Geographical Inequalities and Comorbidities in the Timely Diagnosis of NSCLC: A Real-Life Retrospective Study from a Tertiary Hospital in Western Greece
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. Study Population
2.3. Statistical Analysis
3. Results
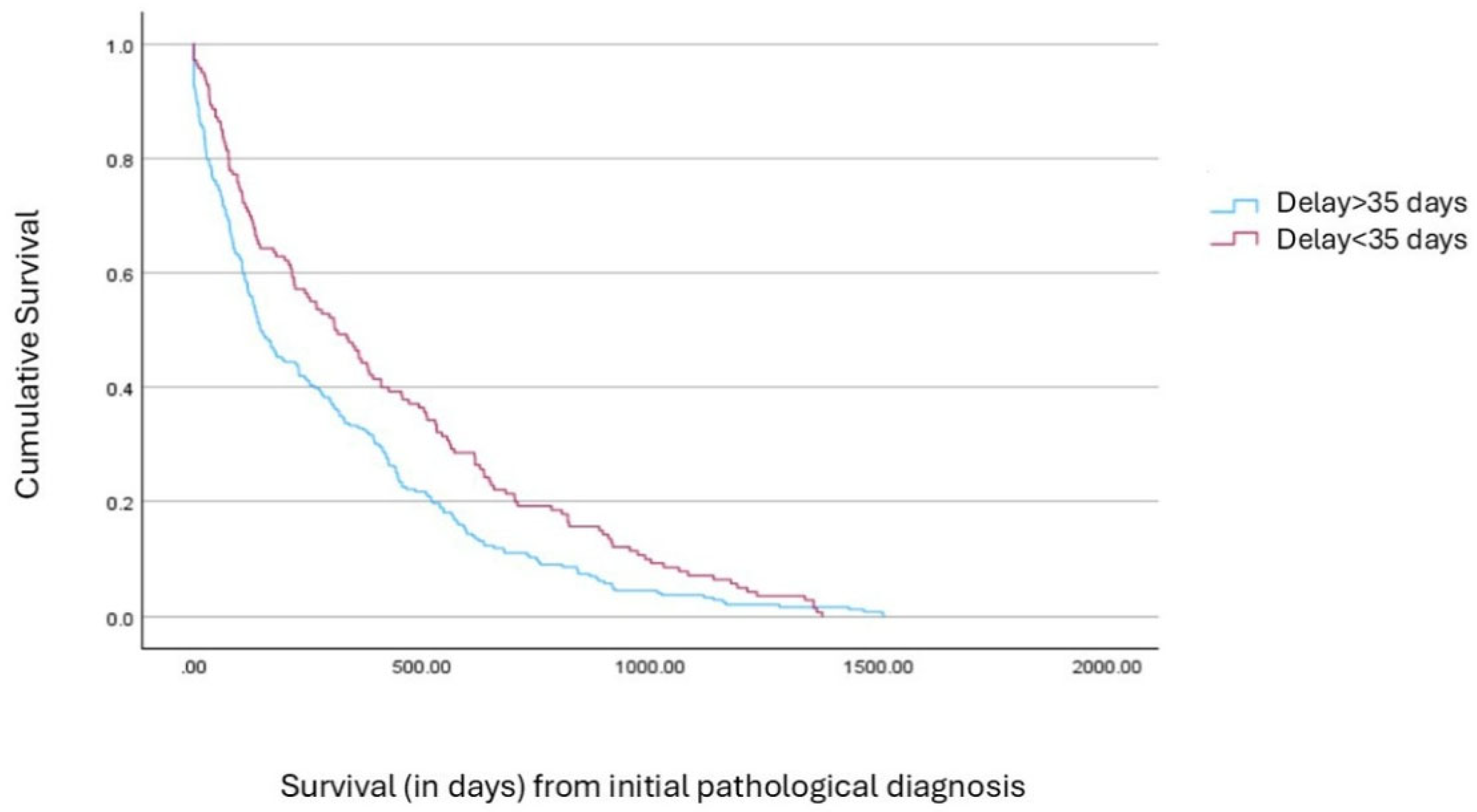
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Shan, T.; Zhang, D.; Ma, F. Nowcasting and forecasting global aging and cancer burden: Analysis of data from the GLOBOCAN and Global Burden of Disease Study. J. Natl. Cancer Cent. 2024, 4, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, D.S.; Conev, N.V.; Donev, I.S.; Tonev, I.D.; Panayotova, T.V.; Dimitrova-Gospodinova, E.G. Impact of travel burden on clinical outcomes in lung cancer. Support. Care Cancer 2022, 30, 5381–5387. [Google Scholar] [CrossRef]
- Fu, K.; Xie, F.; Wang, F.; Fu, L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J. Hematol. Oncol. 2022, 15, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Franchina, T.; Ricciardi, G.; Smiroldo, V.; Picciotto, M.; Zanghì, M.; Rolfo, C.; Adamo, V. Third generation EGFR TKIs in EGFR-mutated NSCLC: Where are we now and where are we going. Crit. Rev. Oncol. 2017, 117, 38–47. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-generation EGFR and ALK inhibitors: Mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Virgilsen, L.F.; Møller, H.; Vedsted, P. Travel distance to cancer-diagnostic facilities and tumour stage. Heal. Place 2019, 60, 102208. [Google Scholar] [CrossRef]
- Scoggins, J.F.; Fedorenko, C.R.; Donahue, S.M.A.; Buchwald, D.; Blough, D.K.; Ramsey, S.D. Is Distance to Provider a Barrier to Care for Medicaid Patients With Breast, Colorectal, or Lung Cancer? J. Rural. Heal. 2011, 28, 54–62. [Google Scholar] [CrossRef]
- Kerr, K.M.; Bibeau, F.; Thunnissen, E.; Botling, J.; Ryška, A.; Wolf, J.; Öhrling, K.; Burdon, P.; Malapelle, U.; Büttner, R. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer 2021, 154, 161–175. [Google Scholar] [CrossRef]
- Waarts, M.R.; Stonestrom, A.J.; Park, Y.C.; Levine, R.L. Targeting mutations in cancer. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Ambroggi, M.; Biasini, C.; Del Giovane, C.; Fornari, F.; Cavanna, L. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. Oncol. 2015, 20, 1378–1385. [Google Scholar] [CrossRef]
- Scott, J.A.; Lennerz, J.; Johnson, M.L.; Gordan, L.N.; Dumanois, R.H.; Quagliata, L.; Ritterhouse, L.L.; Cappuzzo, F.; Wang, B.; Xue, M.; et al. Compromised Outcomes in Stage IV Non–Small-Cell Lung Cancer With Actionable Mutations Initially Treated Without Tyrosine Kinase Inhibitors: A Retrospective Analysis of Real-World Data. JCO Oncol. Pr. 2024, 20, 145–153. [Google Scholar] [CrossRef]
- Logan, C.D.; Feinglass, J.; Halverson, A.L.; Lung, K.; Kim, S.; Bharat, A.; Odell, D.D. Rural-urban survival disparities for patients with surgically treated lung cancer. J. Surg. Oncol. 2022, 126, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Snow, S.; Brezden-Masley, C.; Carter, M.D.; Dhani, N.; Macaulay, C.; Ramjeesingh, R.; Raphael, M.J.; D’angelo, M.S.; Servidio-Italiano, F. Barriers and Unequal Access to Timely Molecular Testing Results: Addressing the Inequities in Cancer Care Delays across Canada. Curr. Oncol. 2024, 31, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.A.; Antonoff, M.B. On the road again: Impact of travel distance on outcomes for lung cancer. JTCVS Open 2023, 16, 976. [Google Scholar] [CrossRef] [PubMed]
- Herb, J.N.; Dunham, L.N.; Mody, G.; Long, J.M.; Stitzenberg, K.B. Lung Cancer Surgical Regionalization Disproportionately Worsens Travel Distance for Rural Patients. J. Rural. Heal. 2020, 36, 496–505. [Google Scholar] [CrossRef]
- Johnson, A.M.; Hines, R.B.; Johnson, J.A.; Bayakly, A.R. Treatment and survival disparities in lung cancer: The effect of social environment and place of residence. Lung Cancer 2014, 83, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sampsonas, F.; Bosgana, P.; Bravou, V.; Tzouvelekis, A.; Dimitrakopoulos, F.-I.; Kokkotou, E. Interstitial Lung Diseases and Non-Small Cell Lung Cancer: Particularities in Pathogenesis and Expression of Driver Mutations. Genes 2024, 15, 934. [Google Scholar] [CrossRef]
- Herrera-Juárez, M.; Serrano-Gómez, C.; Bote-De-Cabo, H.; Paz-Ares, L. Targeted therapy for lung cancer: Beyond EGFR and ALK. Cancer 2023, 129, 1803–1820. [Google Scholar] [CrossRef]
- Hendriks, L.; Kerr, K.; Menis, J.; Mok, T.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.; Solomon, B.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- Takenaka, T.; Inamasu, E.; Yoshida, T.; Toyokawa, G.; Nosaki, K.; Hirai, F.; Yamaguchi, M.; Seto, T.; Takenoyama, M.; Ichinose, Y. Influence of the distance between home and the hospital on patients with surgically resected non-small-cell lung cancer. Eur. J. Cardio-Thoracic Surg. 2015, 49, 842–846. [Google Scholar] [CrossRef]
- Charpidou, A.; Mani, M.; Kokkotou, E.; Stournara, L.; Stathelou, L.; Antoniadou, K.; Argyropoulos, E.; Syrigos, K.N. Molecular Diagnostics and Treatment Patterns in Metastatic Non-small Cell Lung Cancer Patients: Real World Evidence from Greece: LACHESIS Study. Anticancer. Res. 2024, 44, 2063–2072. [Google Scholar] [CrossRef]
- Johnson, K.J.; Wang, X.; Barnes, J.M.; Delavar, A. Associations between geographic residence and US adolescent and young adult cancer stage and survival. Cancer 2021, 127, 3640–3650. [Google Scholar] [CrossRef]
- Bahnassy, A.A.; Ismail, H.; Mohanad, M.; El-Bastawisy, A.; Yousef, H.F. The prognostic role of PD-1, PD-L1, ALK, and ROS1 proteins expression in non-small cell lung carcinoma patients from Egypt. J. Egypt. Natl. Cancer Inst. 2022, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kuhn, M.; Prettner, K.; Yu, F.; Yang, T.; Bärnighausen, T.; E Bloom, D.; Wang, C. The global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020–50: A health-augmented macroeconomic modelling study. Lancet Glob. Heal. 2023, 11, e1183–e1193. [Google Scholar] [CrossRef]
- Kourlaba, G.; Hillas, G.; Vassilakopoulos, T.; Maniadakis, N. The disease burden of chronic obstructive pulmonary disease in Greece. Int. J. Chronic Obstr. Pulm. Dis. 2016, ume 11, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.T.; Kim, T.; Munson, J. Residence in Rural Areas of the United States and Lung Cancer Mortality. Disease Incidence, Treatment Disparities, and Stage-Specific Survival. Ann. Am. Thorac. Soc. 2017, 14, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.F.; Hampel, K.J.; Sidiropoulos, N. Benefits of Implementing Reflex Genomic Analysis for Nonsmall Cell Lung Cancer. J. Appl. Lab. Med. 2024, 9, 28–40. [Google Scholar] [CrossRef]
- Michaelidou, K.; Karniadakis, I.; Pantelaion, V.; Koutoulaki, C.; Boukla, E.; Folinas, K.; Dimaras, P.; A Papadaki, M.; Koutsopoulos, A.V.; Mavroudis, D.; et al. Rapid and reliable testing for clinically actionable EGFR mutations in non-small cell lung cancer using the Idylla TM platform: A real-world two-center experience in Greece. Expert Rev. Mol. Diagn. 2024, 24, 89–98. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Yuan, J.-Q.; Wang, K.-F.; Fu, X.-H.; Han, X.-R.; Threapleton, D.; Yang, Z.-Y.; Mao, C.; Tang, J.-L. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef]
- Fois, S.S.; Paliogiannis, P.; Zinellu, A.; Fois, A.G.; Cossu, A.; Palmieri, G. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 612. [Google Scholar] [CrossRef]
- Veluswamy, R.; Mack, P.C.; Houldsworth, J.; Elkhouly, E.; Hirsch, F.R. KRAS G12C–Mutant Non–Small Cell Lung Cancer. J. Mol. Diagn. 2021, 23, 507–520. [Google Scholar] [CrossRef]
- Tountas, Y.; Oikonomou, N.; Pallikarona, G.; Dimitrakaki, C.; Tzavara, C.; Souliotis, K.; Mariolis, A.; Pappa, E.; Kontodimopoulos, N.; Niakas, D. Sociodemographic and socioeconomic determinants of health services utilization in Greece: The Hellas Health I study. Heal. Serv. Manag. Res. 2011, 24, 8–18. [Google Scholar] [CrossRef]
| Variable | N (%) | Variable | N (%) |
|---|---|---|---|
| Gender | Molecular Test Performed | ||
| Male | 636 (76.7%) | Yes | 736 (79.4%) |
| Female | 193 (23.3%) | No | 191 (20.6%) |
| Stage (I–III vs. IV) | Residence Area | ||
| Stage I–III | 127 (36.3%) | Urban Area | 278 (38.8%) |
| Stage IV | 222 (63.7%) | Rural Area | 439 (61.2%) |
| Psychiatric Disorders (Depression) | Cardiovascular Disease | ||
| Non-psychiatric | 618 (75.2%) | Non-CVD | 404 (51%) |
| Psychiatric | 204 (24.8%) | CVD | 388 (49%) |
| DM II | COPD | ||
| Non-DMII | 677 (84.0%) | Non-COPD | 490 (62.9%) |
| DMII | 129 (16%) | COPD | 289 (37.1%) |
| NSCLC Type | Smoking Status | ||
| Adenocarcinoma | 445 (48.0%) | Non-smoker | 11 (3.8%) |
| Squamous | 276 (29.8%) | Active smoker | 200 (69.7%) |
| NOS/Other/Not defined/Missing | 206 (22.2%) | Ex-smoker | 76 (26.5%) |
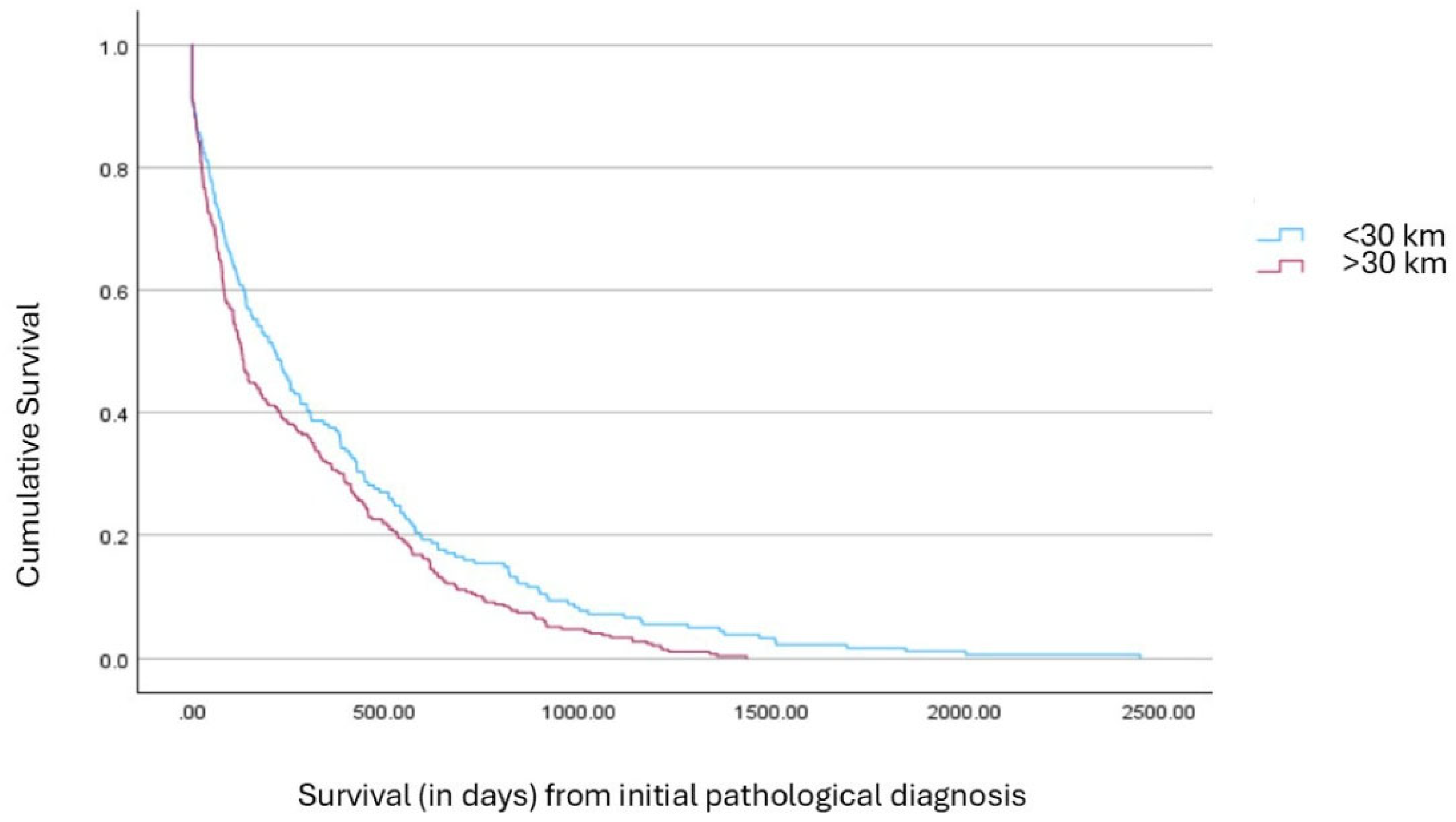
| Variable | <30 km, n (%) or Mean ± SD | >30 km, n (%) or Mean ± SD | Test Statistics | df | p-Value |
|---|---|---|---|---|---|
| Age | 68.6 ± 10.0 (n = 218) | 68.5 ± 9.1 (n = 373) | t = 0.050 | 0.960 | |
| Stage at Diagnosis | χ2 = 0.204 | 1 | 0.651 | ||
| • Stage I–III | 24 (35.3%) | 68 (38.4%) | |||
| • Stage IV | 44 (64.7%) | 109 (61.6%) | |||
| Smoking Status | χ2 = 0.752 | 2 | 0.687 | ||
| • Non-smoker | 4 (7.1%) | 6 (4.2%) | |||
| • Active smoker | 34 (60.7%) | 91 (64.1%) | |||
| • Ex-smoker | 18 (32.2%) | 45 (31.3%) | |||
| Histological Type | χ2 = 4.903 | 2 | 0.086 | ||
| • AdNSCLC | 164 (53.1%) | 231 (58.5%) | |||
| • SqNSCLC | 87 (27.2%) | 108 (27.4%) | |||
| • NOS/other | 62 (20.1%) | 37 (9.4%) | |||
| Molecular Test Performed | χ2 = 6.365 | 1 | 0.012 * | ||
| • Yes | 253 (92%) | 371 (84%) | |||
| • No | 25 (8%) | 68 (16%) | |||
| Psychiatric History (Depression) | χ2 = 1.580 | 1 | 0.209 | ||
| • Yes | 66 (23.8%) | 123 (28%) | |||
| • No | 211 (76.2%) | 315 (72%) | |||
| CVD | χ2 = 0.158 | 1 | 0.691 | ||
| • Yes | 135 (49.2%) | 221 (50.8%) | |||
| • No | 139 (50.8%) | 214 (49.2%) | |||
| DM II | χ2 = 0.658 | 1 | 0.417 | ||
| • Yes | 44 (15.9%) | 80 (18.3%) | |||
| • No | 232 (84.1%) | 357 (81.7%) | |||
| COPD | χ2 = 9.606 | 1 | 0.002 * | ||
| • Yes | 85 (31.2%) | 185 (42.5%) | |||
| • No | 187 (68.8%) | 246 (57.5%) |
| Molecular Marker | Adenocarcinoma (%) | Squamous (%) | NOS/Other/Unclassified (%) | Total Mutated/Valid N | p-Value |
|---|---|---|---|---|---|
| EGFR | 7.8% (30/384) | 0.9% (2/225) | 3.6% (3/84) | 35/693 | <0.001 |
| KRAS G12C | 13.3% (48/361) | 2.4% (5/206) | 13.5% (10/74) | 63/641 | <0.001 |
| ALK | 2.3% (8/351) | 1.0% (2/196) | 1.8% (1/56) | 11/603 | 0.573 |
| BRAF | 3.3% (12/360) | 2.0% (4/197) | 4.2% (3/71) | 19/628 | 0.569 |
| ROS1 | 8.2% (23/281) | 1.9% (3/159) | 4.5% (2/44) | 28/484 | 0.023 |
| PD-L1 ≥1% | 59.9% (203/339) | 62.9% (124/197) | 67.3% (33/49) | 360/585 | 0.534 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampsonas, F.; Bosgana, P.; Psarros, E.; Papaioannou, O.; Tryfona, F.; Mantzouranis, K.; Katsaras, M.; Christopoulos, I.; Tsirikos, G.; Tsiri, P.; et al. Geographical Inequalities and Comorbidities in the Timely Diagnosis of NSCLC: A Real-Life Retrospective Study from a Tertiary Hospital in Western Greece. Cancers 2025, 17, 2701. https://doi.org/10.3390/cancers17162701
Sampsonas F, Bosgana P, Psarros E, Papaioannou O, Tryfona F, Mantzouranis K, Katsaras M, Christopoulos I, Tsirikos G, Tsiri P, et al. Geographical Inequalities and Comorbidities in the Timely Diagnosis of NSCLC: A Real-Life Retrospective Study from a Tertiary Hospital in Western Greece. Cancers. 2025; 17(16):2701. https://doi.org/10.3390/cancers17162701
Chicago/Turabian StyleSampsonas, Fotios, Pinelopi Bosgana, Emmanouil Psarros, Ourania Papaioannou, Fotini Tryfona, Konstantinos Mantzouranis, Matthaios Katsaras, Ioannis Christopoulos, Georgios Tsirikos, Panagiota Tsiri, and et al. 2025. "Geographical Inequalities and Comorbidities in the Timely Diagnosis of NSCLC: A Real-Life Retrospective Study from a Tertiary Hospital in Western Greece" Cancers 17, no. 16: 2701. https://doi.org/10.3390/cancers17162701
APA StyleSampsonas, F., Bosgana, P., Psarros, E., Papaioannou, O., Tryfona, F., Mantzouranis, K., Katsaras, M., Christopoulos, I., Tsirikos, G., Tsiri, P., Komninos, D., Koulousousa, E., Theochari, E., Sotiropoulou, V., Tzelepi, V., Zolota, V., Kokkotou, E., Kouvela, M., Syrigos, K. N., & Tzouvelekis, A. (2025). Geographical Inequalities and Comorbidities in the Timely Diagnosis of NSCLC: A Real-Life Retrospective Study from a Tertiary Hospital in Western Greece. Cancers, 17(16), 2701. https://doi.org/10.3390/cancers17162701








