Quality of Life After Pancreatic Surgery for Neuroendocrine Tumors of the Pancreas: Observational Study of Long-Term Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
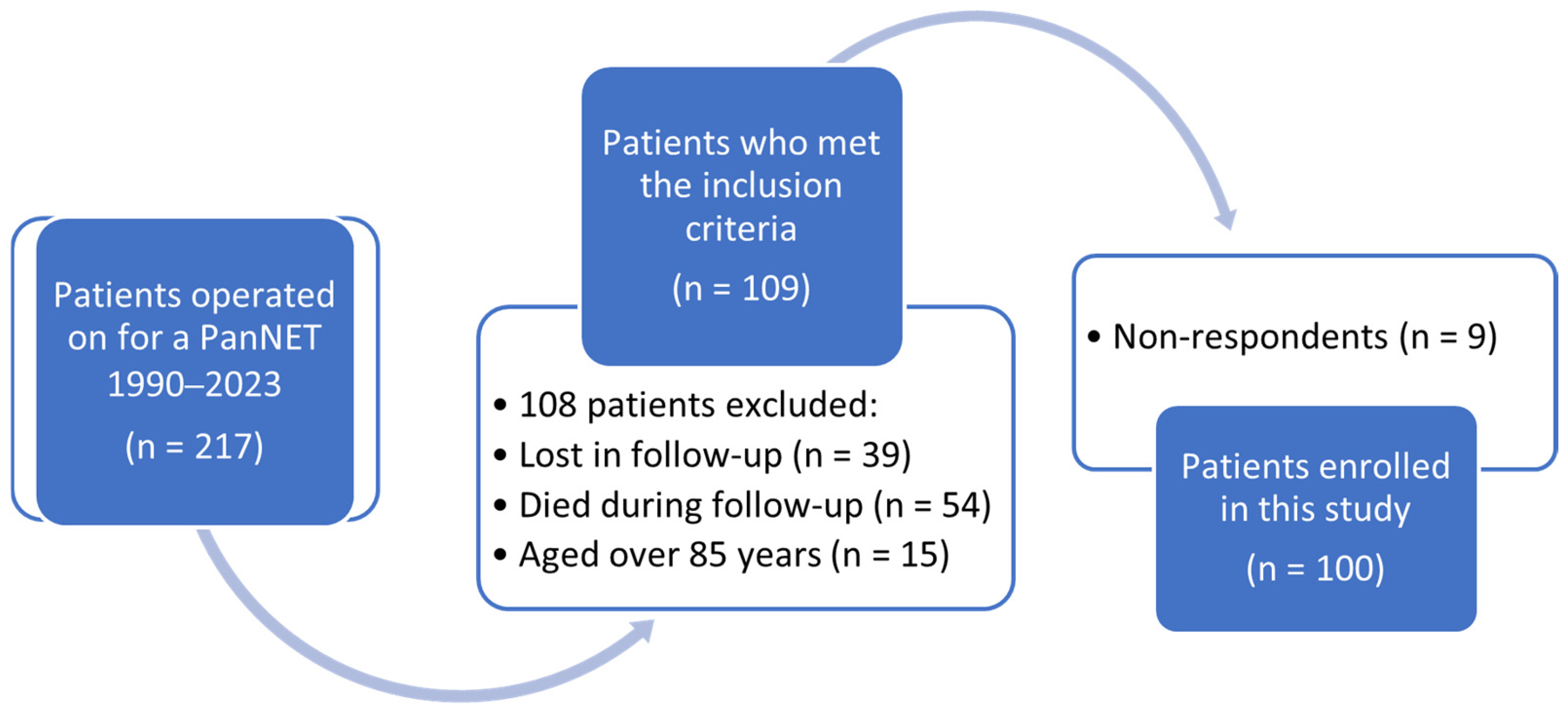
2.1. Study Population
2.2. Health-Related Quality of Life Assessment and Rationale
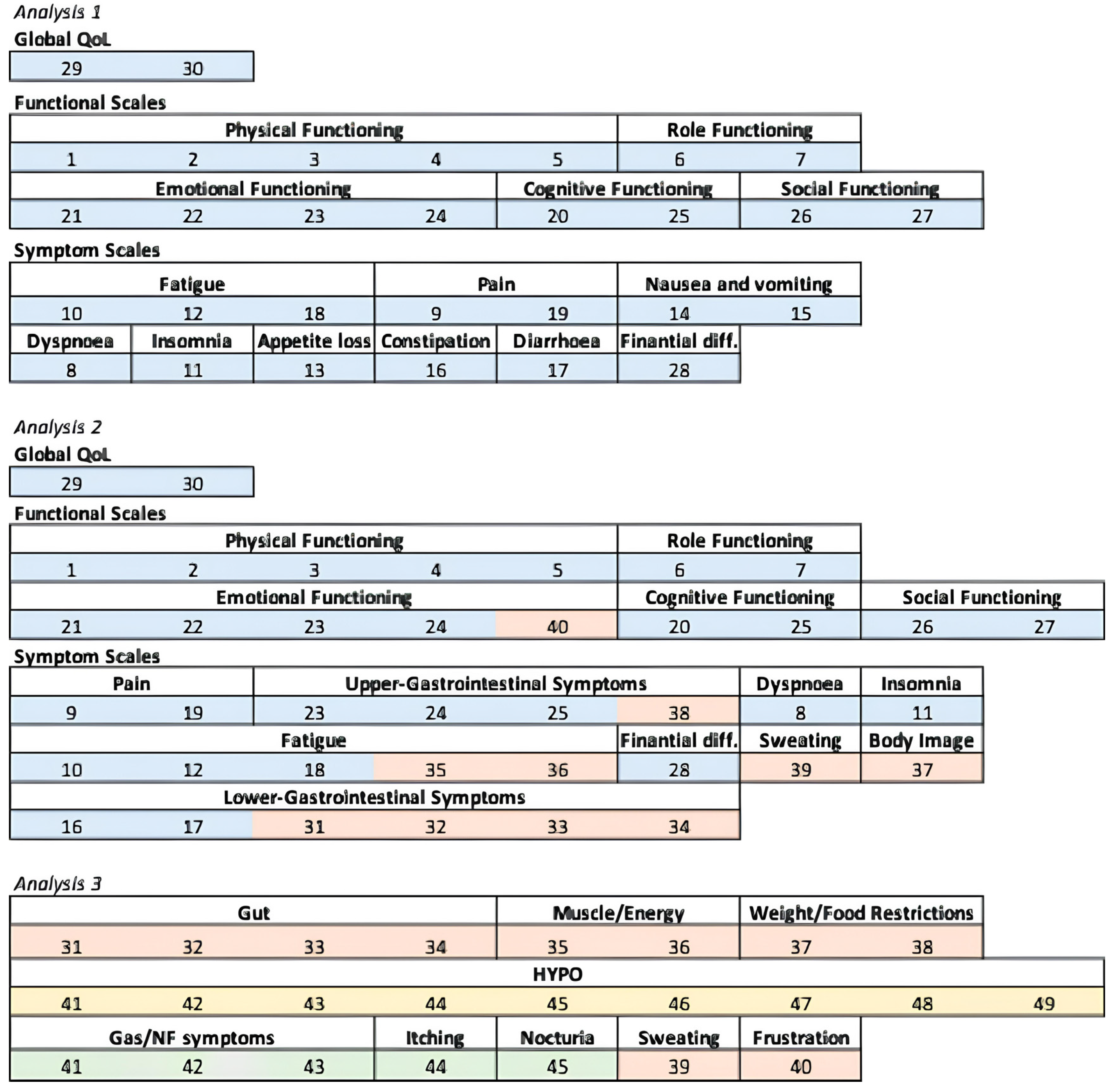
2.3. Domains’ Creation and Three Analyses
2.4. Statistical Analysis
3. Results
3.1. Clinical Data
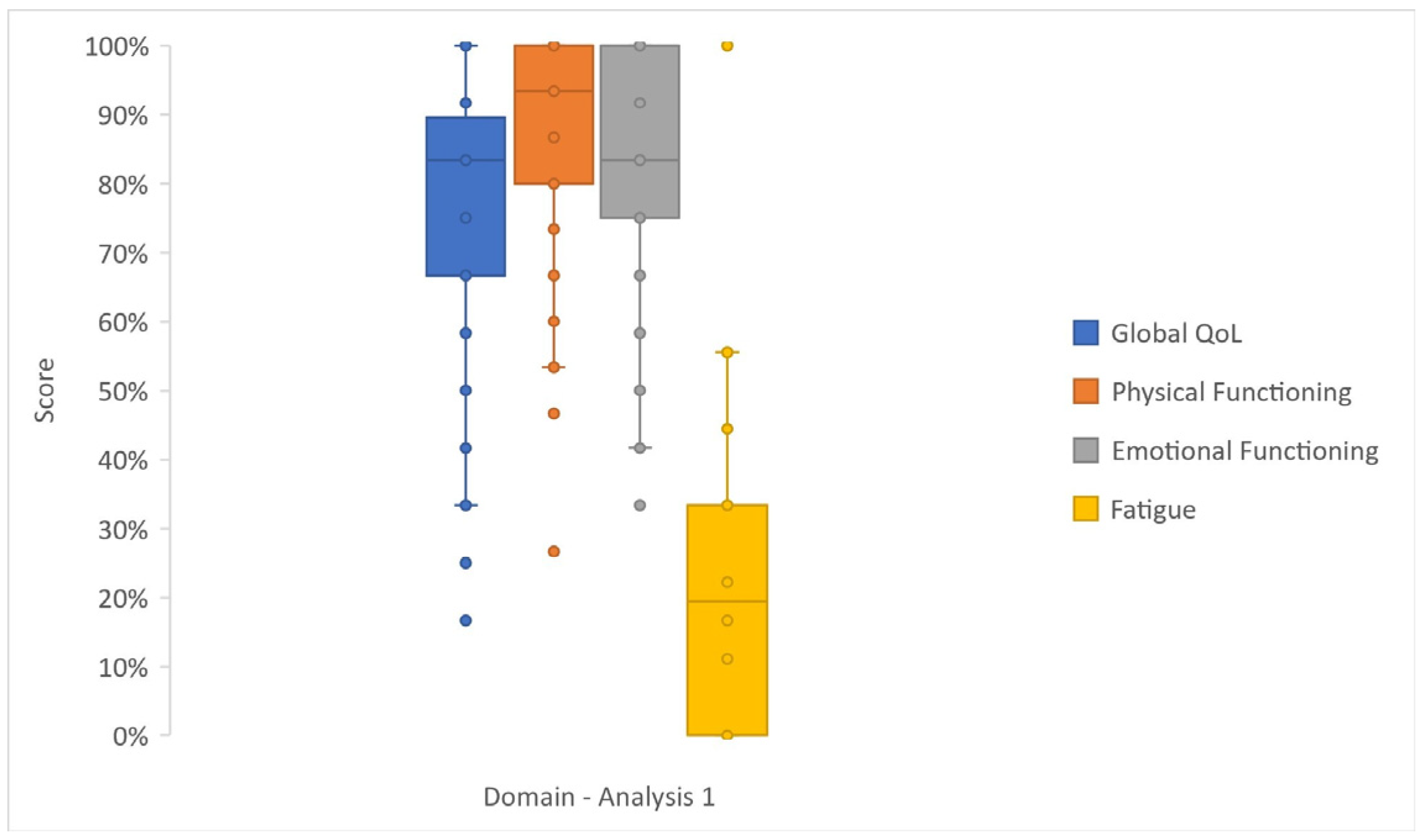
3.2. Analysis 1
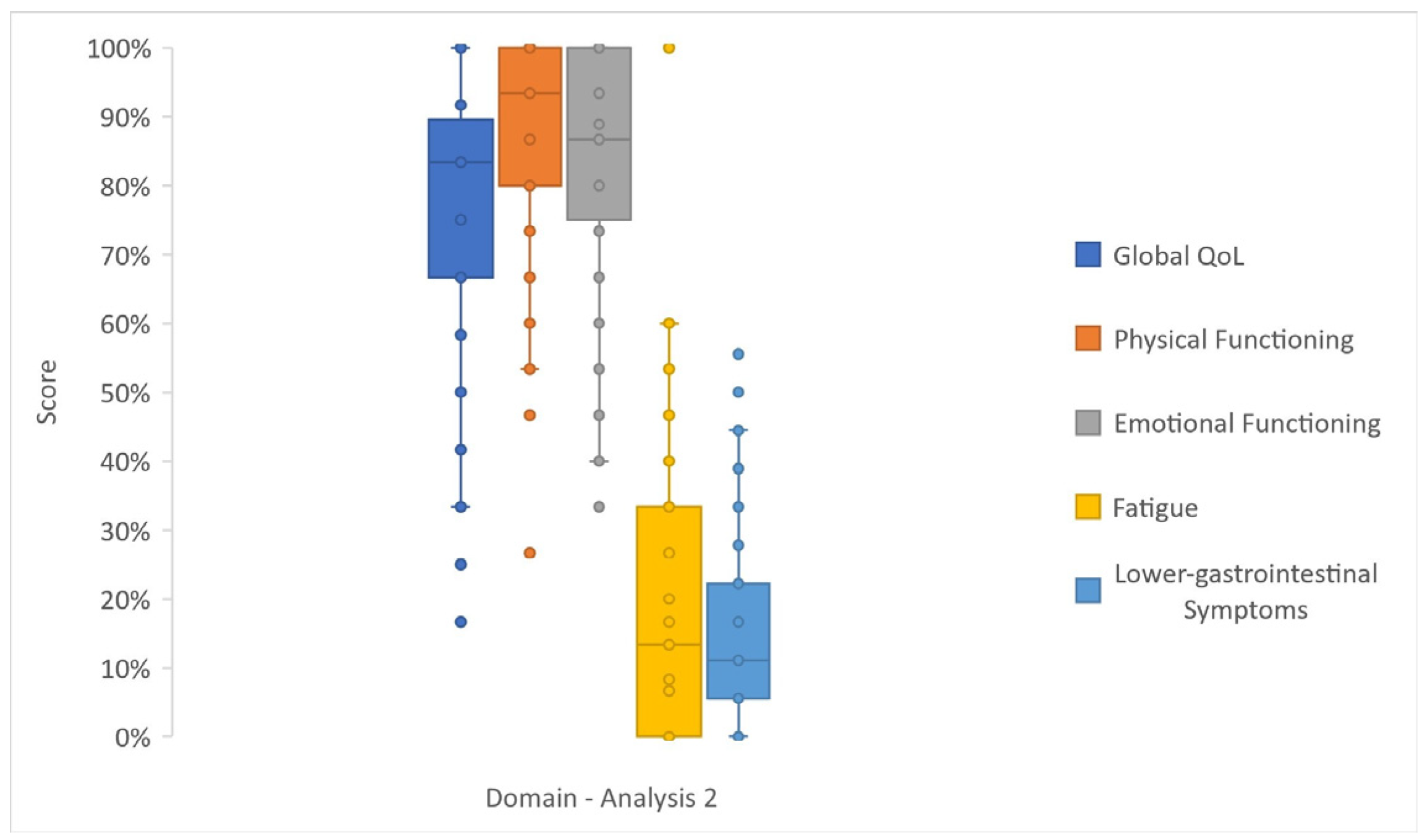
3.3. Analysis 2
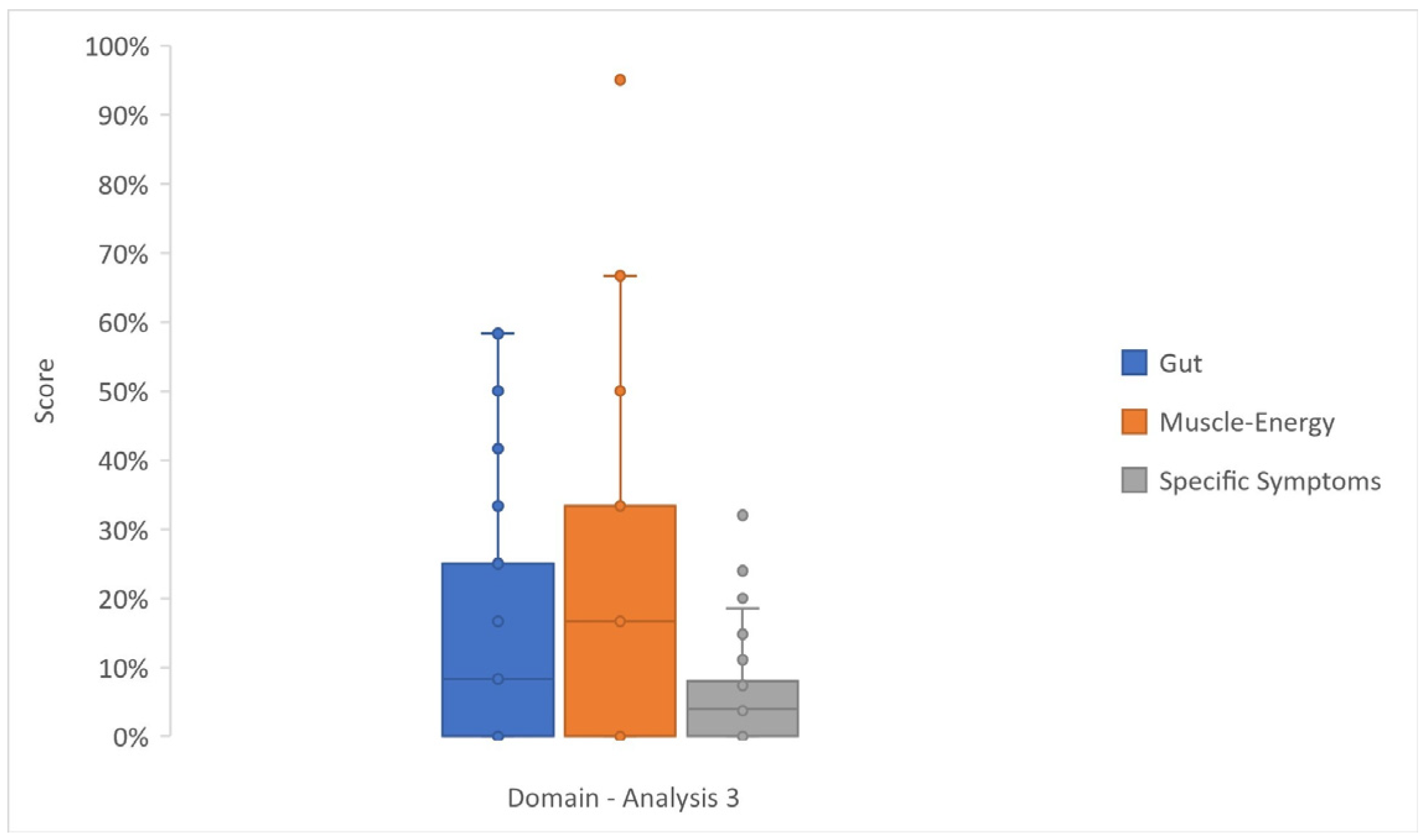
3.4. Analysis 3
3.5. Sensitivity Analysis for Gamma Model
3.6. Structural Validity and Internal Consistency of Analysis 3 Questionnaires
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PanNET | pancreatic neuroendocrine tumor |
| NF-PanNET | non-functioning pancreatic neuroendocrine tumor |
| MEN1 | multiple endocrine neoplasia type 1 |
| HRQoL | health-related quality of life |
| GEP-NET | gastro–entero-pancreatic neuroendocrine tumor |
| EORTC | European Organization for Research and Treatment of Cancer |
| FU | follow-up |
| Gas/NF-PanNET | gastrinoma/non-functioning pancreatic neuroendocrine tumor |
| AME | Average Marginal Effect |
| MCIDs | minimal clinically important differences |
| FDR | false-discovery rate |
| PF2 | physical functioning |
| EF | emotional functioning |
| GI | Gastrointestinal |
| DPPHR | duodenum-preserving pancreatic head resection |
References
- Cazzato, R.L.; Hubelé, F.; De Marini, P.; Ouvrard, E.; Salvadori, J.; Addeo, P.; Garnon, J.; Kurtz, J.-E.; Greget, M.; Mertz, L.; et al. Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures. Cancers 2021, 13, 6368. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Agarwal, S.K.; Perrier, N.D.; E Lines, K.; Valk, G.D.; Thakker, R.V. Multiple Endocrine Neoplasia Type 1: Latest Insights. Endocr. Rev. 2021, 42, 133–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, B.; Chen, J.; Su, Z.; Sun, S. The incidence, prevalence, and survival analysis of pancreatic neuroendocrine tumors in the United States. J. Endocrinol. Investig. 2023, 46, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Bevere, M.; Gkountakos, A.; Martelli, F.M.; Scarpa, A.; Luchini, C.; Simbolo, M. An Insight on Functioning Pancreatic Neuroendocrine Neoplasms. Biomedicines 2023, 11, 303. [Google Scholar] [CrossRef]
- Ronde, E.M.; Heidsma, C.M.; Eskes, A.M.; Schopman, J.E.; van Dijkum, E.J.M.N. Health-related quality of life and treatment effects in patients with well-differentiated gastroenteropancreatic neuroendocrine neoplasms: A systematic review and meta-analysis. Eur. J. Cancer Care 2021, 30, e13504. [Google Scholar] [CrossRef]
- Watson, C.; Tallentire, C.W.; Ramage, J.K.; Srirajaskanthan, R.; Leeuwenkamp, O.R.; Fountain, D. Quality of life in patients with gastroenteropancreatic tumours: A systematic literature review. World J. Gastroenterol. 2020, 26, 3686–3711. [Google Scholar] [CrossRef]
- Jiménez-Fonseca, P.; Carmona-Bayonas, A.; Martín-Pérez, E.; Crespo, G.; Serrano, R.; Llanos, M.; Villabona, C.; García-Carbonero, R.; Aller, J.; Capdevila, J.; et al. Spanish Neuroendocrine Tumor Group (GETNE). Health-related quality of life in well-differentiated metastatic gastroenteropancreatic neuroendocrine tumors. Cancer Metastasis Rev. 2015, 34, 381–400. [Google Scholar] [CrossRef]
- Beaumont, J.L.; Cella, D.; Phan, A.T.; Choi, S.; Liu, Z.; Yao, J.C. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 2012, 41, 461–466. [Google Scholar] [CrossRef]
- Larsson, G.; von Essen, L.; Sjödén, P.-O. Health-related quality of life in patients with endocrine tumours of the gastrointestinal tract. Acta Oncol. 1999, 38, 481–490. [Google Scholar] [CrossRef]
- Peltola, E.; Hannula, P.; Huhtala, H.; Sintonen, H.; Metso, S.; Sand, J.; Laukkarinen, J.; Tiikkainen, M.; Schalin-Jäntti, C.; Sirén, J.; et al. Long-term health-related quality of life in persons diagnosed with an insulinoma in Finland 1980-2010. Clin. Endocrinol. 2021, 94, 250–257. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Milanetto, A.C.; Armellin, C.; Brigiari, G.; Lorenzoni, G.; Pasquali, C. Younger Age and Parenchyma-Sparing Surgery Positively Affected Long-Term Health-Related Quality of Life after Surgery for Pancreatic Neuroendocrine Neoplasms. J. Clin. Med. 2023, 12, 6529. [Google Scholar] [CrossRef] [PubMed]
- Ramage, J.K.; Friend, E.; Randell, J.; King, B.; Ortega, P.F.; McNamara, M.G.; Kaltsas, G.; Falconi, M.; Cwikla, J.; Capdevila, J.; et al. EORTC Quality of Life Group. Development of a quality of life questionnaire for patients with pancreatic neuroendocrine tumours (the PANNET module). J. Neuroendocr. 2022, 34, e13097. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A.; EORTC Quality of Life Group. Scoring procedures. In The EORTC QLQ-C30 Scoring Manual, 3rd ed.; EORTC Quality of Life Group Publications: Brussels, Belgium, 2001; pp. 6–14. [Google Scholar]
- Scott, N.W.; Fayers, P.M.; Aaronson, N.K.; Bottomley, A.; de Graeff, A.; Groenveld, M.; Gundy, C.; Koller, M.; Petersen, M.A.; Sprangers, M.A.G.; et al. EORTC QLQC30 tables of reference values. In The EORTC QLQ-C30 Reference Values Manual; EORTC Quality of Life Group Publications: Brussels, Belgium, 2008; pp. 14–294. [Google Scholar]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis, 2nd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Farewell, V.; Long, D.; Tom, B.; Yiu, S.; Su, L. Two-Part and Related Regression Models for Longitudinal Data. Annu. Rev. Stat. Its Appl. 2017, 4, 283–315. [Google Scholar] [CrossRef]
- Ferrari, S.; Cribari-Neto, F. Beta Regression for Modelling Rates and Proportions. J. Appl. Stat. 2004, 31, 799–815. [Google Scholar] [CrossRef]
- Papke, L.E.; Wooldridge, J.M. Econometric methods for fractional response variables with an application to 401(k) plan participation rates. J. Appl. Econ. 1996, 11, 619–632. [Google Scholar] [CrossRef]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef]
- Costello, A.B.; Osborne, J.W. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pract. Assess. Res. Eval. 2005, 10, 7. [Google Scholar] [CrossRef]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 September 2024).
- Wasserstein, R.L.; Lazar, N.A. The ASA Statement on p-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Marini, F.; Giusti, F.; Tonelli, F.; Brandi, M.L. Management impact: Effects on quality of life and prognosis in MEN1. Endocr. Relat. Cancer 2017, 24, T227–T242. [Google Scholar] [CrossRef]
- Berglund, G.; Lidén, A.; Hansson, M.G.; Öberg, K.; Sjöden, P.O.; Nordin, K. Quality of life in patients with multiple endocrine neoplasia type 1 (MEN 1). Fam. Cancer 2003, 2, 27–33. [Google Scholar] [CrossRef]
- Strømsvik, N.; Nordin, K.; Berglund, G.; Engebretsen, L.F.; Hansson, M.G.; Gjengedal, E. Living with multiple endocrine neoplasia type 1: Decent care-insufficient medical and genetic information: A qualitative study of MEN 1 patients in a Swedish hospital. J. Genet. Couns. 2007, 16, 105–117. [Google Scholar] [CrossRef]
- Giusti, F.; Cioppi, F.; Fossi, C.; Marini, F.; Masi, L.; Tonelli, F.; Brandi, M.L. Quality of life in Italian patients with Multiple endocrine neoplasia type 1 (MEN 1): Results of an extensive survey. Orphanet J. Rare Dis. 2021, 16, 16. [Google Scholar] [CrossRef]
- Teunissen, J.J.; Kwekkeboom, D.J.; Krenning, E.P. Quality of life in patients with gastroenteropancreatic tumors treated with [177Lu-DOTA0, Tyr3]octreotate. J. Clin. Oncol. 2004, 22, 2724–2729. [Google Scholar] [CrossRef]
- Pavel, M.; Unger, N.; Borbath, I.; Ricci, S.; Hwang, T.-L.; Brechenmacher, T.; Park, J.; Herbst, F.; Beaumont, J.L.; Bechter, O. Safety and QOL in patients with advanced NET in a phase 3b expanded access study of everolimus. Target. Oncol. 2016, 11, 667–675. [Google Scholar] [CrossRef]
- Băjenaru, L.; Balog, A.; Dobre, C.; Drăghici, R.; Prada, G.-I. Latent profile analysis for quality of life in older patients. BMC Geriatr. 2022, 22, 848. [Google Scholar] [CrossRef]
- Derogar, M.; van der Schaaf, M.; Lagergren, P. Reference values for the EORTC QLQ-C30 quality of life questionnaire in a random sample of the Swedish population. Acta Oncol. 2012, 51, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.; Hinz, A. Reference data for the quality of life questionnaire EORTC QLQ-C30in the general German population. Eur. J. Cancer 2001, 37, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, A.; Kosaka, H.; Kaibori, M.; Higashi, T.; Ogawa, A. Activities of daily living after surgery among older patients with gastrointestinal and hepatobiliary-pancreatic cancers: A retrospective observational study using nationwide health services utilisation data from Japan. BMJ Open 2023, 13, e070415. [Google Scholar] [CrossRef] [PubMed]
- Jałtuszewska, S.; Basiński, A. Quality of life and functional status of patients treated for neoplastic disease in mid-northern Poland. Appl. Nurs. Res. 2016, 32, 85–90. [Google Scholar] [CrossRef]
- Fu, M.; Yu, L.; Yang, L.; Chen, Y.; Chen, X.; Hu, Q.; Sun, H. Gender differences in pancreatic neuroendocrine neoplasms: A retrospective study based on the population of Hubei Province, China. Front. Endocrinol. 2022, 13, 885895. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fayers, P.M.; Bjordal, K.; Kaasa, S. Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: The QLQ=C30 (+ 3). J. Clin. Oncol. 1998, 16, 1188–1196. [Google Scholar] [CrossRef]
- Michelson, H.; Bolund, C.; Nilsson, B.; Brandberg, Y. Health-related quality of life measured by the EORTC QLQ-C30: Reference values from a large sample of Swedish population. Acta Oncol. 2000, 39, 477–484. [Google Scholar] [CrossRef]
- Milanetto, A.C.; Nordenström, E.; Sundlöv, A.; Almquist, M. Health-Related Quality of Life After Surgery for Small Intestinal Neuroendocrine Tumours. World J. Surg. 2018, 42, 3231–3239. [Google Scholar] [CrossRef]
- Rubin, R.R.; Peyrot, M. Quality of life and diabetes. Diabetes Metab. Res. Rev. 1999, 15, 205–218. [Google Scholar] [CrossRef]
- Shaw, K.; Thomas, A.S.; Rosario, V.; Kwon, W.; Schrope, B.A.; Sugahara, K.; Chabot, J.A.; Genkinger, J.M.; Kluger, M.D. Long term quality of life amongst pancreatectomy patients with diabetes mellitus. Pancreatology 2021, 21, 501–508. [Google Scholar] [CrossRef]
- Latenstein, A.E.; Blonk, L.; Tjahjadi, N.S.; de Jong, N.; Busch, O.R.; de Hingh, I.H.; van Hooft, J.E.; Liem, M.S.; Molenaar, I.Q.; van Santvoort, H.C.; et al. Dutch Pancreatic Cancer Group. Long-term quality of life and exocrine and endocrine insufficiency after pancreatic surgery: A multicenter, cross-sectional study. HPB 2021, 23, 1722–1731. [Google Scholar] [CrossRef]
- Beger, H.G.; Mayer, B.; Vasilescu, C.; Poch, B. Long-term Metabolic Morbidity and Steatohepatosis Following Standard Pancreatic Resections and Parenchyma-sparing, Local Extirpations for Benign Tumor: A Systematic Review and Meta-analysis. Ann. Surg. 2022, 275, 54–66. [Google Scholar] [CrossRef]
- Giuliani, T.; De Pastena, M.; Paiella, S.; Marchegiani, G.; Landoni, L.; Festini, M.; Ramera, M.; Marinelli, V.; Casetti, L.; Esposito, A.; et al. Pancreatic Enucleation Patients Share the Same Quality of Life as the General Population at Long-Term Follow-Up: A Propensity Score-Matched Analysis. Ann. Surg. 2023, 277, e609–e616. [Google Scholar] [CrossRef]
- Li, S.-Z.; Zhen, T.-T.; Wu, Y.; Wang, M.; Qin, T.-T.; Zhang, H.; Qin, R.-Y. Quality of life after pancreatic surgery. World J. Gastroenterol. 2024, 30, 943–955. [Google Scholar] [CrossRef]
| At the Time of Surgery | ||
|---|---|---|
| Gender, n | Female | 60 |
| Male | 40 | |
| Type of NET, n | Gastrinoma/NF a | 68 |
| insulinoma | 32 | |
| Tumor Grade, n | G2 | 23 |
| G1 | 72 | |
| N/A | 5 | |
| Type of Surgery, n | Standard pancreatic resection b | 54 |
| Parenchyma-sparing resection c | 46 | |
| MEN1 syndrome, n | Yes | 17 |
| No | 83 | |
| At the time of the study | ||
| Time from surgery (months) | Median (IQR) | 133 (52−219) |
| Age at questionnaire (years) | Median (IQR) | 65 (55−73) |
| Other diseases, n | Multiple | 63 |
| Single | 23 | |
| No | 14 | |
| Tumor burden, n | Distant metastases d | 5 |
| Local recurrence | 6 | |
| Non-evidence of disease | 89 | |
| Active disease, n | Yes | 21 |
| No | 79 | |
| Other treatments, n | Yes | 15 |
| No | 85 | |
| Pancreatic function, n | Diabetes mellitus | 29 |
| Exocrine insufficiency | 4 | |
| Diabetes mellitus and Exocrine insufficiency | 6 | |
| Normal | 61 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanetto, A.C.; Armellin, C.; Gasparini, D.; Lorenzoni, G.; Pasquali, C. Quality of Life After Pancreatic Surgery for Neuroendocrine Tumors of the Pancreas: Observational Study of Long-Term Outcomes. Cancers 2025, 17, 3205. https://doi.org/10.3390/cancers17193205
Milanetto AC, Armellin C, Gasparini D, Lorenzoni G, Pasquali C. Quality of Life After Pancreatic Surgery for Neuroendocrine Tumors of the Pancreas: Observational Study of Long-Term Outcomes. Cancers. 2025; 17(19):3205. https://doi.org/10.3390/cancers17193205
Chicago/Turabian StyleMilanetto, Anna Caterina, Claudia Armellin, Daniele Gasparini, Giulia Lorenzoni, and Claudio Pasquali. 2025. "Quality of Life After Pancreatic Surgery for Neuroendocrine Tumors of the Pancreas: Observational Study of Long-Term Outcomes" Cancers 17, no. 19: 3205. https://doi.org/10.3390/cancers17193205
APA StyleMilanetto, A. C., Armellin, C., Gasparini, D., Lorenzoni, G., & Pasquali, C. (2025). Quality of Life After Pancreatic Surgery for Neuroendocrine Tumors of the Pancreas: Observational Study of Long-Term Outcomes. Cancers, 17(19), 3205. https://doi.org/10.3390/cancers17193205








