Emerging Therapeutic Approaches to Engage the Androgen Receptor for the Treatment of Castration-Resistant Prostate Cancer
Simple Summary
Abstract
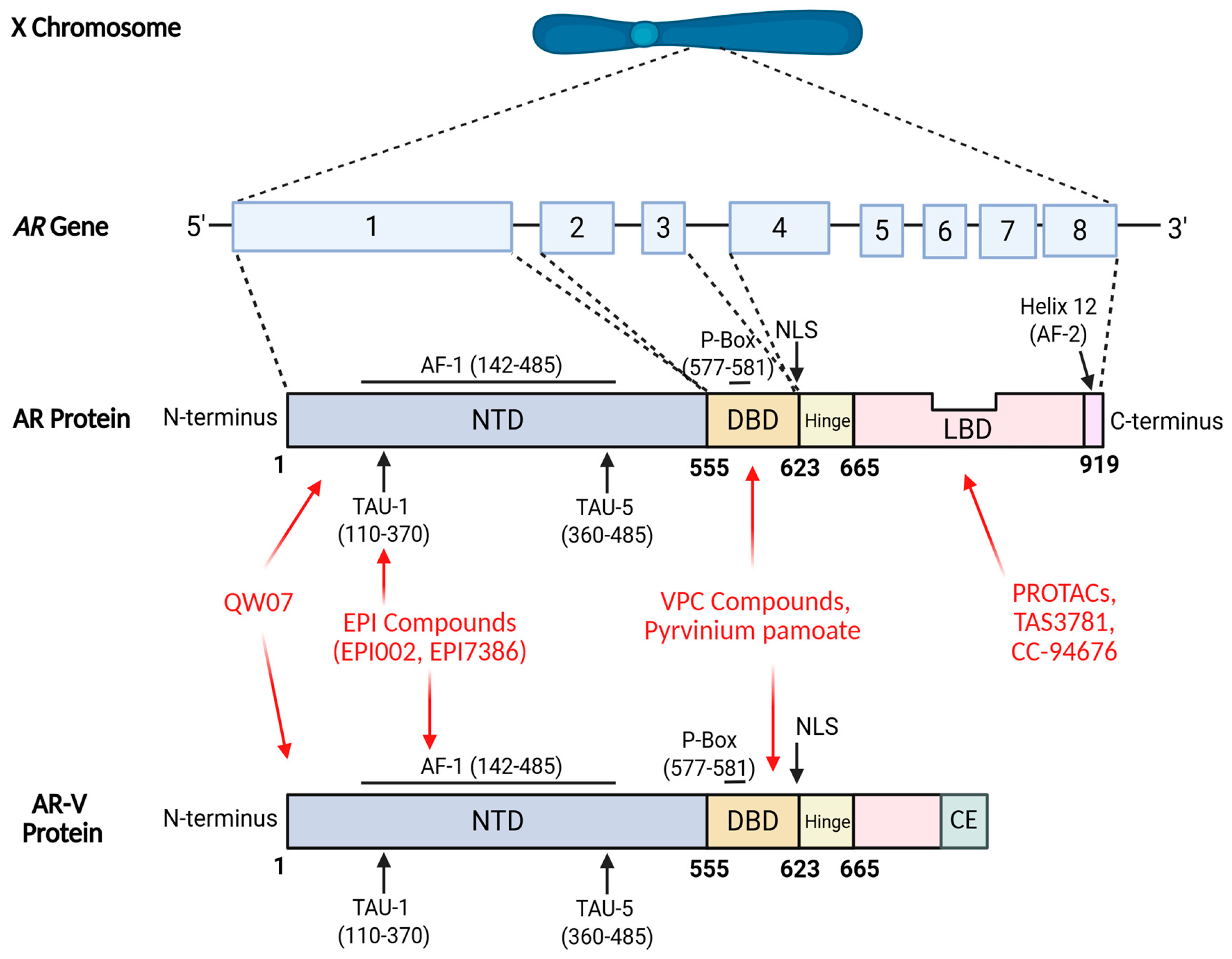
1. Introduction
| Drug | Mechanism of Action | AR Domain Targeted | Effective Mutation/ Variant Coverage | Trial Phase | PSA Response Rate | IC50 Values | Key Limitations |
|---|---|---|---|---|---|---|---|
| EPI Compounds | Bind to Tau-5, preventing interactions between the AF-1 region, CREB-binding protein, and RAP74 [20] | NTD [20] | AR-FL and AR-Vs [21] | Phase I/II (EPI-7386) [22] | 88% of patients achieved a ≥50% decline in PSA when this drug was combined with enzalutamide (EPI-7386) [22] | EPI-7386 has IC50 of 421 nM in LNCaP cell line [23] | Poor pharmacokinetics for EPI-002 [24] and IC50 is too high for EPI-001 [25] |
| QW07 | Prevents interactions between the AR and CREB-binding protein thus inhibiting AR transcriptional activity [26] | NTD [27, 28] | AR-FL and AR-Vs [26] | Pre-clinical [26] | N/A | IC50 of 7.54 µM in 22Rv1 [26] | Lack of clinical data [27] |
| VPC compounds (VPC-17160, VPC-17281 and VPC-14449) | Prevents AR from interacting with chromatin, thereby reducing AR transcriptional activity [29] | DBD [29] | L702H, W742C and W742L at high concentrations [29] | Assumed pre-clinical as no clinical trials have been reported as of yet | Significant decrease in PSA levels [30] | IC50 for VPC-17160 is 2 µM and for VPC-17281 the IC50 value is 6 µM in LNCaP cell lines [30] | VPC-17160 has a short half-life [30] VPC-17281 shown to target AR-null cells [30] VPC-14449 has low efficiency when targeting AR-Vs [29] |
| Pyrvinium pamoate | Non-competitive AR inhibitor [31] | DBD [31] | AR-FL and AR-Vs [31] | Pre-clinical [32] | N/A | IC50 of ~8–30 nM in CWR22Rv1, LNCaP, LNCaP-C4-2, and LAPC4 [31] | Off target effects as targets the DBD (highly conserved across multiple nuclear receptors) [31] |
| PROTACs (ARV-110 and ARV-766) | Degradation of AR via the ubiquitin–proteasome degradation pathway [33] | Mostly LBD [33] | T878, H875 and L702H [34] | Phase I/II [35] | ≥50% PSA declines in 50% of participants [35] | IC50 not publicly available | Many PROTACs are not effective against AR-Vs [36] |
| TAS3681 | AR-LBD antagonist [37] | LBD [37] | F877L/T878, H875Y/T878A and AR-V7 [37] | Phase I [38] | PSA declined over 50% from baseline in a subset of patients [38] | IC50 was 18 nM for LNCaP cell line [37] | Potential short half-life [37] |
| CC-94676 | Heterobifunctional cereblon-mediated ligand-directed degrader [39] | LBD [39] | L702H and H875Y [40] | Phase I/ Phase III [41] | PSA reductions greater than 30% were observed in 34% of patients across all dose levels [42] | IC50 values not publicly available | Ineffective against AR-Vs and less effective in patients who have received chemotherapy [40] |
| RIPTACs (H001, H003 and HLD-0915) | Forms a ternary complex with AR and effector proteins (EP) thereby inhibiting EP function [43, 44] | Undisclosed | AR-FL and AR-Vs [44, 45] | Phase I/II [46] | H001 and H003 significantly reduced PSA levels [43] HLD-0915 showed reduced PSA levels [45] | IC50 not publicly available | Large molecular weight, strict design requirements [43] |
| Asc-J9 | Promotes AR degradation [47] | N/A | AR-FL and AR-V7 [48] | Assumed pre-clinical as no clinical trials have been reported as of yet | Reduced PSA levels [49] | IC50 not publicly available | Short half-life, low oral bioavailability, and limited aqueous solubility [48, 50] |
| ZEN-3694 | BETi, ultimately inhibiting AR transcriptional activation [51] | N/A | AR-FL and AR-V7 [52] | Phase II [51] | 8% of patients achieved a ≥50% decrease in PSA levels from baseline [51] | IC50 not publicly available | Dose dependent toxicities [51] |
| Hairpin pyrrole–imidazole polyamides | Binds minor groove of DNA, thereby inhibiting AR transcriptional activity [53, 54] | N/A | AR-FL and AR-V7 [53, 54] | Assumed pre-clinical as no clinical trials have been reported as of yet | N/A | IC50 not publicly available | May have off-target effects due to also inhibiting GR activity [55] |
| Niclosamide/ PDMX1001 | Multiple suggested: Suppresses the FOXM1-mediated DNA damage response, inhibition of AR-V7, mitochondrial uncoupler and inhibiting mTORC1, STAT3, and Wnt/β-catenin pathways [56, 57] | N/A | AR-V7 and AR-FL [57] | Phase II [57] | 5/9 patients achieved ≥50% PSA reductions when combined with abiraterone/prednisone [58] | IC50 values for niclosamide/PDMX1001 not publicly available | Poor bioavailability if only niclosamide is administered [57] |
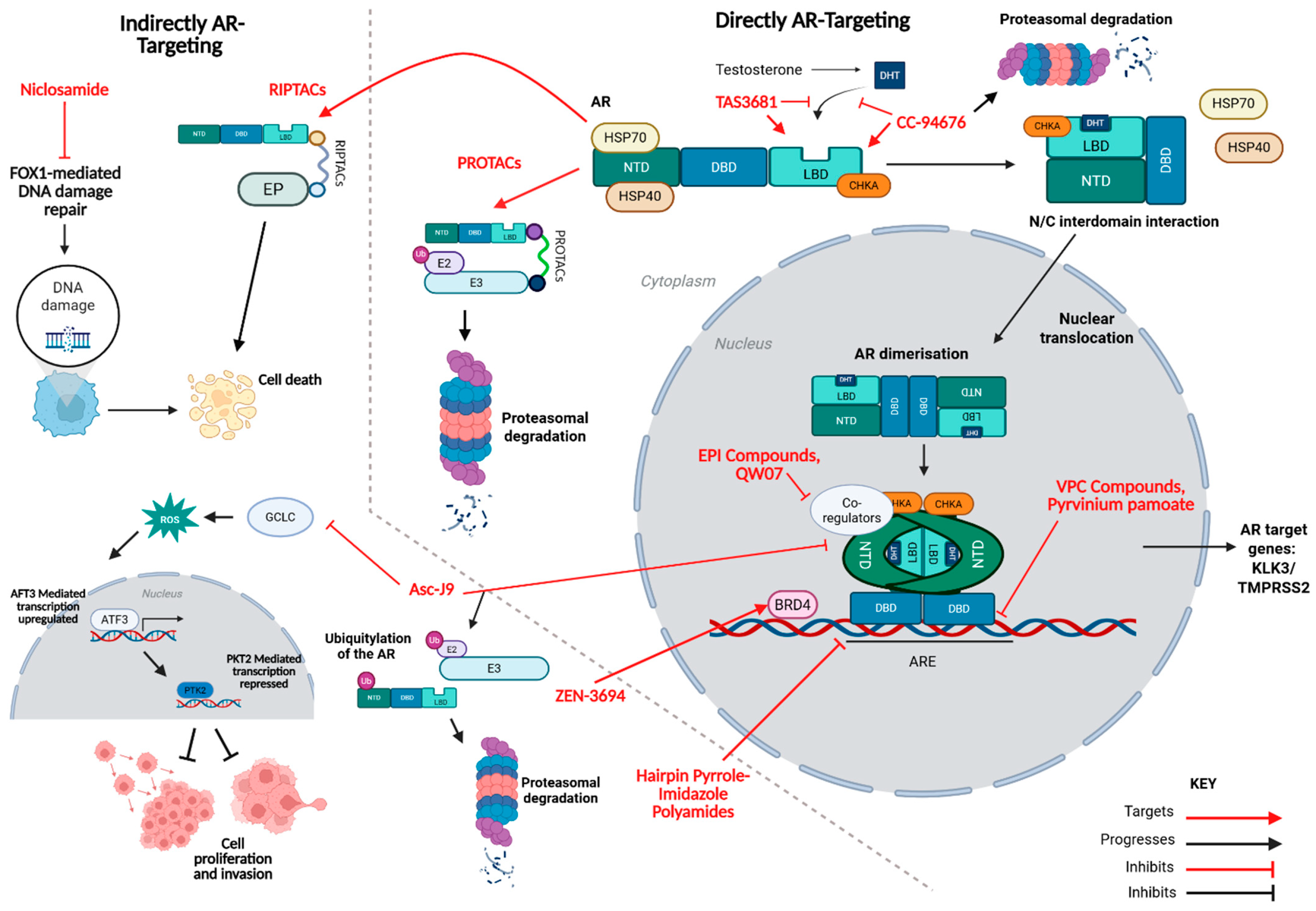
2. Direct AR-Targeting Compounds
2.1. NTD Inhibitors
2.1.1. EPI Compounds
2.1.2. QW07
2.2. DBD Inhibitors
2.2.1. VPC Compounds
2.2.2. Pyrvinium Pamoate
2.3. LBD-Targeting AR Degraders
ARV-110 and ARV-766
2.4. LBD Antagonists
TAS3681
2.5. LBD Antagonist and AR Degraders
CC-94676
3. Indirect AR-Targeting Compounds
3.1. RIPTACs
3.1.1. H001 and H003
3.1.2. HLD-0915
3.2. AR Degradation via Coregulator Disruption
Asc-J9
3.3. Epigenetic Suppression with BET Inhibitors
ZEN-3694
3.4. AR Blockade Through DNA Binding Inhibition
Hairpin Pyrrole–Imidazole Polyamides
3.5. Mitochondrial and Signalling Modulators
Niclosamide
4. Discussion and Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prostate Cancer Statistics. Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer (accessed on 20 January 2025).
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef]
- Brawley, S.; Mohan, R.; Nein, C.D. Localized Prostate Cancer: Treatment Options. Am. Fam. Physician 2018, 97, 798–805. [Google Scholar]
- Nguyen-Nielsen, M.; Borre, M. Diagnostic and Therapeutic Strategies for Prostate Cancer. Semin. Nucl. Med. 2016, 46, 484–490. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.-J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9 (Suppl. S1), S3–S8. [Google Scholar] [PubMed]
- Kunath, F.; Borgmann, H.; Blümle, A.; Keck, B.; Wullich, B.; Schmucker, C.; Sikic, D.; Roelle, C.; Schmidt, S.; Wahba, A.; et al. Gonadotropin-releasing hormone antagonists versus standard androgen suppression therapy for advanced prostate cancer A systematic review with meta-analysis. BMJ Open 2015, 5, e008217. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate-resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 2015, 4, 365–380. [Google Scholar] [CrossRef]
- Messner, E.A.; Steele, T.M.; Tsamouri, M.M.; Hejazi, N.; Gao, A.C.; Mudryj, M.; Ghosh, P.M. The Androgen Receptor in Prostate Cancer: Effect of Structure, Ligands and Spliced Variants on Therapy. Biomedicines 2020, 8, 422. [Google Scholar] [CrossRef]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Tan, M.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Centenera, M.M.; Harris, J.M.; Tilley, W.D.; Butler, L.M. Minireview: The Contribution of Different Androgen Receptor Domains to Receptor Dimerization and Signaling. Mol. Endocrinol. 2008, 22, 2373–2382. [Google Scholar] [CrossRef]
- Le, T.K.; Duong, Q.H.; Baylot, V.; Fargette, C.; Baboudjian, M.; Colleaux, L.; Taïeb, D.; Rocchi, P. Castration-Resistant Prostate Cancer: From Uncovered Resistance Mechanisms to Current Treatments. Cancers 2023, 15, 5047. [Google Scholar] [CrossRef]
- Vellky, J.E.; Ricke, W.A. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia 2020, 22, 566–575. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- Clegg, N.J.; Wongvipat, J.; Joseph, J.; Tran, C.; Ouk, S.; Dilhas, A.; Chen, Y.; Grillot, K.; Bischoff, E.D.; Cai, L.; et al. ARN-509: A novel anti-androgen for prostate cancer treatment. Cancer Res. 2012, 72, 1494–1503. [Google Scholar] [CrossRef]
- Maylin, Z.R.; Nicolescu, R.C.; Pandha, H.; Asim, M. Breaking androgen receptor addiction of prostate cancer by targeting different functional domains in the treatment of advanced disease. Transl. Oncol. 2021, 14, 101115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luo, J. Regulation of androgen receptor variants in prostate cancer. Asian J. Urol. 2020, 7, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Chandhasin, C.; Osbourne, E.; Luo, J.; Sadar, M.D.; Perabo, F. Targeting the N-Terminal Domain of the Androgen Receptor: A New Approach for the Treatment of Advanced Prostate Cancer. Oncol. 2016, 21, 1427–1435. [Google Scholar] [CrossRef]
- Yang, Y.C.; Banuelos, C.A.; Mawji, N.R.; Wang, J.; Kato, M.; Haile, S.; McEwan, I.J.; Plymate, S.; Sadar, M.D. Targeting Androgen Receptor Activation Function-1 with EPI to Overcome Resistance Mechanisms in Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2016, 22, 4466–4477. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.; Chatta, G.S.; Laccetti, A.L.; Iannotti, N.; Sokolova, A.; Hotte, S.J.; Cesano, A. Phase 1/2 trial of oral EPI-7386 (masofaniten) in combination with enzalutamide (Enz) compared to Enz alone in patients with metastatic castration-resistant prostate cancer (mCRPC): Phase 1 (P1) results and phase 2 (P2) design. J. Clin. Oncol. 2024, 42, 141. [Google Scholar] [CrossRef]
- Hong, N.H.; Le Moigne, R.; Banuelos, C.A.; Mawji, N.R.; Tam, T.; Wang, J.; Andersen, R.J.; Cesano, A.; Sadar, M.D.; Zhou, H.-J.; et al. Pre-clinical development of the second-generation N-terminal domain androgen receptor inhibitor, EPI-7386, for the treatment of prostate cancer. Cancer Res. 2020, 80 (Suppl. S16). [Google Scholar] [CrossRef]
- Obst, J.K.; Wang, J.; Jian, K.; Williams, D.E.; Tien, A.H.; Mawji, N.; Tam, T.; Yang, Y.C.; Andersen, R.J.; Chi, K.N.; et al. Revealing Metabolic Liabilities of Ralaniten to Enhance Novel Androgen Receptor Targeted Therapies. ACS Pharmacol. Transl. Sci. 2019, 2, 453–467. [Google Scholar] [CrossRef]
- Brand, L.J.; Olson, M.E.; Ravindranathan, P.; Guo, H.; Kempema, A.M.; Andrews, T.E.; Dehm, S.M. EPI-001 is a selective peroxisome proliferator-activated receptor-gamma modulator with inhibitory effects on androgen receptor expression and activity in prostate cancer. Oncotarget 2015, 6, 3811–3824. [Google Scholar] [CrossRef]
- Peng, S.; Wang, J.; Chen, H.; Hu, P.; He, X.-L.; He, Y.; Wang, M.; Tang, W.; He, Q.; Wang, Y.-Y.; et al. Regression of castration-resistant prostate cancer by a novel compound QW07 targeting androgen receptor N-terminal domain. Cell Biol. Toxicol. 2020, 36, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lan, T. N-terminal domain of androgen receptor is a major therapeutic barrier and potential pharmacological target for treating castration resistant prostate cancer: A comprehensive review. Front. Pharmacol. 2024, 15, 1451957. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, I.; Chen, G.; Piri, K.; Shinkawa, S.; Ashong, D.; Zhang, Q.; Wang, G.; Chen, Q.-H. Tricyclic Diterpenoids Selectively Suppress Androgen Receptor-Positive Prostate Cancer Cells. Molecules 2023, 28, 4743. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.; Che, M.; Que, N.S.; Sharma, A.; Yang, R.; Lallous, N.; Borgmann, H.; Ozistanbullu, D.; Tse, R.; Ban, F.; et al. Bypassing Drug Resistance Mechanisms of Prostate Cancer with Small Molecules that Target Androgen Receptor–Chromatin Interactions. Mol. Cancer Ther. 2017, 16, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Radaeva, M.; Ban, F.; Zhang, F.; LeBlanc, E.; Lallous, N.; Rennie, P.S.; Gleave, M.E.; Cherkasov, A. Development of Novel Inhibitors Targeting the D-Box of the DNA Binding Domain of Androgen Receptor. Int. J. Mol. Sci. 2021, 22, 2493. [Google Scholar] [CrossRef]
- Lim, M.; Otto-Duessel, M.; He, M.; Su, L.; Nguyen, D.; Chin, E.; Alliston, T.; Jones, J.O. Ligand-independent and tissue-selective androgen receptor inhibition by pyrvinium. ACS Chem. Biol. 2014, 9, 692–702. [Google Scholar] [CrossRef]
- Schultz, C.W.; Nevler, A. Pyrvinium Pamoate: Past, Present, and Future as an Anti-Cancer Drug. Biomedicines 2022, 10, 3249. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Munoz, E.; Ashong, D. Insight into Recent Advances in Degrading Androgen Receptor for Castration-Resistant Prostate Cancer. Cancers 2024, 16, 663. [Google Scholar] [CrossRef]
- Zhang, Y.; Ming, A.; Wang, J.; Chen, W.; Fang, Z. PROTACs targeting androgen receptor signaling: Potential therapeutic agents for castration-resistant prostate cancer. Pharmacol. Res. 2024, 205, 107234. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; McKean, M.; Lang, J.M.; Gao, X.; Dreicer, R.; Geynisman, D.M.; Shore15, N.D. ARV-766, a Proteolysis Targeting Chimera (PROTAC) Androgen Receptor (AR) Degrader, in Metastatic Castration-Resistant Prostate Cancer (mCRPC): Initial Results of a Phase 1/2 Study. J. Clin. Oncol. 2024, 42, 5011. [Google Scholar] [CrossRef]
- Burke, M.R.; Smith, A.R.; Zheng, G. Overcoming Cancer Drug Resistance Utilizing PROTAC Technology. Front. Cell Dev. Biol. 2022, 10, 872729. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kajiwara, D.; Seki, M.; Tayama, M.; Tanaka, Y.; Mizutani, H.; Fujita, R.; Yamamura, K.; Okajima, S.; Asai, M.; et al. TAS3681, an androgen receptor antagonist, prevents drug resistance driven by aberrant androgen receptor signaling in prostate cancer. Mol. Oncol. 2024, 18, 1980–2000. [Google Scholar] [CrossRef]
- De Bono, J.S.; Cook, N.; Yu, E.Y.; Lara, P.L.N.; Wang, J.S.; Yamasaki, Y.; Yamamiya, I.; Gao, P.; Calleja, E.M.; Rathkopf, D.E. First-in-human study of TAS3681, an oral androgen receptor (AR) antagonist with AR and AR splice variant (AR-SV) downregulation activity, in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) refractory to abiraterone (ABI) and/or enzalutamide (ENZ) and chemotherapy (CT). J. Clin. Oncol. 2021, 39, 5031. [Google Scholar] [CrossRef]
- Nayak, S.; Norris, J.D.; Ammirante, M.; Rychak, E.; Wardell, S.E.; Liao, D.; Toyama, B.; Kandimalla, R.; Christoforou, A.; Tsuji, T.; et al. Discovery of BMS-986365, a First-in-Class Dual Androgen Receptor Ligand-Directed Degrader and Antagonist, for the Treatment of Advanced Prostate Cancer. Clin. Cancer Res. 2025, OF1–OF18. [Google Scholar] [CrossRef]
- Rathkopf, D.E.; Patel, M.R.; Choundhury, A.D.; Rasco, D.; Lakhani, N.; Hawley, J.E.; Srinivas, S.; Aparicio, A.; Narayan, V.; Runcie, K.D.; et al. Safety and clinical activity of BMS-986365 (CC-94676), a dual androgen receptor ligand-directed degrader and antagonist, in heavily pretreated patients with metastatic castration-resistant prostate cancer. Ann. Oncol. 2025, 36, 76–88. [Google Scholar] [CrossRef]
- Celgene, A Phase 3, Two-Part, Randomized, Open-label, Adaptive Study Comparing BMS-986365 Versus Investigator’s Choice of Therapy Comprising Either Docetaxel or Second Androgen Receptor Pathway Inhibitor (ARPI), in Participants with Metastatic Castration-Resistant Prostate Cancer (mCRPC)—rechARge. Clinicaltrials.gov, Clinical Trial Registration NCT06764485. Oct. 2025. Available online: https://clinicaltrials.gov/study/NCT06764485 (accessed on 30 October 2025).
- Rathkopf, D.E.; Patel, M.R.; Choundhury, A.D.; Rasco, D.W.; Lakhani, N.J.; Hawley, J.E.; Aparicio, A.; Narayan, V.; Srinivas, S.; Runcie, K.; et al. First-in-human phase 1 study of CC-94676, a first-in-class androgen receptor (AR) ligand-directed degrader (LDD), in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2024, 42, 134. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, C.; Shen, Q.; Zhou, J. RIPTACs for Precision Cancer Therapy: A Novel Modality with the Inspiration of HLD-0915 as the First Candidate in Clinical Trials. J. Med. Chem. 2025, 68, 10503–10506. [Google Scholar] [CrossRef]
- Raina, K.; Forbes, C.D.; Stronk, R.; Rappi, J.P., Jr.; Eastman, K.J.; Zaware, N.; Yu, X.; Li, H.; Bhardwaj, A.; Gerritz, S.W.; et al. Regulated induced proximity targeting chimeras-RIPTACs-A heterobifunctional small molecule strategy for cancer selective therapies. Cell Chem. Biol. 2024, 31, 1490–1502.e42. [Google Scholar] [CrossRef]
- Therapeutics, H. Halda Therapeutics Announces First-in-Human Results for HLD-0915, an Oral RIPTACTM Therapeutic Demonstrating Encouraging Safety and Anti-Tumor Activity in Metastatic Castration-Resistance Prostate Cancer (mCRPC). Halda Therapeutics. Available online: https://haldatx.com/halda-therapeutics-announces-first-in-human-results-for-hld-0915-an-oral-riptac-therapeutic-demonstrating-encouraging-safety-and-anti-tumor-activity-in-metastatic-castration-resistance-prost/ (accessed on 30 October 2025).
- Ma, Z.; Bolinger, A.A.; Zhou, J. RIPTACs: A groundbreaking approach to drug discovery. Drug Discov. Today 2023, 28, 103774. [Google Scholar] [CrossRef]
- Chou, F.-J.; Chen, Y.; Chen, D.; Niu, Y.; Li, G.; Keng, P.; Yeh, S.; Chang, C. Preclinical study using androgen receptor (AR) degradation enhancer to increase radiotherapy efficacy via targeting radiation-increased AR to better suppress prostate cancer progression. EBioMedicine 2019, 40, 504–516. [Google Scholar] [CrossRef]
- Hu, H.; Zhou, H.; Xu, D. A review of the effects and molecular mechanisms of dimethylcurcumin (ASC-J9) on androgen receptor-related diseases. Chem. Biol. Drug Des. 2021, 97, 821–835. [Google Scholar] [CrossRef]
- Yamashita, S.; Lai, K.-P.; Chuang, K.-L.; Xu, D.; Miyamoto, H.; Tochigi, T.; Pang, S.-T.; Li, L.; Arai, Y.; Kung, H.-J.; et al. ASC-J9 Suppresses Castration-Resistant Prostate Cancer Growth through Degradation of Full-length and Splice Variant Androgen Receptors. Neoplasia 2012, 14, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Kumar, S.; Kumar, S.; Kumar, R.; Prasad, A.-K. Chemical Features and Therapeutic Applications of Curcumin (A Review). Russ. J. Gen. Chem. 2022, 92, 1785–1805. [Google Scholar] [CrossRef]
- Aggarwal, R.R.; Schweizer, M.T.; Nanus, D.M.; Pantuck, A.J.; Heath, E.I.; Campeau, E.; Attwell, S.; Norek, K.; Snyder, M.; Bauman, L.; et al. A Phase Ib/IIa Study of the Pan-BET Inhibitor ZEN-3694 in Combination with Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2020, 26, 5338–5347. [Google Scholar] [CrossRef] [PubMed]
- Attwel, S.; Jahagirdar, R.; Norek, K.; Calosing, C.; Tsujikawa, L.; Kharenko, O.A.; Patel, R.G.; Gesner, E.M.; Corey, E.; Nguyen, H.M.; et al. Abstract LB-207: Preclinical characterization of ZEN-3694, a novel BET bromodomain inhibitor entering phase I studies for metastatic castration-resistant prostate cancer (mCRPC). Cancer Res. 2016, 76, LB-207. [Google Scholar] [CrossRef]
- Yang, F.; Nickols, N.G.; Li, B.C.; Marinov, G.K.; Said, J.W.; Dervan, P.B. Antitumor activity of a pyrrole-imidazole polyamide. Proc. Natl. Acad. Sci. USA 2013, 110, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Kurmis, A.A.; Yang, F.; Welch, T.R.; Nickols, N.G.; Dervan, P.B. A pyrrole-imidazole polyamide is active against enzalutamide-resistant prostate cancer. Cancer Res. 2017, 77, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Nickols, N.G.; Li, B.C.; Szablowski, J.O.; Hamilton, S.R.; Meier, J.L.; Dervan, P.B. Animal Toxicity of Hairpin Pyrrole-Imidazole Polyamides Varies with the Turn Unit. J. Med. Chem. 2013, 56, 7449–7457. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Jung, A.R.; Shin, D.; Kwon, H.; Cho, H.J.; Ha, U.-S.; Hong, S.-H.; Lee, J.Y.; Kim, S.W.; Park, Y.H. Niclosamide exerts anticancer effects through inhibition of the FOXM1-mediated DNA damage response in prostate cancer. Am. J. Cancer Res. 2021, 11, 2944–2959. [Google Scholar] [PubMed]
- Sakellakis, M. Niclosamide in prostate cancer: An inhibitor of AR-V7, a mitochondrial uncoupler, or more? Cancer Treat. Res. Commun. 2023, 35, 100685. [Google Scholar] [CrossRef]
- Parikh, M.; Liu, C.; Wu, C.-Y.; Evans, C.P.; Dall’Era, M.; Robles, D.; Lara, P.N.; Agarwal, N.; Gao, A.C.; Pan, C.-X. Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer. Sci. Rep. 2021, 11, 6377. [Google Scholar] [CrossRef]
- Siu, L.L.; Rasco, D.W.; Vinay, S.P.; Romano, P.M.; Menis, J.; Opdam, F.L.; Heinhuis, K.M.; Egger, J.L.; Gorman, S.A.; Parasrampuria, R.; et al. 438O—METEOR-1: A phase I study of GSK3326595, a first-in-class protein arginine methyltransferase 5 (PRMT5) inhibitor, in advanced solid tumours. Ann. Oncol. 2019, 30, v159. [Google Scholar] [CrossRef]
- Nicolescu, R.C.B.; Maylin, Z.R.; Pérez-Areales, F.J.; Iegre, J.; Pandha, H.S.; Asim, M.; Spring, D.R. Hybrid Androgen Receptor Inhibitors Outperform Enzalutamide and EPI-001 in in vitro Models of Prostate Cancer Drug Resistance. Chemmedchem 2023, 18, e202200548. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Asim, M.; Massie, C.E.; Orafidiya, F.; Pértega-Gomes, N.; Warren, A.Y.; Esmaeili, M.; Selth, L.A.; Zecchini, H.I.; Luko, K.; Qureshi, A.; et al. Choline Kinase Alpha as an Androgen Receptor Chaperone and Prostate Cancer Therapeutic Target. J Natl Cancer Inst. 2015, 108, djv371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clinical Trial: NCT02566772—My Cancer Genome. Available online: https://www.mycancergenome.org/content/clinical_trials/NCT02566772/ (accessed on 30 October 2025).
- Wang, Z.-Q.; Zhang, Z.-C.; Wu, Y.-Y.; Pi, Y.-N.; Lou, S.H.; Liu, T.-B.; Lou, G.; Yang, C. Bromodomain and extraterminal (BET) proteins: Biological functions, diseases and targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 420. [Google Scholar] [CrossRef]
- Jia, X.; Han, X. Targeting androgen receptor degradation with PROTACs from bench to bedside. Biomed. Pharmacother. 2023, 158, 114112. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Y.; Shi, G.; Wang, X.; Ye, W.; Shan, C.; Wang, D.; Zhang, D.; He, W.; Jiang, J.; et al. A novel inhibitor of ARfl and ARv7 induces protein degradation to overcome enzalutamide resistance in advanced prostate cancer. Acta Pharm. Sin. B 2022, 12, 4165–4179. [Google Scholar] [CrossRef]
- Watson, P.A.; Chen, Y.F.; Balbas, M.D.; Wongvipat, J.; Socci, N.D.; Viale, A.; Kim, K.; Sawyers, C.L. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc. Natl. Acad. Sci. USA 2010, 107, 16759–16765. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-P.; Huang, C.-K.; Chang, Y.-J.; Chung, C.-Y.; Yamashita, S.; Li, L.; Lee, S.-O.; Yeh, S.; Chang, C. New Therapeutic Approach to Suppress Castration-Resistant Prostate Cancer Using ASC-J9 via Targeting Androgen Receptor in Selective Prostate Cells. Am. J. Pathol. 2013, 182, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Chou, F.-J.; Tian, J.; Zhang, Y.; You, B.; Huang, C.-P.; Yeh, S.; Niu, Y.; Chang, C. ASC-J9® suppresses prostate cancer cell proliferation and invasion via altering the ATF3-PTK2 signaling. J. Exp. Clin. Cancer Res. CR 2021, 40, 3. [Google Scholar] [CrossRef]
- Ali, H.A.; Li, Y.; Bilal, A.H.M.; Qin, T.; Yuan, Z.; Zhao, W. A Comprehensive Review of BET Protein Biochemistry, Physiology, and Pathological Roles. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Zen-3694—My Cancer Genome. Available online: https://www.mycancergenome.org/content/drugs/zen-3694/ (accessed on 22 October 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, I.; Foreman, R.; Balachandran, L.; Mortimer, E.; Asim, M. Emerging Therapeutic Approaches to Engage the Androgen Receptor for the Treatment of Castration-Resistant Prostate Cancer. Cancers 2025, 17, 3755. https://doi.org/10.3390/cancers17233755
Henry I, Foreman R, Balachandran L, Mortimer E, Asim M. Emerging Therapeutic Approaches to Engage the Androgen Receptor for the Treatment of Castration-Resistant Prostate Cancer. Cancers. 2025; 17(23):3755. https://doi.org/10.3390/cancers17233755
Chicago/Turabian StyleHenry, Isla, Rebecca Foreman, Lakshana Balachandran, Ethan Mortimer, and Mohammad Asim. 2025. "Emerging Therapeutic Approaches to Engage the Androgen Receptor for the Treatment of Castration-Resistant Prostate Cancer" Cancers 17, no. 23: 3755. https://doi.org/10.3390/cancers17233755
APA StyleHenry, I., Foreman, R., Balachandran, L., Mortimer, E., & Asim, M. (2025). Emerging Therapeutic Approaches to Engage the Androgen Receptor for the Treatment of Castration-Resistant Prostate Cancer. Cancers, 17(23), 3755. https://doi.org/10.3390/cancers17233755







