Exploring the Pivotal Functions of Tertiary Lymphoid Structures in Cancer Prognosis and Immunotherapy Outcomes
Simple Summary
Abstract
1. Introduction
2. TLS Formation and Function
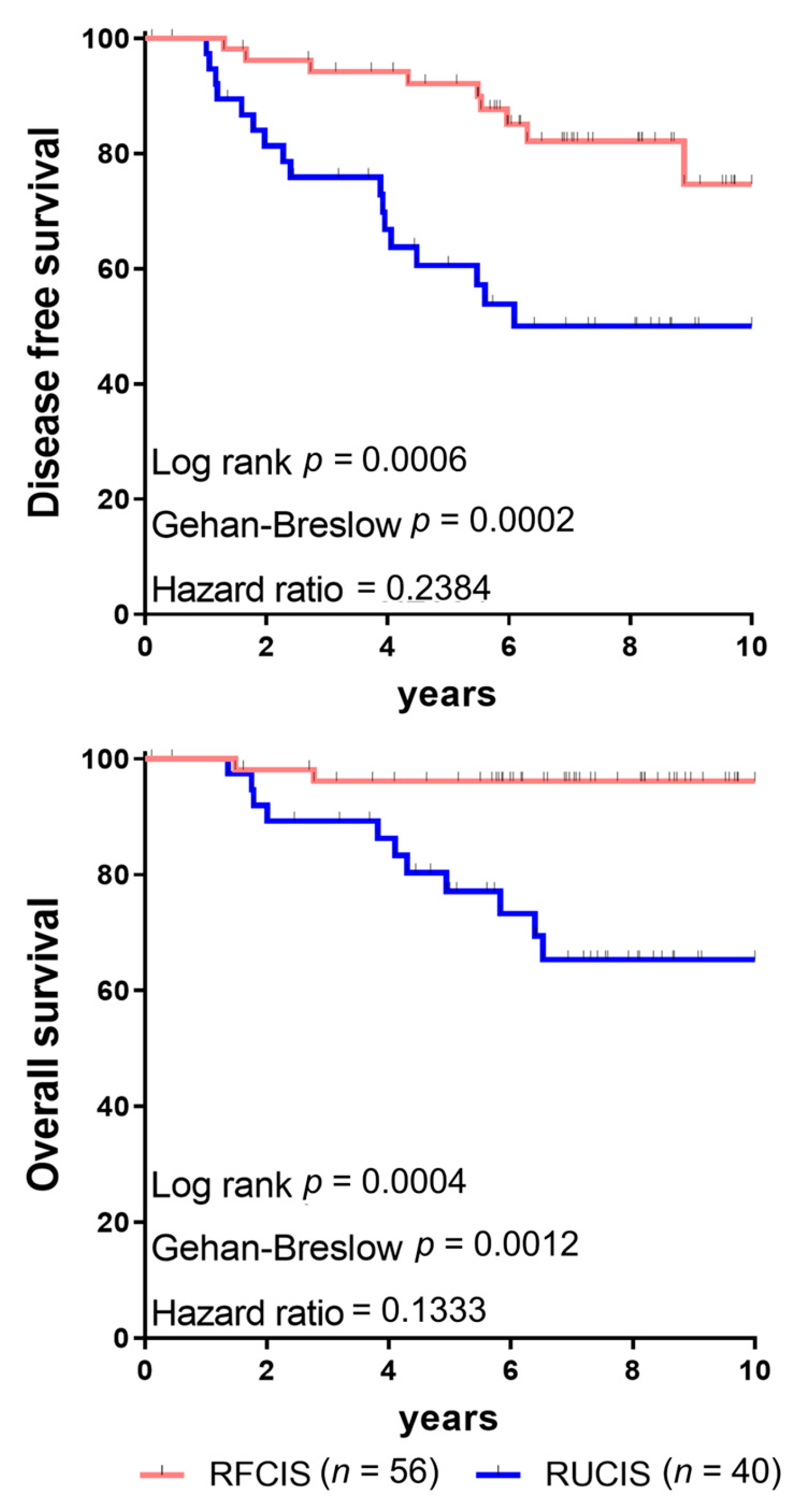
3. The Prognostic Role of TLS
4. The Combined Assessment of TILs and TLS as Indicators for Prognosis
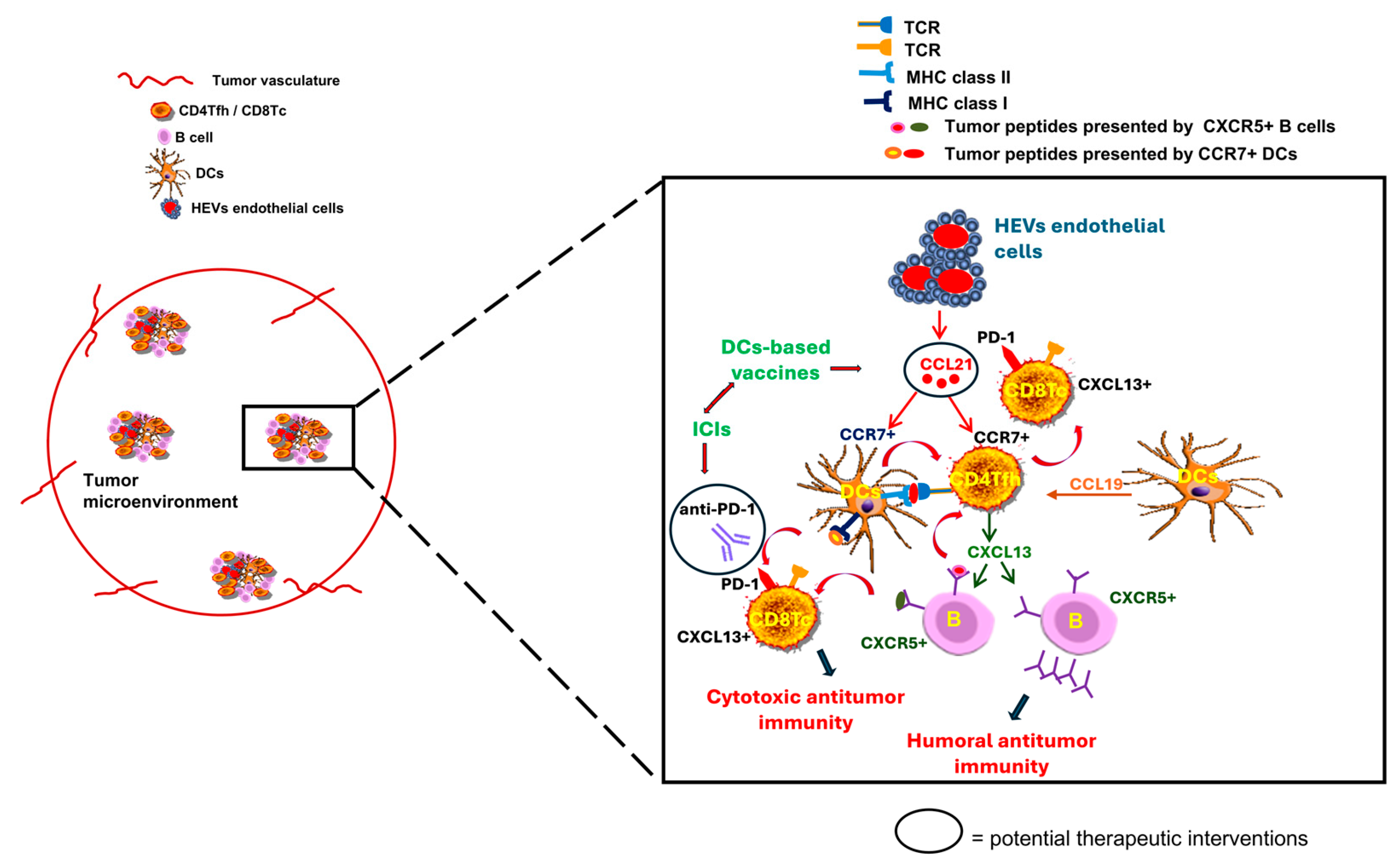
5. Chemokine-Driven TLS: Enhancing Antitumor Immunity and Synergy with Checkpoint Inhibitors
6. TLS as Predictive Biomarkers for Tailored Cancer Immunotherapy
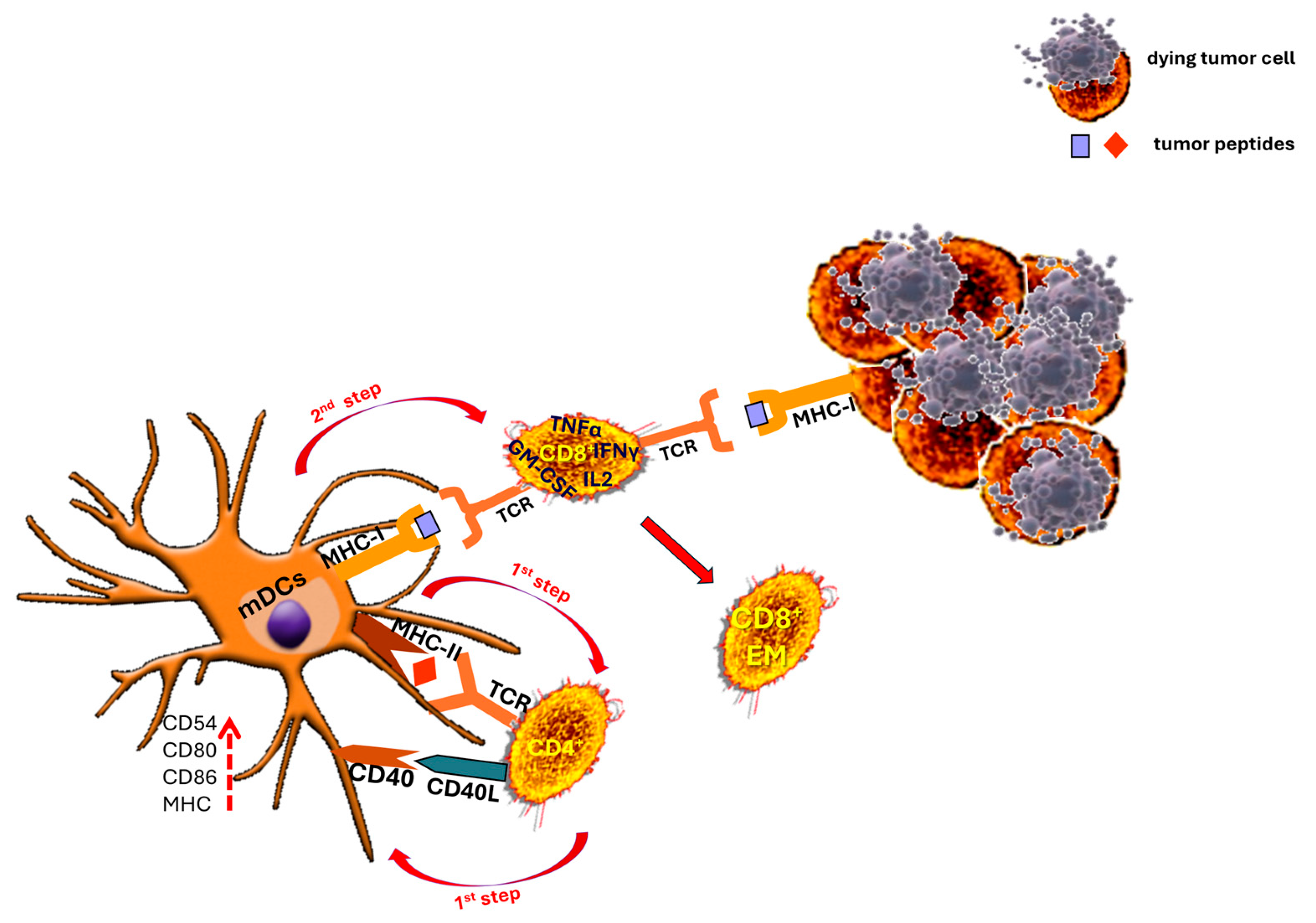
7. The Critical Contribution of CD4+ T Cells to the Development of Robust Antitumor CD8+ T Cell Responses
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLS | tertiary lymphoid structures |
| TME | tumor microenvironment |
| DCs | dendritic cells |
| GCs | germinal centers |
| HEVs | high endothelial venules |
| BCa | breast cancer |
| TNBC | triple-negative breast cancer |
| TILs | tumor infiltrating lymphocytes |
| PD-1 | programmed cell death protein-1 |
| PD-L1 | programmed cell death protein-1 ligand |
| NSCLC | non-small cell lung cancer |
| CXCL | chemokine C-X-C motif ligand |
| CCL | chemokine (C-C motif) ligand |
| ICIs | immune checkpoint inhibitors |
| FDCs | follicular dendritic cells |
| FRCs | fibroblastic reticular cells |
| Tfh | follicular helper T cell |
| IL | interleukin |
| NAC | neoadjuvant chemotherapy |
| TC | tumor center |
| IM | invasive margin |
| OS | overall survival |
| DFS | disease-free survival |
| FCIS | favorable combined immune signature |
| UCIS | unfavorable combined immune signature |
| RFCIS | reinforced favorable combined immune signature |
| RUCIS | reinforced unfavorable combined immune signature |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| IDO-1 | indoleamine 2,3-dioxygenase 1 |
| CIN | cervical intraepithelial neoplasia |
| HCMV | human cytomegalovirus |
| IE1 | immediate-early protein 1 |
| PDAC | pancreatic ductal adenocarcinoma |
References
- Fridman, W.H.; Meylan, M.; Pupier, G.; Calvez, A.; Hernandez, I.; Sautès-Fridman, C. Tertiary Lymphoid Structures and B Cells: An Intratumoral Immunity Cycle. Immunity 2023, 56, 2254–2269. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès–Fridman, C.; Kroemer, G. The Immune Contexture in Cancer Prognosis and Treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Zhan, J.; Kipp, M.; Han, W.; Kaddatz, H. Ectopic Lymphoid Follicles in Progressive Multiple Sclerosis: From Patients to Animal Models. Immunology 2021, 164, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, J.; Marodon, G.; Teillaud, J.; Dieu-Nosjean, M. The Sunrise of Tertiary Lymphoid Structures in Cancer. Immunol. Rev. 2025, 332, e70046. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary Lymphoid Structures in the Era of Cancer Immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Lawand, M.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Dieu-Nosjean, M.-C. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front. Immunol. 2016, 7, 407. [Google Scholar] [CrossRef]
- Finkin, S.; Yuan, D.; Stein, I.; Taniguchi, K.; Weber, A.; Unger, K.; Browning, J.L.; Goossens, N.; Nakagawa, S.; Gunasekaran, G.; et al. Ectopic Lymphoid Structures Function as Microniches for Tumor Progenitor Cells in Hepatocellular Carcinoma. Nat. Immunol. 2015, 16, 1235–1244. [Google Scholar] [CrossRef]
- Teillaud, J.-L.; Houel, A.; Panouillot, M.; Riffard, C.; Dieu-Nosjean, M.-C. Tertiary Lymphoid Structures in Anticancer Immunity. Nat. Rev. Cancer 2024, 24, 629–646. [Google Scholar] [CrossRef]
- Fridman, W.H.; Meylan, M.; Petitprez, F.; Sun, C.-M.; Italiano, A.; Sautès-Fridman, C. B Cells and Tertiary Lymphoid Structures as Determinants of Tumour Immune Contexture and Clinical Outcome. Nat. Rev. Clin. Oncol. 2022, 19, 441–457. [Google Scholar] [CrossRef]
- Liu, C.; Cao, J. The Pivotal Role of Tertiary Lymphoid Structures in the Tumor Immune Microenvironment. Front. Oncol. 2025, 15, 1616904. [Google Scholar] [CrossRef]
- Su, X.; Kang, D.; Wang, J.; Li, L.; Huang, R.; Zou, Z. Tertiary Lymphoid Structures Associated with Improved Survival and Enhanced Antitumor Immunity in Acral Melanoma. NPJ Precis. Oncol. 2025, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, M.; Ren, Y.; Ba, Y.; Liu, S.; Zuo, A.; Xu, H.; Weng, S.; Han, X.; Liu, Z. Tertiary Lymphoid Structural Heterogeneity Determines Tumour Immunity and Prospects for Clinical Application. Mol. Cancer 2024, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, S.; Wang, S.; Zhang, Z.; Wang, X.; Chen, Z.; Wang, X.; Huang, S.; Zhang, D.; Wu, H. Tertiary Lymphoid Structures in Diseases: Immune Mechanisms and Therapeutic Advances. Signal Transduct. Target. Ther. 2024, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Dieu-Nosjean, M.-C.; Goc, J.; Giraldo, N.A.; Sautès-Fridman, C.; Fridman, W.H. Tertiary Lymphoid Structures in Cancer and Beyond. Trends Immunol. 2014, 35, 571–580. [Google Scholar] [CrossRef]
- Baxevanis, C.N.; Fortis, S.P.; Perez, S.A. The Balance between Breast Cancer and the Immune System: Challenges for Prognosis and Clinical Benefit from Immunotherapies. Semin. Cancer Biol. 2021, 72, 76–89. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, I.A.; Song, I.H.; Shin, S.-J.; Kim, J.Y.; Yu, J.H.; Gong, G. Tertiary Lymphoid Structures: Prognostic Significance and Relationship with Tumour-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. J. Clin. Pathol. 2016, 69, 422–430. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.Y.; Park, I.A.; Song, I.H.; Yu, J.H.; Ahn, J.-H.; Gong, G. Prognostic Significance of Tumor-Infiltrating Lymphocytes and the Tertiary Lymphoid Structures in HER2-Positive Breast Cancer Treated with Adjuvant Trastuzumab. Am. J. Clin. Pathol. 2015, 144, 278–288. [Google Scholar] [CrossRef]
- Liu, X.; Tsang, J.Y.S.; Hlaing, T.; Hu, J.; Ni, Y.-B.; Chan, S.K.; Cheung, S.Y.; Tse, G.M. Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers. Oncologist 2017, 22, 1316–1324. [Google Scholar] [CrossRef]
- Figenschau, S.L.; Fismen, S.; Fenton, K.A.; Fenton, C.; Mortensen, E.S. Tertiary Lymphoid Structures Are Associated with Higher Tumor Grade in Primary Operable Breast Cancer Patients. BMC Cancer 2015, 15, 101. [Google Scholar] [CrossRef]
- Sofopoulos, M.; Fortis, S.P.; Vaxevanis, C.K.; Sotiriadou, N.N.; Arnogiannaki, N.; Ardavanis, A.; Vlachodimitropoulos, D.; Perez, S.A.; Baxevanis, C.N. The Prognostic Significance of Peritumoral Tertiary Lymphoid Structures in Breast Cancer. Cancer Immunol. Immunother. 2019, 68, 1733–1745. [Google Scholar] [CrossRef]
- Liu, X.; Lv, W.; Huang, D.; Cui, H. The Predictive Role of Tertiary Lymphoid Structures in the Prognosis and Response to Immunotherapy of Lung Cancer Patients: A Systematic Review and Meta-Analysis. BMC Cancer 2025, 25, 87. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wu, X.; Zhang, C.; Liang, Y.; Cheng, S.; Zhang, H.; Shen, L.; Chen, Y. Analyzing the Associations between Tertiary Lymphoid Structures and Postoperative Prognosis, along with Immunotherapy Response in Gastric Cancer: Findings from Pooled Cohort Studies. J. Cancer Res. Clin. Oncol. 2024, 150, 153. [Google Scholar] [CrossRef] [PubMed]
- Spicer, J.; Cascone, T.; Kar, G.; Zheng, Y.; Blando, J.; Tan, T.H.; Cheng, M.; Mager, R.; Hamid, O.; Soo-Hoo, Y.; et al. Platform Study of Neoadjuvant Durvalumab (D) Alone or Combined with Novel Agents in Patients (Pts) with Resectable, Early-Stage Non-Small Cell Lung Cancer (NSCLC): Pharmacodynamic Correlates and Circulating Tumor DNA (CtDNA) Dynamics in the NeoCOAST Study. Ann. Oncol. 2022, 33, S971. [Google Scholar] [CrossRef]
- Gulubova, M.V. Tertiary Lymphoid Structures in Colorectal Cancer—Organization and Immune Cell Interactions. Am. J. Clin. Exp. Immunol. 2024, 13, 236–245. [Google Scholar] [CrossRef]
- Bao, X.; Lin, X.; Xie, M.; Yao, J.; Song, J.; Ma, X.; Zhang, X.; Zhang, Y.; Liu, Y.; Han, W.; et al. Mature Tertiary Lymphoid Structures: Important Contributors to Anti-Tumor Immune Efficacy. Front. Immunol. 2024, 15, 1413067. [Google Scholar] [CrossRef]
- Hegoburu, A.; Amer, M.; Frizelle, F.; Purcell, R. B Cells and Tertiary Lymphoid Structures in Cancer Therapy Response. BJC Rep. 2025, 3, 40. [Google Scholar] [CrossRef]
- Becht, E.; Giraldo, N.A.; Germain, C.; de Reyniès, A.; Laurent-Puig, P.; Zucman-Rossi, J.; Dieu-Nosjean, M.-C.; Sautès-Fridman, C.; Fridman, W.H. Immune Contexture, Immunoscore, and Malignant Cell Molecular Subgroups for Prognostic and Theranostic Classifications of Cancers. Adv. Immunol. 2016, 130, 95–190. [Google Scholar]
- Vanhersecke, L.; Brunet, M.; Guégan, J.-P.; Rey, C.; Bougouin, A.; Cousin, S.; Le Moulec, S.; Besse, B.; Loriot, Y.; Larroquette, M.; et al. Mature Tertiary Lymphoid Structures Predict Immune Checkpoint Inhibitor Efficacy in Solid Tumors Independently of PD-L1 Expression. Nat. Cancer 2021, 2, 794–802. [Google Scholar] [CrossRef]
- Kim, H.M.; Bruno, T.C. An Introduction to Tertiary Lymphoid Structures in Cancer. In Tertiary Lymphoid Structures. Methods in Molecular Biology; Humana: New York, NY, USA, 2025; pp. 1–19. [Google Scholar]
- Shu, D.H.; Sidiropoulos, D.N. Maturation of Tertiary Lymphoid Structures. In Tertiary Lymphoid Structures. Methods in Molecular Biology; Humana: New York, NY, USA, 2025; pp. 43–55. [Google Scholar]
- Vella, G.; Hua, Y.; Bergers, G. High Endothelial Venules in Cancer: Regulation, Function, and Therapeutic Implication. Cancer Cell 2023, 41, 527–545. [Google Scholar] [CrossRef]
- Omotesho, Q.A.; Escamilla, A.; Pérez-Ruiz, E.; Frecha, C.A.; Rueda-Domínguez, A.; Barragán, I. Epigenetic Targets to Enhance Antitumor Immune Response through the Induction of Tertiary Lymphoid Structures. Front. Immunol. 2024, 15, 1348156. [Google Scholar] [CrossRef]
- Langouo Fontsa, M.; Padonou, F.; Willard-Gallo, K. Tumor-Associated Tertiary Lymphoid Structures in Cancer: Implications for Immunotherapy. Expert Rev. Clin. Immunol. 2024, 20, 839–847. [Google Scholar] [CrossRef]
- Cui, X.; Gu, X.; Li, D.; Wu, P.; Sun, N.; Zhang, C.; He, J. Tertiary Lymphoid Structures as a Biomarker in Immunotherapy and beyond: Advancing towards Clinical Application. Cancer Lett. 2025, 613, 217491. [Google Scholar] [CrossRef]
- Cui, C.; Craft, J.; Joshi, N.S. T Follicular Helper Cells in Cancer, Tertiary Lymphoid Structures, and Beyond. Semin. Immunol. 2023, 69, 101797. [Google Scholar] [CrossRef]
- Ruffin, A.T.; Cillo, A.R.; Tabib, T.; Liu, A.; Onkar, S.; Kunning, S.R.; Lampenfeld, C.; Atiya, H.I.; Abecassis, I.; Kürten, C.H.L.; et al. B Cell Signatures and Tertiary Lymphoid Structures Contribute to Outcome in Head and Neck Squamous Cell Carcinoma. Nat. Commun. 2021, 12, 3349. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Ito, M.; Ohmura, H.; Hanamura, F.; Nakano, M.; Tsuchihashi, K.; Nagai, S.; Ariyama, H.; Kusaba, H.; Yamamoto, H.; et al. Helper T Cell-Dominant Tertiary Lymphoid Structures Are Associated with Disease Relapse of Advanced Colorectal Cancer. Oncoimmunology 2020, 9, 1724763. [Google Scholar] [CrossRef] [PubMed]
- Teillaud, J.-L.; Dieu-Nosjean, M.-C. Intratumoral Plasma Cells: More than a Predictive Marker of Response to Anti-PD-L1 Treatment in Lung Cancer? Cancer Cell 2022, 40, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Chandnani, N.; Gupta, I.; Mandal, A.; Sarkar, K. Participation of B Cell in Immunotherapy of Cancer. Pathol. Res. Pract. 2024, 255, 155169. [Google Scholar] [CrossRef]
- Yoshimitsu, M.; Nakamura, M.; Kano, S.; Magara, T.; Kato, H.; Sakai, A.; Sugiyama, M.; Mizokami, M.; Morita, A. CXCL13 and CCL21 Induce Tertiary Lymphoid Structures and Enhance the Efficacy of Immunotherapy for Melanoma. Cancer Sci. 2025, 116, 2075–2085. [Google Scholar] [CrossRef]
- Laumont, C.M.; Nelson, B.H. B Cells in the Tumor Microenvironment: Multi-Faceted Organizers, Regulators, and Effectors of Anti-Tumor Immunity. Cancer Cell 2023, 41, 466–489. [Google Scholar] [CrossRef]
- Shalapour, S.; Font-Burgada, J.; Di Caro, G.; Zhong, Z.; Sanchez-Lopez, E.; Dhar, D.; Willimsky, G.; Ammirante, M.; Strasner, A.; Hansel, D.E.; et al. Immunosuppressive Plasma Cells Impede T-Cell-Dependent Immunogenic Chemotherapy. Nature 2015, 521, 94–98. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Becht, E.; Vano, Y.; Petitprez, F.; Lacroix, L.; Validire, P.; Sanchez-Salas, R.; Ingels, A.; Oudard, S.; Moatti, A.; et al. Tumor-Infiltrating and Peripheral Blood T-Cell Immunophenotypes Predict Early Relapse in Localized Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 4416–4428. [Google Scholar] [CrossRef]
- Becht, E.; Giraldo, N.A.; Beuselinck, B.; Job, S.; Marisa, L.; Vano, Y.; Oudard, S.; Zucman-Rossi, J.; Laurent-Puig, P.; Sautès-Fridman, C.; et al. Prognostic and Theranostic Impact of Molecular Subtypes and Immune Classifications in Renal Cell Cancer (RCC) and Colorectal Cancer (CRC). Oncoimmunology 2015, 4, e1049804. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; Fossati, N.; Vano, Y.; Freschi, M.; Becht, E.; Lucianò, R.; Calderaro, J.; Guédet, T.; Lacroix, L.; Rancoita, P.M.V.; et al. PD-L1 Expression and CD8+ T-Cell Infiltrate Are Associated with Clinical Progression in Patients with Node-Positive Prostate Cancer. Eur. Urol. Focus 2019, 5, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Fraune, C.; Blessin, N.C.; Lennartz, M.; Kluth, M.; Hube-Magg, C.; Lindhorst, L.; Dahlem, R.; Fisch, M.; Eichenauer, T.; et al. Tumor Cell PD-L1 Expression Is a Strong Predictor of Unfavorable Prognosis in Immune Checkpoint Therapy-Naive Clear Cell Renal Cell Cancer. Int. Urol. Nephrol. 2021, 53, 2493–2503. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Q.; Chen, X.; Zhang, B.; Liang, J.; Zhang, B. B Cells and Tertiary Lymphoid Structures in Tumors: Immunity Cycle, Clinical Impact, and Therapeutic Applications. Theranostics 2025, 15, 605–631. [Google Scholar] [CrossRef]
- Vella, G.; Guelfi, S.; Bergers, G. High Endothelial Venules: A Vascular Perspective on Tertiary Lymphoid Structures in Cancer. Front. Immunol. 2021, 12, 736670. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Sautès-Fridman, C. Tertiary Lymphoid Structures, Drivers of the Anti-tumor Responses in Human Cancers. Immunol. Rev. 2016, 271, 260–275. [Google Scholar] [CrossRef]
- Baxevanis, C.N.; Voutsas, I.F.; Tsitsilonis, O.E.; Gritzapis, A.D.; Sotiriadou, R.; Papamichail, M. Tumor-Specific CD4+ T Lymphocytes from Cancer Patients Are Required for Optimal Induction of Cytotoxic T Cells Against the Autologous Tumor. J. Immunol. 2000, 164, 3902–3912. [Google Scholar] [CrossRef]
- Weng, Y.; Yuan, J.; Cui, X.; Wang, J.; Chen, H.; Xu, L.; Chen, X.; Peng, M.; Song, Q. The Impact of Tertiary Lymphoid Structures on Tumor Prognosis and the Immune Microenvironment in Non-Small Cell Lung Cancer. Sci. Rep. 2024, 14, 16246. [Google Scholar] [CrossRef]
- Shen, S.; Sckisel, G.; Sahoo, A.; Lalani, A.; Otter, D.D.; Pearson, J.; DeVoss, J.; Cheng, J.; Casey, S.C.; Case, R.; et al. Engineered IL-21 Cytokine Muteins Fused to Anti-PD-1 Antibodies Can Improve CD8+ T Cell Function and Anti-Tumor Immunity. Front. Immunol. 2020, 11, 832. [Google Scholar] [CrossRef]
- Hennequin, A.; Derangère, V.; Boidot, R.; Apetoh, L.; Vincent, J.; Orry, D.; Fraisse, J.; Causeret, S.; Martin, F.; Arnould, L.; et al. Tumor Infiltration by Tbet+ Effector T Cells and CD20+ B Cells Is Associated with Survival in Gastric Cancer Patients. Oncoimmunology 2016, 5, e1054598. [Google Scholar] [CrossRef] [PubMed]
- Origoni, M.; Parma, M.; Dell’Antonio, G.; Gelardi, C.; Stefani, C.; Salvatore, S.; Candiani, M. Prognostic Significance of Immunohistochemical Phenotypes in Patients Treated for High-Grade Cervical Intraepithelial Neoplasia. Biomed Res. Int. 2013, 2013, 831907. [Google Scholar] [CrossRef]
- Calderaro, J.; Petitprez, F.; Becht, E.; Laurent, A.; Hirsch, T.Z.; Rousseau, B.; Luciani, A.; Amaddeo, G.; Derman, J.; Charpy, C.; et al. Intra-Tumoral Tertiary Lymphoid Structures Are Associated with a Low Risk of Early Recurrence of Hepatocellular Carcinoma. J. Hepatol. 2019, 70, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, J.; Xie, M.; Liang, Y.; Lin, X.; Song, J.; Bao, X.; Ma, X.; Wang, Y.; Zhang, Y.; et al. Tertiary Lymphoid Structures as Potential Biomarkers for Cancer Prediction and Prognosis. Int. Immunopharmacol. 2024, 140, 112790. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Meng, X.; Tang, X.; Zou, W.; He, Y. Intratumoral Tertiary Lymphoid Structures Promote Patient Survival and Immunotherapy Response in Head Neck Squamous Cell Carcinoma. Cancer Immunol. Immunother. 2023, 72, 1505–1521. [Google Scholar] [CrossRef]
- Karjula, T.; Niskakangas, A.; Mustonen, O.; Puro, I.; Elomaa, H.; Ahtiainen, M.; Kuopio, T.; Mecklin, J.-P.; Seppälä, T.T.; Wirta, E.-V.; et al. Tertiary Lymphoid Structures in Pulmonary Metastases of Microsatellite Stable Colorectal Cancer. Virchows Arch. 2023, 483, 21–32. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, X.; An, R.; Bai, J.; Dong, J.; Cai, H.; Zhu, H.; Zhong, W.; Chen, W.; Liu, A.; et al. Peritumoral Tertiary Lymphoid Structure and Tumor Stroma Percentage Predict the Prognosis of Patients with Non-Metastatic Colorectal Cancer. Front. Immunol. 2022, 13, 962056. [Google Scholar] [CrossRef]
- Deguchi, S.; Tanaka, H.; Suzuki, S.; Natsuki, S.; Mori, T.; Miki, Y.; Yoshii, M.; Tamura, T.; Toyokawa, T.; Lee, S.; et al. Clinical Relevance of Tertiary Lymphoid Structures in Esophageal Squamous Cell Carcinoma. BMC Cancer 2022, 22, 699. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B Cells and Tertiary Lymphoid Structures Promote Immunotherapy Response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Marliot, F.; Pagès, F.; Galon, J. Usefulness and Robustness of Immunoscore for Personalized Management of Cancer Patients. Oncoimmunology 2020, 9, 1832324. [Google Scholar] [CrossRef]
- Galon, J.; Angell, H.K.; Bedognetti, D.; Marincola, F.M. The Continuum of Cancer Immunosurveillance: Prognostic, Predictive, and Mechanistic Signatures. Immunity 2013, 39, 11–26. [Google Scholar] [CrossRef]
- Sayaman, R.W.; Saad, M.; Thorsson, V.; Hu, D.; Hendrickx, W.; Roelands, J.; Porta-Pardo, E.; Mokrab, Y.; Farshidfar, F.; Kirchhoff, T.; et al. Germline Genetic Contribution to the Immune Landscape of Cancer. Immunity 2021, 54, 367–386.e8. [Google Scholar] [CrossRef] [PubMed]
- Berthe, J.; Poudel, P.; Segerer, F.J.; Jennings, E.C.; Ng, F.; Surace, M.; Andoni, A.; Testori, M.; Saraiya, M.; Vuko, M.; et al. Exploring the Impact of Tertiary Lymphoid Structures Maturity in NSCLC: Insights from TLS Scoring. Front. Immunol. 2024, 15, 1422206. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Koop, K.; Weigert, A. Heterogeneity of Tertiary Lymphoid Structures in Cancer. Front. Immunol. 2023, 14, 1286850. [Google Scholar] [CrossRef] [PubMed]
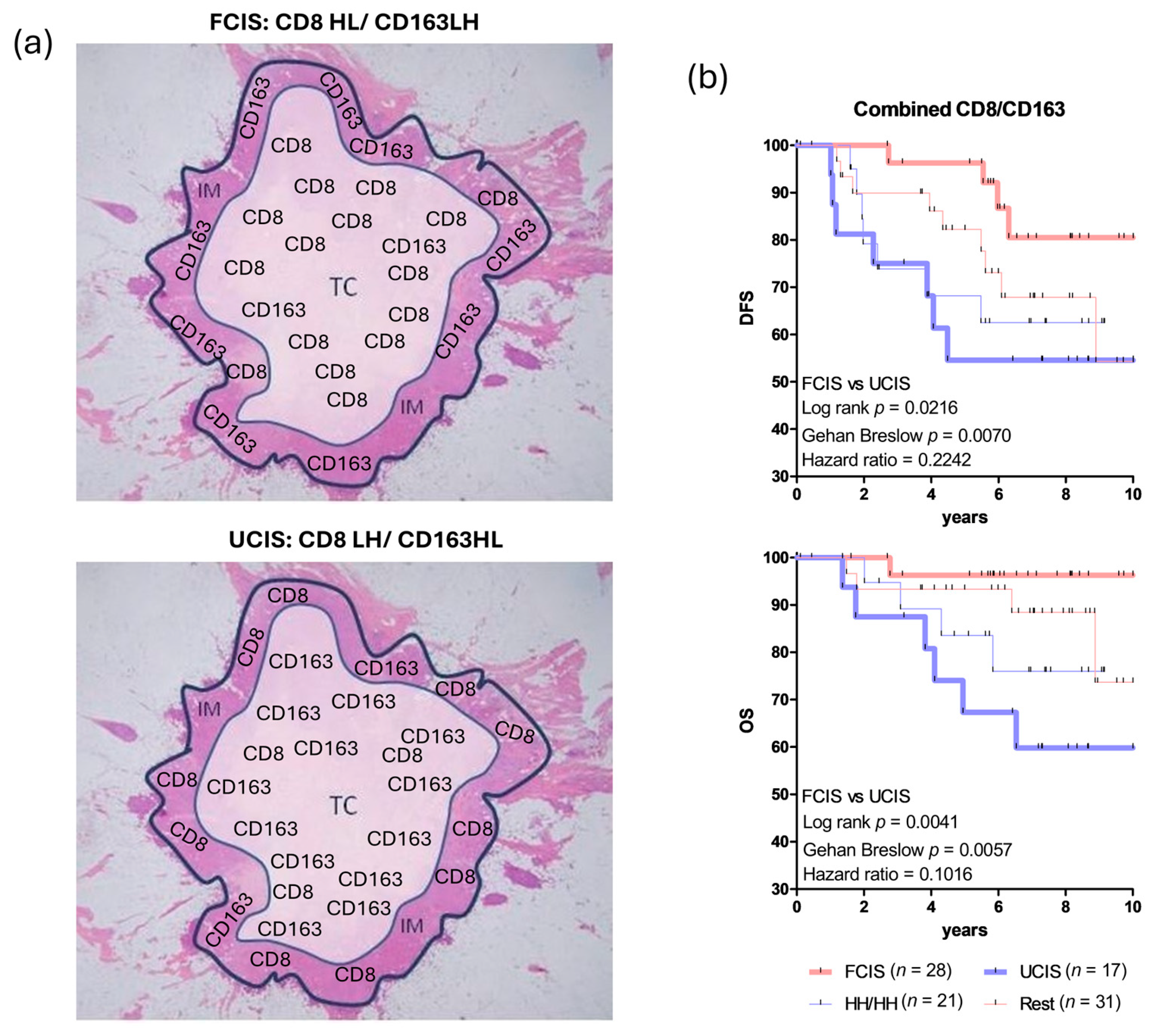
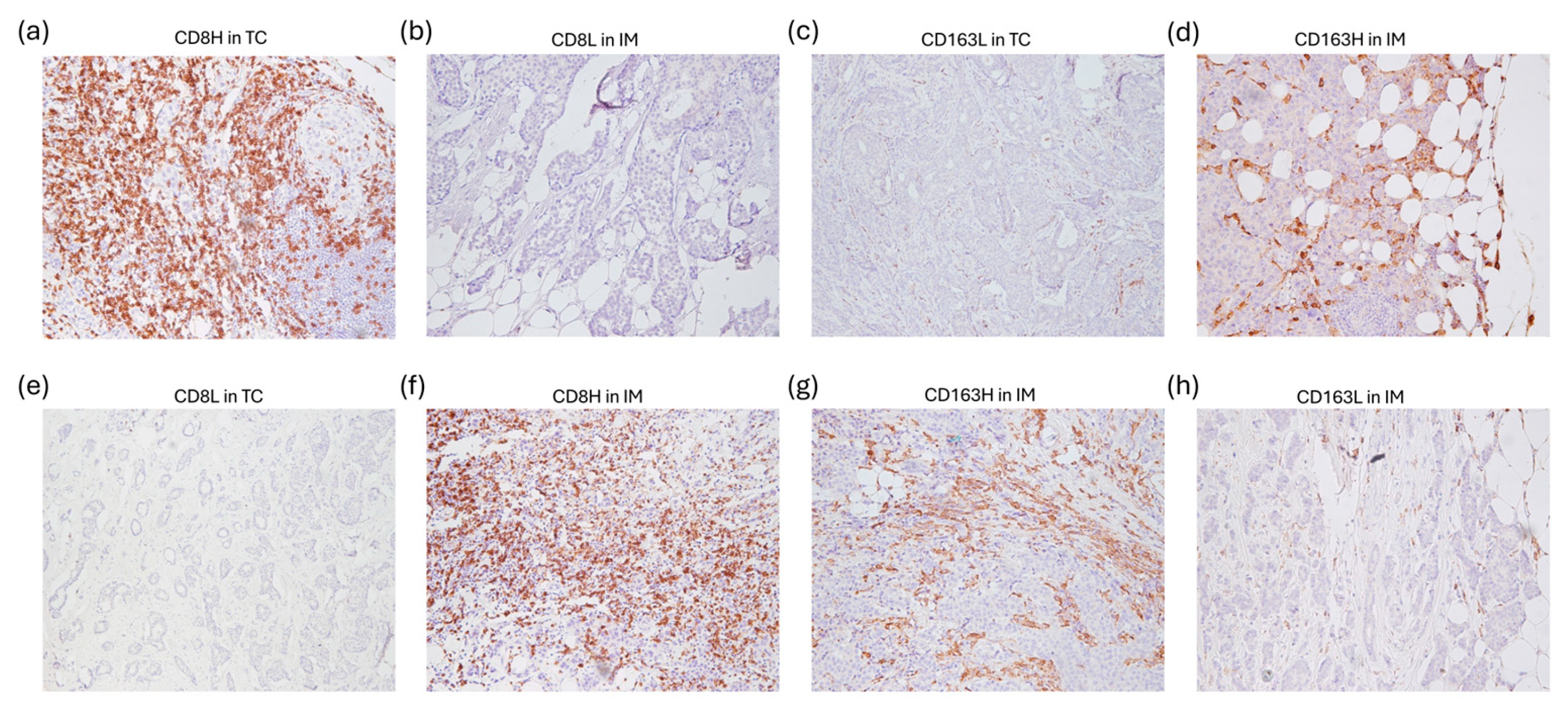
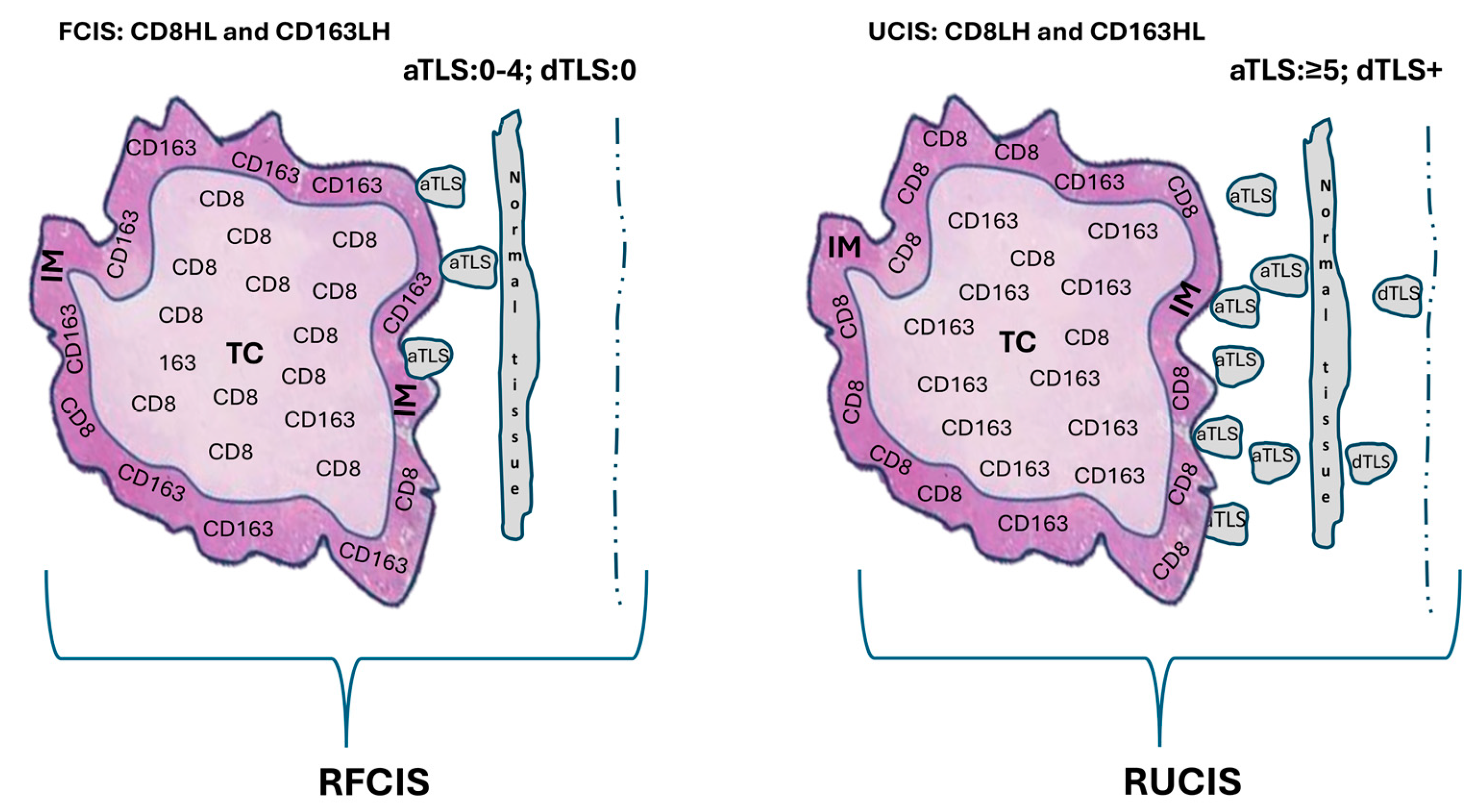
- Fortis, S.P.; Sofopoulos, M.; Sotiriadou, N.N.; Haritos, C.; Vaxevanis, C.K.; Anastasopoulou, E.A.; Janssen, N.; Arnogiannaki, N.; Ardavanis, A.; Pawelec, G.; et al. Differential Intratumoral Distributions of CD8 and CD163 Immune Cells as Prognostic Biomarkers in Breast Cancer. J. Immunother. Cancer 2017, 5, 39. [Google Scholar] [CrossRef]
- Martinet, L.; Filleron, T.; Le Guellec, S.; Rochaix, P.; Garrido, I.; Girard, J.-P. High Endothelial Venule Blood Vessels for Tumor-Infiltrating Lymphocytes Are Associated with Lymphotoxin β–Producing Dendritic Cells in Human Breast Cancer. J. Immunol. 2013, 191, 2001–2008. [Google Scholar] [CrossRef]
- Varghese, A.; Hess, S.M.; Chilakapati, S.; Conejo-Garcia, J.R.; McGray, A.J.R.; Zsiros, E. Tertiary Lymphoid Structures: Exploring Opportunities to Improve Immunotherapy in Ovarian Cancer. Front. Immunol. 2025, 16, 1473969. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.-J.; Zhang, B.; Lin, W.-P.; Li, S.-J.; Xiong, D.; Wang, Q.; Wang, W.-D.; Yang, Q.-C.; Huang, C.-F.; et al. Mature Tertiary Lymphoid Structures Evoke Intra-Tumoral T and B Cell Responses via Progenitor Exhausted CD4+ T Cells in Head and Neck Cancer. Nat. Commun. 2025, 16, 4228. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Liu, D.; Yang, K.; Xiong, J.; Fang, Z. Multiple Mechanisms and Applications of Tertiary Lymphoid Structures and Immune Checkpoint Blockade. J. Exp. Clin. Cancer Res. 2025, 44, 84. [Google Scholar] [CrossRef]
- Messina, J.L.; Fenstermacher, D.A.; Eschrich, S.; Qu, X.; Berglund, A.E.; Lloyd, M.C.; Schell, M.J.; Sondak, V.K.; Weber, J.S.; Mulé, J.J. 12-Chemokine Gene Signature Identifies Lymph Node-like Structures in Melanoma: Potential for Patient Selection for Immunotherapy? Sci. Rep. 2012, 2, 765. [Google Scholar] [CrossRef]
- Korbecki, J.; Kojder, K.; Barczak, K.; Simińska, D.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Hypoxia Alters the Expression of CC Chemokines and CC Chemokine Receptors in a Tumor–A Literature Review. Int. J. Mol. Sci. 2020, 21, 5647. [Google Scholar] [CrossRef]
- Korbecki, J.; Kojder, K.; Simińska, D.; Bohatyrewicz, R.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. Int. J. Mol. Sci. 2020, 21, 8412. [Google Scholar] [CrossRef]
- Karapetyan, L.; Li, A.; Vargas De Stefano, D.; Abushukair, H.M.; Al-Bzour, A.N.; Knight, A.; Layding, C.; Wang, H.; Xu, J.; Yao, J.; et al. Differences in the Pathological, Transcriptomic, and Prognostic Implications of Lymphoid Structures between Primary and Metastatic Cutaneous Melanomas. J. Immunother. Cancer 2024, 12, e009231. [Google Scholar] [CrossRef]
- Li, J.-P.; Wu, C.-Y.; Chen, M.-Y.; Liu, S.-X.; Yan, S.-M.; Kang, Y.-F.; Sun, C.; Grandis, J.R.; Zeng, M.-S.; Zhong, Q. PD-1+CXCR5−CD4+ Th-CXCL13 Cell Subset Drives B Cells into Tertiary Lymphoid Structures of Nasopharyngeal Carcinoma. J. Immunother. Cancer 2021, 9, e002101. [Google Scholar] [CrossRef] [PubMed]
- Petroni, G.; Pillozzi, S.; Antonuzzo, L. Exploiting Tertiary Lymphoid Structures to Stimulate Antitumor Immunity and Improve Immunotherapy Efficacy. Cancer Res. 2024, 84, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Ukita, M.; Hamanishi, J.; Yoshitomi, H.; Yamanoi, K.; Takamatsu, S.; Ueda, A.; Suzuki, H.; Hosoe, Y.; Furutake, Y.; Taki, M.; et al. CXCL13-Producing CD4+ T Cells Accumulate in the Early Phase of Tertiary Lymphoid Structures in Ovarian Cancer. JCI Insight 2022, 7, e157215. [Google Scholar] [CrossRef] [PubMed]
- Workel, H.H.; Lubbers, J.M.; Arnold, R.; Prins, T.M.; van der Vlies, P.; de Lange, K.; Bosse, T.; van Gool, I.C.; Eggink, F.A.; Wouters, M.C.A.; et al. A Transcriptionally Distinct CXCL13+CD103+CD8+ T-Cell Population Is Associated with B-Cell Recruitment and Neoantigen Load in Human Cancer. Cancer Immunol. Res. 2019, 7, 784–796. [Google Scholar] [CrossRef]
- Ding, L.; Sun, L.; Bu, M.T.; Zhang, Y.; Scott, L.N.; Prins, R.M.; Su, M.A.; Lechner, M.G.; Hugo, W. Antigen Presentation by Clonally Diverse CXCR5+ B Cells to CD4 and CD8 T Cells Is Associated with Durable Response to Immune Checkpoint Inhibitors. Front. Immunol. 2023, 14, 1176994. [Google Scholar] [CrossRef]
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-Analysis of Tumor- and T Cell-Intrinsic Mechanisms of Sensitization to Checkpoint Inhibition. Cell 2021, 184, 596–614.e14. [Google Scholar] [CrossRef]
- Lowery, F.J.; Krishna, S.; Yossef, R.; Parikh, N.B.; Chatani, P.D.; Zacharakis, N.; Parkhurst, M.R.; Levin, N.; Sindiri, S.; Sachs, A.; et al. Molecular Signatures of Antitumor Neoantigen-Reactive T Cells from Metastatic Human Cancers. Science 2022, 375, 877–884. [Google Scholar] [CrossRef]
- Kadam, P.; Singh, R.P.; Davoodi, M.; Lee, J.M.; St. John, M.; Sharma, S. Immune Checkpoint Blockade Enhances Immune Activity of Therapeutic Lung Cancer Vaccine. Vaccines 2020, 8, 655. [Google Scholar] [CrossRef]
- Sharma, S.; Kadam, P.; Dubinett, S. CCL21 Programs Immune Activity in Tumor Microenvironment. In Tumor Microenvironment. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; pp. 67–78. [Google Scholar]
- Wu, S.-Y.; Zhang, S.-W.; Ma, D.; Xiao, Y.; Liu, Y.; Chen, L.; Song, X.-Q.; Ma, X.-Y.; Xu, Y.; Chai, W.-J.; et al. CCL19+ Dendritic Cells Potentiate Clinical Benefit of Anti-PD-(L)1 Immunotherapy in Triple-Negative Breast Cancer. Med 2023, 4, 373–393.e8. [Google Scholar] [CrossRef] [PubMed]
- Kadam, P.; Sharma, S. PD-1 Immune Checkpoint Blockade Promotes Therapeutic Cancer Vaccine to Eradicate Lung Cancer. Vaccines 2020, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, M.-H.; Garon, E.; Goldman, J.W.; Salehi-Rad, R.; Baratelli, F.E.; Schaue, D.; Wang, G.; Rosen, F.; Yanagawa, J.; et al. Phase I Trial of Intratumoral Injection of CCL21 Gene–Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8+ T-Cell Infiltration. Clin. Cancer Res. 2017, 23, 4556–4568. [Google Scholar] [CrossRef] [PubMed]
- Baratelli, F.; Takedatsu, H.; Hazra, S.; Peebles, K.; Luo, J.; Kurimoto, P.S.; Zeng, G.; Batra, R.K.; Sharma, S.; Dubinett, S.M.; et al. Pre-Clinical Characterization of GMP Grade CCL21-Gene Modified Dendritic Cells for Application in a Phase I Trial in Non-Small Cell Lung Cancer. J. Transl. Med. 2008, 6, 38. [Google Scholar] [CrossRef]
- Salehi-Rad, R.; Lim, R.J.; Du, Y.; Tran, L.M.; Li, R.; Ong, S.L.; Ling Huang, Z.; Dumitras, C.; Zhang, T.; Park, S.J.; et al. CCL21-DC in Situ Vaccination in Murine NSCLC Overcomes Resistance to Immunotherapy and Generates Systemic Tumor-Specific Immunity. J. Immunother. Cancer 2023, 11, e006896. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, H.; Pu, N.; Zhang, J.; Zhao, G.; Lou, W.; Wu, W. Chemokine C-C Motif Ligand 21 Synergized with Programmed Death-ligand 1 Blockade Restrains Tumor Growth. Cancer Sci. 2021, 112, 4457–4469. [Google Scholar] [CrossRef]
- Oh, M.S.; Dumitras, C.; Salehi-Rad, R.; Tran, L.M.; Krysan, K.; Lim, R.J.; Jing, Z.; Tappuni, S.; Lisberg, A.; Garon, E.B.; et al. Characteristics of a CCL21 Gene–Modified Dendritic Cell Vaccine Utilized for a Clinical Trial in Non–Small Cell Lung Cancer. Mol. Cancer Ther. 2025, 24, 286–298. [Google Scholar] [CrossRef]
- Choi, J.; Crotty, S.; Choi, Y.S. Cytokines in Follicular Helper T Cell Biology in Physiologic and Pathologic Conditions. Immune Netw. 2024, 24, e8. [Google Scholar] [CrossRef]
- Yang, F.; Yang, J.; Wu, M.; Chen, C.; Chu, X. Tertiary Lymphoid Structures: New Immunotherapy Biomarker. Front. Immunol. 2024, 15, 1394505. [Google Scholar] [CrossRef]
- Voabil, P.; de Bruijn, M.; Roelofsen, L.M.; Hendriks, S.H.; Brokamp, S.; van den Braber, M.; Broeks, A.; Sanders, J.; Herzig, P.; Zippelius, A.; et al. An Ex Vivo Tumor Fragment Platform to Dissect Response to PD-1 Blockade in Cancer. Nat. Med. 2021, 27, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary Lymphoid Structures Improve Immunotherapy and Survival in Melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Makino, T.; Sato, E.; Ohshima, K.; Nogi, Y.; Kanemura, T.; Honma, K.; Yamashita, K.; Saito, T.; Tanaka, K.; et al. Density and Maturity of Peritumoral Tertiary Lymphoid Structures in Oesophageal Squamous Cell Carcinoma Predicts Patient Survival and Response to Immune Checkpoint Inhibitors. Br. J. Cancer 2023, 128, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B Cells Are Associated with Survival and Immunotherapy Response in Sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Italiano, A.; Bessede, A.; Pulido, M.; Bompas, E.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Bertucci, F.; Toulmonde, M.; Bellera, C.; et al. Pembrolizumab in Soft-Tissue Sarcomas with Tertiary Lymphoid Structures: A Phase 2 PEMBROSARC Trial Cohort. Nat. Med. 2022, 28, 1199–1206. [Google Scholar] [CrossRef]
- Rodriguez, A.B.; Peske, J.D.; Woods, A.N.; Leick, K.M.; Mauldin, I.S.; Meneveau, M.O.; Young, S.J.; Lindsay, R.S.; Melssen, M.M.; Cyranowski, S.; et al. Immune Mechanisms Orchestrate Tertiary Lymphoid Structures in Tumors via Cancer-Associated Fibroblasts. Cell Rep. 2021, 36, 109422. [Google Scholar] [CrossRef]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 Combination Blockade Expands Infiltrating T Cells and Reduces Regulatory T and Myeloid Cells within B16 Melanoma Tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef]
- Vaccaro, A.; van de Walle, T.; Ramachandran, M.; Essand, M.; Dimberg, A. Of Mice and Lymphoid Aggregates: Modeling Tertiary Lymphoid Structures in Cancer. Front. Immunol. 2023, 14, 1275378. [Google Scholar] [CrossRef]
- Lynch, K.T.; Young, S.J.; Meneveau, M.O.; Wages, N.A.; Engelhard, V.H.; Slingluff, C.L., Jr.; Mauldin, I.S. Heterogeneity in Tertiary Lymphoid Structure B-Cells Correlates with Patient Survival in Metastatic Melanoma. J. Immunother. Cancer 2021, 9, e002273. [Google Scholar] [CrossRef]
- Sun, X.; Liu, W.; Sun, L.; Mo, H.; Feng, Y.; Wu, X.; Li, C.; Chen, C.; Li, J.; Xin, Y.; et al. Maturation and Abundance of Tertiary Lymphoid Structures Are Associated with the Efficacy of Neoadjuvant Chemoimmunotherapy in Resectable Non-Small Cell Lung Cancer. J. Immunother. Cancer 2022, 10, e005531. [Google Scholar] [CrossRef]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical Efficacy and Biomarker Analysis of Neoadjuvant Atezolizumab in Operable Urothelial Carcinoma in the ABACUS Trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef]
- Melssen, M.M.; Pollack, K.E.; Meneveau, M.O.; Smolkin, M.E.; Pinczewski, J.; Koeppel, A.F.; Turner, S.D.; Sol-Church, K.; Hickman, A.; Deacon, D.H.; et al. Characterization and Comparison of Innate and Adaptive Immune Responses at Vaccine Sites in Melanoma Vaccine Clinical Trials. Cancer Immunol. Immunother. 2021, 70, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Lutz, E.R.; Wu, A.A.; Bigelow, E.; Sharma, R.; Mo, G.; Soares, K.; Solt, S.; Dorman, A.; Wamwea, A.; Yager, A.; et al. Immunotherapy Converts Nonimmunogenic Pancreatic Tumors into Immunogenic Foci of Immune Regulation. Cancer Immunol. Res. 2014, 2, 616–631. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, L.; Teague, J.E.; Morrow, M.P.; Jotova, I.; Wu, T.C.; Wang, C.; Desmarais, C.; Boyer, J.D.; Tycko, B.; Robins, H.S.; et al. Intramuscular Therapeutic Vaccination Targeting HPV16 Induces T Cell Responses That Localize in Mucosal Lesions. Sci. Transl. Med. 2014, 6, 221ra13. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, S.; Liu, F.; Li, Z.; Liu, W.; Zhang, X.; Nan, F.; Li, J.; Yu, M.; Wang, Y.; et al. HCMV IE1/IE1mut Therapeutic Vaccine Induces Tumor Regression via Intratumoral Tertiary Lymphoid Structure Formation and Peripheral Immunity Activation in Glioblastoma Multiforme. Mol. Neurobiol. 2024, 61, 5935–5949. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Han, Y.; Deng, Y.; Li, J.; Jiang, Y. The Presence of Tertiary Lymphoid Structures Provides New Insight Into the Clinicopathological Features and Prognosis of Patients with Breast Cancer. Front. Immunol. 2022, 13, 868155. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, X.; Xi, L.; Dong, M.; Song, P.; Miao, J.; Lu, C.; Sun, S.; Li, Q.; Yu, C.; et al. Prediction Values of Tertiary Lymphoid Structures in the Prognosis of Patients with Left- and Right-Sided Colon Cancer: A Multicenter Propensity Score-Matched Study. Int. J. Surg. 2023, 109, 2344–2358. [Google Scholar] [CrossRef]
- Mori, T.; Tanaka, H.; Deguchi, S.; Yamakoshi, Y.; Miki, Y.; Yoshii, M.; Tamura, T.; Toyokawa, T.; Lee, S.; Muguruma, K.; et al. Clinical Efficacy of Nivolumab Is Associated with Tertiary Lymphoid Structures in Surgically Resected Primary Tumors of Recurrent Gastric Cancer. PLoS ONE 2022, 17, e0262455. [Google Scholar] [CrossRef]
- Siliņa, K.; Soltermann, A.; Attar, F.M.; Casanova, R.; Uckeley, Z.M.; Thut, H.; Wandres, M.; Isajevs, S.; Cheng, P.; Curioni-Fontecedro, A.; et al. Germinal Centers Determine the Prognostic Relevance of Tertiary Lymphoid Structures and Are Impaired by Corticosteroids in Lung Squamous Cell Carcinoma. Cancer Res. 2018, 78, 1308–1320. [Google Scholar] [CrossRef]
- Fukuhara, M.; Muto, S.; Inomata, S.; Yamaguchi, H.; Mine, H.; Takagi, H.; Ozaki, Y.; Watanabe, M.; Inoue, T.; Yamaura, T.; et al. The Clinical Significance of Tertiary Lymphoid Structure and Its Relationship with Peripheral Blood Characteristics in Patients with Surgically Resected Non-Small Cell Lung Cancer: A Single-Center, Retrospective Study. Cancer Immunol. Immunother. 2022, 71, 1129–1137. [Google Scholar] [CrossRef]
- Wen, S.; Chen, Y.; Hu, C.; Du, X.; Xia, J.; Wang, X.; Zhu, W.; Wang, Q.; Zhu, M.; Chen, Y.; et al. Combination of Tertiary Lymphoid Structure and Neutrophil-to-Lymphocyte Ratio Predicts Survival in Patients with Hepatocellular Carcinoma. Front. Immunol. 2022, 12, 788640. [Google Scholar] [CrossRef]
- Zhang, T.; Lei, X.; Jia, W.; Li, J.; Nie, Y.; Mao, Z.; Wang, Y.; Tao, K.; Song, W. Peritumor Tertiary Lymphoid Structures Are Associated with Infiltrating Neutrophils and Inferior Prognosis in Hepatocellular Carcinoma. Cancer Med. 2023, 12, 3068–3078. [Google Scholar] [CrossRef]
- Ding, G.-Y.; Ma, J.-Q.; Yun, J.-P.; Chen, X.; Ling, Y.; Zhang, S.; Shi, J.-Y.; Chang, Y.-Q.; Ji, Y.; Wang, X.-Y.; et al. Distribution and Density of Tertiary Lymphoid Structures Predict Clinical Outcome in Intrahepatic Cholangiocarcinoma. J. Hepatol. 2022, 76, 608–618. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.-Y.; Zuo, J.-L.; Wang, X.-F.; Feng, X.-W.; Zhang, B.; Li, Y.-T.; Yi, C.-H.; Zhang, P.; Ma, X.-C.; et al. Localization and Density of Tertiary Lymphoid Structures Associate with Molecular Subtype and Clinical Outcome in Colorectal Cancer Liver Metastases. J. Immunother. Cancer 2023, 11, e006425. [Google Scholar] [CrossRef]
- Xu, W.; Lu, J.; Liu, W.-R.; Anwaier, A.; Wu, Y.; Tian, X.; Su, J.-Q.; Qu, Y.-Y.; Yang, J.; Zhang, H.; et al. Heterogeneity in Tertiary Lymphoid Structures Predicts Distinct Prognosis and Immune Microenvironment Characterizations of Clear Cell Renal Cell Carcinoma. J. Immunother. Cancer 2023, 11, e006667. [Google Scholar] [CrossRef]
- Kasikova, L.; Rakova, J.; Hensler, M.; Lanickova, T.; Tomankova, J.; Pasulka, J.; Drozenova, J.; Mojzisova, K.; Fialova, A.; Vosahlikova, S.; et al. Tertiary Lymphoid Structures and B Cells Determine Clinically Relevant T Cell Phenotypes in Ovarian Cancer. Nat. Commun. 2024, 15, 2528. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, S.-Y.; He, S.; Chi, D.-M.; Wang, X.-Z.; Wen, Y.-F.; Ma, D.; Nie, R.-C.; Xiang, P.; Zhou, Y.; et al. Single-Cell and Spatial Transcriptome Analyses Reveal Tertiary Lymphoid Structures Linked to Tumour Progression and Immunotherapy Response in Nasopharyngeal Carcinoma. Nat. Commun. 2024, 15, 7713. [Google Scholar] [CrossRef] [PubMed]
- Reste, M.; Ajazi, K.; Sayi-Yazgan, A.; Jankovic, R.; Bufan, B.; Brandau, S.; Bækkevold, E.S.; Petitprez, F.; Lindstedt, M.; Adema, G.J.; et al. The Role of Dendritic Cells in Tertiary Lymphoid Structures: Implications in Cancer and Autoimmune Diseases. Front. Immunol. 2024, 15, 1439413. [Google Scholar] [CrossRef] [PubMed]
- Topchyan, P.; Lin, S.; Cui, W. The Role of CD4 T Cell Help in CD8 T Cell Differentiation and Function During Chronic Infection and Cancer. Immune Netw. 2023, 23, e41. [Google Scholar] [CrossRef] [PubMed]
- Huff, A.L.; Longway, G.; Mitchell, J.T.; Andaloori, L.; Davis-Marcisak, E.; Chen, F.; Lyman, M.R.; Wang, R.; Mathew, J.; Barrett, B.; et al. CD4 T Cell–Activating Neoantigens Enhance Personalized Cancer Vaccine Efficacy. JCI Insight 2023, 8, e174027. [Google Scholar] [CrossRef]
- Rock, K.L.; Shen, L. Cross-presentation: Underlying Mechanisms and Role in Immune Surveillance. Immunol. Rev. 2005, 207, 166–183. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-Presentation by Dendritic Cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
| Location | Findings/Outcomes | Reference(s) |
|---|---|---|
| TLS in TME | High frequencies of CD8+ PD-1+, cytokine-producing Tfh CD4+ cells, and DCs within TLS B-cell zones generating antitumor mechanistic pathways during immunotherapy. | [4,92,93] |
| High frequencies of TLS-associated PD1+ CD39+ CD103+ T memory cells correlate with responses to anti-PD-1. | [94] | |
| Memory B cells within TLS regulate clinical outcomes with ICIs in melanoma and renal cell carcinoma patients. | [61] | |
| TLS infiltrated by high numbers of TILs (CD8+, CD20+, Tnaive, Tmemory) and expressing an activated gene signature correlate with favorable clinical responses with ICIs in melanoma. | [95] | |
| High density of mature TLS predicted responses to anti-PD-1 and prolonged OS. | [96] | |
| Favorable clinical outcomes with PD-1 inhibitors in patients with soft tissue sarcomas having TLS with strong infiltration by CD8+ cells, B cells, and high PD-1 expression. | [97,98] | |
| Favorable response to neoadjuvant chemoimmunotherapy in NSCLC in the presence of high numbers mature TLS. | [103] | |
| Favorable response to neoadjuvant immunotherapy I urothelial carcinoma in the presence of TLS expressing high levels of an 8-effector gene signature. | [104] | |
| High densities of TLS expressing a 9-gene signature. Favorable clinical outcomes in breast cancer. | [109] | |
| High average level of TLS abundance predict favorable clinical responses in HCC, GC, colon cancer, and squamous lung cancer. | [110,111,112,113] | |
| TLS in peritumoral regions of melanoma metastatic lesions | Improved clinical outcomes with TLS-infiltrated B cells possessing Ig somatic hypermutations and worse outcomes associated with high densities of CD21+ B cells. | [102] |
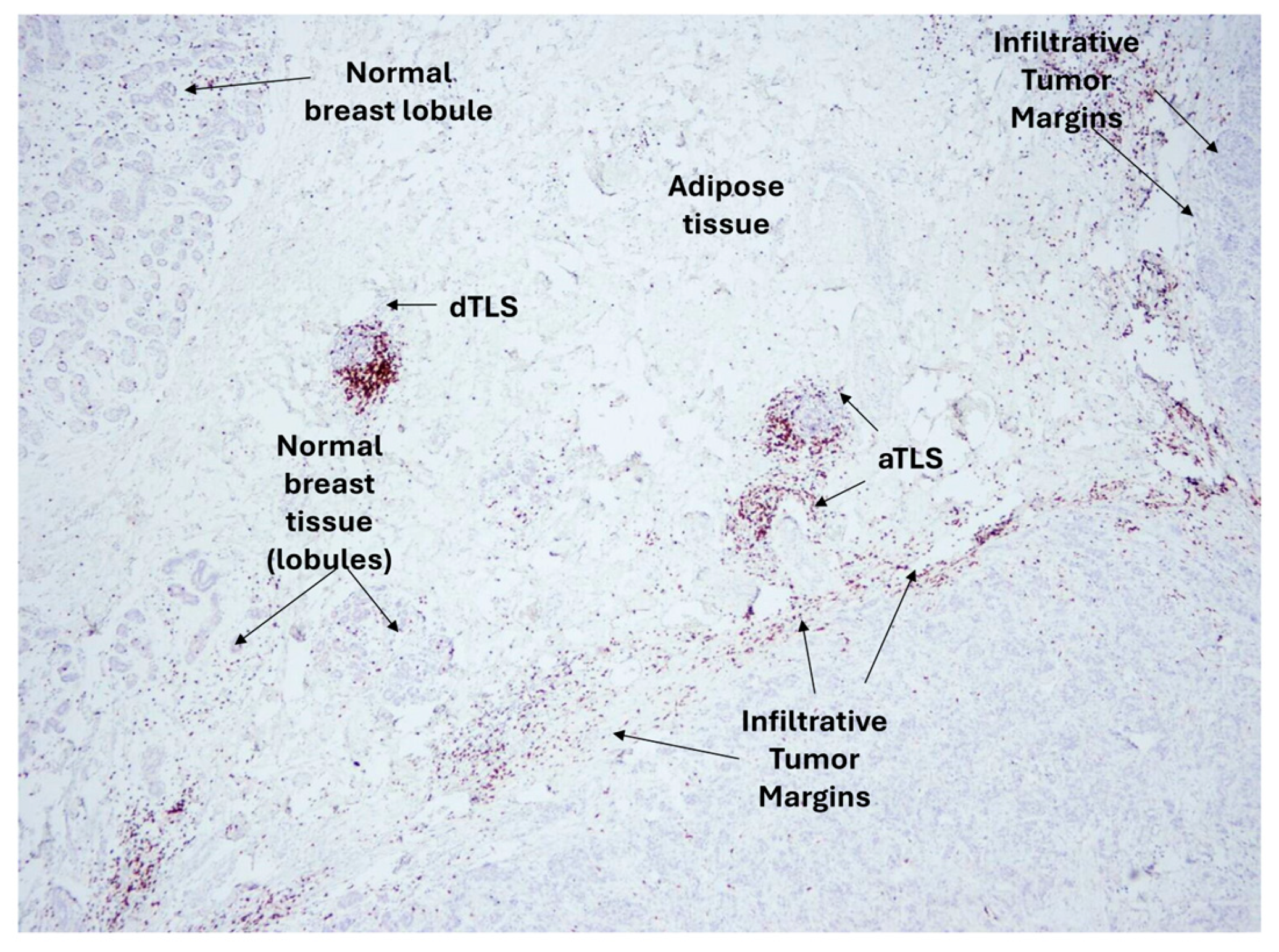
| TLS peritumoral | Increased DFS in breast and NSCLC patients associated with high TLS densities. | [20,114] |
| Increased TLS densities associated with progressively worse outcomes in HCC. | [115] | |
| High peritumoral TLS density positively correlated with unfavorable clinical responses whereas abundant intratumoral TLS density positively correlated with increased survival in CCA as well as in ccRCC and metastatic colorectal cancer. | [116,117,118] | |
| TLS in TME (vaccine-induced) | Generation of TLS with high PD-1+, IDO+ expression upon vaccination with a multivalent vaccine or with recombinant MAGE in melanoma patients. Presence of an effector multigene signature among vaccine responders. | [105] |
| High TLS aggregates with increased PD-1/PD-L1 signaling as centers for generation of adaptive antitumor responses upon vaccination with GVAX in patients with PDAC. Implications for combining GVAX with ICIs. | [106] | |
| Murine glioblastoma regression associated with large sized TLS infiltrated by high numbers of effector-memory T cells and by activated B cells upon vaccination with HCMV-IE1. | [108] | |
| TLS in TME (ICIs-induced) | Reduced melanoma growth upon ICIs therapy via development of TLS infiltrated by T cells, B cells, and other tumor reactive immune elements upon therapy with ICIs. | [99,100,101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baxevanis, C.N.; Sofopoulos, M.; Tsitsilonis, O.E.; Gritzapis, A.D. Exploring the Pivotal Functions of Tertiary Lymphoid Structures in Cancer Prognosis and Immunotherapy Outcomes. Cancers 2025, 17, 3754. https://doi.org/10.3390/cancers17233754
Baxevanis CN, Sofopoulos M, Tsitsilonis OE, Gritzapis AD. Exploring the Pivotal Functions of Tertiary Lymphoid Structures in Cancer Prognosis and Immunotherapy Outcomes. Cancers. 2025; 17(23):3754. https://doi.org/10.3390/cancers17233754
Chicago/Turabian StyleBaxevanis, Constantin N., Michael Sofopoulos, Ourania E. Tsitsilonis, and Angelos D. Gritzapis. 2025. "Exploring the Pivotal Functions of Tertiary Lymphoid Structures in Cancer Prognosis and Immunotherapy Outcomes" Cancers 17, no. 23: 3754. https://doi.org/10.3390/cancers17233754
APA StyleBaxevanis, C. N., Sofopoulos, M., Tsitsilonis, O. E., & Gritzapis, A. D. (2025). Exploring the Pivotal Functions of Tertiary Lymphoid Structures in Cancer Prognosis and Immunotherapy Outcomes. Cancers, 17(23), 3754. https://doi.org/10.3390/cancers17233754







