Lymph Node Reporting and Data System (LN-RADS)—Retrospective Evaluation for Ultrasound Classification of Superficial Lymph Nodes
Simple Summary
Abstract
1. Introduction
- Significant impact of nodal burden on survival: The study highlights that the number of affected LNs is a critical predictor of mortality across various solid cancers. An increased nodal burden is consistently associated with poorer survival outcomes, underscoring the importance of comprehensive nodal evaluation in cancer staging and prognosis.
- Nodal burden as a superior prognostic factor: In certain cancers, nodal burden was found to be a stronger predictor of patient outcomes than traditional prognostic factors, such as tumour size. This suggests that the extent of LN involvement may be more indicative of disease progression and survival than other commonly used metrics.
- Implications for personalized therapy: The findings suggest that a detailed assessment of LN involvement could inform more personalized treatment strategies. By quantifying nodal burden, clinicians may better stratify patients according to risk and tailor therapies to improve survival outcomes.
- Staging Phase: Relying on a rigid size criterion may result in an underestimated stage of disease, leading to a limited surgical scope, insufficiently aggressive systemic therapy, or even the omission of adjuvant therapies, such as radiotherapy, increasing the risk of disease recurrence.
- Treatment Phase: Neglecting macrometastases (Figure 1) during active treatment can delay necessary interventions by weeks or even months, allowing for the potential spread of cancer to an extent that may evade control and treatment.
- Post-Treatment Surveillance: Similarly, during follow-up, there is a risk of overlooking macrometastases despite the technical capability to detect them, which may hinder timely therapeutic interventions and negatively impact patient prognosis.
2. Materials and Methods
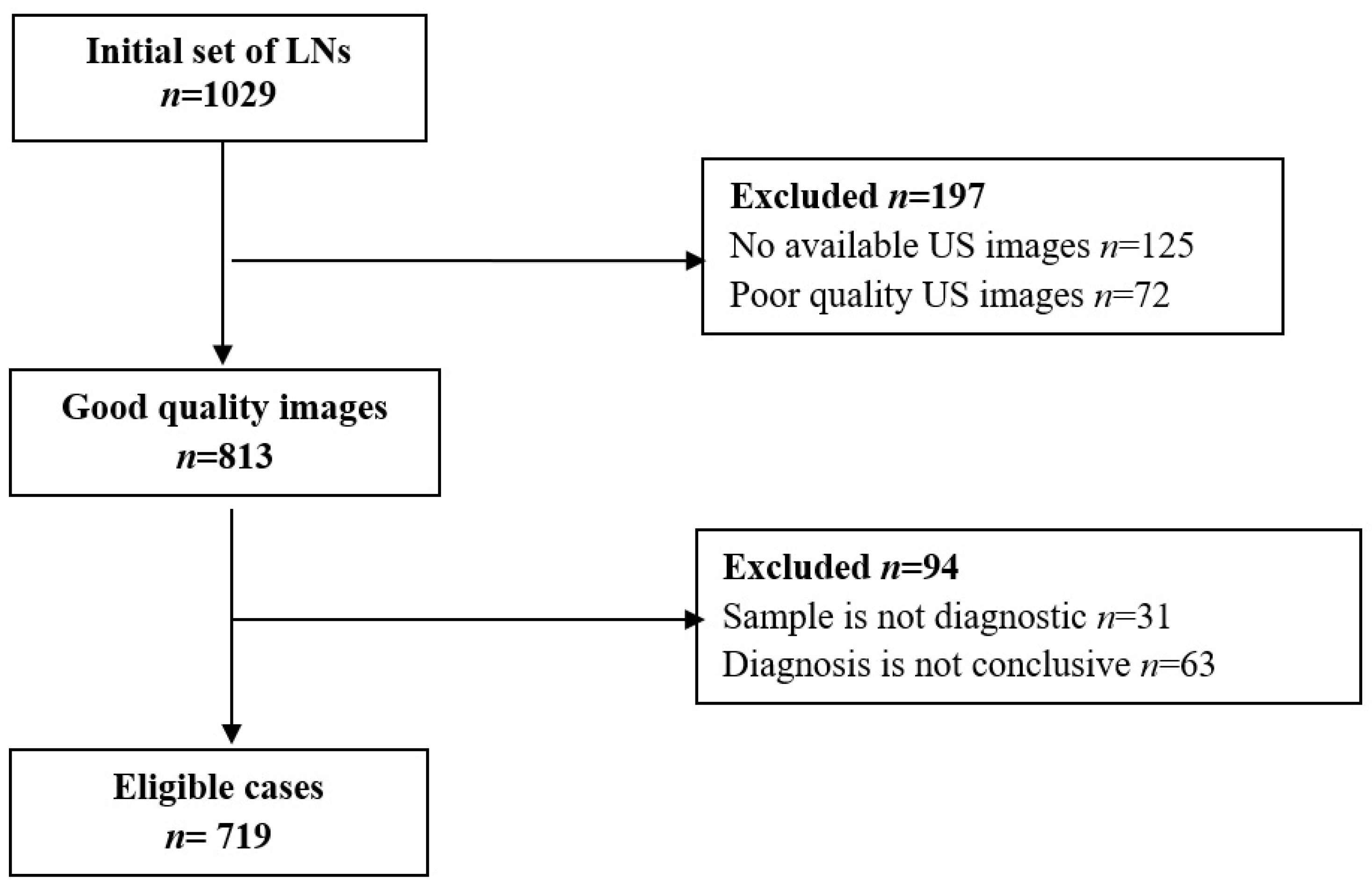
2.1. Patient Population and LNs’ Characteristics
2.2. Imaging Data Analysis
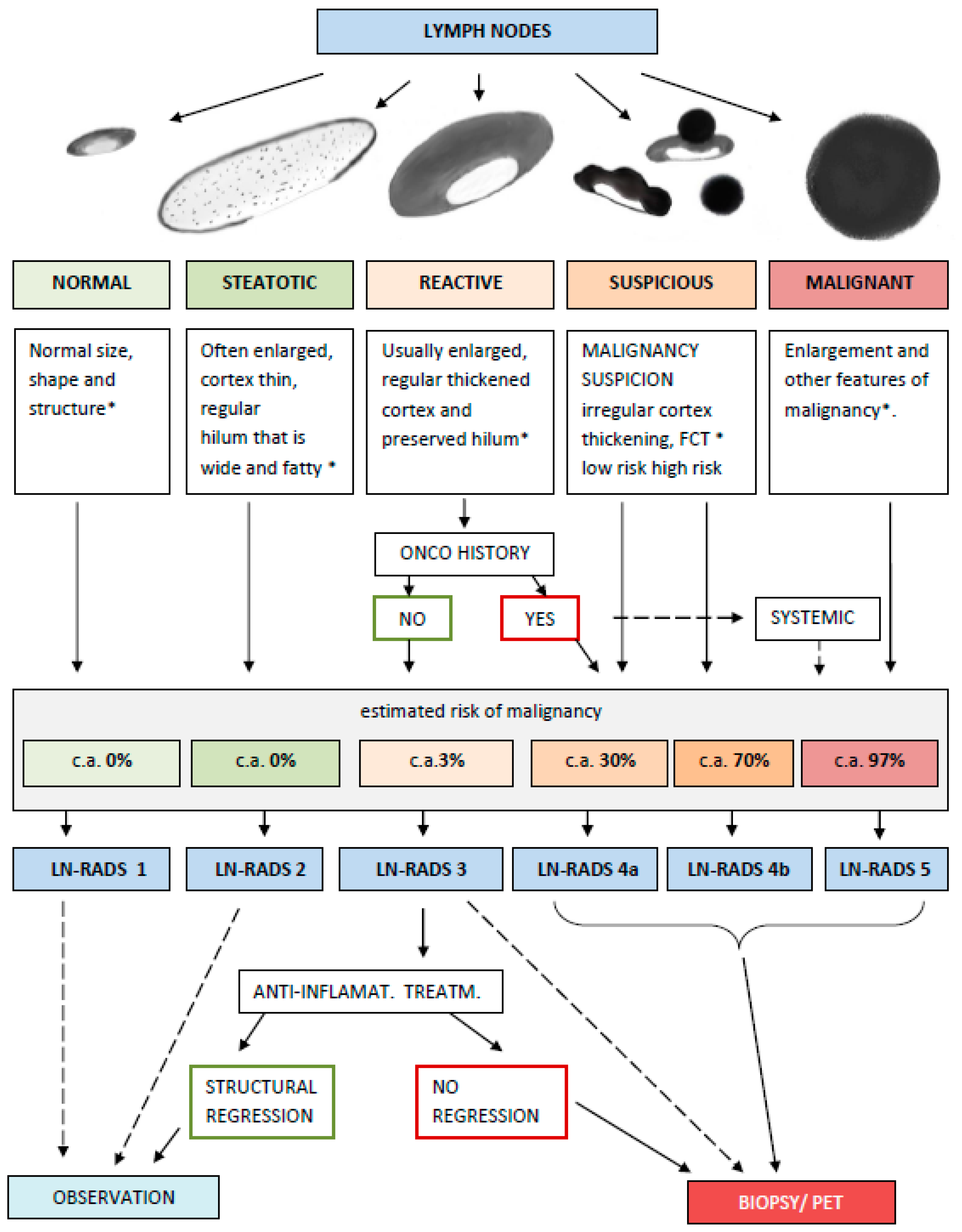
2.3. LN-RADS Assessment Guidelines
- LN-RADS 1: Normal. No enlargement (recommended max SAD up to 6–7 mm), oval shape (L/S-ratio > 2), regular cortex with maximum thickness ≤ 3 mm, cortex echogenicity similar or higher to the background fatty tissue, smooth margins, no other changes in architecture (no calcifications, no fluid collections, no necrosis, and no FCT [focal cortical thickening] or LCT [local cortical thickening]), and no pathological peripheral or chaotic vascularization
- LN-RADS 2: Steatotic. LNs can be enlarged in one or both axes, with regular cortex with a maximum thickness of ≤3 mm, hilum hyperechoic (steatotic) with no size limits, no other changes in architecture (no calcifications, no fluid collections, no necrosis, no FCT, and no LCT), and no pathological peripheral or chaotic vascularization.
- LN-RADS 3: Reactive. Probably due to an inflammatory process or vaccination. Dominant feature: thickened cortex > 3 mm, regular or with discrete irregularity, general enlargement in one or two axes, preserved oval shape (L/S-ratio > 2), preserved medulla, no other changes in architecture (no calcifications, no fluid collections, no necrosis, and no focal cortical thickening (FCT)), cortex echogenicity similar to or moderately lower than the background fatty tissue, well-defined margins, no pathologic peripheral or chaotic vascularization (small vessels in hilum can be seen), no oncological or haematological history, and no laboratory oncological abnormalities.
- LN-RADS 4: Suspicious for malignancy, thus LNs that morphologically do not match group 1, 2, 3, or 5 or have additional radiological or clinical factors increasing probability of malignancy in LNs categorized as LN-RADS 3, i.e., high or increasing laboratory markers (i.e., PSA for inguinal LNs); active neoplasm in the region (i.e., breast cancer for axillary LNs); another metastatic or systemic LN in the region; and clinical symptoms suggesting oncological or systemic hematological disease. The main rule of selecting LNs for group 4 is “better check than miss”. The LN-RADS 4 category is divided into two subcategories:
- -
- 4a: low suspicion for malignancy—size may be normal in SAD and LAD, cortex with thickening over 3 mm, and moderate irregularity, especially LCT. It is assumed that all 4a LNs should be verified by biopsy or PET. If biopsy is not possible, they should be treated as suspected and malignant.
- -
- 4b: high suspicion of malignancy—size may be normal in SAD and LAD; cortex thickening over 4 mm and irregularity, especially FCT, or no hilum; shape is more round than oval (L/S-ratio ≤ 2); hypoechogenicity to background fatty tissue, especially nearly anechoic “black hole sign”; micro-calcifications; fluid collections; necrosis; abnormal peripheral or chaotic vascularization architecture; ill-defined/blurred margins.
- LN-RADS 5: Definitely malignant. Enlargement in SAD and more malignancy features: cortex thickening over 6 mm, lack of hilum, hypoechogenicity to the background fatty tissue or “black hole sign”, evident cortex irregularity (LCT and FCT), shape more round than oval (L/S-ratio ≤ 2), micro-calcifications, fluid collections, necrosis, abnormal peripheral or chaotic vascularization architecture, ill-defined/blurred borders, or signs of extracapsular infiltration.
2.4. The Assessment Based on the LN-RADS Scale
2.5. Statistical Analysis
3. Results
3.1. Assessment of the Accuracy of LN-RADS for Differentiation Between Malignant and Benign Superficial LNs in US
3.2. Assessment of the Risk of Malignancy in Each Group of LN-RADS
- LN-RADS-1—33 normal LNs—0% risk of malignancy.
- LN-RADS-2—46 steatotic LNs—0% risk of malignancy.
- LN-RADS-3—109 reactive LNs—2% risk of malignancy.
- LN-RADS-4—320 LNs with suspicion of malignancy further divided into the following categories:
- 5.
- LN-RADS-5—211 definitely malignant LNs, with a 97% risk of malignancy.
3.3. The Assessment of the Agreement Between the Readers
Inter-Observer Agreement
3.4. The Assessment of Morphological Features Allows for Identifying the Predictors of Benign or Malignant LNs—Data Are Presented in Table 3 and Figure 5
Cohorts Analysis
- 233 LNs with cancer—27 LNs classified as 4a, 99 as 4b, and 107 as 5, yielding a sensitivity of 88%.
- 93 LNs with leukemias/lymphoma—1 LN classified as 3, 8 as 4a, 22 as 4b, and 62 as 5, yielding a sensitivity of 90%.
- 48 LNs with melanoma/sarcoma—3 LNs classified as 4a, 15 as 4b, and 30 as 5, yielding a sensitivity of 94%.
- 17 nonspecific neoplastic LNs—6 LNs classified as 4a, 6 as 4b, and 5 as 5, yielding a sensitivity of 64%.
| Parameter | Threshold Value | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|
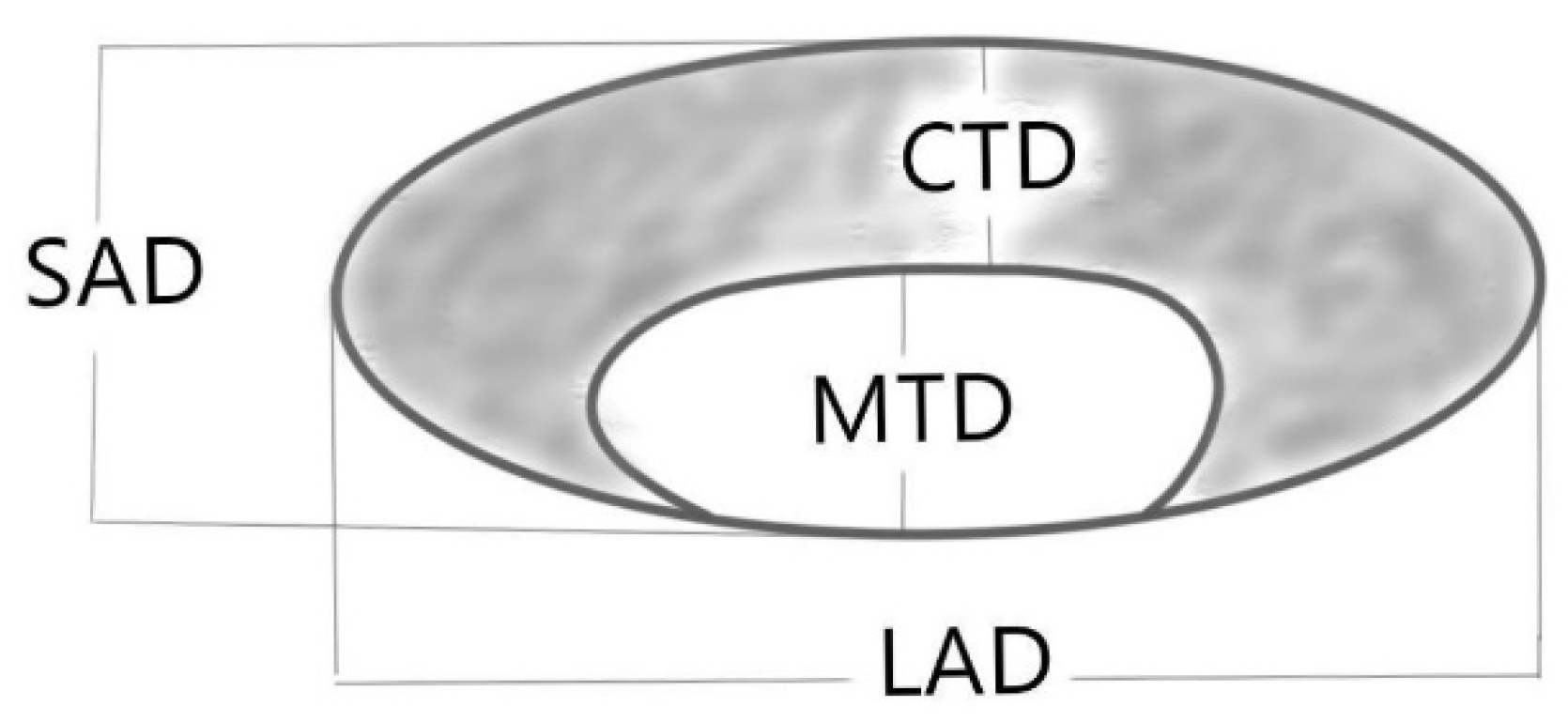
| LAD | 17 mm | 55% | 58% | 57% | 61% | 52% |
| SAD | 9 mm | 69% | 75% | 72% | 76% | 67% |
| S/L ratio | 0.51 | 80% | 62% | 72% | 71% | 73% |
| CTD | 6 mm | 89% | 74% | 82% | 80% | 85% |
| MTD/(MTD + CTD) ratio | 0.24 | 78% | 78% | 78% | 81% | 75% |
| Cortex echogenicity * | (-) * | 68% | 82% | 74% | 81% | 68% |
| Cortex irregularity * | (-) * | 90% | 68% | 80% | 77% | 86% |
| Margins * | (-) * | 8% | 98% | 49% | 83% | 47% |
| Inhomogeneity * | (-) * | 25% | 92% | 56% | 79% | 51% |
| Shape * | (-) * | 75% | 76% | 75% | 79% | 72% |
| Vascular architecture ** | (-) * | 77% | 70% | 74% | 84% | 60% |
| Total LN-RADS | 89% | 85% | 87% | 88% | 86% |
| Feature | Cancers [mm] | Leukemias/ Lymphoma [mm] | Melanomas /Sarcoma [mm] | Undifferentiated [mm] | Benign [mm] | p |
|---|---|---|---|---|---|---|
| LAD | 17.2 (10.0) | 29.9 (11.7) | 23.3 (14.7) | 16.6 (9.2) | 17.2 (9.1) | 0.0001 |
| SAD | 12.0 (7.7) | 18.2 (7.6) | 15.8 (11.6) | 10.0 (6.9) | 7.4 (3.3) | 0.0001 |
| CTD | 11.5 (7.9) | 15.4 (8.4) | 15.8 (11.6) | 10.1 (7.1) | 4.5 (3.2) | 0.0001 |
| MTD | 0.9 (1.8) | 2.0 (2.5) | 0.9 (1.9) | 1.1 (1.6) | 2.9 (2.4) | 0.0001 |

4. Discussion
- The single rigid criterion model, e.g., 10 mm SAD;
- The multiparametric model with strictly defined features and rigid cutoff values—the so-called calculation system, e.g., Node RADS;
- The open, flexible system utilizing a wide range of available radiological and clinical data based on quick heuristic evaluation—e.g., LN-RADS.
4.1. Analysis of Features as Predictors
4.2. Limitations
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent Diffusion Coefficient. |
| AUC | Area Under the Curve. |
| BI-RADS | Breast Imaging Reporting and Data System. |
| BMI | Body Mass Index. |
| BRI | Body Roundness Index. |
| CNB | Core Needle Biopsy. |
| CT | Computed Tomography. |
| DWI | Diffusion-Weighted Imaging. |
| FNB | Fine-Needle Biopsy. |
| H-P | Histopathological. |
| LAD | Long-Axis Diameter. |
| LN | Lymph Node. |
| LN-RADS | Lymph Node Reporting and Data System. |
| MRI | Magnetic Resonance Imaging. |
| SAD | Short-Axis Diameter. |
| US | Ultrasound. |
| VAB | Vacuum-Assisted Biopsy. |
References
- Nguyen, A.T.; Luu, M.; Nguyen, V.P.; Lu, D.J.; Shiao, S.L.; Kamrava, M.; Atkins, K.M.; Mita, A.C.; Scher, K.S.; Spratt, D.E.; et al. Quantitative Nodal Burden and Mortality Across Solid Cancers. J. Natl. Cancer Inst. 2022, 114, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Woolgar, J.A.; Rogers, S.N.; Lowe, D.; Brown, J.S.; Vaughan, E.D. Cervical lymph node metastasis in oral cancer: The importance of even microscopic extracapsular spread. Oral Oncol. 2003, 39, 130–137. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Li, S.; Bai, F.; Xie, H.; Shan, H.; Liu, Z.; Ma, T.; Tang, X.; Tang, H.; et al. Risk Factors of Lymph Node Metastasis and Its Prognostic Significance in Early Gastric Cancer: A Multicenter Study. Front. Oncol. 2021, 11, 649035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Ma, L. Nomograms predict survival of patients with lymph node-positive, luminal a breast cancer. BMC Cancer 2021, 21, 965. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, S.D. Insights into the mechanisms of lymph node metastasis. Cancer 2003, 98, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Doe, A. Lymph node involvement is associated with overall survival for elderly patients with non-metastatic gallbladder adenocarcinoma. J. Clin. Oncol. 2022, 40, 123–130. [Google Scholar]
- Ho, A.S.; Kim, S.; Tighiouart, M.; Gudino, C.; Mita, A.; Scher, K.S.; Laury, A.; Prasad, R.; Shiao, S.L.; Van Eyk, J.E.; et al. Metastatic lymph node burden and survival in oral cavity cancer. J. Clin. Oncol. 2017, 35, 3601–3609. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J. Completion dissection or observation for sentinel-node metastasis in melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Patel, D.N.; Luu, M.; Zumsteg, Z.S.; Daskivich, T.J. Development and validation of an im-proved pathological nodal staging system for urothelial carcinoma of the bladder. Eur. Urol. Oncol. 2019, 2, 656–663. [Google Scholar] [CrossRef]
- Fortuin, A.S.; Meijer, H.; Thompson, L.C.; Witjes, J.A.; Barentsz, J.O. Ferumoxtran-10 Ultrasmall Superparamagnetic Iron Oxide–Enhanced Diffusion-weighted Imaging Magnetic Resonance Imaging for Detection of Metastases in Normal-sized Lymph Nodes in Patients with Bladder and Prostate Cancer: Do We Enter the Era After Extended Pelvic Lymph Node Dissection? Eur. Urol. 2013, 64, 961–963. [Google Scholar]
- Glazer, G.M.; Gross, B.H.; Quint, L.E.; Francis, I.R.; Bookstein, F.L.; Orringer, M.B. Normal mediastinal lymph nodes: Number and size according to American Thoracic Society mapping. Am. J. Poentgenol. 1985, 144, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, R.E.; Alpern, M.B.; Gross, B.H.; Sandler, M.A. Upper abdominal lymph nodes: Criteria for normal size determined with CT. Padiology 1991, 180, 319–322. [Google Scholar] [CrossRef]
- Callen, P.W.; Korobkin, M.; Isherwood, I. Computed tomography evaluation of the retrocrural prevertebral space. Am. J. Poentgenol. 1977, 129, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Vinnicombe, S.; Norman, A.; Nicolson, V.; Husband, J.E. Normal pelvic lymph nodes: Evaluation with CT after bipedal lymphangiography. Padiology 1995, 194, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.G.; Rieger, J.; Baghi, M.; Bisdas, S.; Vogl, T.J. Cervical lymph nodes. Eur. J. Radiol. 2008, 66, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Hövels, A.M.; Heesakkers, R.A.M.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–956. [Google Scholar] [CrossRef]
- van den Brekel, M.W.; Castelijns, J.A.; Snow, G.B. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: How reliable is it? Am. J. Neuroradiol. 1998, 19, 695–700. [Google Scholar] [PubMed] [PubMed Central]
- Kramer, H.; Groen, H.J. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann. Surg. 2003, 238, 180–188. [Google Scholar] [CrossRef]
- Jager, G.J.; Barentsz, J.O.; Oosterhof, G.O.; Witjes, J.A.; Ruijs, S.J. Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional TI-weighted magnetization-prepared-rapid gradient-echo sequence. Am. J. Roentgenol. 1996, 167, 1503–1507. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Leung, K.J.; O’Neill, A.; Jayender, J.; Lee, T.C. Jugulodigastric lymph node size by age on CT in an adult cancer-free population. Clin. Imaging 2018, 47, 30–33, ISSN 0899-7071. [Google Scholar]
- Placke, J.M.; Reis, H.; Hadaschik, E.; Roesch, A.; Schadendorf, D.; Stoffels, I.; Klode, J. Coronavirus disease 2019 vaccine mimics lymph node metastases in patients undergoing skin cancer follow-up: A monocentre study. Eur. J. Cancer 2021, 154, 167–174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryu, K.H.; Lee, K.H.; Ryu, J.; Baek, H.J.; Kim, S.J.; Jung, H.K.; Kim, S.M. Cervical Lymph Node Imaging Reporting and Data System for Ultrasound of Cervical Lymphadenopathy: A Pilot Study. Am. J. Roentgenol. 2013, 206, 1286–1291. [Google Scholar] [CrossRef]
- Chung, M.S.; Choi, Y.J.; Kim, S.O.; Lee, Y.S.; Hong, J.Y.; Lee, J.H.; Baek, J.H. A Scoring System for Prediction of Cervical Lymph Node Metastasis in Patients with Head and Neck Squamous Cell Carcinoma. Am. J. Neuroradiol. 2019, 40, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Xie, C.; Wei, W.; Pan, C.; Wang, W.; Lv, N.; Wu, P. A new, preoperative, MRI-based scoring system for diagnosing malignant axillary lymph nodes in women evaluated for breast cancer. Eur. J. Radiol. 2012, 81, 2602–2612, ISSN 0720-048X. [Google Scholar] [CrossRef] [PubMed]
- Elsholtz, F.H.J.; Asbach, P.; Haas, M.; Becker, M.; Beets-Tan, R.G.H.; Thoeny, H.C.; Padhani, A.R.; Hamm, B. Introducing the Node Reporting and Data System 1.0 (Node-RADS): A concept for standardized assessment of lymph nodes in cancer. Eur. Radiol. 2016, 31, 6116–6124. [Google Scholar] [CrossRef]
- Chudobiński, C.; Świderski, B.; Antoniuk, I.; Kurek, J. Enhancements in Radiological Detection of Metastatic Lymph Nodes Utilizing AI-Assisted Ultrasound Imaging Data and the Lymph Node Reporting and Data System Scale. Cancers 2024, 16, 1564. [Google Scholar] [CrossRef]
- López-Ratón, M.; Rodríguez-Álvarez, M.X.; Cadarso-Suárez, C.; Gude-Sampedro, F. Optimal Cutpoints: An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- Gamer, M.; Lemon, J.; Fellows, I.; Singh, P. Various Coefficients of Interrater Reliability and Agreement. [R Package]. Available online: https://CRAN.R-project.org/package=irr (accessed on 16 September 2024).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Libshitz, H.I.; McKenna Jr, R.J. Mediastinal lymph node size in lung cancer. Am. J. Roentgenol. 1984, 143, 715–718. [Google Scholar] [CrossRef]
- Som, P.M. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. Am. J. Roentgenol. 1992, 158, 961–969. [Google Scholar] [CrossRef] [PubMed]
- van der Brekel, M.W.; Stel, H.V.; Castelijns, J.A.; Nauta, J.J.; van der Waal, I.; Valk, J.; Meyer, C.J.; Snow, G.B. Cervical lymph node metastasis: Assessment of radiological criteria. Radiology 1990, 177, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Hilden, P.; Coiffier, B.; Hagenbeek, A.; Salles, G.; Wilson, W.; Seymour, J.F.; Kelly, K.; Gribben, J.; Pfreunschuh, M.; et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann. Oncol. 2017, 28, 1436–1447. [Google Scholar] [CrossRef]
- Song, S.E.; Seo, B.K.; Lee, S.H.; Yie, A.; Lee, K.Y.; Cho, K.R.; Woo, O.H.; Cha, S.H.; Kim, B.H. Classification of Metastatic versus Non-Metastatic Axillary Nodes in Breast Cancer Patients: Value of Cortex-Hilum Area Ratio with Ultrasound. J. Breast Cancer 2012, 15, 65–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, Y.J.; Ko, E.Y.; Han, B.K.; Shin, J.H.; Kang, S.S.; Hahn, S.Y. High-resolution ultrasonographic features of axillary lymph node metastasis in patients with breast cancer. Breast 2009, 18, 119–122. [Google Scholar] [CrossRef]
- Ahuja, A.; Ying, M. An overview of neck node sonography. Investig. Radiol. 2002, 37, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Rubaltelli, L.; Proto, E.; Salmaso, R.; Bortoletto, P.; Candiani, F.; Cagol, P. Sonography of abnormal lymph nodes in vitro: Correlation of sonographic and histologic findings. Am. J. Roentgenol. 1990, 155, 1241–1244. [Google Scholar] [CrossRef]
- Sutton, R.T.; Reading, C.C.; Charboneau, J.W.; James, E.M.; Grant, C.S.; Hay, I.D. US-guided biopsy of neck masses in postoperative management of patients with thyroid cancer. Radiology 1998, 168, 769–772. [Google Scholar] [CrossRef]
- Ahuja, A.T.; Ying, M.; Ho, S.Y.; Antonio, G.; Lee, Y.P.; King, A.D.; Wong, K.T. Ultrasound of malignant cervical lymph nodes. Cancer Imaging 2008, 8, 48. [Google Scholar] [CrossRef]
- Giandola, T.; Maino, C.; Marrapodi, G.; Ratti, M.; Ragusi, M.; Bigiogera, V.; Talei Franzesi, C.; Corso, R.; Ippolito, D. Imaging in Gastric Cancer: Current Practice and Future Perspectives. Diagnostics 2023, 28, 1276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chikui, T.; Yonetsu, K.; Nakamura, T. Multivariate feature analysis of sonographic findings of metastatic cervical lymph nodes: Contribution of blood flow features revealed by power Doppler sonography for predicting metastasis. Am. J. Neuroradiol. 2000, 21, 561–567. [Google Scholar] [PubMed]
- Ariji, Y.; Kimura, Y.; Hayashi, N.; Onitsuka, T.; Yonetsu, K.; Hayashi, K.; Ariji, E.; Kobayashi, T.; Nakamura, T. Power Doppler sonographyof cervical lymph nodes in patients with head and neck cancer. Am. J. Neuroradiol. 1998, 19, 303–308. [Google Scholar] [PubMed]
- Tschammler, A.; Ott, G.; Schang, T.; Seelback-Goebel, B.; Schwager, K.; Hahn, D. Lymphadenopathy: Differentiation of benign from malignant disease: Color Doppler US assessment of intranodal angioarchitecture. Radiology 1998, 208, 117–123. [Google Scholar] [CrossRef]
- Tschammler, A.; Beer, M.; Hahn, D. Differential diagnosis of lymphadenopathy: Power Doppler vs color Doppler sonography. Eur. Radiol. 2002, 12, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, H.J.; Wissgott, C.; Rademaker, J.; Felix, R. Current status of power Doppler and color Doppler sonography in the differential diagnosis of lymph node lesions. Eur. Radiol. 2002, 12, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Naito, K.; Horiguchi, J.; Fukuda, H.; Tachikake, T.; Ito, K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: Comparison with conventional B-mode sonography. Am. J. Roentgenol. 2008, 191, 604–610. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Q.; Wang, J.Y.; Yu, Y.; Yi, A.J.; Cui, X.W.; Dietrich, C.F. Ultrasound Elastography for the Evaluation of Lymph Nodes. Front. Oncol. 2021, 11, 714660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, G.-X.; Xiao, X.-Y.; Xu, X.-L.; Yang, H.-Y.; Cai, Y.-C.; Liu, X.-D.; Tian, J.; Luo, B.-M. Diagnostic value of ultrasound elastography for differentiation of benign and malignant axillary lymph nodes: A meta-analysis. Clin. Radiol. 2020, 75, 481.e9–481.e16, ISSN 0009-9260. [Google Scholar] [CrossRef]
- Choi, J.J.; Kang, B.J.; Kim, S.H.; Lee, J.H.; Jeong, S.H.; Yim, H.W.; Song, B.J.; Jung, S.S. Role of sonographic elastography in the differential diagnosis of axillary lymph nodes in breast cancer. J. Ultrasound Med. 2011, 30, 429–436. [Google Scholar] [CrossRef]
- Taylor, K.; O’Keeffe, S.; Britton, P.D.; Wallis, M.G.; Treece, G.M.; Housden, J.; Parashar, D.; Bond, S.; Sinnatamby, R. Ultrasound elastography as an adjuvant to conventional ultrasound in the preoperative assessment of axillary lymph nodes in suspected breast cancer: A pilot study. Clin. Radiol. 2011, 66, 1064–1071. [Google Scholar] [CrossRef]
- Zhang, Q.; Suo, J.; Chang, W.; Shi, J.; Chen, M. Dual-modal computer-assisted evaluation of axillary lymph node metastasis in breast cancer patients on both real-time elastography and B-mode ultrasound. Eur. J. Radiol. 2017, 95, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Hou, Y.; Zheng, H.M.; Lin, X.; Xie, Z.L.; Hu, Y.P. Real-time elastography for the differentiation of benign and malignant superficial lymph nodes: A meta-analysis. Eur. J. Radiol. 2012, 81, 2576–2584. [Google Scholar] [CrossRef] [PubMed]

| Grid | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Feature | ||||||
| Shape |  |  |  |  |  | |
| Cortex Irregularity |  |  |  |  |  | |
| Cortex Echogenicity |  |  |  |  |  | |
| Cortex Inhomogenity |  |  |  |  |  | |
| Borders |  |  |  |  |  | |
| Vascular pattern |  |  |  |  |  | |
| Benign | Malignant | ||||
|---|---|---|---|---|---|
| Category | TN | FN | Category | TP | FP |
| LN-RADS 1 | 33 | 0 | LN-RADS 4b | 142 | 42 |
| LN-RADS 2 | 46 | 0 | LN-RADS 5 | 204 | 7 |
| LN-RADS 3 | 107 | 2 | Sum | 346 | 49 |
| LN-RADS 4a | 95 | 41 | |||
| Sum | 281 | 43 | |||
| Vascular Pattern Type Description | Scheme | Malignancy Probability | Benign in HP | Malignant in HP |
|---|---|---|---|---|
| Vascular-grid-1 No visible blood flow |  | 53% | 8 | 9 |
| Vascular-grid-2 Hilar flow (small tree) | 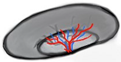 | 29% | 36 | 15 |
| Vascular-grid-3 Hilar-cortical flow (big tree) |  | 54% | 11 | 13 |
| Vascular-grid-4 Peripheral vasculature | 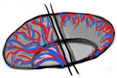 | 78% | 17 | 60 |
| Vascular-grid-5 Chaotic vascular architecture |  | 92% | 7 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudobiński, C.; Pasicz, K.; Hanke, M.; Kaczmarek, A.; Pajdziński, M.; Kołacińska-Wow, A.; Gottwald, L.; Kuncman, W.; Podgórski, M.; Cieszanowski, A. Lymph Node Reporting and Data System (LN-RADS)—Retrospective Evaluation for Ultrasound Classification of Superficial Lymph Nodes. Cancers 2025, 17, 2030. https://doi.org/10.3390/cancers17122030
Chudobiński C, Pasicz K, Hanke M, Kaczmarek A, Pajdziński M, Kołacińska-Wow A, Gottwald L, Kuncman W, Podgórski M, Cieszanowski A. Lymph Node Reporting and Data System (LN-RADS)—Retrospective Evaluation for Ultrasound Classification of Superficial Lymph Nodes. Cancers. 2025; 17(12):2030. https://doi.org/10.3390/cancers17122030
Chicago/Turabian StyleChudobiński, Cezary, Katarzyna Pasicz, Małgorzata Hanke, Adam Kaczmarek, Mateusz Pajdziński, Agnieszka Kołacińska-Wow, Leszek Gottwald, Wojciech Kuncman, Michał Podgórski, and Andrzej Cieszanowski. 2025. "Lymph Node Reporting and Data System (LN-RADS)—Retrospective Evaluation for Ultrasound Classification of Superficial Lymph Nodes" Cancers 17, no. 12: 2030. https://doi.org/10.3390/cancers17122030
APA StyleChudobiński, C., Pasicz, K., Hanke, M., Kaczmarek, A., Pajdziński, M., Kołacińska-Wow, A., Gottwald, L., Kuncman, W., Podgórski, M., & Cieszanowski, A. (2025). Lymph Node Reporting and Data System (LN-RADS)—Retrospective Evaluation for Ultrasound Classification of Superficial Lymph Nodes. Cancers, 17(12), 2030. https://doi.org/10.3390/cancers17122030






