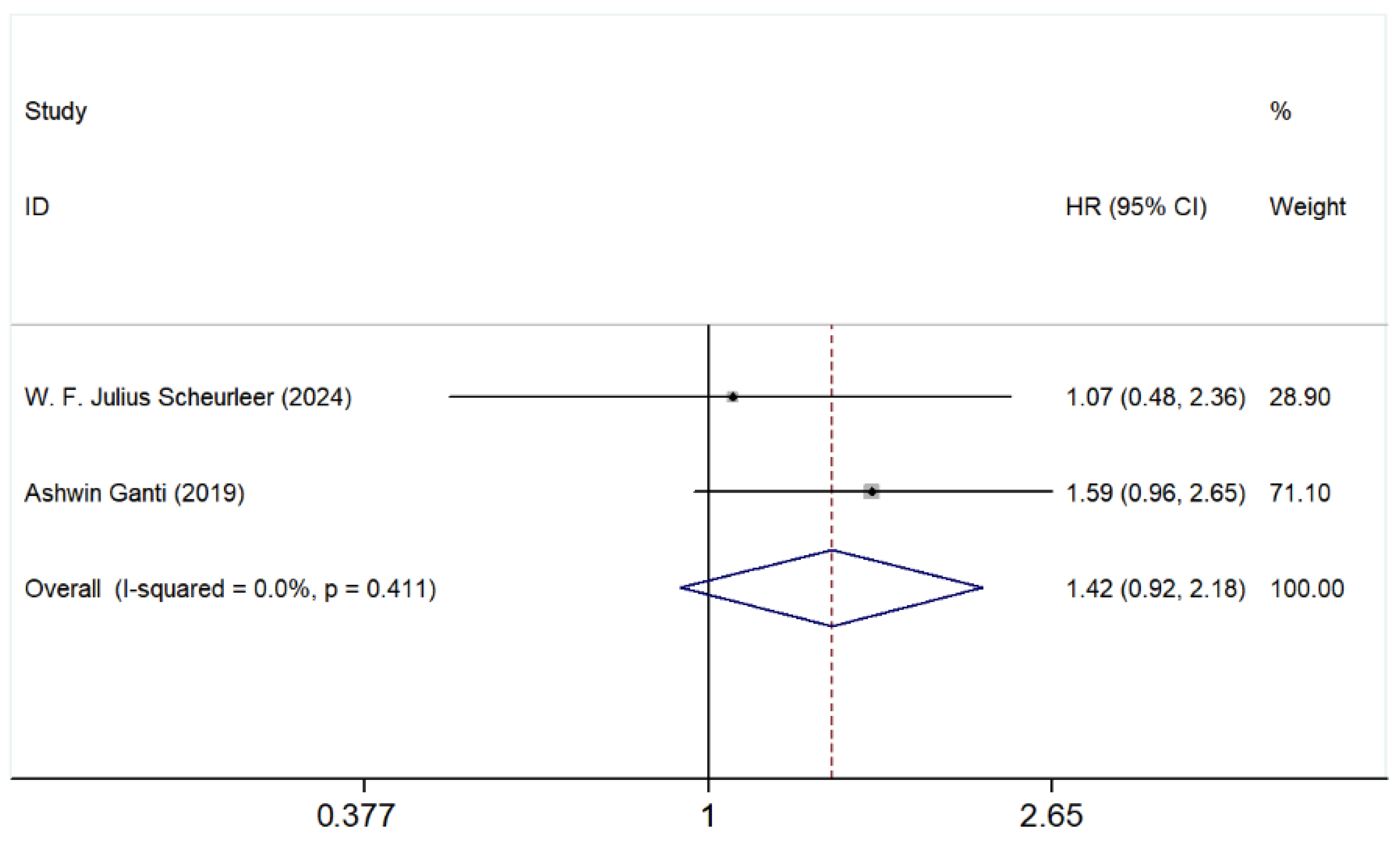
3.5.3. Ethmoid Sinus vs. Nasal Cavity
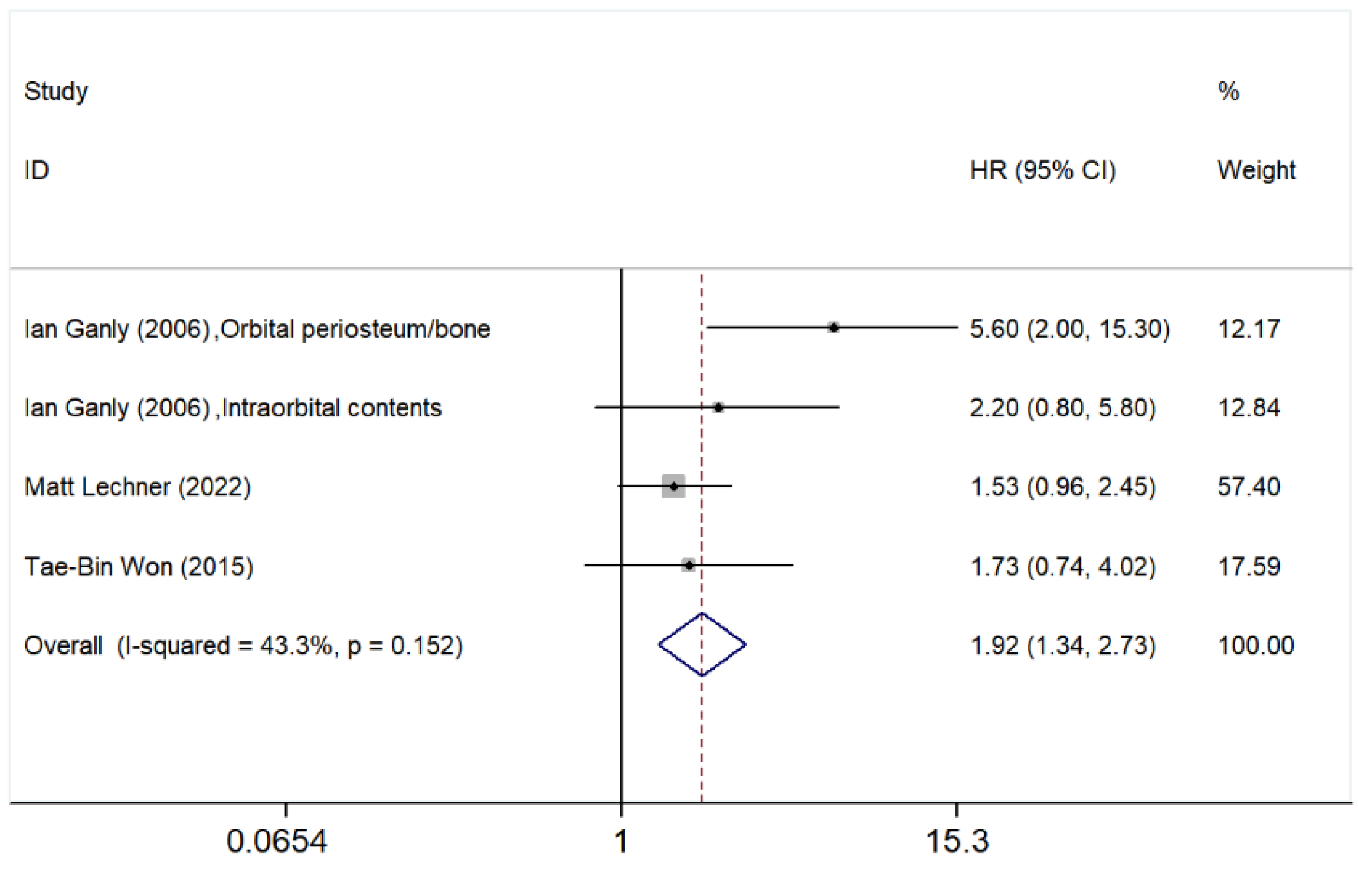
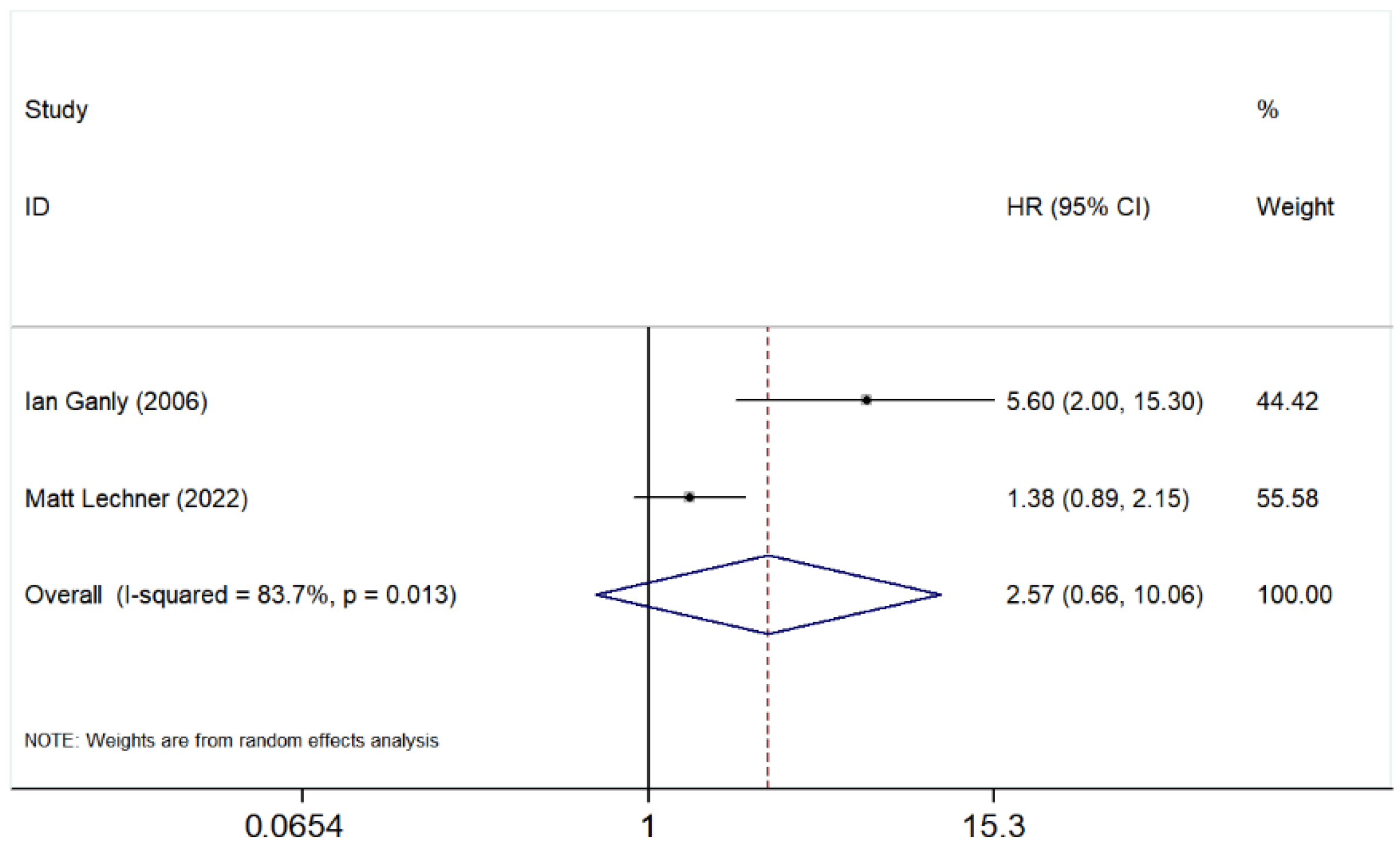
3.5.4. Orbital Involvement
3.5.5. Bone Involvement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SNMM | Sinonasal mucosal melanoma |
| HR | Hazard ratio |
| CI | Confidence interval |
| OS | Overall survival |
| S | Surgery |
| CFR | Craniofacial resection |
| Gy | Gray |
| PORT | Postoperative radiotherapy |
| ICI | Immune checkpoint inhibitor |
| TNM | Tumor Node Metastasis |
| PFS | Progression-free survival |
| DFS | Disease-free survival |
| DSS | Disease-specific survival |
| MM | Mucosal melanoma |
| HNMM | Head and neck mucosal melanoma |
| MSS | Melanoma-specific survival |
| RFS | Recurrence-free survival |
| LR | Local recurrence |
| RR | Regional recurrence |
| DM | Distant metastasis |
| RS | Relative survival |
| POCRT | Postoperative chemoradiotherapy |
| RT | Radiotherapy |
| SD | Standard deviation |
| IQR | Interquartile range |
| NR | Not reported |
| NCDB | National Cancer Database |
| SEER | Surveillance, Epidemiology, and End Results database |
| NCR | Netherlands Cancer Registry |
| REFCOR | Réseau d’Expertise Français sur les Cancers ORL Rares |
| NOS | Not otherwise specified |
Appendix A
Appendix A.1. Search Strategy
Appendix A.2. Risk of Bias Assessment
| Study | Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis and Reporting |
|---|---|---|---|---|---|---|
| Sayed et al., 2017. [20] | Moderate | Low | Low | Low | Moderate | Low |
| Ganly et al., 2006. [40] | Moderate | Low | Moderate | Low | Moderate | Low |
| Lechner et al., 2022. [41] | Low | Moderate | Low | Low | Moderate | Low |
| Amit et al., 2018. [21] | Low | Low | Low | Low | Low | Low |
| Manton et al., 2019. [39] | Low | High | Low | Low | Moderate | Low |
| Scheurleer et al., 2024. [6] | Low | High | Low | Low | High | Moderate |
| Ganti et al., 2020. [3] | Low | High | Low | Low | Moderate | Low |
| Won et al., 2015. [22] | Low | High | Low | Low | Moderate | Moderate |
| Sun et al., 2023. [17] | Low | High | Low | Low | Low | Low |
| Tsushima et al., 2023. [4] | Low | High | Low | Low | Moderate | Low |
| Koivunen et al., 2012. [38] | Low | Low | Moderate | Low | High | Moderate |
| Yang et al., 2022. [11] | Low | Low | Moderate | Low | Low | Low |
| Gras-Cabrerizo et al., 2015. [31] | Moderate | Low | Low | Low | Moderate | Low |
| Abt et al., 2021. [23] | Low | Moderate | Low | Low | Moderate | Low |
| Low et al., 2020. [12] | Low | Low | Moderate | Low | Moderate | Low |
| Moya-Plana et al., 2019. [24] | Low | Low | Low | Low | Low | Low |
| Roth et al., 2010. [10] | Moderate | Low | Moderate | Low | High | Moderate |
| Martin et al., 2004. [32] | Moderate | Low | Low | Low | Moderate | Low |
| Wang et al., 2022. [18] | Low | Moderate | Low | Low | Moderate | Low |
| Houette et al., 2016. [25] | Moderate | Low | Low | Low | High | Moderate |
| Lundberg et al., 2019. [26] | Low | Low | Low | Low | Moderate | Moderate |
| Letievant et al., 2016. [19] | Moderate | Moderate | Moderate | Low | High | Moderate |
| Khan et al., 2014. [42] | Low | Low | Low | Low | Moderate | Low |
| Wang et al., 2020. [27] | Low | Moderate | Low | Low | Moderate | Moderate |
| Dréno et al., 2017. [28] | Moderate | Moderate | Low | Low | High | Moderate |
| Vandenhende et al., 2012. [36] | Moderate | Moderate | Low | Low | High | Moderate |
| Liétin et al., 2010. [37] | High | Moderate | Low | Low | High | Moderate |
| Rojas-Lechuga et al., 2022. [33] | Low | Low | Low | Low | Low | Low |
| Amit et al., 2017. [5] | Low | Low | Low | Low | Moderate | Low |
| Sun et al., 2014. [34] | Low | Low | Low | Low | Moderate | Low |
| Dauer et al., 2008. [35] | Moderate | High | Moderate | Low | Moderate | Moderate |
| Ajmani et al., 2017. [29] | Low | Low | Low | Low | Moderate | Low |
| Zhu et al., 2024. [49] | Low | Low | Low | Low | Low | Low |
| Yu et al., 2015. [30] | Moderate | Low | Moderate | Low | High | Moderate |
References
- Rojas-Lechuga, M.J.; Jubés, S.; Molina-García, M.; da Silva-Júnior, R.M.P.; Sampieri, C.; Langdon, C.; Gras-Cabrerizo, J.R.; Bernal-Sprekelsen, M.; Puig, S.; Alobid, I. Survival Outcomes in Sinonasal Mucosal Melanoma: Systematic Review and Meta-Analysis. J. Pers. Med. 2024, 14, 1120. [Google Scholar] [CrossRef]
- Gal, T.J.; Silver, N.; Huang, B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope 2011, 121, 2026–2033. [Google Scholar] [CrossRef]
- Ganti, A.; Raman, A.; Shay, A.; Kuhar, H.N.; Auger, S.R.; Patel, T.; Kuan, E.C.; Diaz, A.Z.; Batra, P.S.; Tajudeen, B.A. Treatment modalities in sinonasal mucosal melanoma: A national cancer database analysis. Laryngoscope 2019, 130, 275–282. [Google Scholar] [CrossRef]
- Tsushima, N.; Kano, S.; Yasuda, K.; Suzuki, T.; Hamada, S.; Nakamaru, Y.; Suzuki, M.; Uchinami, Y.; Aoyama, H.; Homma, A. Treatment outcomes of the patient with sinonasal mucosal melanoma: The role of endoscopic resection and postoperative radiotherapy. Int. J. Clin. Oncol. 2023, 28, 1218–1226. [Google Scholar] [CrossRef]
- Abdelmeguid, A.S.; Kupferman, M.E.; Su, S.Y.; Raza, S.M.; DeMonte, F.; Amit, M.; Tam, S.; Hanna, E.Y. Role of Adjuvant Treatment in Sinonasal Mucosal Melanoma. J. Neurol. Surg. Part. B Skull Base 2017, 78, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Scheurleer, W.F.J.; van de Velde, L.J.; Devriese, L.A.; de Ridder, M.; Louwman, M.W.J.; Breimer, G.E.; de Bree, R.; van Dijk, B.A.C.; Rijken, J.A. Sinonasal mucosal melanoma in The Netherlands between 2001 and 2021: A clinical and epidemio-logical overview of 320 cases. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 5437–5446. [Google Scholar] [CrossRef]
- Salem, A.F.; Chen, M.M.; Williams, M.D.; Swanson, D.M.; McQuade, J.L.; Amaria, R.N.; Hanna, E.Y.; Bishop, A.J.; Farooqi, A.S.; Guadagnolo, B.A.; et al. Resectable Sinonasal Mucosal Melanoma in the Immunotherapy Era: Upfront Surgery vs. Neoadjuvant Therapy. Head. Neck 2025, 47, 1848–1856. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Moya-Plana, A.; Mangin, D.; Dercle, L.; Taouachi, R.; Casiraghi, O.; Ammari, S.; Nguyen, F.; Temam, S.; Robert, C.; Gorphe, P. Risk-based stratification in head and neck mucosal melanoma. Oral. Oncol. 2019, 97, 44–49. [Google Scholar] [CrossRef]
- Roth, T.N.; Gengler, C.; Huber, G.F.; Holzmann, D. Outcome of sinonasal melanoma: Clinical experience and review of literature. Head. Neck 2010, 32, 1385–1392. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Lai, Y.; Liu, Q.; Sun, X.; Wang, D.; Yu, H. A nomogram for predicting overall survival of patients with sinonasal melanoma: A population-based study. Laryngoscope Investig. Otolaryngol. 2022, 7, 1837–1848. [Google Scholar] [CrossRef]
- Low, C.M.; Price, D.L.; Moore, E.J.; Stokken, J.K.; Van Abel, K.M.; Janus, J.R.; Choby, G. Nodal and distant metastases in sinonasal mucosal melanoma: A population-based analysis. Laryngoscope 2019, 130, 622–627. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 160. [Google Scholar] [CrossRef]
- Hayden, J.A.; Van Der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing Bias in Studies of Prognostic Factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Hayden, J.A.; Côté, P.; Bombardier, C. Evaluation of the Quality of Prognosis Studies in Systematic Reviews. Ann. Intern. Med. 2006, 144, 427–437. [Google Scholar] [CrossRef]
- Yin, S.; Zhu, F.; Liu, Y.; Chen, Q. Effects of silymarin on insulin resistance and sensitivity: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2025, 220, 112008. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, Y.; Huang, X.; Wang, K.; Qu, Y.; Wu, R.; Chen, X.; Wang, J.; Zhang, J.; Luo, J.; et al. Sinonasal mucosal melanoma: Is there a need for elective neck irradiation. Radiother. Oncol. 2023, 185, 109642. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H. PD-L1 expression in 117 sinonasal mucosal melanomas and its association with clinical outcome. Ann. Diagn. Pathol. 2022, 60, 151976. [Google Scholar] [CrossRef] [PubMed]
- Letievant, J.-C.; Poupart, M.; Ambrun, A.; Colin, C.; Pignat, J.-C. Single-center retrospective series of fourteen patients with mucosal melanoma of the nasal cavity and paranasal sinuses. Eur. Ann. Otorhinolaryngol. Head. Neck Dis. 2016, 133, 387–391. [Google Scholar] [CrossRef]
- Sayed, Z.; Migliacci, J.C.; Cracchiolo, J.R.; Barker, C.A.; Lee, N.Y.; McBride, S.M.; Tabar, V.S.; Ganly, I.; Patel, S.G.; Morris, L.T.; et al. Association of Surgical Approach and Margin Status with Oncologic Outcomes Following Gross Total Resection for Sinonasal Melanoma. Arch. Otolaryngol. Neck Surg. 2017, 143, 1220–1227. [Google Scholar] [CrossRef]
- Amit, M.; Tam, S.; Abdelmeguid, A.S.; Kupferman, M.E.; Su, S.Y.; Raza, S.M.; DeMonte, F.; Hanna, E.Y. Patterns of Treatment Failure in Patients with Sinonasal Mucosal Melanoma. Ann. Surg. Oncol. 2018, 25, 1723–1729. [Google Scholar] [CrossRef]
- Won, T.; Choi, K.Y.; Rhee, C.; Jin, H.; Yi, J.S.; Dhong, H.; Kim, S.W.; Choi, J.H.; Kim, J.K.; Chung, Y.; et al. Treatment outcomes of sinonasal malignant melanoma: A Korean multicenter study. Int. Forum Allergy Rhinol. 2015, 5, 950–959. [Google Scholar] [CrossRef]
- Abt, N.B.; Miller, L.E.; Mokhtari, T.E.; Lin, D.T.; Richmon, J.D.; Deschler, D.G.; Varvares, M.A.; Puram, S.V. Nasal and paranasal sinus mucosal melanoma: Long-term survival outcomes and prognostic factors. Am. J. Otolaryngol. 2021, 42, 103070. [Google Scholar] [CrossRef] [PubMed]
- Moya-Plana, A.; Aupérin, A.; Obongo, R.; Ferrand, F.; Baujat, B.; Saroul, N.; Casiraghi, O.; Vergez, S.; Herman, P.; Janot, F.; et al. Oncologic outcomes, prognostic factor analysis and therapeutic algorithm evaluation of head and neck mucosal melanomas in France. Eur. J. Cancer 2019, 123, 1–10. [Google Scholar] [CrossRef]
- Houette, A.; Gilain, L.; Mulliez, A.; Mom, T.; Saroul, N. Prognostic value of two tumour staging classifications in patients with sinonasal mucosal melanoma. Eur. Ann. Otorhinolaryngol. Head. Neck Dis. 2016, 133, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Haapaniemi, A.; Hagstrom, J.; Juteau, S.; Hernberg, M.; Makitie, A.; Vento, S. Similar survival outcome after endoscopic and open approaches for sinonasal mucosal melanoma. Rhinol. J. 2019, 57, 132–138. [Google Scholar] [CrossRef]
- Wang, T.; Huang, Y.; Lu, J.; Xiang, M. Sinonasal mucosal melanoma: A 10-year experience of 36 cases in China. Ann. Transl. Med. 2020, 8, 1022. [Google Scholar] [CrossRef]
- Dréno, M.; Georges, M.; Espitalier, F.; Ferron, C.; Charnolé, A.; Dréno, B.; Malard, O. Sinonasal mucosal melanoma: A 44-case study and literature analysis. Eur. Ann. Otorhinolaryngol. Head. Neck Dis. 2017, 134, 237–242. [Google Scholar] [CrossRef]
- Ajmani, G.S.; Liederbach, E.; Kyrillos, A.; Wang, C.-H.; Pinto, J.M.; Bhayani, M.K. Adjuvant radiation and survival following surgical resection of sinonasal melanoma. Am. J. Otolaryngol. 2017, 38, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, G. Clinical analysis of 29 cases of nasal mucosal malignant melanoma. Oncol. Lett. 2015, 10, 1166–1170. [Google Scholar] [CrossRef]
- Gras-Cabrerizo, J.R.; León-Vintró, X.; Tarruella, M.M.; Sarria, G.P.; Gonzalez, C.B.; Montserrat-Gili, J.R.; Kolanczak, K.; Massegur-Solench, H. Management of Sinonasal Mucosal Melanomas and Comparison of Classification Staging Systems. Am. J. Rhinol. Allergy 2015, 29, e37–e40. [Google Scholar] [CrossRef]
- Martin, J.M.; Porceddu, S.; Weih, L.; Corry, J.; Peters, L.J. Outcomes in sinonasal mucosal melanoma. ANZ J. Surg. 2004, 74, 838–842. [Google Scholar] [CrossRef]
- Rojas-Lechuga, M.J.; Gras-Cabrerizo, J.; Aviles-Jurado, F.; Malvehy, J.; Arance, A.; Castillo, P.; Barreiro, A.; Podlipnik, S.; Lopez-Chacon, M.; Alobid, I.; et al. Sinonasal mucosal melanomas: Defining profiles for better survival outcomes. Rhinol. J. 2022, 60, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Z.; Li, Q.-L.; Hu, Z.-D.; Jiang, Y.-E.; Song, M.; Yang, A.-K. Treatment and prognosis in sinonasal mucosal melanoma: A retrospective analysis of 65 patients from a single cancer center. Head. Neck 2013, 36, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Dauer, E.H.; Lewis, J.E.; Rohlinger, A.L.; Weaver, A.L.; Olsen, K.D. Sinonasal melanoma: A clinicopathologic review of 61 cases. Otolaryngol. Neck Surg. 2008, 138, 347–352. [Google Scholar] [CrossRef]
- Vandenhende, C.; Leroy, X.; Chevalier, D.; Mortuaire, G. Sinonasal mucosal melanoma: Retrospective survival study of 25 patients. J. Laryngol. Otol. 2011, 126, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Liétin, B.; Montalban, A.; Louvrier, C.; Kemeny, J.-L.; Mom, T.; Gilain, L. Sinonasal mucosal melanomas. Eur. Ann. Otorhinolaryngol.-Head Neck Dis. 2010, 127, 70–76. [Google Scholar] [CrossRef]
- Koivunen, P.; Bäck, L.; Pukkila, M.; Laranne, J.; Kinnunen, I.; Grénman, R.; Mäkitie, A.A. Accuracy of the current TNM classification in predicting survival in patients with sinonasal mucosal melanoma. Laryngoscope 2012, 122, 1734–1738. [Google Scholar] [CrossRef]
- Manton, T.; Tillman, B.; McHugh, J.; Bellile, E.; McLean, S.; McKean, E. Sinonasal Melanoma: A Single Institutional Analysis and Future Directions. J. Neurol. Surg. Part. B Skull Base 2018, 80, 484–492. [Google Scholar] [CrossRef]
- Ganly, I.; Patel, S.G.; Singh, B.; Kraus, D.H.; Bridger, P.G.; Cantu, G.; Cheesman, A.; De Sa, G.; Donald, P.; Fliss, D.M.; et al. Craniofacial resection for malignant melanoma of the skull base: Report of an international collaborative study. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 73–78. [Google Scholar] [CrossRef]
- Lechner, M.; Takahashi, Y.; Turri-Zanoni, M.; Ferrari, M.; Liu, J.; Counsell, N.; Mattavelli, D.; Rampinelli, V.; Vermi, W.; Lombardi, D.; et al. International Multicenter Study of Clinical Outcomes of Sinonasal Melanoma Shows Survival Benefit for Patients Treated with Immune Checkpoint Inhibitors and Potential Improvements to the Current TNM Staging System. J. Neurol. Surg. Part B Skull Base 2022, 84, 307–319. [Google Scholar] [CrossRef]
- Khan, M.N.; Kanumuri, V.V.; Raikundalia, M.D.; Vazquez, A.; Govindaraj, S.; Baredes, S.; Eloy, J.A. Sinonasal melanoma: Survival and prognostic implications based on site of involvement. Int. Forum Allergy Rhinol. 2013, 4, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Stammberger, H.; Nicolai, P.; Castelnuovo, P.; Beal, T.; Beham, A.; Bernal-Sprekelsen, M.; Braun, H.; Cappabianca, P.; Carrau, R.; et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol. Suppl. 2010, 22, 1–143. [Google Scholar]
- Khademi, B.; Soltani, S.; Yousefi, A.; Heidari, P.; Mardani, Z.; Yousufzai, S. Ocular invasion in sinonasal malignant melanoma: A case report and review of literature. Int. J. Surg. Case Rep. 2025, 127, 110904. [Google Scholar] [CrossRef]
- Shraddha, J.; Priya, M.; Sunil, K. Mucosal Malignant Melanoma of Sino-Nasal Region with Orbital Involvement: Case Report. Indian J. Otolaryngol. Head Neck Surg. 2012, 65, 178–181. [Google Scholar] [CrossRef]
- Scheurleer, W.F.J.; Braunius, W.W.; de Ridder, M.; Voormolen, E.H.J.; de Bree, R.; Bleys, R.L.A.W.; Kalmann, R.; Breimer, G.E.; Rijken, J.A. Exploring Potential Orbital Metastatic Pathways in Sinonasal Mucosal Melanoma: A Case Report. Case Rep. Otolaryngol. 2024, 2024, 6637565. [Google Scholar] [CrossRef]
- Fusetti, M.; Eibenstein, A.; Lupi, E.; Iacomino, E.; Pieramici, T.; Fioretti, A. Clinical management of a patient with advanced mucosal malignant melanoma in the sinonasal area. Ear Nose Throat J. 2014, 93, E1–E5. [Google Scholar]
- Prasad, M.L.; Patel, S.G.; Huvos, A.G.; Shah, J.P.; Busam, K.J. Primary mucosal melanoma of the head and neck: A proposal for microstaging localized, Stage I (lymph node-negative) tumors. Cancer 2004, 100, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, W.; Zha, Y.; Wang, X.; Aodeng, S.; Wang, L.; Liu, Y.; Lv, W. Development and validation of a nomogram for predicting overall survival in patients with sinonasal mucosal melanoma. BMC Cancer 2024, 24, 184. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.R.; Wieneke, J.A.; Miettinen, M. Sinonasal Tract and Nasopharyngeal Melanomas: A Clinicopathologic Study of 115 Cases with a Proposed Staging System. Am. J. Surg. Pathol. 2003, 27, 594–611. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Ruiz Cifuentes, M.; Horvatovich, P.; Guryev, V.; Baltás, E.; Diercks, G.F.H.; van Pijkeren, A.; Wegner, I.; Halmos, G.B. The Effect of Tumor Location and Extension on Survival in Patients with Sinonasal Mucosal Melanoma: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 3757. https://doi.org/10.3390/cancers17233757
Yang F, Ruiz Cifuentes M, Horvatovich P, Guryev V, Baltás E, Diercks GFH, van Pijkeren A, Wegner I, Halmos GB. The Effect of Tumor Location and Extension on Survival in Patients with Sinonasal Mucosal Melanoma: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(23):3757. https://doi.org/10.3390/cancers17233757
Chicago/Turabian StyleYang, Fan, Marina Ruiz Cifuentes, Peter Horvatovich, Victor Guryev, Eszter Baltás, Gilles F H Diercks, Alienke van Pijkeren, Inge Wegner, and Gyorgy B Halmos. 2025. "The Effect of Tumor Location and Extension on Survival in Patients with Sinonasal Mucosal Melanoma: A Systematic Review and Meta-Analysis" Cancers 17, no. 23: 3757. https://doi.org/10.3390/cancers17233757
APA StyleYang, F., Ruiz Cifuentes, M., Horvatovich, P., Guryev, V., Baltás, E., Diercks, G. F. H., van Pijkeren, A., Wegner, I., & Halmos, G. B. (2025). The Effect of Tumor Location and Extension on Survival in Patients with Sinonasal Mucosal Melanoma: A Systematic Review and Meta-Analysis. Cancers, 17(23), 3757. https://doi.org/10.3390/cancers17233757








