Advances in Endoscopic Ultrasonography-Based Diagnosis of Pancreatic Lesions: Narrative Review
Simple Summary
Abstract
1. Introduction
2. Method
Literature Search and Review
- I.
- Advances in EUS for diagnosis of pancreatic diseases
- Advances in EUS (Figure 1)

- (i)
- B mode imaging
- (ii)
- Assessment of vascularity
- (iii)
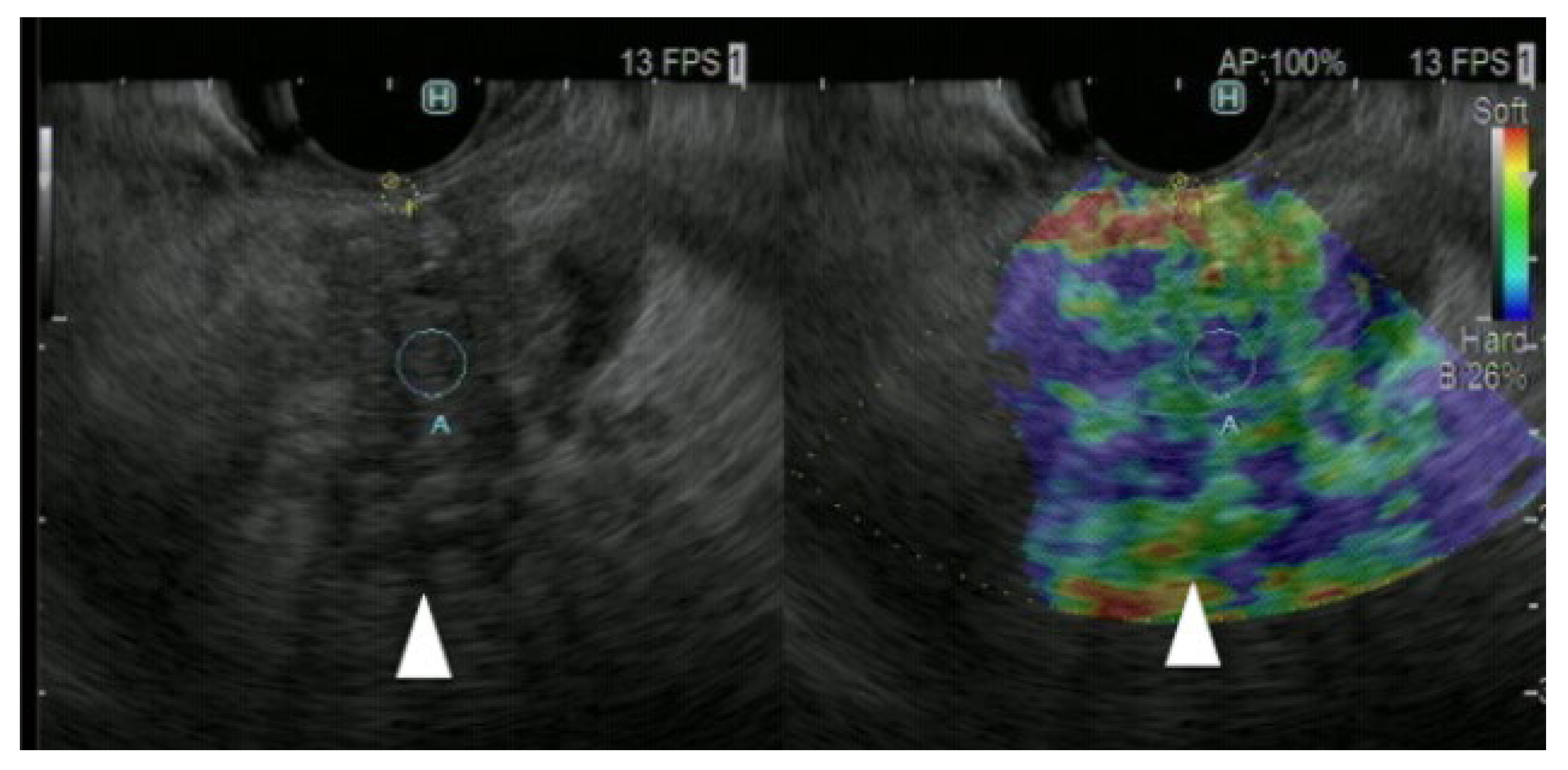
- Assessment of tissue elasticity
- (iv)
- EUS-guided tissue acquisition (EUS-TA)
- II.
- Utility of EUS for diagnosis of Pancreatic solid lesion (Table 1)
- (A)
- Conventional EUS
- (B)
- Doppler EUS
- (C)
- CH-EUS
- (D)
- Strain elastography
- (E)
- Shear wave elastography
- (F)
- EUS-TA
- (G)
- EUS-FNA combined with CH-EUS or EUS elastography
- (H)
- CH-EUS for the treatment efficacy of endoscopic radiofrequency ablation (RFA)
- III.
- Utility of EUS for diagnosis of pancreatic cysts
- (A)
- Conventional EUS
- (B)
- CH-EUS
- (C)
- DFI-EUS
- (D)
- EUS-TA
- IV.
- Future of echoendoscopy
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statement
Data availability statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Center for Cancer Control and Information Services. Tokyo: National Cancer Center Japan. Available online: https://ganjoho.jp/reg_stat/statistics/stat/summary.html (accessed on 14 March 2023).
- Boicean, A.; Prisca, D.; Bratu, D.G.; Bacila, C.I.; Tanasescu, C.; Chicea, R.; Fleaca, S.R.; Birsan, S.A.; Ichim, C.; Mohor, C.I.; et al. Uncommon Presentation of Gastric Duplication Cyst with Left-Sided Portal Hypertension: A Case Report and Literature Review. Diagnostics 2024, 14, 675. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Sahai, A.V.; D’Onofrio, M.; Will, U.; Arcidiacono, P.G.; Petrone, M.C.; Hocke, M.; Braden, B.; Burmester, E.; Möller, K.; et al. Differential diagnosis of small solid pancreatic lesions. Gastrointest. Endosc. 2016, 84, 933–940. [Google Scholar] [CrossRef]
- Kato, T.; Tsukamoto, Y.; Naitoh, Y.; Hirooka, Y.; Furukawa, T.; Hayakawa, T. Ultrasonographic and endoscopic ultrasonographic angiography in pancreatic mass lesions. Acta Radiol. 1995, 36, 381–387. [Google Scholar] [CrossRef]
- Hirooka, Y.; Itoh, A.; Hashimoto, S.; Kawashima, H.; Hara, K.; Kanamori, A.; Uchida, H.; Goto, J.; Ishikawa, S.; Ohmiya, N.; et al. Utility of EUS: Elastography in the diagnosis of pancreatic diseases. Gastrointest. Endosc. 2005, 61, AB282. [Google Scholar] [CrossRef]
- Vilmann, P.; Jacobsen, G.K.; Henriksen, F.W.; Hancke, S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest. Endosc. 1992, 38, 172–173. [Google Scholar] [CrossRef]
- Wiersema, M.J.; Levy, M.J.; Harewood, G.C.; Vazquez-Sequeiros, E.; Jondal, M.L.; Wiersema, L.M. Initial experience with EUS-guided trucut needle biopsies of perigastric organs. Gastrointest. Endosc. 2002, 56, 275–278. [Google Scholar] [CrossRef]
- Queneau, P.E.; Sauvé, G.; Koch, S.; Thibault, P.; Cléau, D.; Heyd, B.; Mantion, G.; Carayon, P. The impact on clinical practice of endoscopic ultrasonography used for the diagnosis and staging of pancreatic adenocarcinoma. JOP 2001, 2, 98–104. [Google Scholar]
- Li, H.; Hu, Z.; Chen, J.; Guo, X. Comparison of ERCP, EUS, and ERCP combined with EUS in diagnosing pancreatic neoplasms: A systematic review and meta-analysis. Tumour. Biol. 2014, 35, 8867–8874. [Google Scholar] [CrossRef]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Todor, S.B.; Hasegan, A.; Bacila, C.; Solomon, A.; Cristian, A.; Dura, H. Predictors of Post-ERCP Pancreatitis (P.E.P.) in Choledochal Lithiasis Extraction. J. Pers. Med. 2023, 13, 1356. [Google Scholar] [CrossRef]
- Krishna, S.G.; Rao, B.B.; Ugbarugba, E.; Shah, Z.K.; Blaszczak, A.; Hinton, A.; Conwell, D.L.; Hart, P.A. Diagnostic performance of endoscopic ultrasound for detection of pancreatic malignancy following an indeterminate multidetector CT scan: A systemic review and meta-analysis. Surg. Endosc. 2017, 31, 4558–4567. [Google Scholar] [CrossRef] [PubMed]
- Toft, J.; Hadden, W.J.; Laurence, J.M.; Lam, V.; Yuen, L.; Janssen, A.; Pleass, H. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: A systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur. J. Radiol. 2017, 92, 17–23. [Google Scholar] [CrossRef]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Ito, Y.; Mizuno, N.; Hanada, K.; Ozaka, M.; Morizane, C.; Takeyama, Y.; et al. Clinical Practice Guidelines for Pancreatic Cancer 2022 from the Japan Pancreas Society: A synopsis. Int. J. Clin. Oncol. 2023, 28, 493–511. [Google Scholar] [CrossRef]
- Wolff, R.A.; Wolpin, B.M.; Lynn, B.; George, G.V. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar]
- Denecke, T.; Grieser, C.; Neuhaus, P.; Bahra, M. Radiologic resectability assessment in pancreatic cancer. Rofo 2014, 186, 23–29. [Google Scholar] [CrossRef]
- Kurita, Y.; Hara, K.; Kobayashi, N.; Kuwahara, T.; Mizuno, N.; Okuno, N.; Haba, S.; Yagi, S.; Hasegawa, S.; Sato, T.; et al. Detection rate of endoscopic ultrasound and computed tomography in diagnosing pancreatic neuroendocrine neoplasms including small lesions: A multicenter study. J. Hepatobiliary Pancreat. Sci. 2022, 29, 950–959. [Google Scholar] [CrossRef]
- James, P.D.; Tsolakis, A.V.; Zhang, M.; Belletrutti, P.J.; Mohamed, R.; Roberts, D.J.; Heitman, S.J. Incremental benefit of preoperative EUS for the detection of pancreatic neuroendocrine tumors: A meta-analysis. Gastrointest. Endosc. 2015, 81, 848–856. [Google Scholar] [CrossRef]
- Săftoiu, A.; Popescu, C.; Cazacu, S.; Dumitrescu, D.; Georgescu, C.V.; Popescu, M.; Ciurea, T.; Gorunescu, F. Power Doppler endoscopic ultrasonography for the differential diagnosis between pancreatic cancer and pseudotumoral chronic pancreatitis. J. Ultrasound Med. 2006, 25, 363–372. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kato, J.; Ueda, K.; Nakamura, Y.; Kawaji, Y.; Abe, H.; Nuta, J.; Tamura, T.; Itonaga, M.; Yoshida, T.; et al. Contrast-Enhanced Endoscopic Ultrasonography for Pancreatic Tumors. Biomed. Res. Int. 2015, 2015, 491782. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimokawa, T.; Ashida, R.; Napoléon, B.; Lisotti, A.; Fusaroli, P.; Gincul, R.; Dietrich, C.F.; Omoto, S.; Kitano, M. Comparison of endoscopic ultrasonography with and without contrast enhancement for characterization of pancreatic tumors: A meta-analysis. Endosc. Int. Open. 2022, 10, E369–E377. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanioka, K.; Kawaji, Y.; Tamura, T.; Nuta, J.; Hatamaru, K.; Itonaga, M.; Yoshida, T.; Ida, Y.; Maekita, T.; et al. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Early Diagnosis of Small Pancreatic Cancer. Diagnostics 2020, 10, 23. [Google Scholar] [CrossRef]
- Palazzo, M.; Napoléon, B.; Gincul, R.; Pioche, M.; Pujol, B.; Lefort, C.; Fumex, F.; Hautefeuille, V.; Fabre, M.; Cros, J.; et al. Contrast harmonic EUS for the prediction of pancreatic neuroendocrine tumor aggressiveness (with videos). Gastrointest. Endosc. 2018, 87, 1481–1488. [Google Scholar] [CrossRef]
- Iglesias-Garcia, J.; Larino-Noia, J.; Abdulkader, I.; Forteza, J.; Dominguez-Munoz, J.E. EUS elastography for the characterization of solid pancreatic masses. Gastrointest. Endosc. 2009, 70, 1101–1108. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, F.; Li, P.; Yu, S.; Zhao, Y.; Li, M. Endoscopic ultrasound elastography in the diagnosis of pancreatic masses: A meta-analysis. Pancreatology 2018, 18, 833–840. [Google Scholar] [CrossRef]
- Ohno, E.; Kawashima, H.; Ishikawa, T.; Iida, T.; Suzuki, H.; Uetsuki, K.; Yashika, J.; Yamada, K.; Yoshikawa, M.; Gibo, N.; et al. Diagnostic performance of endoscopic ultrasonography-guided elastography for solid pancreatic lesions: Shear-wave measurements versus strain elastography with histogram analysis. Dig. Endosc. 2021, 33, 629–638. [Google Scholar] [CrossRef]
- Van Riet, P.A.; Erler, N.S.; Bruno, M.J.; Cahen, D.L. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasonography-guided sampling of solid lesions: A systemic review and meta-analysis. Endoscopy 2021, 53, 411–423. [Google Scholar]
- Madhoun, M.F.; Wani, S.B.; Rastogi, A.; Early, D.; Gaddam, S.; Tierney, W.M.; Maple, J.T. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: A meta-analysis. Endoscopy 2013, 45, 86–92. [Google Scholar] [CrossRef]
- Kanno, A.; Yasuda, I.; Irisawa, A.; Hara, K.; Ashida, R.; Iwashita, T.; Takenaka, M.; Katanuma, A.; Takikawa, T.; Kubota, K.; et al. Adverse events of endoscopic ultrasound-guided fine-needle aspiration for histologic diagnosis in Japanese tertiary centers: Multicenter retrospective study. Dig. Endosc. 2021, 33, 1146–1157. [Google Scholar] [CrossRef]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative diagnostic performance of different techniques for EUS-guided fine-needle biopsy sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2023, 97, 839–848. [Google Scholar] [CrossRef]
- Kandel, P.; Nassar, A.; Gomez, V.; Raimondo, M.; Woodward, T.A.; Crook, J.E.; Fares, N.S.; Wallace, M.B. Comparison of endoscopic ultrasonography-guided fine-needle biopsy versus fine-needle aspiration for genomic profiling and DNA yield in PC: A randomized crossover trial. Endoscopy 2021, 53, 376–382. [Google Scholar]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef]
- Kameta, E.; Sugimori, K.; Kaneko, T.; Ishii, T.; Miwa, H.; Sato, T.; Ishii, Y.; Sue, S.; Sasaki, T.; Yamashita, Y.; et al. Diagnosis of pancreatic lesions collected by endoscopic ultrasound-guided fine-needle aspiration using next-generation sequencing. Oncol. Lett. 2016, 12, 3875–3881. [Google Scholar] [CrossRef]
- Elhanafi, S.; Mahmud, N.; Vergara, N.; Kochman, M.L.; Das, K.K.; Ginsberg, G.G.; Rajala, M.; Chandrasekhara, V. Comparison of endoscopic ultrasound tissue acquisition methods for genomic analysis of pancreatic cancer. J. Gastroenterol. Hepatol. 2019, 34, 907–913. [Google Scholar] [CrossRef]
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 821–828. [Google Scholar] [CrossRef]
- Kongkam, P.; Lakananurak, N.; Navicharern, P.; Chantarojanasiri, T.; Aye, K.; Ridtitid, W.; Kritisin, K.; Angsuwatcharakon, P.; Aniwan, S.; Pittayanon, R.; et al. Combination of EUS-FNA and elastography (strain ratio) to exclude malignant solid pancreatic lesions: A prospective single-blinded study. J. Gastroenterol. Hepatol. 2015, 30, 1683–1689. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, D.W.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasound for the Guidance and Monitoring of Endoscopic Radiofrequency Ablation. Gut Liver 2020, 14, 826–832. [Google Scholar] [CrossRef]
- Kamata, K.; Kitano, M.; Kudo, M.; Sakamoto, H.; Kadosaka, K.; Miyata, T.; Imai, H.; Maekawa, K.; Chikugo, T.; Kumano, M.; et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 2014, 46, 22–29. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Fernandez-Del Castillo, C.; Furukawa, T.; Hijioka, S.; Jang, J.Y.; Lennon, A.M.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2024, 24, 255–270. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ueda, K.; Itonaga, M.; Yoshida, T.; Maeda, H.; Maekita, T.; Iguchi, M.; Tamai, H.; Ichinose, M.; Kato, J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: A single-center prospective study. J. Ultrasound Med. 2013, 32, 61–68. [Google Scholar] [CrossRef]
- Lisotti, A.; Napoleon, B.; Facciorusso, A.; Cominardi, A.; Crinò, S.F.; Brighi, N.; Gincul, R.; Kitano, M.; Yamashita, Y.; Marchegiani, G.; et al. Contrast-enhanced EUS for the characterization of mural nodules within pancreatic cystic neoplasms: Systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 881–889. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ashida, R.; Tamura, T.; Shimokawa, T.; Yamazaki, H.; Kawaji, Y.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Kitano, M. Novel Technique of Endoscopic Ultrasonography for the Differential Diagnosis of Gallbladder Lesions and Intraductal Papillary Mucinous Neoplasms: A Single-Center Prospective Study. Diagnostics 2023, 13, 2132. [Google Scholar] [CrossRef]
- Maire, F.; Voitot, H.; Aubert, A.; Palazzo, L.; O’Toole, D.; Couvelard, A.; Levy, P.; Vidaud, M.; Sauvanet, A.; Ruszniewski, P.; et al. Intraductal papillary mucinous neoplasms of the pancreas: Performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am. J. Gastroenterol. 2008, 103, 2871–2877. [Google Scholar] [CrossRef]
- McCarty, T.R.; Garg, R.; Rustagi, T. Pancreatic cyst fluid glucose in differentiating mucinous from nonmucinous pancreatic cysts: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 698–712. [Google Scholar] [CrossRef]
- Serinelli, S.; Khurana, K.K. Intraductal papillary mucinous neoplasms of the pancreas: Cytologic-histologic correlation study and evaluation of the cytologic accuracy in identifying high-grade dysplasia/invasive adenocarcinoma. Cytojournal 2024, 21, 6. [Google Scholar] [CrossRef]
- Gopakumar, H.; Puli, S.R. Value of Endoscopic Ultrasound-Guided Through-the-Needle Biopsy in Pancreatic Cystic Lesions. A Systematic Review and Meta-Analysis. J. Gastrointest. Cancer. 2024, 55, 15–25. [Google Scholar] [CrossRef]
- Facciorusso, A.; Kovacevic, B.; Yang, D.; Vilas-Boas, F.; Martínez-Moreno, B.; Stigliano, S.; Rizzatti, G.; Sacco, M.; Arevalo-Mora, M.; Villarreal-Sanchez, L.; et al. Predictors of adverse events after endoscopic ultrasound-guided through-the-needle biopsy of pancreatic cysts: A recursive partitioning analysis. Endoscopy 2022, 54, 1158–1168. [Google Scholar] [CrossRef]
- Konda, V.J.; Meining, A.; Jamil, L.H.; Giovannini, M.; Hwang, J.H.; Wallace, M.B.; Chang, K.J.; Siddiqui, U.D.; Hart, J.; Lo, S.K.; et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy 2013, 45, 1006–1013. [Google Scholar] [CrossRef]
- Giovannini, M.; Caillol, F.; Poizat, F.; Bories, E.; Pesenti, C.; Monges, G.; Raoul, J.L. Feasibility of intratumoral confocal microscopy under endoscopic ultrasound guidance. Endosc. Ultrasound 2012, 1, 80–83. [Google Scholar] [CrossRef]
- Napoléon, B.; Lemaistre, A.I.; Pujol, B.; Caillol, F.; Lucidarme, D.; Bourdariat, R.; Morellon-Mialhe, B.; Fumex, F.; Lefort, C.; Lepilliez, V.; et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: Needle-based confocal laser endomicroscopy. Endoscopy 2015, 47, 26–32. [Google Scholar] [CrossRef]
- Rift, C.V.; Melchior, L.C.; Kovacevic, B.; Klausen, P.; Toxværd, A.; Grossjohann, H.; Karstensen, J.G.; Brink, L.; Hassan, H.; Kalaitzakis, E.; et al. Targeted next-generation sequencing of EUS-guided through-the-needle-biopsy sampling from pancreatic cystic lesion. Gastrointest. Endosc. 2023, 97, 50–58. [Google Scholar] [CrossRef]
- Konikoff, T.; Loebl, N.; Benson, A.A.; Green, O.; Sandler, H.; Gingold-Belfer, R.; Levi, Z.; Perl, L.; Dotan, I.; Shamah, S. Enhancing detection of various pancreatic lesions on endoscopic ultrasound through artificial intelligence: A basis for computer-aided detection systems. J. Gastroenterol. Hepatol. 2024; Online ahead of print. [Google Scholar] [CrossRef]





| Pancreatic Cancer | Inflammatory Mass | Neuroendocrine Neoplasm | |
|---|---|---|---|
| B mode | Heterogeneous | Heterogeneous | Homogeneous, but heterogeneous (malignant) |
| Hypoechoic | Calcification | Round | |
| Irregular margin | Cysts | Hypoechoic | |
| Peripancreatic echo-rich stranding | Clearly demarcated | ||
| Contrast-enhanced | Hypovascular | Isovascular | Hypervascular |
| Elastography (strain) | Heterogeneous blue pattern | Green to blue pattern according to the degree of fibrosis | Homogeneous blue pattern |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, Y.; Yamazaki, H.; Nakahata, A.; Emori, T.; Kawaji, Y.; Tamura, T.; Itonaga, M.; Ashida, R.; Kitano, M. Advances in Endoscopic Ultrasonography-Based Diagnosis of Pancreatic Lesions: Narrative Review. Cancers 2025, 17, 172. https://doi.org/10.3390/cancers17020172
Yamashita Y, Yamazaki H, Nakahata A, Emori T, Kawaji Y, Tamura T, Itonaga M, Ashida R, Kitano M. Advances in Endoscopic Ultrasonography-Based Diagnosis of Pancreatic Lesions: Narrative Review. Cancers. 2025; 17(2):172. https://doi.org/10.3390/cancers17020172
Chicago/Turabian StyleYamashita, Yasunobu, Hirofumi Yamazaki, Akiya Nakahata, Tomoya Emori, Yuki Kawaji, Takashi Tamura, Masahiro Itonaga, Reiko Ashida, and Masayuki Kitano. 2025. "Advances in Endoscopic Ultrasonography-Based Diagnosis of Pancreatic Lesions: Narrative Review" Cancers 17, no. 2: 172. https://doi.org/10.3390/cancers17020172
APA StyleYamashita, Y., Yamazaki, H., Nakahata, A., Emori, T., Kawaji, Y., Tamura, T., Itonaga, M., Ashida, R., & Kitano, M. (2025). Advances in Endoscopic Ultrasonography-Based Diagnosis of Pancreatic Lesions: Narrative Review. Cancers, 17(2), 172. https://doi.org/10.3390/cancers17020172








