Exploratory Evaluation of Topical Tacrolimus for Prevention of Breast Cancer-Related Arm Lymphedema: A Multicenter Non-Randomized Pilot Study
Simple Summary
Abstract
1. Background
2. Methods
2.1. Trial Design and Registration
2.2. Sample Size Calculation
2.3. Participants
2.4. Intervention
2.5. Endpoints Measures
2.6. Baseline Characteristics
2.7. Lymphedema Diagnosis
2.8. Water Displacement Volumetry (WDV)
2.9. Patient Reported Outcome Measures (PROMs)
2.10. Bioimpedance Spectroscopy (BIS)
2.11. Safety
2.12. Statistical Analysis
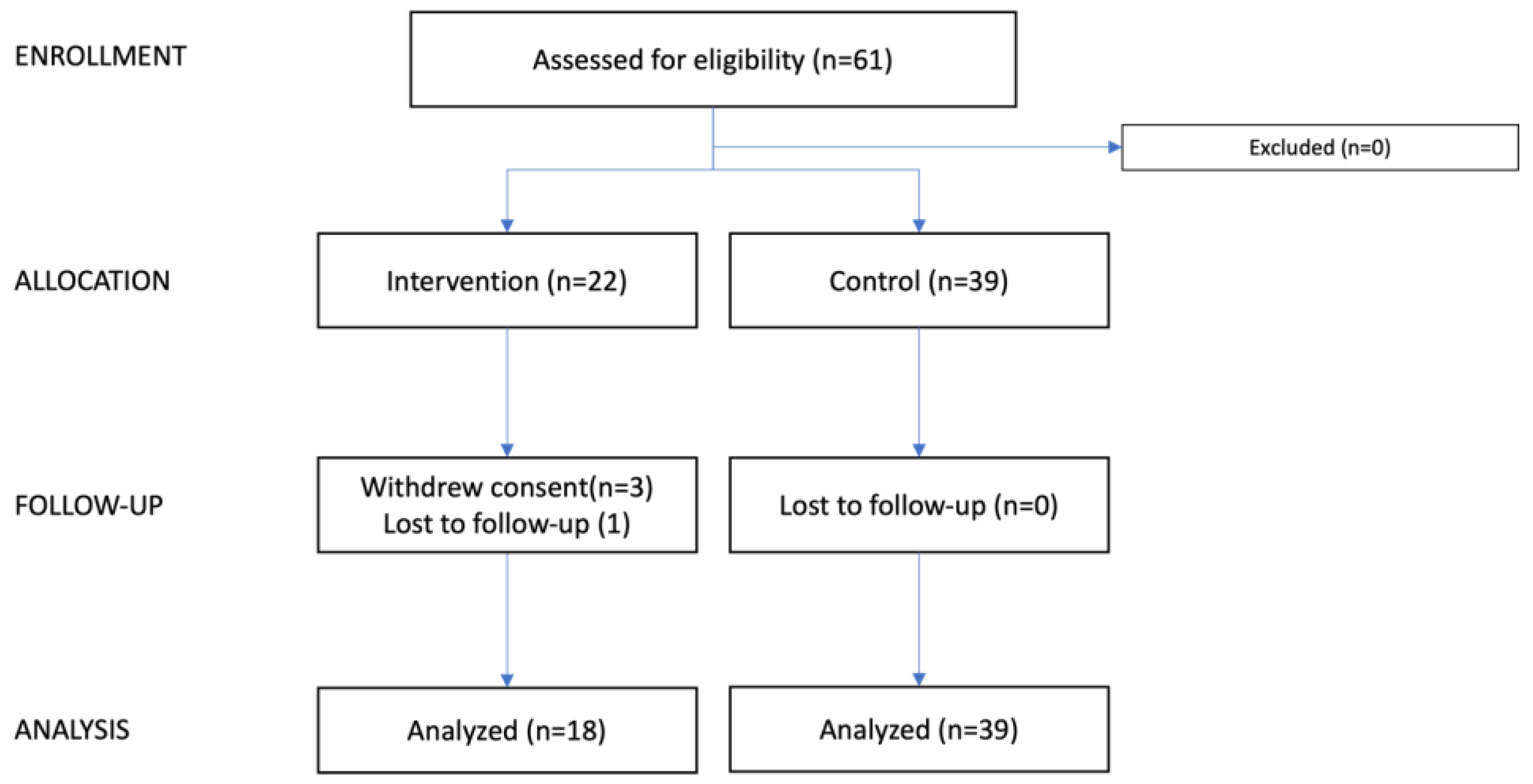
3. Results
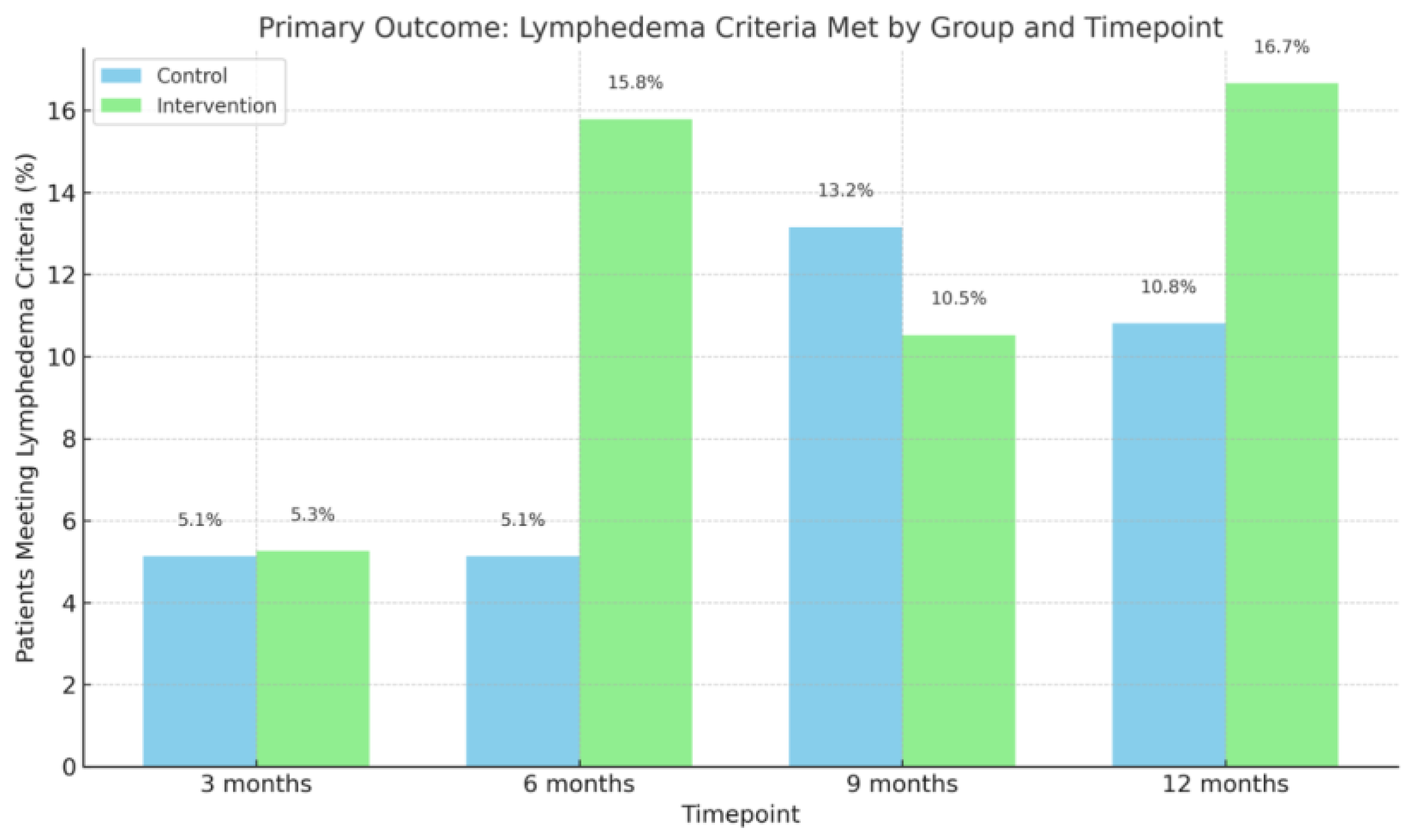
3.1. Lymphedema Diagnosis
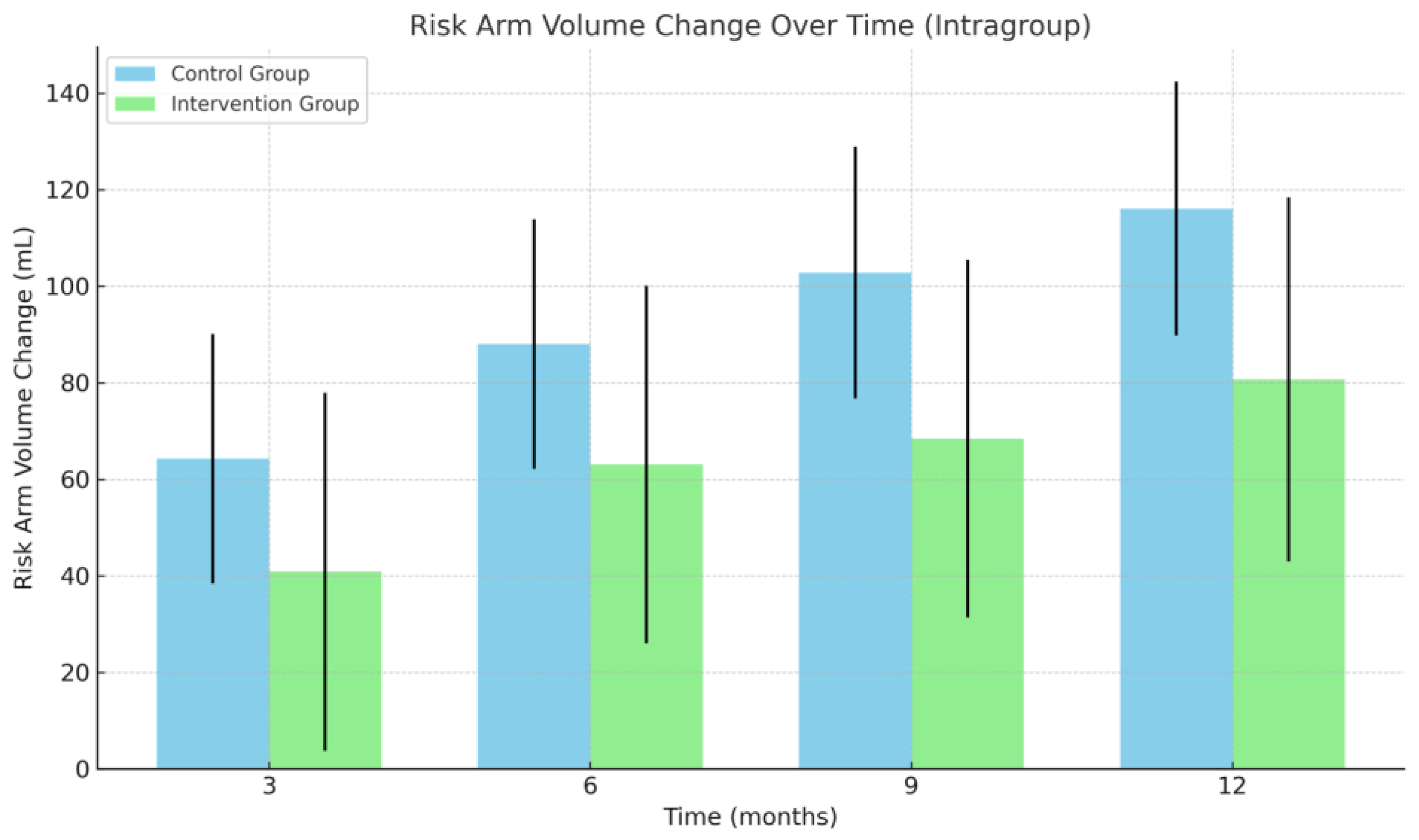
3.2. Arm Volume
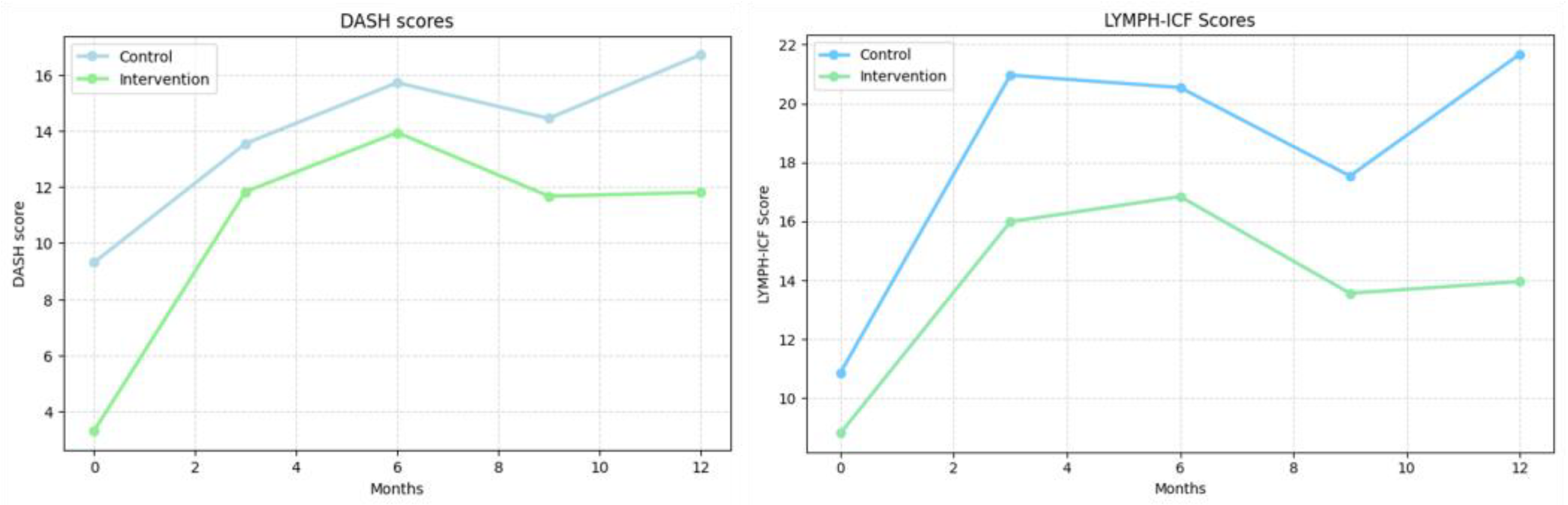
3.3. Patient Reported Outcome Measures
3.4. Lymphedema Index
3.5. Adherence to Intervention
3.6. Safety (Adverse Events)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AE | Adverse Event |
| ALND | Axillary Lymph Node Dissection |
| BCRL | Breast Cancer-Related Lymphedema |
| BIS | Bioimpedance Spectroscopy |
| BMI | Body Mass Index |
| DASH | Disabilities of the Arm, Shoulder, and Hand Questionnaire |
| EORTC QLQ-BR23 | EORTC Breast Cancer-specific Quality of Life Questionnaire module QLQ-BR23 |
| L-Dex | Lymphedema Index |
| LYMPH-ICF | Lymphedema Functioning, Disability and Health Questionnaire |
| OPEN | Open Patient Data Explorative Network |
| PROM | Patient Reported Outcome Measure |
| QOL | Quality of Life |
| SE | Standard Error |
| WDV | Water Displacement Volumetry |
References
- Warren, A.G.; Brorson, H.; Borud, L.J.; Slavin, S.A. Lymphedema: A comprehensive review. Ann. Plast. Surg. 2007, 59, 464–472. [Google Scholar] [CrossRef]
- McEvoy, M.P.; Gomberawalla, A.; Smith, M.; Boccardo, F.M.; Holmes, D.; Djohan, R.; Thiruchelvam, P.; Klimberg, S.; Dietz, J.; Feldman, S. The prevention and treatment of breast cancer- related lymphedema: A review. Front. Oncol. 2022, 12, 1062472. [Google Scholar] [CrossRef]
- Shah, C.; Arthur, D.; Riutta, J.; Whitworth, P.; Vicini, F.A. Breast-cancer related lymphedema: A review of procedure-specific incidence rates, clinical assessment AIDS, treatment paradigms, and risk reduction. Breast J. 2012, 18, 357–361. [Google Scholar] [CrossRef]
- Norman, S.A.; Localio, A.R.; Potashnik, S.L.; Simoes Torpey, H.A.; Kallan, M.J.; Weber, A.L.; Miller, L.T.; Demichele, A.; Solin, L.J. Lymphedema in breast cancer survivors: Incidence, degree, time course, treatment, and symptoms. J. Clin. Oncol. 2009, 27, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Petrek, J.A.; Senie, R.T.; Peters, M.; Rosen, P.P. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001, 92, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.G.; Toyserkani, N.M.; Hansen, F.G.; Bygum, A.; Sorensen, J.A. The impact of lymphedema on health-related quality of life up to 10 years after breast cancer treatment. NPJ Breast Cancer 2021, 7, 70. [Google Scholar] [CrossRef]
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar] [CrossRef]
- Donahue, P.M.C.; MacKenzie, A.; Filipovic, A.; Koelmeyer, L. Advances in the prevention and treatment of breast cancer-related lymphedema. Breast Cancer Res. Treat. 2023, 200, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rafn, B.S.; Christensen, J.; Larsen, A.; Bloomquist, K. Prospective Surveillance for Breast Cancer-Related Arm Lymphedema: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2022, 40, 1009–1026. [Google Scholar] [CrossRef]
- Shah, C.; Arthur, D.W.; Wazer, D.; Khan, A.; Ridner, S.; Vicini, F. The impact of early detection and intervention of breast cancer-related lymphedema: A systematic review. Cancer Med. 2016, 5, 1154–1162. [Google Scholar] [CrossRef]
- Ghanta, S.; Cuzzone, D.A.; Torrisi, J.S.; Albano, N.J.; Joseph, W.J.; Savetsky, I.L.; Gardenier, J.C.; Chang, D.; Zampell, J.C.; Mehrara, B.J. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am. J. Physiol.-Heart Circ. Physiol. 2015, 308, H1065–H1077. [Google Scholar] [CrossRef]
- Ogata, F.; Fujiu, K.; Matsumoto, S.; Nakayama, Y.; Shibata, M.; Oike, Y.; Koshima, I.; Watabe, T.; Nagai, R.; Manabe, I. Excess Lymphangiogenesis Cooperatively Induced by Macrophages and CD4(+) T Cells Drives the Pathogenesis of Lymphedema. J. Investig. Dermatol. 2016, 136, 706–714. [Google Scholar] [CrossRef]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013, 27, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef] [PubMed]
- Gardenier, J.C.; Kataru, R.P.; Hespe, G.E.; Savetsky, I.L.; Torrisi, J.S.; Nores, G.D.; Jowhar, D.K.; Nitti, M.D.; Schofield, R.C.; Carlow, D.C.; et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat. Commun. 2017, 8, 14345. [Google Scholar] [CrossRef] [PubMed]
- Malecic, N.; Young, H. Tacrolimus for the management of psoriasis: Clinical utility and place in therapy. Psoriasis 2016, 6, 153–163. [Google Scholar] [CrossRef]
- Cury Martins, J.; Martins, C.; Aoki, V.; Gois, A.F.; Ishii, H.A.; da Silva, E.M. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst. Rev. 2015, 7. [Google Scholar] [CrossRef]
- Mancuso, G.; Berdondini, R.M. Localized scleroderma: Response to occlusive treatment with tacrolimus ointment. Br. J. Dermatol. 2005, 152, 180–182. [Google Scholar] [CrossRef]
- Harvey, L.A. REDCap: Web-based software for all types of data storage and collection. Spinal Cord 2018, 56, 625. [Google Scholar] [CrossRef]
- (DBCG) TDBCG. Clinical Guidelines on Breast Cancer Treatment. Available online: https://www.dmcg.dk/Kliniske-retningslinjer/kliniske-retningslinjer-opdelt-paa-dmcg/brystcancer/ (accessed on 14 October 2025).
- Damstra, R.J.; Glazenburg, E.J.; Hop, W.C. Validation of the inverse water volumetry method: A new gold standard for arm volume measurements. Breast Cancer Res. Treat. 2006, 99, 267–273. [Google Scholar] [CrossRef]
- Tan, M.L.; Idris, D.B.; Teo, L.W.; Loh, S.Y.; Seow, G.C.; Chia, Y.Y.; Tin, A.S. Validation of EORTC QLQ-C30 and QLQ-BR23 questionnaires in the measurement of quality of life of breast cancer patients in Singapore. Asia-Pac. J. Oncol. Nurs. 2014, 1, 22–32. [Google Scholar] [CrossRef]
- Grarup, K.R.; Devoogdt, N.; Strand, L.I. The Danish version of Lymphoedema Functioning, Disability and Health Questionnaire (Lymph-ICF) for breast cancer survivors: Translation and cultural adaptation followed by validity and reliability testing. Physiother. Theory Pract. 2019, 35, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Puentes Gutierrez, A.B.; Garcia Bascones, M.; Jimenez Diaz, F.; Cuena Boy, R.; Puentes Gutierrez, R. Validity and reliability of DASH questionnaire in women who suffer from lymphedema as a side effect of a breast cancer treatment. Rehabilitacion 2023, 57, 100780. [Google Scholar] [CrossRef] [PubMed]
- Ogino, R.; Yokooji, T.; Hayashida, M.; Suda, S.; Yamakawa, S.; Hayashida, K. Emerging Anti-Inflammatory Pharmacotherapy and Cell-Based Therapy for Lymphedema. Int. J. Mol. Sci. 2022, 23, 7614. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Campbell, A.C.; Kuonqui, K.; Sarker, A.; Park, H.J.; Shin, J.; Kataru, R.P.; Coriddi, M.; Dayan, J.H.; Mehrara, B.J. The Future of Lymphedema: Potential Therapeutic Targets for Treatment. Curr. Breast Cancer Rep. 2023, 15, 233–241. [Google Scholar] [CrossRef]
- Hsu, J.F.; Yu, R.P.; Stanton, E.W.; Wang, J.; Wong, A.K. Current Advancements in Animal Models of Postsurgical Lymphedema: A Systematic Review. Adv. Wound Care 2022, 11, 399–418. [Google Scholar] [CrossRef]
- Jorgensen, M.G.; Toyserkani, N.M.; Hansen, C.R.; Hvidsten, S.; Baun, C.; Hejbol, E.K.; Schroder, H.D.; Sorensen, J.A. Quantification of Chronic Lymphedema in a Revised Mouse Model. Ann. Plast. Surg. 2018, 81, 594–603. [Google Scholar] [CrossRef]
- Gulmark Hansen, F.C.; Jorgensen, M.G.; Sorensen, J.A. Treatment of Breast Cancer-Related Lymphedema with Topical Tacrolimus: A Prospective, Open-Label, Single-Arm, Phase II Pilot Trial. J. Breast Cancer 2023, 26, 46. [Google Scholar] [CrossRef]
- Nakamura, K.; Radhakrishnan, K.; Wong, Y.M.; Rockson, S.G. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS ONE 2009, 4, e8380. [Google Scholar] [CrossRef]
- Rockson, S.G.; Tian, W.; Jiang, X.; Kuznetsova, T.; Haddad, F.; Zampell, J.; Mehrara, B.; Sampson, J.P.; Roche, L.; Kim, J.; et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight 2018, 3, e123775. [Google Scholar] [CrossRef]
- Brown, S.; Dayan, J.H.; Coriddi, M.; McGrath, L.; Kataru, R.P.; Mehrara, B.J. Doxycycline for the treatment of breast cancer-related lymphedema. Front. Pharmacol. 2022, 13, 1028926. [Google Scholar] [CrossRef]
- Brubaker, D.K.; Lauffenburger, D.A. Translating preclinical models to humans. Science 2020, 367, 742–743. [Google Scholar] [CrossRef]
| Variable | Control Group (n = 39) | Intervention Group (n = 22) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 53.8 ± 11.43 | 52.8 ± 9.10 | >0.05 |
| In relationship n (%) | 20 (71.43%) | 12 (66.67%) | >0.05 |
| Smoking n (%) | 4 (13.79%) | 3 (15.79%) | >0.05 |
| Alcohol consumption (units per week, mean ± SD) | 1.61 ± 3.20 | 2.63 ± 3.53 | >0.05 |
| Employment status (working, n (%)) | 22 (64.71%) | 13 (68.42%) | >0.05 |
| Body Mass Index (BMI, mean ± SD) | 27.92 ± 5.12 | 24.38 ± 3.22 | <0.01 |
| Surgical treatment | <0.01 | ||
| Lumpectomy, n (%) | 27 (69.23%) | 4 (21.05%) | |
| Mastectomy, n (%) | 10 (30.77%) | 15 (78.95%) | |
| Sentinel node biopsy, n (%) | 32 (82.05%) | 7 (36.84%) | <0.01 |
| Number of lymph nodes resected (mean ± SD) | 14.65 ± 6.03 | 22.5 ± 7.01 | <0.001 |
| Number of lymph nodes with metastasis (mean ± SD) | 3.08 ± 6.05 | 3.78 ± 3.78 | >0.05 |
| Oncologic treatment, n (%) | |||
| Radiotherapy | 36 (92.31%) | 18 (100%) | >0.05 |
| Chemotherapy | 28 (71.80%) | 17 (94.44%) | >0.05 |
| Neo-adjuvant chemotherapy | 24 (61.54%) | 10 (55.56%) | >0.05 |
| Endocrine therapy | 36 (96.31%) | 12 (66.67%) | <0.05 |
| Biological therapy | 4 (10.26%) | 2 (12.50%) | >0.05 |
| Zolendronic acid | 20 (51.28%) | 10 (55.56%) | >0.05 |
| Arm volume difference (mL, mean ± SD) | 5.90 ± 115.87 | 22.84 ± 95.69 | >0.05 |
| Operated in dominant side, n (%) | 18 (46.15%) | 8 (42.11%) | >0.05 |
| Outcome | Control Group | Intervention Group | p-Value |
|---|---|---|---|
| Lymphedema diagnosis acording to medical journal (yes) | |||
| 3 months, n (%) | 2 (5.13%) | 1 (5.26%) | >0.05 |
| 6 months, n (%) | 6 (15.38%) | 3 (15.79%) | >0.05 |
| 9 months, n (%) | 10 (26.32%) | 6 (31.58%) | >0.05 |
| 12 months, n (%) | 13 (35.14%) | 7 (38.89%) | >0.05 |
| Lymphedema criteria met? (yes) (≥10% volume change compared to baseline) | |||
| 3 months, n (%) | 2 (5.13%) | 1 (5.26%) | >0.05 |
| 6 months, n (%) | 2 (5.13%) | 3 (15.79%) | >0.05 |
| 9 months, n (%) | 5 (13.16%) | 2 (10.53%) | >0.05 |
| 12 months, n (%) | 4 (10.81%) | 3 (16.67%) | >0.05 |
| Risk arm volume change | |||
| 3 months (mean ± SE) | 64.26 ± 25.87 mL * | 40.84 ± 37.06 mL | >0.05 |
| 6 months (mean ± SE) | 88.00 ± 25.87 mL * | 63.05 ± 37.06 mL | >0.05 |
| 9 months (mean ± SE) | 102.79 ± 26.09 mL * | 68.42 ± 37.06 mL | >0.05 |
| 12 months (mean ± SE) | 116.07 ± 26.31 mL * | 80.68 ± 37.70 mL * | >0.05 |
| Healthy arm volume change | |||
| 3 months (mean ± SE) | 19.67 ± 20.18 mL | 7.21 ± 26.60 mL | >0.05 |
| 6 months (mean ± SE) | 36.77 ± 20.18 mL | −2.74 ± 26.60 mL | >0.05 |
| 9 months (mean ± SE) | 31.03 ± 20.35 mL | −6.53 ± 26.60 mL | >0.05 |
| 12 months (mean ± SE) | 40.23 ± 20.53 mL * | −15 ± 27.05 mL | >0.05 |
| Lymphedema Volume change | |||
| 3 months (mean ± SE) | 42.03 ± 23.99 mL | −13.74 ± 34.37 mL | >0.05 |
| 6 months (mean ± SE) | 51.23 ± 23.99 mL * | 65.79 ± 34.37 mL | >0.05 |
| 9 months (mean ± SE) | 71.47 ± 24.18 mL * | 74.95 ± 34.37 mL * | >0.05 |
| 12 months (mean ± SE) | 76.26 ± 24.38 mL * | 95.22 ± 34.93 mL * | >0.05 |
| Time to lymphedema diagnosis | |||
| Time (days, mean ± SE) | 162.54 ± 26.69 days | 184 ± 39.70 days | >0.05 |
| Outcome | Control Group | Intervention Group | p-Value |
|---|---|---|---|
| L-Dex score (Intervention group compared to baseline measurements) | |||
| 3 months (mean ± SE) | 7.22 ± 2.47 * | <0.01 ** | |
| 6 months (mean ± SE) | 8.75 ± 2.47 * | <0.001 ** | |
| 9 months (mean ± SE) | 9.04 ± 2.47 * | <0.001 ** | |
| 12 months (mean ± SE) | 5.98 ± 2.51 * | <0.05 * | |
| DASH—(0 = best, 100 = worst) | |||
| 3 months (mean ± SE) | 4.24 ± 1.51 * | 8.54 ± 2.15 * | >0.05 |
| 6 months (mean ± SE) | 6.42 ± 1.50 * | 10.65 ± 2.15 * | >0.05 |
| 9 months (mean ± SE) | 5.14 ± 1.53 * | 8.38 ± 2.15 * | >0.05 |
| 12 months (mean ± SE) | 7.41 ± 1.53 * | 8.51 ± 2.18 * | >0.05 |
| LYMPH-ICF—(0 = best, 100 = worst) | |||
| 3 months (mean ± SE) | 10.09 ± 2.35 * | 7.17 ± 33.34 * | >0.05 |
| 6 months (mean ± SE) | 9.67 ± 2.33 * | 8.03 ± 3.34 * | >0.05 |
| 9 months (mean ± SE) | 6.67 ± 2.39 * | 4.75 ± 3.34 | >0.05 |
| 12 months (mean ± SE) | 10.80 ± 2.37 * | 5.14 ± 3.40 | >0.05 |
| EORTC-BR23—Symptoms: Systemic Therapy Side Effects (0 = best, 100 = worst) | |||
| 3 months (mean ± SE) | −0.51 ± 2.92 | −4.51 ± 4.15 | >0.05 |
| 6 months (mean ± SE) | 4.32 ± 2.92 | 4.26 ± 4.15 | >0.05 |
| 9 months (mean ± SE) | 7.09 ± 2.97 * | 7.02 ± 4.15 | >0.05 |
| 12 months (mean ± SE) | 9.44 ± 3.21 * | 6.80 ± 4.42 | >0.05 |
| EORTC-BR23—Symptoms: Upset by Hair Loss (0 = best, 100 = worst) | |||
| 3 months (mean ± SE) | 16.65 ± 9.74 | 21.42 ± 17.67 | >0.05 |
| 6 months (mean ± SE) | 14.17 ± 9.84 | 41.58 ± 25.75 | >0.05 |
| 9 months (mean ± SE) | 9.16 ± 11.54 | 2.50 ± 18.36 | >0.05 |
| 12 months (mean ± SE) | 35.86 ± 11.70 * | Not answered | N/A |
| EORTC-BR23—Symptoms: Arm Symptoms (0 = best, 100 = worst) | |||
| 3 months (mean ± SE) | 9.98 ± 2.77 * | 11.11 ± 3.93 * | >0.05 |
| 6 months (mean ± SE) | 13.30 ± 2.76 * | 15.79 ± 3.93 * | >0.05 |
| 9 months (mean ± SE) | 13.32 ± 2.82 * | 15.20 ± 3.93 * | >0.05 |
| 12 months (mean ± SE) | 14.75 ± 3.06 * | 11.56 ± 4.00 * | >0.05 |
| EORTC-BR23—Symptoms: Breast Symptoms (0 = best, 100 = worst) | |||
| 3 months (mean ± SE) | 17.13 ± 3.06 * | 14.47 ± 4.35 * | >0.05 |
| 6 months (mean ± SE) | 11.41 ± 3.06 * | 15.35 ± 4.35 * | >0.05 |
| 9 months (mean ± SE) | 9.47 ±3.12 * | 14.47± 4.35 * | >0.05 |
| 12 months (mean ± SE) | 12.36 ± 3.38 * | 11.86 ± 4.42 * | >0.05 |
| EORTC-BR23—Functional: Body Image (0 = worst, 100 = best) | |||
| 3 months (mean ± SE) | 8.67 ± 3.38 * | 1.75 ± 4.80 | >0.05 |
| 6 months (mean ± SE) | 5.02 ± 3.37 | 2.63 ± 4.80 | >0.05 |
| 9 months (mean ± SE) | −2.87 ± 3.44 | 3.95 ± 4.80 | >0.05 |
| 12 months (mean ± SE) | −0.11 ± 3.73 | −1.26 ± 4.88 | >0.05 |
| EORTC-BR23—Functional: Future Perspective (0 = worst, 100 = best) | |||
| 3 months (mean ± SE) | −15.09 ± 4.49 * | −15.79 ± 6.37 * | >0.05 |
| 6 months (mean ± SE) | −19.16 ± 4.48 * | −22.81 ± 6.37 * | >0.05 |
| 9 months (mean ± SE) | −23.17 ± 4.56 * | −24.56 ± 6.37 * | >0.05 |
| 12 months (mean ± SE) | −26.38 ± 4.95 * | −22.32 ± 6.48 * | >0.05 |
| EORTC-BR23—Functional: Sexual Functioning (0 = worst, 100 = best) | |||
| 3 months (mean ± SE) | 2.74 ± 3.38 | 0.52 ± 4.92 | >0.05 |
| 6 months (mean ± SE) | −3.41 ± 3.36 | 0.52 ± 4.92 | >0.05 |
| 9 months (mean ± SE) | −1.09 ± 3.45 | −1.54 ± 4.92 | >0.05 |
| 12 months (mean ± SE) | −3.39 ± 3.72 | 0.35 ± 5.12 | >0.05 |
| EORTC-BR23: Functional: Sexual Enjoyment (0 = worst, 100 = best) | |||
| 3 months (mean ± SE) | 2.29 ± 7.05 | 9.26 ± 7.12 | >0.05 |
| 6 months (mean ± SE) | 7.13 ± 6.49 | 8.05 ± 7.28 | >0.05 |
| 9 months (mean ± SE) | 12.95 ± 6.56 * | −0.96 ± 6.90 | >0.05 |
| 12 months (mean ± SE) | 12.12 ± 7.06 | 1.49 ± 7.51 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulmark Hansen, F.; Jørgensen, M.G.; Gordon, K.; Kjær, C.; Carstensen, L.F.; Tambour, M.; Gram, B.; Thomsen, J.B.; Sørensen, J.A. Exploratory Evaluation of Topical Tacrolimus for Prevention of Breast Cancer-Related Arm Lymphedema: A Multicenter Non-Randomized Pilot Study. Cancers 2025, 17, 3753. https://doi.org/10.3390/cancers17233753
Gulmark Hansen F, Jørgensen MG, Gordon K, Kjær C, Carstensen LF, Tambour M, Gram B, Thomsen JB, Sørensen JA. Exploratory Evaluation of Topical Tacrolimus for Prevention of Breast Cancer-Related Arm Lymphedema: A Multicenter Non-Randomized Pilot Study. Cancers. 2025; 17(23):3753. https://doi.org/10.3390/cancers17233753
Chicago/Turabian StyleGulmark Hansen, Frederik, Mads Gustaf Jørgensen, Kim Gordon, Christina Kjær, Lena Felicia Carstensen, Mette Tambour, Bibi Gram, Jørn Bo Thomsen, and Jens Ahm Sørensen. 2025. "Exploratory Evaluation of Topical Tacrolimus for Prevention of Breast Cancer-Related Arm Lymphedema: A Multicenter Non-Randomized Pilot Study" Cancers 17, no. 23: 3753. https://doi.org/10.3390/cancers17233753
APA StyleGulmark Hansen, F., Jørgensen, M. G., Gordon, K., Kjær, C., Carstensen, L. F., Tambour, M., Gram, B., Thomsen, J. B., & Sørensen, J. A. (2025). Exploratory Evaluation of Topical Tacrolimus for Prevention of Breast Cancer-Related Arm Lymphedema: A Multicenter Non-Randomized Pilot Study. Cancers, 17(23), 3753. https://doi.org/10.3390/cancers17233753






