Risk Factors for Long-Term Delayed Gastric Emptying and Its Impact on the Quality of Life After Laparoscopic Pylorus-Preserving Gastrectomy in Patients with Gastric Cancer: Secondary Analysis of the Prospective Multicenter Trial KLASS-04
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Statistical Analysis
3. Results
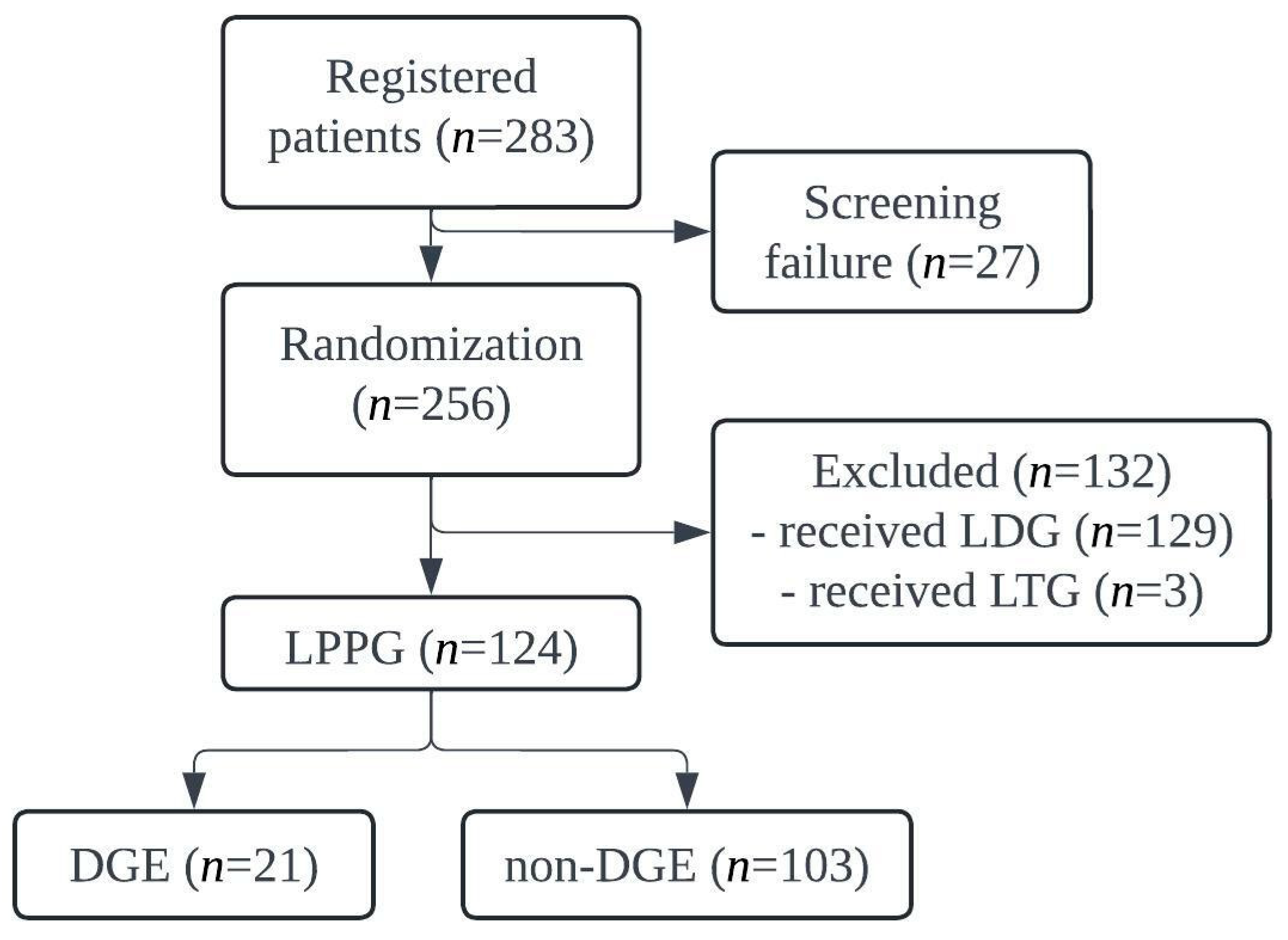
3.1. Patient Recruitment
3.2. Clinicopathological Features
3.3. Surgical Outcomes
3.4. Logistic Regression Analysis
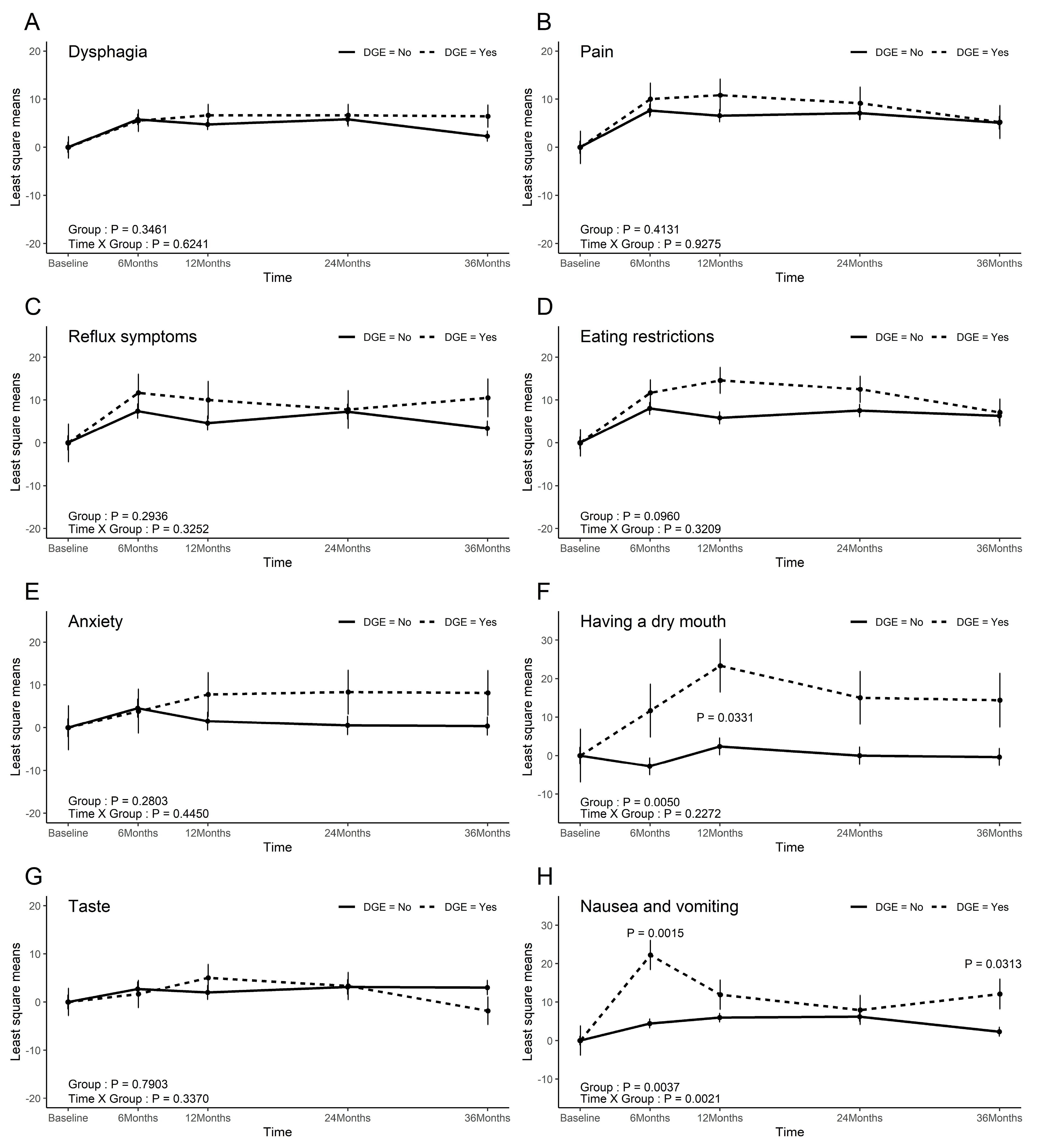
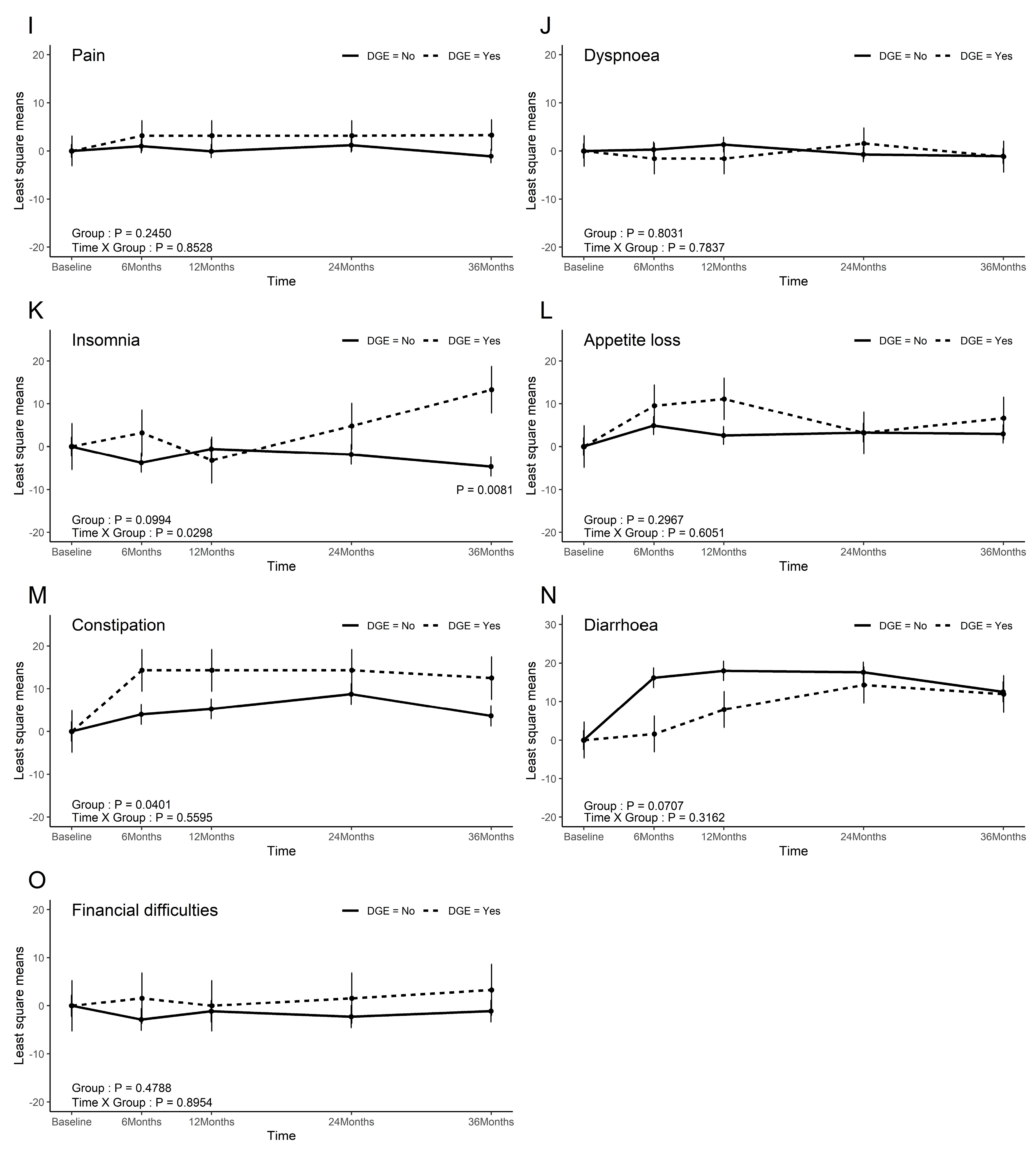
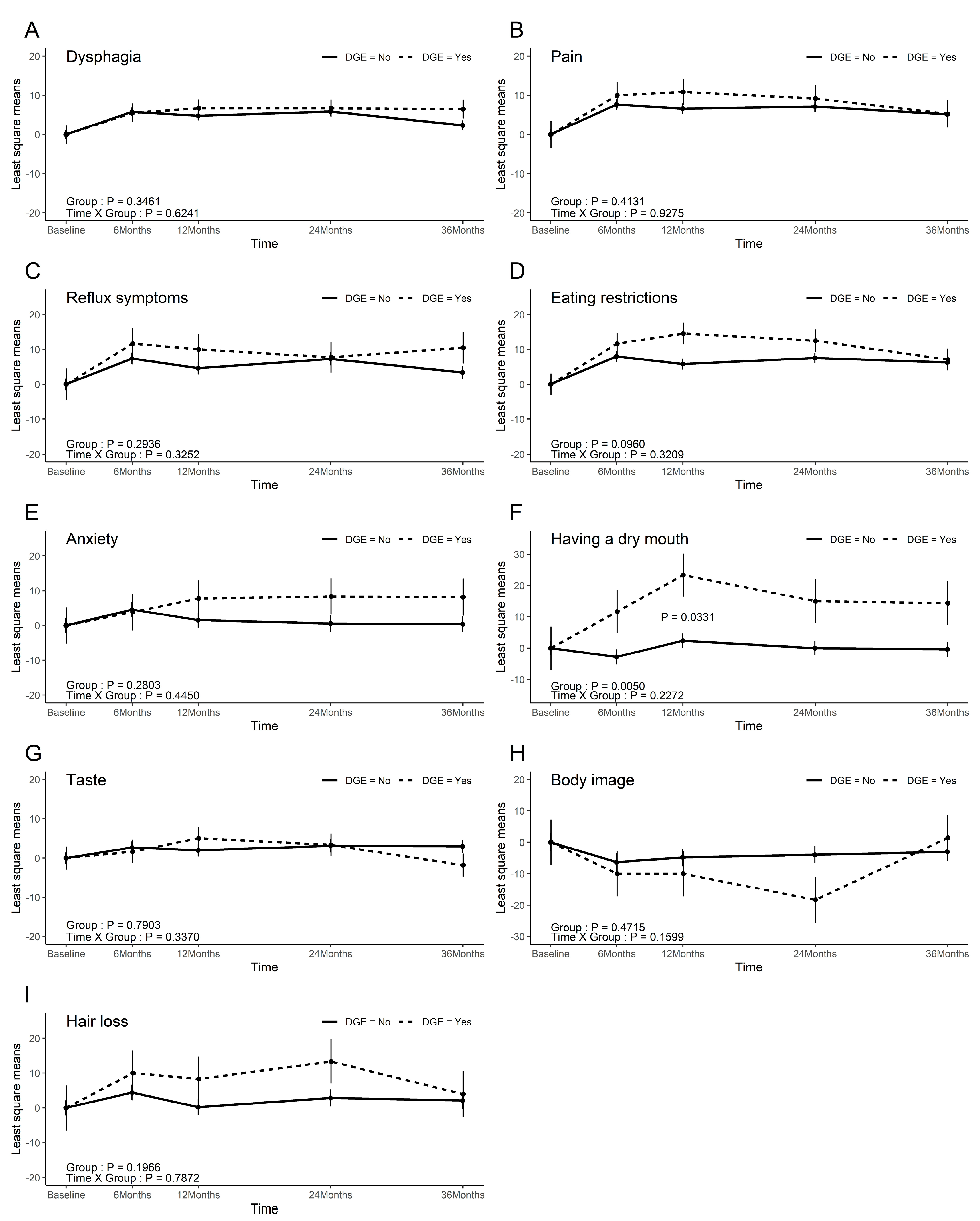
3.5. QoL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DGE | delayed gastric emptying |
| LPPG | laparoscopic pylorus-preserving gastrectomy |
| LDG | laparoscopic distal gastrectomy |
| QoL | quality of life |
| EGC | early gastric cancer |
| HBVN | hepatic branch of the vagus nerve |
| IPA | infrapyloric artery |
| IPV | infrapyloric vein |
| ECOG | eastern cooperative oncology group |
| BMI | body mass index |
| DM | diabetes mellitus |
| LN | lymph node |
| OR | odds ratio |
| CI | confidence interval |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kang, M.J.; Yun, E.H.; Jung, K.W. Epidemiology of Gastric Cancer in Korea: Trends in Incidence and Survival Based on Korea Central Cancer Registry Data (1999–2019). J. Gastric Cancer 2022, 22, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Song, J.H.; Park, J.H.; Kim, S.; Park, S.H.; Shin, C.M.; Kwak, Y.; Bang, K.; Gong, C.-S.; Oh, S.E.; et al. Korean Gastric Cancer Association-Led Nationwide Survey on Surgically Treated Gastric Cancers in 2023. J. Gastric Cancer 2025, 25, 115–132. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.D.; Graham, P.J.; Bathe, O.F. Quality of life: A critical outcome for all surgical treatments of gastric cancer. World J. Gastroenterol. 2016, 22, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, Y.W.; Park, D.J.; Han, S.U.; Ryu, K.W.; Kim, H.H.; Hyung, W.J.; Park, J.-H.; Suh, Y.-S.; Kwon, O.-K.; et al. Laparoscopic Pylorus Preserving Gastrectomy versus Distal Gastrectomy for Early Gastric Cancer; A Multicenter Randomized Controlled Trial (KLASS-04). Ann. Surg. 2024, 281, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Nunobe, S.; Sasako, M.; Saka, M.; Fukagawa, T.; Katai, H.; Sano, T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer 2007, 10, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Shibata, C.; Saijo, F.; Kakyo, M.; Kinouchi, M.; Tanaka, N.; Sasaki, I.; Aikou, T.; The Society for the Study of Postoperative Morbidity After Gastrectomy. Current Status of Pylorus-Preserving Gastrectomy for the Treatment of Gastric Cancer: A Questionnaire Survey and Review of Literatures. World J. Surg. 2012, 36, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, T.; Hiki, N.; Nunobe, S.; Honda, M.; Ohashi, M.; Sano, T. Preserving infrapyloric vein reduces postoperative gastric stasis after laparoscopic pylorus-preserving gastrectomy. Langenbeck’s Arch. Surg. 2017, 402, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Nakane, Y.; Michiura, T.; Inoue, K.; Sato, M.; Nakai, K.; Yamamichi, K. Length of the antral segment in pylorus-preserving gastrectomy. Br. J. Surg. 2002, 89, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hiki, N.; Nunobe, S.; Fukunaga, T.; Kumagai, K.; Nohara, K.; Sano, T.; Yamaguchi, T. Postoperative outcomes and complications after laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Ann. Surg. 2011, 253, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Chedid, V.; Ford, A.C.; Haruma, K.; Horowitz, M.; Jones, K.L.; Low, P.A.; Park, S.-Y.; Parkman, H.P.; Stanghellini, V. Gastroparesis. Nat. Rev. Dis. Primers 2018, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Sasako, M.; Gotoda, T.; Ono, H.; Fujishiro, M.; Saito, D.; Sano, T.; Katai, H. Endoscopic evaluation of the remnant stomach after gastrectomy: Proposal for a new classification. Gastric Cancer 2002, 5, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Parkman, H.P.; Sharkey, E.; McCallum, R.W.; Hasler, W.L.; Koch, K.L.; Sarosiek, I.; Abell, T.L.; Kuo, B.; Shulman, R.J.; Grover, M.; et al. Constipation in Patients With Symptoms of Gastroparesis: Analysis of Symptoms and Gastrointestinal Transit. Clin. Gastroenterol. Hepatol. 2022, 20, 546–558.e5. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Yun, H.Y.; Song, Y.J.; Ryu, D.H.; Han, H.S.; Han, J.H.; Kim, K.B.; Yoon, S.M.; Youn, S.J. Clinical features of gastric emptying after distal gastrectomy. Ann. Surg. Treat. Res. 2017, 93, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Hiki, N.; Nunobe, S.; Sekikawa, S.; Chiba, T.; Kiyokawa, T.; Jiang, X.; Tanimura, S.; Sano, T.; Yamaguchi, T. Totally laparoscopic pylorus-preserving gastrectomy for early gastric cancer in the middle stomach: Technical report and surgical outcomes. Gastric Cancer 2015, 18, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, K.; Park, J.H.; Lee, H.J.; Park, S.H.; Choi, J.H.; Wang, C.; Alzahrani, F.; Suh, Y.-S.; Kong, S.-H.; Park, D.J.; et al. Short-term Outcomes of Pylorus-Preserving Gastrectomy for Early Gastric Cancer: Comparison Between Extracorporeal and Intracorporeal Gastrogastrostomy. J. Gastric Cancer 2022, 22, 135–144. [Google Scholar] [CrossRef] [PubMed]
| Delayed Gastric Emptying | p-Value | ||
|---|---|---|---|
| No | No | ||
| n = 103 | n = 103 | ||
| Age (years), mean ± standard deviation | 55.2 ± 10.1 | 57.8 ± 13.3 | 0.31 * |
| Sex ratio (M:F) | 50:53:00 | 8:13 | 0.382 |
| BMI, mean ± standard deviation | 23.5 ± 2.7 | 23.6 ± 2.4 | 0.896 * |
| ECOG | |||
| 0 | 100 | 19 | 0.199 |
| 1 | 3 | 2 | |
| DM | |||
| No | 94 | 20 | >0.99 |
| Yes | 9 | 1 | |
| Previous surgical history | |||
| No | 70 | 20 | 0.011 |
| Yes | 33 | 1 | |
| Tumor location | |||
| Upper | 1 | 0 | 0.376 |
| Middle | 80 | 14 | |
| Low | 20 | 7 | |
| Tumor size (mm), median | 20 (0–75) | 16 (0.8–55) | 0.05 # |
| (Min–Max) | |||
| pT classification | |||
| T1a | 62 | 13 | 0.853 |
| T1b | 35 | 7 | |
| T2 | 3 | 1 | |
| T3 | 1 | 0 | |
| pN classification | |||
| N0 | 91 | 19 | >0.99 |
| N+ | 11 | 2 | |
| LN dissection level | |||
| D1 | 0 | 1 | 0.169 |
| D1+ | 103 | 20 | |
| Pathologic stage | |||
| IA | 88 | 18 | 0.504 |
| IB | 12 | 2 | |
| IIA | 1 | 1 | |
| IIB | 1 | 0 | |
| Delayed Gastric Emptying | p-Value | ||
|---|---|---|---|
| No | Yes | ||
| (n = 103) | (n = 21) | ||
| Operation time (min) * | 199 (105–275) | 190 (130–243) | 0.08 |
| Blood loss (cc) * | 31.5 (5–1000) | 50 (5–220) | 0.248 |
| IPA type | |||
| ASPDA (distal) | 26 | 4 | 0.944 ‡ |
| RGEA (caudal) | 39 | 9 | |
| GDA (proximal) | 34 | 7 | |
| None or unknown | 4 | 1 | |
| IPA injury | |||
| No | 101 | 21 | >0.99 ‡ |
| Yes | 1 | 0 | |
| IPV injury | |||
| No | 98 | 21 | >0.99 ‡ |
| Yes | 4 | 0 | |
| HBVN injury | |||
| No | 100 | 20 | >0.99 ‡ |
| Yes | 2 | 1 | |
| Length of antral cuff * | 4 (3–8.6) | 4 (3–6) | 0.919 |
| Proximal margin * | 2.2 (0.2–13.5) | 2.2 (1.1–5) | 0.954 |
| Distal margin * | 2.7 (0.2–13.8) | 3.6 (0.2–11) | 0.427 |
| Preservation of the CBVN | |||
| No | 78 | 14 | 0.346 † |
| Yes | 24 | 7 | |
| Resected LN 6 * | 6 (0–20) | 5 (1–14) | 0.526 |
| Resected LN 9 * | 2 (0–11) | 2.5 (0–5) | 0.471 |
| Resected total LN * | 34 (16–88) | 39 (15–82) | 0.113 |
| Morbidity | |||
| No | 84 | 15 | 0.227 ‡ |
| Yes | 17 | 6 | |
| N | DGE | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Age | 124 | 21 | 1.024 (0.979–1.07) | 0.309 | ||
| Sex | ||||||
| Female | 66 | 13 | 1 (ref) | |||
| Male | 58 | 8 | 0.652 (0.249–1.707) | 0.384 | ||
| BMI | 124 | 21 | 1.012 (0.845–1.213) | 0.895 | ||
| ECOG | ||||||
| 0 | 119 | 19 | 1 (ref) | |||
| 1 | 5 | 2 | 3.509 (0.549–22.432) | 0.185 | ||
| DM | ||||||
| No | 114 | 20 | 1 (ref) | |||
| Yes | 10 | 1 | 0.522 (0.063–4.358) | 0.548 | ||
| Previous surgical history | ||||||
| No | 90 | 20 | 1 (ref) | 1 (ref) | ||
| Yes | 34 | 1 | 0.106 (0.014–0.824) | 0.032 | 0.094 (0.012–0.757) | 0.0263 |
| Tumor location | ||||||
| Low | 27 | 7 | 1 (ref) | −0.39 | ||
| Middle | 94 | 14 | 0.492 (0.177–1.366) | 0.584 | ||
| Upper | 1 | 0 | 0.913 (0.009–91.151) | 0.91 | ||
| Tumor size | 123 | 21 | 0.973 (0.935–1.012) | 0.165 | ||
| pT classification | ||||||
| T1a | 75 | 13 | 1 (ref) | −0.936 | ||
| T1b | 42 | 7 | 0.978 (0.362–2.640) | 0.671 | ||
| T2 | 4 | 1 | 1.984 (0.216–18.250) | 0.687 | ||
| T3 | 1 | 0 | 1.573 (0.017–147.919) | 0.921 | ||
| pN classification | ||||||
| N0 | 110 | 19 | 1 (ref) | −0.722 | ||
| N1 | 10 | 1 | 0.741 (0.114–4.815) | 0.45 | ||
| N2 | 2 | 1 | 4.694 (0.281–78.349) | 0.364 | ||
| N3b | 1 | 0 | 1.615 (0.018–147.941) | 0.979 | ||
| Pathologic stage | ||||||
| IA | 106 | 18 | 1 (ref) | −0.743 | ||
| IB | 14 | 2 | 0.957 (0.215–4.258) | 0.526 | ||
| IIA | 2 | 1 | 4.785 (0.286–80.046) | 0.385 | ||
| IIB | 1 | 0 | 1.639 (0.018–151.093) | 0.996 | ||
| Operation time (min) | 124 | 21 | 0.989 (0.977–1.002) | 0.093 | 0.989 (0.976–1.002) | 0.1002 |
| Blood loss (cc) | 124 | 21 | 1 (0.996–1.004) | 0.919 | ||
| IPA type | ||||||
| ASPDA (distal) | 30 | 4 | 1 (ref) | −0.936 | ||
| RGEA (caudal) | 48 | 9 | 1.5 (0.418–5.383) | 0.798 | ||
| GDA (proximal) | 41 | 7 | 1.338 (0.354–5.06) | 0.992 | ||
| No or unknown | 5 | 1 | 1.625 (0.143–18.473) | 0.825 | ||
| IPA injury | ||||||
| No | 122 | 21 | 1 (ref) | |||
| Yes | 1 | 0 | 1.577 (0.017–148.94) | 0.844 | ||
| IPV injury | ||||||
| No | 119 | 21 | 1 (ref) | |||
| Yes | 4 | 0 | 0.509 (0.019–13.8) | 0.689 | ||
| HBVN injury | ||||||
| No | 120 | 20 | 1 (ref) | |||
| Yes | 3 | 1 | 2.5 (0.216–28.913) | 0.463 | ||
| Length of antral cuff | 123 | 21 | 0.911 (0.516–1.609) | 0.748 | ||
| Proximal margin | 122 | 21 | 0.951 (0.728–1.243) | 0.716 | ||
| Distal margin | 122 | 21 | 1.079 (0.913–1.274) | 0.373 | ||
| CBVN preservation | ||||||
| No | 92 | 14 | 1 (ref) | |||
| Yes | 31 | 7 | 1.625 (0.588–4.489) | 0.349 | ||
| Resected LN 6 | 123 | 21 | 0.96 (0.847–1.089) | 0.528 | ||
| Resected LN 9 | 121 | 20 | 0.895 (0.719–1.114) | 0.322 | ||
| Resected total LN | 123 | 21 | 1.029 (0.997–1.061) | 0.074 | 1.031 (0.997–1.065) | 0.0726 |
| Morbidity | ||||||
| No | 99 | 15 | 1 (ref) | |||
| Yes | 23 | 6 | 1.976 (0.671–5.824) | 0.217 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhee, Y.S.; Eom, S.S.; Eom, B.W.; Lee, D.-e.; Kim, S.-H.; Lee, H.-J.; Kim, Y.-W.; Yang, H.-K.; Park, D.J.; Han, S.U.; et al. Risk Factors for Long-Term Delayed Gastric Emptying and Its Impact on the Quality of Life After Laparoscopic Pylorus-Preserving Gastrectomy in Patients with Gastric Cancer: Secondary Analysis of the Prospective Multicenter Trial KLASS-04. Cancers 2025, 17, 2527. https://doi.org/10.3390/cancers17152527
Rhee YS, Eom SS, Eom BW, Lee D-e, Kim S-H, Lee H-J, Kim Y-W, Yang H-K, Park DJ, Han SU, et al. Risk Factors for Long-Term Delayed Gastric Emptying and Its Impact on the Quality of Life After Laparoscopic Pylorus-Preserving Gastrectomy in Patients with Gastric Cancer: Secondary Analysis of the Prospective Multicenter Trial KLASS-04. Cancers. 2025; 17(15):2527. https://doi.org/10.3390/cancers17152527
Chicago/Turabian StyleRhee, Young Shick, Sang Soo Eom, Bang Wool Eom, Dong-eun Lee, Sa-Hong Kim, Hyuk-Joon Lee, Young-Woo Kim, Han-Kwang Yang, Do Joong Park, Sang Uk Han, and et al. 2025. "Risk Factors for Long-Term Delayed Gastric Emptying and Its Impact on the Quality of Life After Laparoscopic Pylorus-Preserving Gastrectomy in Patients with Gastric Cancer: Secondary Analysis of the Prospective Multicenter Trial KLASS-04" Cancers 17, no. 15: 2527. https://doi.org/10.3390/cancers17152527
APA StyleRhee, Y. S., Eom, S. S., Eom, B. W., Lee, D.-e., Kim, S.-H., Lee, H.-J., Kim, Y.-W., Yang, H.-K., Park, D. J., Han, S. U., Kim, H.-H., Hyung, W. J., Park, J.-H., Suh, Y.-S., Kwon, O. K., Kim, W., Park, Y.-K., Yoon, H. M., Ahn, S.-H., ... Ryu, K. W. (2025). Risk Factors for Long-Term Delayed Gastric Emptying and Its Impact on the Quality of Life After Laparoscopic Pylorus-Preserving Gastrectomy in Patients with Gastric Cancer: Secondary Analysis of the Prospective Multicenter Trial KLASS-04. Cancers, 17(15), 2527. https://doi.org/10.3390/cancers17152527





