The Role of Radiotherapy in Octogenarian Cancer Patients
Simple Summary
Abstract
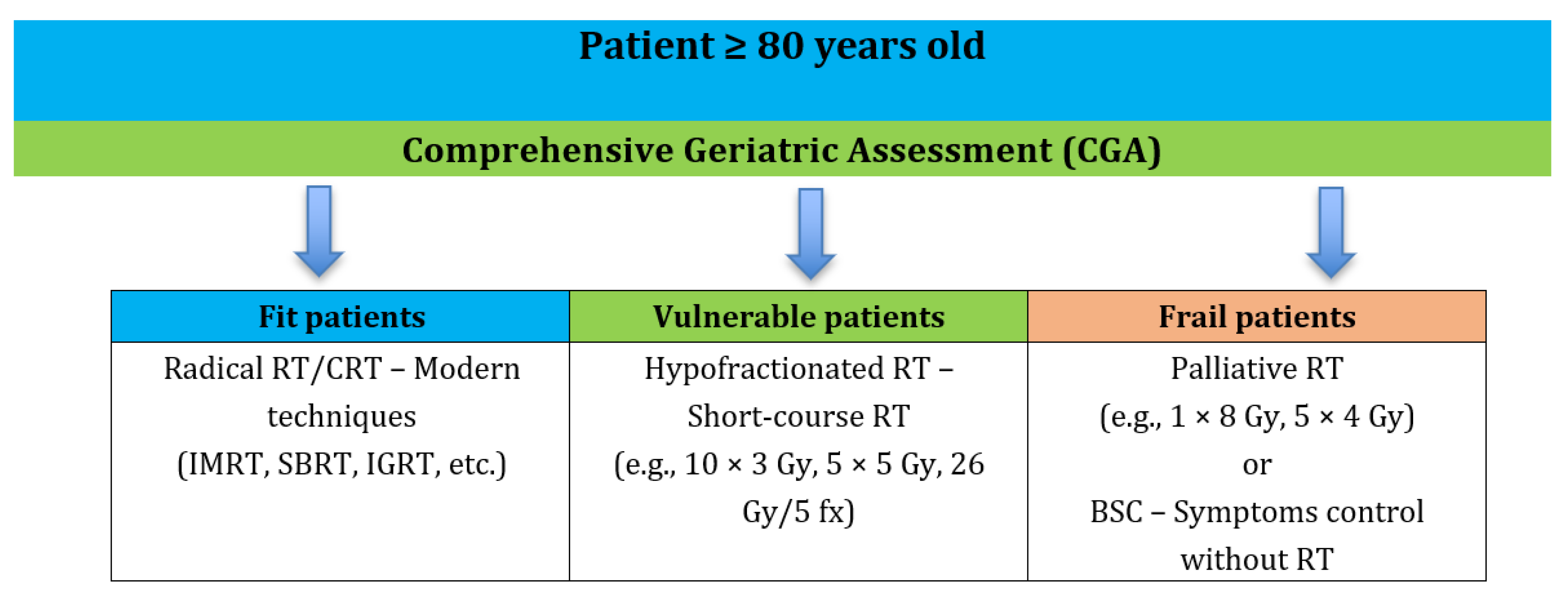
1. Introduction
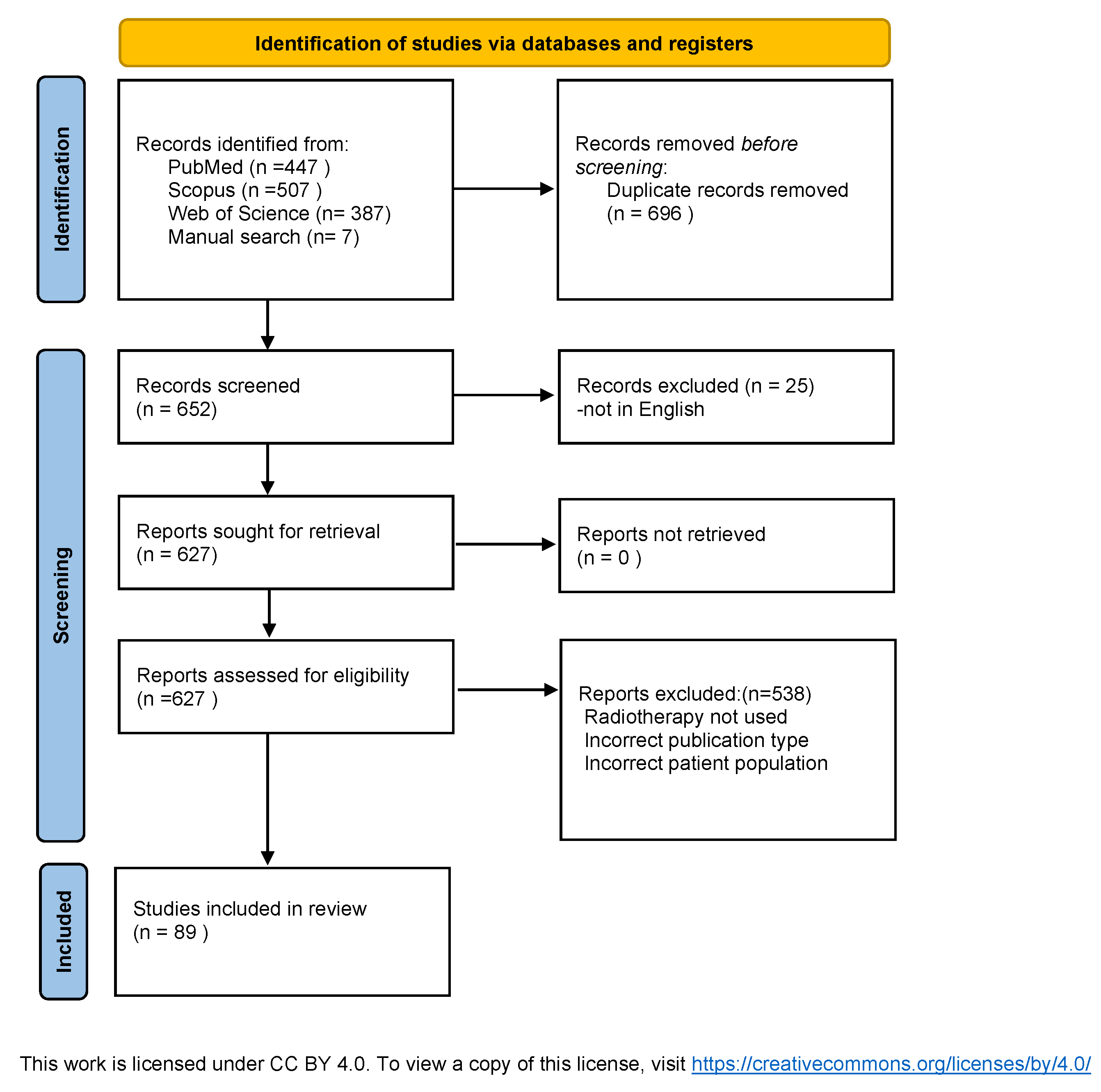
2. Materials and Methods
3. Discussion
3.1. Breast Cancer
| References | Radiotherapy Approach | Outcomes/Tolerability | Guidelines |
|---|---|---|---|
| Fonseca et al. [13] | Meta-analysis of RT in >70 yrs | In >80 yrs RT used in only 3–25%; no geriatric assessment | Need for improved evaluation of older adults |
| Murray Brunt et al. [18] | Post-BCS RT; hypofractionation | Comparable control and toxicity; ≥80 yrs = 3% of cohort | Recommended HF-RT 26 Gy/5 fractions |
| McGale et al. [20] | Post-mastectomy RT (PMRT) | Limited data in >70; 20-year mortality reduction 9.3% with ≥4 nodes | Standard for ≥4 positive nodes |
| Vaidya et al. [22,23] Veronesi et al. [24] | Partial RT (APBI, IORT) vs. WBRT | Higher recurrence with IORT (3.3% vs. 1.3%), lower toxicity | Boost recommended only for high-risk ≥60 yrs |
| Huges et al. [26,27] | RT omission after BCS in >70 yrs | 12-year recurrence 10% without RT vs. 2% with RT; no OS difference | RT may be omitted in ER+ with endocrine therapy |
| Rashmi et al. [28] | Criteria for RT omission after BCS | RT omission possible: ≥70 yrs, HR+, HER2–, pT1, cN0 | NCCN v6.2024 |
| Meattini et al. [29] | RT vs. endocrine therapy | Better QoL after RT; ≥80 yrs = 26–28% | RT as alternative to long-term endocrine therapy |
| Biganzoli et al. [30] | RT after BCS; HF and PBI | RT halves recurrence risk; may be omitted in low-risk | EUSOMA–SIOG 2021 |
3.2. Lung Cancer
| Reference | Radiotherapy Approach | Outcomes/Tolerability | Guidelines/Authors’ Comments |
|---|---|---|---|
| Bei et al. [39] | SBRT (T1–T2b) in ≥80 yrs | 3-yr: OS 65.3%, PFS 58%, RRFS 73.9%, LRFS 85.3% | SBRT effective; incorporate geriatric assessment and biomarkers |
| Pergolizzi et al. [42] | RT monotherapy (median 60 Gy, 2.0 Gy/fx)—stage IIIA, median age 77 | 3-yr OS 18%; mild esophagitis 70%; | Definitive RT monotherapy is a radical option for selected patients |
| Lonardi et al. [44] | RT 50 Gy (1.8–2.0 Gy/fx)—stage IIIA/B (75–85 yrs) | 2-yr OS 10%; total dose considered suboptimal | Consider higher/definitive doses in fit patients |
| Hayashi et al. [45] | CIRT (carbon ions) for locally advanced NSCLC in >80 yrs | All completed therapy; no ≥G4 toxicity; 2-yr LC 83.5%, PFS 46.7%, OS 68% | CIRT safe and effective but with very limited availability |
| He et al. [46] | Proton therapy vs. photon RT in early-stage NSCLC | No significant differences in 1- and 3-yr OS or 3-yr PFS; similar toxicity | Evidence insufficient to claim superiority of proton therapy in early-stage disease |
| Shiono et al. [47] | LD-SCLC: concurrent vs. sequential CRT in ≥75 yrs | No PFS/OS differences; concurrent more toxic | Offer concurrent CRT in fit octogenarians |
| Christodoulou et al. [48] | LD-SCLC: standard hyperfractionation 45 Gy/21 days + platinum chemo | In >80 yrs higher ≥G3 toxicity; small sample | PCI should not be routine in this subgroup |
| Kim et al. [49] | PCI in >70 yrs with tumor >5 cm | No 2-yr OS improvement; not recommended routinely |
3.3. Prostate Cancer
| Reference | Radiotherapy Approach | Outcomes/Tolerability | Guidelines/Authors’ Comments |
|---|---|---|---|
| Nguyen et al. [54] | 3D-RT: pelvis 45 Gy + prostate 69.5 Gy in >80 with comorbidities | 5-year OS 77%. | RT is feasible for selected octogenarians |
| Okonogi et al. [55] | IMRT 78 Gy + ADT; >80 vs. younger in intermediate/high-risk | 3-year OS 92% (>80) vs. 99.4% (<80); small sample limits conclusions | IMRT + ADT feasible in old adults; larger studies needed |
| Le Tuo et al. [56] | RT (median 78 Gy) + ADT in >80; 72.9% high-risk; 27.1% intermediate-risk | 5-year OS 91.4%, 10-year OS 67.2%; acceptable GI/GU toxicity; 97.2% received ADT | Selected >80 with localized PC benefit from RT + ADT |
| Arcangeli et al. [57] | Hypofractionation 62 Gy/20 fx vs. conventional 80 Gy/40 fx (3D conformal) | No differences in GU frequency/severity or late GI toxicity | HF offers shorter treatment with comparable toxicity—valuable in older adults |
| Fransson et al. [59] | Ultra-hypofractionation 42.7 Gy/7 fx vs. conventional 78 Gy/39 fx. | Higher acute toxicity with ultra-HF; QoL similar; >75 yrs not eligible | Use caution extrapolating to >75; evidence in the oldest is limited |
| Syndicus et al. [61] | 74 Gy/37 fx vs. 60 Gy/30 fx vs. 57 Gy/19 fx (moderate HF) | 60 Gy non-inferior to 74 Gy; no late-toxicity differences at 10 yrs | 60 Gy/30 fx recommended in practice, suitable for well-selected older adults |
3.4. Colorectal Cancer
| Reference | Radiotherapy Approach | Outcomes/Tolerability | Guidelines/Authors’ Comments |
|---|---|---|---|
| Pasetto et al. [69] | Preop CRT: 3D RT 50.4 Gy (45 Gy/25 fx + 5.4 Gy/3 fx boost) + 5-FU | In >70 (70–82): chemo stopped in 36%; surgery after 6 weeks in 32/36; median PFS not reached at 37-month follow-up | Use geriatric scales (Charlson 0–2) for selection |
| Margalit et al. [70] | Pre- vs. postoperative CRT in old adults | 92% received planned RT dose; 25% required a break, 11% hospitalized; 33% needed dose-mod/cessation of chemo; only 17% completed as planned. Preop better tolerated than postop | Avoid intensification without aggressive comorbidity management; prefer preop CRT when feasible |
| Socha et al. [71] Beets et al. [72] | Short-course RT 5 × 5 Gy with deferred surgery; “watch & wait” after complete response | Beneficial for unresectable/borderline- resectable and frail/chemo-ineligible; W&W feasible after CR—important in frail older adults | Consider 5 × 5 Gy + delay as organ-sparing strategy in older chemo-ineligible patients |
| Bujko et al. [73] | 5 × 5 Gy with delay surgery also for early-stage cases prior to planned resection when chemo-ineligible | May be used routinely in this population | Standard option for older chemo-ineligible in unresectable and early-stage pre-resection settings |
3.5. Head and Neck Cancer
| Reference | Radiotherapy Approach | Outcomes/Tolerability | Guidelines/Authors’ Comments |
|---|---|---|---|
| Kim et al. [76] | Retrospective analysis (26% >80); comparison: surgery, RT, CRT | >80 preferred RT; >50% discontinued treatment; no OS difference between modalities | Individualized treatment selection necessary |
| Lacas et al. [78] | CRT vs. RT in squamous cell HNC | CRT improved OS in locally advanced disease; >70 yrs—worse outcomes | Avoid CRT in older adults; favor RT alone or less toxic regimens |
| Zachariah et al. [79] | Radical or palliative RT in ≥80 | CR in 65% of HNC; 81% response in palliative | RT is effective and well tolerated in ≥80; age not a contraindication for radical RT |
| Wasil et al. [80] | Radical/palliative RT in >80 | 77% completed RT; 36% required interruptions; overall safe | RT feasible and effective both in radical and palliative settings in older adults |
| Desai et al. [81] | 3D-RT vs. single-modality RT in ≥80 | 3D-RT: OS 23 months; single-modality RT: OS 8.5 months | Advanced techniques (3D-RT) improve OS and are well tolerated in the older adults |
3.6. Glioblastoma
| Reference | Radiotherapy Approach | Outcomes/Tolerability | Guidelines/Authors’ Comments |
|---|---|---|---|
| Perry et al. [86] | HF-RT 40 Gy/15 fx + adjuvant TMZ vs. RT alone in ≥65 with glioma | Better OS for HF-RT + TMZ vs. RT alone | Combine HF-RT + TMZ for fit older adults |
| Roa et al. [88] | Very short RT 25 Gy/5 fx (1 wk) vs. 40 Gy/15 fx (3 wks) in elderly/frail newly diagnosed GBM | Non-inferior: median PFS 4.2 mo both arms; median OS 7.9 mo (25 Gy) vs. 6.4 mo (40 Gy); QoL comparable | 25 Gy/5 fx is a recommended option for older adults/frail |
| Nieder et al. [89] | CNS metastases in ≥80: SRS vs. WBRT; other modalities (surgery, systemic, BSC) | SRS associated with longer survival than WBRT in selected cases; need for octogenarian-focused prospective data | Prefer SRS when feasible; individualize by disease burden and performance status |
| Stadlbauer et al. [90] | WBRT in ≥80 with brain metastases: propose survival score (fitness, lesion number, extracranial disease) | Median OS 2.0 mo; score 7 (poor) → all died ≤2 mo (BSC recommended); score 10 → OS 2 mo, 3-mo OS 25%, 6-mo 13% (also BSC favored); score 13–17 → 6-mo 50%, 12-mo 27%; suggested RT: 5 × 4 Gy/10 × 3 Gy/20 × 2 Gy | Simple clinical score aids WBRT vs. BSC decision and fractionation choice |
| Chen et al. [91] | WBRT vs. SRS in >70 (including >80 = 21%) | Median OS from brain mets diagnosis: 4.3 mo (WBRT) vs. 14.4 mo (SRS); better tolerability with SRS; age >80 not linked to higher toxicity; selection bias likely | Favor SRS when feasible (limited volume/lesions); avoid routine WBRT in toxicity-sensitive |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cancer Tomorrow. Available online: https://gco.iarc.who.int/tomorrow/en/dataviz/tables?cancers=27&age_start=16 (accessed on 23 January 2025).
- Potyra, M.; Góral-Radziszewska, K.; Waśkiewicz, K.; Gawińska-Drużba, E. Statistical Analyses; 2024; ISSN 1507-1340. Available online: www.stat.gov.pl (accessed on 23 January 2025).
- IARC—International Agency for Research on Cancer. Available online: https://www.iarc.who.int/ (accessed on 28 January 2025).
- Pallis, A.G.; Fortpied, C.; Wedding, U.; Van Nes, M.C.; Penninckx, B.; Ring, A.; Lacombe, D.; Monfardini, S.; Scalliet, P.; Wildiers, H. EORTC Elderly Task Force Position Paper: Approach to the Older Cancer Patient. Eur. J. Cancer 2010, 46, 1502–1513. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tesarova, P. Breast Cancer in the Elderly—Should It Be Treated Differently? Rep. Pract. Oncol. Radiother. 2012, 18, 26–33. [Google Scholar] [CrossRef]
- Bertolo, A.; Rosso, C.; Voutsadakis, I.A. Breast Cancer in Patients 80 Years-Old and Older. Eur. J. Breast Health 2020, 16, 208–212. [Google Scholar] [CrossRef]
- Jenkins, E.O.; Deal, A.M.; Anders, C.K.; Prat, A.; Perou, C.M.; Carey, L.A.; Muss, H.B. Age-Specific Changes in Intrinsic Breast Cancer Subtypes: A Focus on Older Women. Oncologist 2014, 19, 1076–1083. [Google Scholar] [CrossRef]
- Anderson, W.F.; Katki, H.A.; Rosenberg, P.S. Incidence of Breast Cancer in the United States: Current and Future Trends. J. Natl. Cancer Inst. 2011, 103, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Tsoutsou, P.G.; Vozenin, M.C.; Durham, A.D.; Bourhis, J. How Could Breast Cancer Molecular Features Contribute to Locoregional Treatment Decision Making? Crit. Rev. Oncol. Hematol. 2017, 110, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Van der Leij, F.; van Werkhoven, E.; Bosma, S.; Linn, S.C.; Rutgers, E.J.; van de Vijver, M.J.; Bartelink, H.; Elkhuizen, P.H.M.; Scholten, A. Low Risk of Recurrence in Elderly Patients Treated with Breast Conserving Therapy in a Single Institute. Breast 2016, 30, 19–25. [Google Scholar] [CrossRef]
- Bastiaannet, E.; Liefers, G.J.; De Craen, A.J.M.; Kuppen, P.J.K.; Van De Water, W.; Portielje, J.E.A.; Van Der Geest, L.G.M.; Janssen-Heijnen, M.L.G.; Dekkers, O.M.; Van De Velde, C.J.H.; et al. Breast Cancer in Elderly Compared to Younger Patients in the Netherlands: Stage at Diagnosis, Treatment and Survival in 127,805 Unselected Patients. Breast Cancer Res. Treat 2010, 124, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.C.; Sidiropoulou, Z. Geriatric Breast Cancer: Staging, Molecular Surrogates, and Treatment. A Review & Meta-Analysis. Aging Dis. 2024, 15, 1602–1618. [Google Scholar]
- Kunkler, I.H.; Audisio, R.; Belkacemi, Y.; Betz, M.; Gore, E.; Hoffe, S.; Kirova, Y.; Koper, P.; Lagrange, J.L.; Markouizou, A.; et al. Review of Current Best Practice and Priorities for Research in Radiation Oncology for Elderly Patients with Cancer: The International Society of Geriatric Oncology (SIOG) Task Force. Ann. Oncol. 2014, 25, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Pignol, J.-P.; Levine, M.N.; Julian, J.A.; MacKenzie, R.; Parpia, S.; Shelley, W.; Grimard, L.; Bowen, J.; Lukka, H.; et al. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. N. Engl. J. Med. 2010, 362, 513–520. [Google Scholar] [CrossRef]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) Trials of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: 10-Year Follow-up Results of Two Randomised Controlled Trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated Breast Radiotherapy for 1 Week Versus 3 Weeks (FAST-Forward): 5-Year Efficacy and Late Normal Tissue Effects Results from a Multicentre, Non-Inferiority, Randomised, Phase 3 Trial. Lancet 2020, 395, 1613. [Google Scholar] [CrossRef]
- Smith, B.D.; Haffty, B.G.; Hurria, A.; Galusha, D.H.; Gross, C.P. Postmastectomy Radiation and Survival in Older Women with Breast Cancer. J. Clin. Oncol. 2006, 24, 4901–4907. [Google Scholar] [CrossRef] [PubMed]
- McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; Whelan, T.; et al. Effect of Radiotherapy after Mastectomy and Axillary Surgery on 10-Year Recurrence and 20-Year Breast Cancer Mortality: Meta-Analysis of Individual Patient Data for 8135 Women in 22 Randomised Trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef]
- Kunkler, I.H.; Canney, P.; van Tienhoven, G.; Russell, N.S. Elucidating the Role of Chest Wall Irradiation in “intermediate-Risk” Breast Cancer: The MRC/EORTC SUPREMO Trial. Clin. Oncol. 2008, 20, 31–34. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Joseph, D.J.; Tobias, J.S.; Bulsara, M.; Wenz, F.; Saunders, C.; Alvarado, M.; Flyger, H.L.; Massarut, S.; Eiermann, W.; et al. Targeted Intraoperative Radiotherapy versus Whole Breast Radiotherapy for Breast Cancer (TARGIT-A Trial): An International, Prospective, Randomised, Non-Inferiority Phase 3 Trial. Lancet 2010, 376, 91–102. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Wenz, F.; Bulsara, M.; Tobias, J.S.; Joseph, D.J.; Keshtgar, M.; Flyger, H.L.; Massarut, S.; Alvarado, M.; Saunders, C.; et al. Risk-Adapted Targeted Intraoperative Radiotherapy Versus Whole-Breast Radiotherapy for Breast Cancer: 5-Year Results for Local Control and Overall Survival from the TARGIT-A Randomised Trial. Lancet 2014, 383, 603–613. [Google Scholar] [CrossRef]
- Veronesi, U.; Orecchia, R.; Maisonneuve, P.; Viale, G.; Rotmensz, N.; Sangalli, C.; Luini, A.; Veronesi, P.; Galimberti, V.; Zurrida, S.; et al. Intraoperative Radiotherapy versus External Radiotherapy for Early Breast Cancer (ELIOT): A Randomised Controlled Equivalence Trial. Lancet Oncol. 2013, 14, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, H.; Maingon, P.; Poortmans, P.; Weltens, C.; Fourquet, A.; Jager, J.; Schinagl, D.; Oei, B.; Rodenhuis, C.; Horiot, J.C.; et al. Whole-Breast Irradiation With or Without a Boost for Patients Treated with Breast-Conserving Surgery for Early Breast Cancer: 20-Year Follow-up of a Randomised Phase 3 Trial. Lancet Oncol. 2015, 16, 47–56. [Google Scholar] [CrossRef]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy plus Tamoxifen With or Without Irradiation in Women Age 70 Years or Older with Early Breast Cancer: Long-Term Follow-up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.S.; Schnaper, L.A.; Berry, D.; Cirrincione, C.; McCormick, B.; Shank, B.; Wheeler, J.; Champion, L.A.; Smith, T.J.; Smith, B.L.; et al. Lumpectomy plus Tamoxifen with or without Irradiation in Women 70 Years of Age or Older with Early Breast Cancer. N. Engl. J. Med. 2004, 351, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Rashmi Kumar, N.; Schonfeld, R.; Gradishar, W.J.; Lurie, R.H.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; et al. NCCN Guidelines Version 6.2024 Breast Cancer; NCCN: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Meattini, I.; De Santis, M.C.; Visani, L.; Scorsetti, M.; Fozza, A.; Meduri, B.; De Rose, F.; Bonzano, E.; Prisco, A.; Masiello, V.; et al. Single-Modality Endocrine Therapy versus Radiotherapy after Breast-Conserving Surgery in Women Aged 70 Years and Older with Luminal A-like Early Breast Cancer (EUROPA): A Preplanned Interim Analysis of a Phase 3, Non-Inferiority, Randomised Trial. Lancet Oncol. 2025, 26, 37–50. [Google Scholar] [CrossRef]
- Biganzoli, L.; Battisti, N.M.L.; Wildiers, H.; McCartney, A.; Colloca, G.; Kunkler, I.H.; Cardoso, M.J.; Cheung, K.L.; de Glas, N.A.; Trimboli, R.M.; et al. Updated Recommendations Regarding the Management of Older Patients with Breast Cancer: A Joint Paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021, 22, e327–e340. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Deng, H.Y.; Xu, K.; Lin, M.Y.; Li, P.; Yuan, C.; Zhou, Q. Initial Treatment of Early-Stage Small-Sized Non-Small Cell Lung Cancer for Octogenarians: A Population-Based Study. Updates Surg. 2022, 74, 1461–1470. [Google Scholar] [CrossRef]
- Li, S.; Ge, Y.; Ma, R.; Wang, J.; Ma, T.; Sun, T.; Feng, S.; Zhang, C.; Zhang, H. Comparison of Wedge Resection and Anatomical Lung Resection in Elderly Patients with Early-Stage Nonsmall Cell Lung Cancer With Visceral Pleural Invasion: A Population-Based Study. Thorac. Cancer 2025, 16, 15532. [Google Scholar] [CrossRef]
- Ni, L.; Lin, G.; Zhang, Z.; Sun, D.; Liu, Z.; Liu, X. Surgery versus Radiotherapy in Octogenarians with Stage Ia Non-small Cell Lung Cancer: Propensity Score Matching Analysis of the SEER Database. BMC Pulm. Med. 2022, 22, 411. [Google Scholar] [CrossRef]
- Robinson, C.G.; Dewees, T.A.; El Naqa, I.M.; Creach, K.M.; Olsen, J.R.; Crabtree, T.D.; Meyers, B.F.; Puri, V.; Bell, J.M.; Parikh, P.J.; et al. Patterns of Failure after Stereotactic Body Radiation Therapy or Lobar Resection for Clinical Stage I Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 192–201. [Google Scholar] [CrossRef]
- Stokes, W.A.; Bronsert, M.R.; Meguid, R.A.; Blum, M.G.; Jones, B.L.; Koshy, M.; Sher, D.J.; Louie, A.V.; Palma, D.A.; Senan, S.; et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 642–651. [Google Scholar] [CrossRef]
- Franks, K.N.; McParland, L.; Webster, J.; Baldwin, D.R.; Sebag-Montefiore, D.; Evison, M.; Booton, R.; Faivre-Finn, C.; Naidu, B.; Ferguson, J.; et al. SABRTooth: A Randomised Controlled Feasibility Study of Stereotactic Ablative Radiotherapy (SABR) with Surgery in Patients with Peripheral Stage I Nonsmall Cell Lung Cancer Considered to Be at Higher Risk of Complications from Surgical Resection. Eur. Respir. J. 2020, 56, 2000118. [Google Scholar] [CrossRef]
- Cassidy, R.J.; Patel, P.R.; Zhang, X.; Press, R.H.; Switchenko, J.M.; Pillai, R.N.; Owonikoko, T.K.; Ramalingam, S.S.; Fernandez, F.G.; Force, S.D.; et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer in Patients 80 Years and Older: A Multi-Center Analysis. Clin. Lung Cancer 2017, 18, 551–558.e6. [Google Scholar] [CrossRef]
- Bei, Y.; Murakami, N.; Nakayama, Y.; Kae, O.; Kashihara, T.; Raturi, V.P.; Okamoto, H.; Takahashi, K.; Inaba, K.; Igaki, H.; et al. Stereotactic Body Radiation Therapy for Early-Stage Non–Small-Cell Lung Cancer in Octogenarians and Older: An Alternative Treatment. J. Radiat. Res. 2020, 61, 586. [Google Scholar] [CrossRef]
- Movsas, B.; Scott, C.; Sause, W.; Byhardt, R.; Komaki, R.; Cox, J.; Johnson, D.; Lawton, C.; Dar, A.R.; Wasserman, T.; et al. The Benefit of Treatment Intensification Is Age and Histology-Dependent in Patients with Locally Advanced Non-Small Cell Lung Cancer (NSCLC): A Quality-Adjusted Survival Analysis of Radiation Therapy Oncology Group (RTOG) Chemoradiation Studies. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 1143–1149. [Google Scholar] [CrossRef]
- Werner-Wasik, M.; Scott, C.; Cox, J.D.; Sause, W.T.; Byhardt, R.W.; Asbell, S.; Russell, A.; Komaki, R.; Lee, J.S. Recursive Partitioning Analysis of 1999 Radiation Therapy Oncology Group (RTOG) Patients with Locally-Advanced Non-Small-Cell Lung Cancer (LA-NSCLC): Identification of Five Groups with Different Survival. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1475–1482. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Santacaterina, A.; De Renzis, C.; Settineri, N.; Gaeta, M.; Frosina, P.; Russi, E.G.; Altavilla, G. Older People with Non Small Cell Lung Cancer in Clinical Stage IIIA and Co-Morbid Conditions: Is Curative Irradiation Feasible? Final Results of a Prospective Study. Lung Cancer 2002, 37, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Geinitz, H.; Zimmermann, F.B.; Molls, M. Radiotherapy of the Elderly Patient. Radiotherapy Tolerance and Results in Older Patients. Strahlenther. Onkol. 1999, 175, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lonardi, F.; Coeli, M.; Pavanato, G.; Adami, F.; Gioga, G.; Campostrini, F. Radiotherapy for Non-Small Cell Lung Cancer in Patients Aged 75 and over: Safety, Effectiveness and Possible Impact on Survival. Lung Cancer 2000, 28, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamamoto, N.; Nakajima, M.; Nomoto, A.; Ishikawa, H.; Ogawa, K.; Tsuji, H. Carbon-Ion Radiotherapy for Octogenarians with Locally Advanced Non-Small-Cell Lung Cancer. Jpn. J. Radiol. 2021, 39, 703. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Zhang, X.; Li, B.; Yang, L.; Wang, H.; Wang, S.; Yu, J.; Wang, L. Comparison of Proton Therapy and Photon Therapy for Early-Stage Non-Small Cell Lung Cancer: A Meta-Analysis. Biomark. Res. 2024, 12, 90. [Google Scholar] [CrossRef]
- Shiono, A.; Imai, H.; Endo, S.; Katayama, K.; Sato, H.; Hashimoto, K.; Miura, Y.; Okazaki, S.; Abe, T.; Mouri, A.; et al. A Retrospective Evaluation of Therapeutic Efficacy and Safety of Chemoradiotherapy in Older Patients (Aged ≥75 Years) with Limited-Disease Small Cell Lung Cancer: Insights from Two Institutions and Review of the Literature. Radiol. Oncol. 2024, 58, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.; Blackhall, F.; Mistry, H.; Leylek, A.; Knegjens, J.; Remouchamps, V.; Martel-Lafay, I.; Farré, N.; Zwitter, M.; Lerouge, D.; et al. Compliance and Outcome of Elderly Patients Treated in the Concurrent Once-Daily Versus Twice-Daily Radiotherapy (CONVERT) Trial. J. Thorac. Oncol. 2019, 14, 63–71. [Google Scholar] [CrossRef]
- Kim, T.G.; Pyo, H.; Ahn, Y.C.; Noh, J.M.; Oh, D. Role of Prophylactic Cranial Irradiation for Elderly Patients with Limited-Disease Small-Cell Lung Cancer: Inverse Probability of Treatment Weighting Using Propensity Score. J. Radiat. Res. 2019, 60, 630–638. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer—2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Droz, J.P.; Albrand, G.; Gillessen, S.; Hughes, S.; Mottet, N.; Oudard, S.; Payne, H.; Puts, M.; Zulian, G.; Balducci, L.; et al. Management of Prostate Cancer in Elderly Patients: Recommendations of a Task Force of the International Society of Geriatric Oncology. Eur. Urol. 2017, 72, 521–531. [Google Scholar] [CrossRef]
- Predict Prostate. Available online: https://prostate.predict.cam/ (accessed on 24 January 2025).
- Daskivich, T.J.; Chamie, K.; Kwan, L.; Labo, J.; Palvolgyi, R.; Dash, A.; Greenfield, S.; Litwin, M.S. Overtreatment of Men with Low-Risk Prostate Cancer and Significant Comorbidity. Cancer 2011, 117, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Azria, D.; Brochon, D.; Poortmans, P.; Miller, R.C.; Scandolaro, L.; Majewski, W.; Krengli, M.; Abacioglu, U.; Moretti, L.; et al. Curative External Beam Radiotherapy in Patients over 80 Years of Age with Localized Prostate Cancer: A Retrospective Rare Cancer Network Study. Crit. Rev. Oncol. Hematol. 2010, 74, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, N.; Katoh, H.; Kawamura, H.; Tamaki, T.; Kaminuma, T.; Murata, K.; Ohkubo, Y.; Takakusagi, Y.; Onishi, M.; Sekihara, T.; et al. Clinical Outcomes of Helical Tomotherapy for Super-Elderly Patients with Localized and Locally Advanced Prostate Cancer: Comparison with Patients under 80 Years of Age. J. Radiat. Res. 2015, 56, 889–896. [Google Scholar] [CrossRef]
- Le, T.; Armstrong, S.; Shakespeare, T.P. Outcomes of Dose-Escalated IMRT and ADT in Octogenarians with Prostate Cancer. J. Med. Imaging Radiat. Oncol. 2023, 67, 539–545. [Google Scholar] [CrossRef]
- Arcangeli, G.; Fowler, J.; Gomellini, S.; Arcangeli, S.; Saracino, B.; Petrongari, M.G.; Benassi, M.; Strigari, L. Acute and Late Toxicity in a Randomized Trial of Conventional versus Hypofractionated Three-Dimensional Conformal Radiotherapy for Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1013–1021. [Google Scholar] [CrossRef]
- Qureshy, S.A.; Diven, M.A.; Ma, X.; Marciscano, A.E.; Hu, J.C.; McClure, T.D.; Barbieri, C.; Nagar, H. Differential Use of Radiotherapy Fractionation Regimens in Prostate Cancer. JAMA Netw. Open 2023, 6, E2337165. [Google Scholar] [CrossRef]
- Fransson, P.; Nilsson, P.; Gunnlaugsson, A.; Beckman, L.; Tavelin, B.; Norman, D.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; et al. Ultra-Hypofractionated Versus Conventionally Fractionated Radiotherapy for Prostate Cancer (HYPO-RT-PC): Patient-Reported Quality-of-Life Outcomes of a Randomised, Controlled, Non-Inferiority, Phase 3 Trial. Lancet Oncol. 2021, 22, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Syndikus, I.; Griffin, C.; Philipps, L.; Tree, A.; Khoo, V.; Birtle, A.J.; Choudhury, A.; Ferguson, C.; O’Sullivan, J.M.; Panades, M.; et al. 10-Year Efficacy and Co-Morbidity Outcomes of a Phase III Randomised Trial of Conventional vs. Hypofractionated High Dose Intensity Modulated Radiotherapy for Prostate Cancer (CHHiP; CRUK/06/016). J. Clin. Oncol. 2023, 41, 304. [Google Scholar] [CrossRef]
- Morton, G.; Vigneault, E.; Barkati, M.; Helou, J.; Niazi, T.; Robinson, J.; Loblaw, D.; Tseng, C.; Chung, H.; Delouya, G.; et al. A Randomized Phase II Trial of High Dose-Rate (HDR) and Low Dose-Rate (LDR) Brachytherapy as Monotherapy in Localized Prostate Cancer: Analysis of Initial Arms of Canadian Cancer Trials Group PR19 (NCT02960087). Radiother. Oncol. 2025, 212, 111124. [Google Scholar] [CrossRef]
- Li, B.; Kirshenbaum, E.J.; Blackwell, R.H.; Gange, W.S.; Saluk, J.; Zapf, M.A.C.; Kothari, A.N.; Flanigan, R.C.; Gupta, G.N. Thirty-Day Hospital Revisits After Prostate Brachytherapy: Who Is at Risk? Prostate Int. 2019, 7, 68–72. [Google Scholar] [CrossRef]
- Yamazaki, H.; Masui, K.; Suzuki, G.; Shimizu, D.; Aibe, N.; Yamada, K.; Fujihara, A.; Shiraishi, T.; Okihara, K.; Ukimura, O.; et al. Radiotherapy for Elder Patients Aged ≥80 with Clinically Localized Prostate Cancer—Brachytherapy Enhanced Late GU Toxicity Especially in Elderly. Clin. Transl. Radiat. Oncol. 2020, 25, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kotake, K.; Asano, M.; Ozawa, H.; Kobayashi, H.; Sugihara, K. Tumour Characteristics, Treatment Patterns and Survival of Patients Aged 80 Years or Older with Colorectal Cancer. Colorectal Dis. 2015, 17, 205–215. [Google Scholar] [CrossRef]
- Kunitake, H.; Zingmond, D.S.; Ryoo, J.; Ko, C.Y. Caring for Octogenarian and Nonagenarian Patients with Colorectal Cancer: What Should Our Standards and Expectations Be? Dis. Colon Rectum. 2010, 53, 735–743. [Google Scholar] [CrossRef]
- Goldvaser, H.; Shroitman, N.K.; Ben-Aharon, I.; Purim, O.; Kundel, Y.; Shepshelovich, D.; Shochat, T.; Sulkes, A.; Brenner, B. Octogenarian Patients with Colorectal Cancer: Characterizing an Emerging Clinical Entity. World J. Gastroenterol. 2017, 23, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yu, W.; Ma, Y.; Xu, P.; Yao, Q.; Sun, Q.; Ren, J.; Wang, D. Evaluation of the Safety and Efficacy of Perform Enterectomy in Colorectal Cancer Patients Aged 80 or Older. A Meta-Analysis and a Systematic Review. Int. J. Colorectal Dis. 2023, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Claassen, Y.H.M.; Vermeer, N.C.A.; Iversen, L.H.; van Eycken, E.; Guren, M.G.; Mroczkowski, P.; Martling, A.; Cazador, A.C.; Johansson, R.; Vandendael, T.; et al. Treatment and Survival of Rectal Cancer Patients over the Age of 80 Years: A EURECCA International Comparison. Br. J. Cancer 2018, 119, 517–522. [Google Scholar] [CrossRef]
- Pasetto, L.M.; Friso, M.L.; Pucciarelli, S.; Basso, U.; Falci, C.; Bortolami, A.; Toppan, P.; Agostini, M.; Rugge, M.; Serpentini, S.; et al. Rectal Cancer Neoadjuvant Treatment in Elderly Patients. Anticancer Res. 2006, 26, 3913–3923. [Google Scholar]
- Margalit, D.N.; Mamon, H.J.; Ancukiewicz, M.; Kobayashi, W.; Ryan, D.P.; Blaszkowsky, L.S.; Clark, J.; Willett, C.G.; Hong, T.S. Tolerability of Combined Modality Therapy for Rectal Cancer in Elderly Patients Aged 75 Years and Older. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e735–e741. [Google Scholar] [CrossRef]
- Socha, J.; Bujko, K. A Watch-and-Wait Strategy or Local Excision in Complete Clinical Responders after Radiation for Early-Stage Rectal Cancer. Lancet Healthy Longev. 2022, 3, e807–e808. [Google Scholar] [CrossRef]
- Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Van De Velde, C.J.H. A New Paradigm for Rectal Cancer: Organ Preservation: Introducing the International Watch & Wait Database (IWWD). Eur. J. Surg. Oncol. 2015, 41, 1562–1564. [Google Scholar] [CrossRef]
- Bujko, K.; Partycki, M.; Pietrzak, L. Neoadjuvant Radiotherapy (5 × 5 Gy): Immediate versus Delayed Surgery. Recent Results Cancer Res. 2014, 203, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Wyrwicz, L.; Rutkowski, A.; Malinowska, M.; Pietrzak, L.; Kryński, J.; Michalski, W.; Oledzki, J.; Kuśnierz, J.; Zajac, L.; et al. Long-Course Oxaliplatin-Based Preoperative Chemoradiation versus 5 × 5 Gy and Consolidation Chemotherapy for CT4 or Fixed CT3 Rectal Cancer: Results of a Randomized Phase III Study. Ann. Oncol. 2016, 27, 834–842. [Google Scholar] [CrossRef]
- Ciseł, B.; Pietrzak, L.; Michalski, W.; Wyrwicz, L.; Rutkowski, A.; Kosakowska, E.; Cencelewicz, A.; Spałek, M.; Polkowski, W.; Jankiewicz, M.; et al. Long-Course Preoperative Chemoradiation versus 5 × 5 Gy and Consolidation Chemotherapy for Clinical T4 and Fixed Clinical T3 Rectal Cancer: Long-Term Results of the Randomized Polish II Study. Ann. Oncol. 2019, 30, 1298–1303. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.D.; Shim, Y.J.; Lee, S.Y.; Sung, M.W.; Kim, K.H.; Hah, J.H. Is There Any Age Cutoff to Treat Elderly Patients with Head and Neck Cancer? Comparing with Septuagenarians and Octogenarians. J. Korean Med. Sci. 2016, 31, 1300–1306. [Google Scholar] [CrossRef]
- VanderWalde, N.A.; Fleming, M.; Weiss, J.; Chera, B.S. Treatment of Older Patients with Head and Neck Cancer: A Review. Oncologist 2013, 18, 568–578. [Google Scholar] [CrossRef]
- Lacas, B.; Carmel, A.; Landais, C.; Wong, S.J.; Licitra, L.; Tobias, J.S.; Burtness, B.; Ghi, M.G.; Cohen, E.E.W.; Grau, C.; et al. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update on 107 Randomized Trials and 19,805 Patients, on Behalf of MACH-NC Group. Radiother. Oncol. 2021, 156, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, B.; Balducci, L.; Venkattaramanabalaji, G.V.; Casey, L.; Greenberg, H.M.; DelRegato, J.A. Radiotherapy for Cancer Patients Aged 80 and Older: A Study of Effectiveness and Side Effects. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 1125–1129. [Google Scholar] [CrossRef]
- Wasil, T.; Lichtman, S.M.; Gupta, V.; Rush, S. Radiation Therapy in Cancer Patients 80 Years of Age and Older. Am. J. Clin. Oncol. 2000, 23, 526–530. [Google Scholar] [CrossRef]
- Desai, P.A.; Saksena, A.; Miller, F.R.; Karnad, A.B.; Cervantez, S.R. Head and Neck Cancer in Octogenarians: A Single Center Experience. J. Clin. Oncol. 2018, 36, e18044. [Google Scholar] [CrossRef]
- Abdullah, K.G.; Ramayya, A.; Thawani, J.P.; Macyszyn, L.; Martinez-Lage, M.; O’Rourke, D.M.; Brem, S. Factors Associated with Increased Survival after Surgical Resection of Glioblastoma in Octogenarians. PLoS ONE 2015, 10, e0127202. [Google Scholar] [CrossRef] [PubMed]
- Malmström, A.; Oppong, F.B.; O’Callaghan, C.J.; Wick, W.; Laperriere, N.; Gorlia, T.; Weller, M.; Henriksson, R.; Mason, W.; Platten, M.; et al. Prognostic Factors for Overall Survival in Elderly Patients with Glioblastoma: Analysis of the Pooled NOA-08 and Nordic Trials with the CCTG-EORTC (CE.6) Trial. Neurooncol. Adv. 2024, 6, vdae211. [Google Scholar] [CrossRef]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus Standard 6-Week Radiotherapy versus Hypofractionated Radiotherapy in Patients Older than 60 Years with Glioblastoma: The Nordic Randomised, Phase 3 Trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Platten, M.; Meisner, C.; Felsberg, J.; Tabatabai, G.; Simon, M.; Nikkhah, G.; Papsdorf, K.; Steinbach, J.P.; Sabel, M.; et al. Temozolomide Chemotherapy Alone versus Radiotherapy Alone for Malignant Astrocytoma in the Elderly: The NOA-08 Randomised, Phase 3 Trial. Lancet Oncol. 2012, 13, 707–715. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Roa, W.; Brasher, P.M.A.; Bauman, G.; Anthes, M.; Bruera, E.; Chan, A.; Fisher, B.; Fulton, D.; Gulavita, S.; Hao, C.; et al. Abbreviated Course of Radiation Therapy in Older Patients with Glioblastoma Multiforme: A Prospective Randomized Clinical Trial. J. Clin. Oncol. 2004, 22, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Roa, W.; Kepka, L.; Kumar, N.; Sinaika, V.; Matiello, J.; Lomidze, D.; Hentati, D.; De Castro, D.G.; Dyttus-Cebulok, K.; Drodge, S.; et al. International Atomic Energy Agency Randomized Phase III Study of Radiation Therapy in Elderly and/or Frail Patients with Newly Diagnosed Glioblastoma Multiforme. J. Clin. Oncol. 2015, 33, 4145–4150. [Google Scholar] [CrossRef]
- Nieder, C.; Andratschke, N.H.; Grosu, A.L. How We Treat Octogenarians with Brain Metastases. Front. Oncol. 2023, 13, 1213122. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Rades, D.; Delikanli, C.; Schild, S.E.; Kristiansen, C.; Tvilsted, S.; Janssen, S. The First Survival Score for Patients Aged ≥80 Years Irradiated for Brain Metastases. Biology 2022, 11, 1434. [Google Scholar] [CrossRef]
- Chen, L.; Shen, C.; Redmond, K.J.; Page, B.R.; Kummerlowe, M.; Mcnutt, T.; Bettegowda, C.; Rigamonti, D.; Lim, M.; Kleinberg, L. Use of Stereotactic Radiosurgery in Elderly and Very Elderly Patients with Brain Metastases to Limit Toxicity Associated with Whole Brain Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Ocanto, A.; Cantero, R.; Morera, R.; Ramírez, R.; Rodríguez, I.; Castillo, K.; Couñago, F.; Samper, P. Results of Radical Treatment of Locally Advanced Rectal Cancer in Geriatric and Non-Geriatric Patients. Rep. Pract. Oncol. Radiother. 2025, 30, 54–61. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebiedzińska, A.; Wasilewska-Teśluk, E.; Sopel, A.; Nawrocki, S. The Role of Radiotherapy in Octogenarian Cancer Patients. Cancers 2025, 17, 3758. https://doi.org/10.3390/cancers17233758
Lebiedzińska A, Wasilewska-Teśluk E, Sopel A, Nawrocki S. The Role of Radiotherapy in Octogenarian Cancer Patients. Cancers. 2025; 17(23):3758. https://doi.org/10.3390/cancers17233758
Chicago/Turabian StyleLebiedzińska, Aneta, Ewa Wasilewska-Teśluk, Agnieszka Sopel, and Sergiusz Nawrocki. 2025. "The Role of Radiotherapy in Octogenarian Cancer Patients" Cancers 17, no. 23: 3758. https://doi.org/10.3390/cancers17233758
APA StyleLebiedzińska, A., Wasilewska-Teśluk, E., Sopel, A., & Nawrocki, S. (2025). The Role of Radiotherapy in Octogenarian Cancer Patients. Cancers, 17(23), 3758. https://doi.org/10.3390/cancers17233758





