The Role of the Extracellular Matrix in Cancer Prevention
Simple Summary
Abstract
1. Introduction
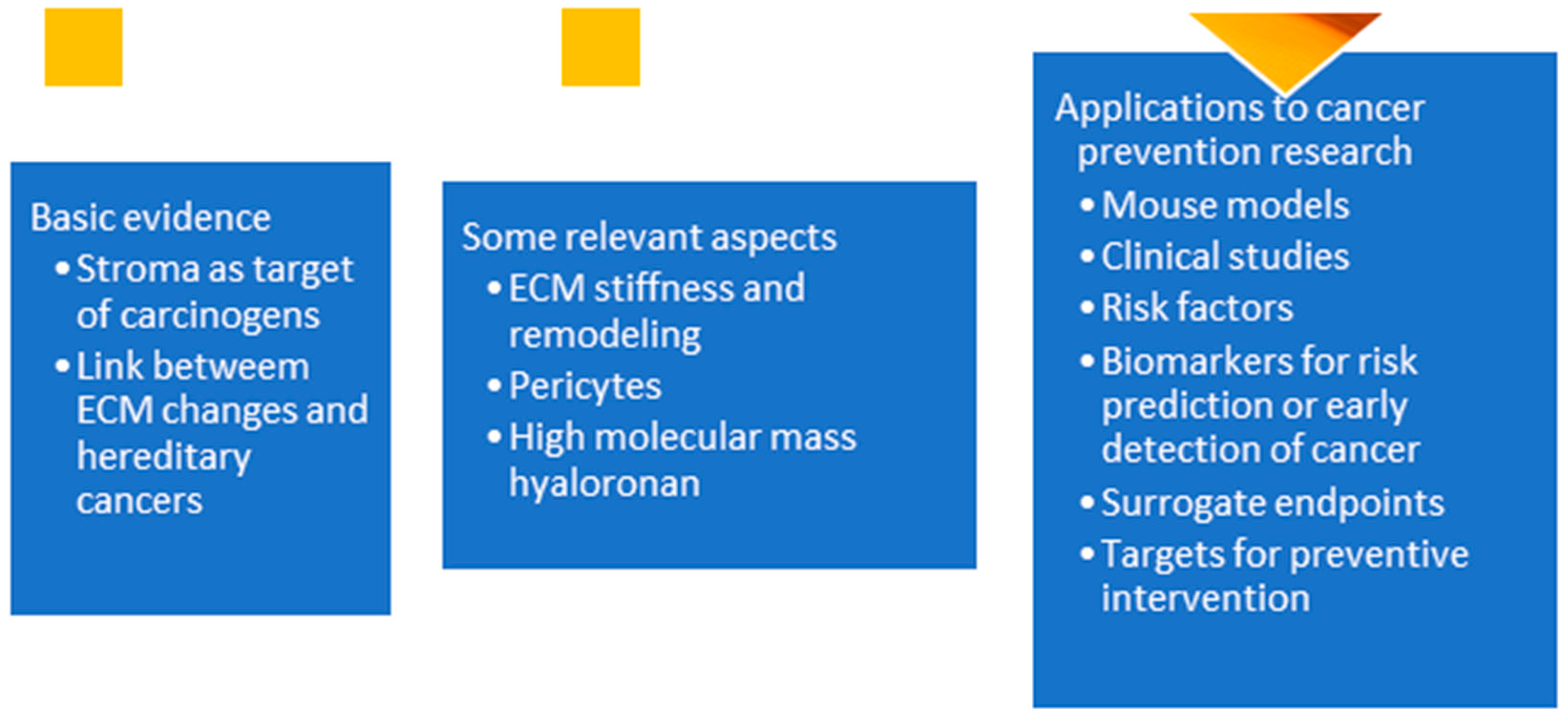
2. Basic Evidence
2.1. ECM and Tumor Initiation
2.2. ECM and Hereditary Cancers
3. Relevant Aspects of the ECM
3.1. ECM Stiffness and Remodeling
3.2. Pericytes
- (1)
- (2)
- Some detached pericytes develop into myofibroblasts, which leads to alterations of the ECM. Supporting evidence comes from fate-tracing studies that show that pericytes can develop into myofibroblasts [25].
- (3)
- (4)
- Some MSCs adhere to the altered ECM. Supporting evidence comes from mouse experiments showing that MSCs adhering to microbeads develop into sarcomas [28].
- (5)
- The altered ECM disrupts normal tissue regulatory controls, causing the MSCs to develop into sarcomas or carcinomas. Supporting evidence comes from studies showing that MSCs can differentiate into epithelial cells in culture [29] and induce epithelial proliferation in the presence of chronic inflammation [30]. Also, transplant studies showed that MSCs can develop into epithelial tumors [31].
3.3. Hyaluronan
4. New Insights and Directions
4.1. Mouse Experiments
4.2. Clinical Studies
4.3. Risk Factors
4.4. Biomarkers for Risk Prediction or Early Detection of Cancer
4.5. Surrogate Endpoints
4.6. Targets for Preventive Intervention
4.6.1. Fibrosis Inhibitors
4.6.2. Pericyte Targets
4.6.3. Hyaluronidase Inhibitors
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Models Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, J.; Sun, H.; Zhang, Y.; Zou, D. New insights into fibrosis from the ECM degradation perspective: The macrophage-MMP-ECM interaction. Cell Biosci. 2022, 12, 117. [Google Scholar]
- Maffini, M.V.; Soto, A.M.; Calabro, J.M.; Ucci, A.A.; Sonnenschein, C. The stroma as a crucial target in rat mammary gland carcinogenesis. J. Cell Sci. 2004, 117, 1495–1502. [Google Scholar] [CrossRef]
- Newcombe, R.G. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Nee, K.; Ma, D.; Nguyen, Q.H.; Pein, M.; Pervolarakis, N.; Insua-Rodríguez, J.; Kessenbrock, K. Preneoplastic stromal cells promote BRCA1-mediated breast tumorigenesis. Nat. Genet. 2023, 55, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Lutgens, M.W.; Vleggaar, F.P.; Schipper, M.E.; Stokkers, P.C.; van der Woude, C.J.; Hommes, D.W.; Samsom, M. High frequency of early colorectal cancer in inflammatory bowel disease. Gut 2008, 57, 1246–1251. [Google Scholar] [CrossRef]
- Derkacz, A.; Olczyk, P.; Olczyk, K.; Komosinska-Vassev, K. The role of extracellular matrix components in inflammatory bowel diseases. J. Clin. Med. 2021, 10, 1122. [Google Scholar] [CrossRef]
- Ishihara, S.; Haga, H. Matrix stiffness contributes to cancer progression by regulating transcription factors. Cancers 2022, 14, 1049. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Koshy, S.T.; Branco da Cunha, C.; Shin, J.W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Tlsty, T.D.; Pan, D.; Gascard, P.; Caruso, J.; Ferri, L.; Chen-Tanyolac, C. Inflammation-induced mechanotransduction is necessary and sufficient to create pre-cancerous squamous lung metaplasias and necessary to drive progression to dysplasia. In Proceedings of the AACR Special Conference in Cancer Research: Tumor-body Interactions: The Roles of Micro- and Macroenvironment in Cancer, Boston, MA, USA, 17–20 November 2024. [Google Scholar]
- Chin, L.; Xia, Y.; Discher, D.E.; Janmey, P.A. Mechanotransduction in cancer. Curr. Opin. Chem. Eng. 2016, 11, 77–84. [Google Scholar] [CrossRef]
- Liu, L.; Liu, M.; Xie, D.; Liu, X.; Yan, H. Role of the extracellular matrix and YAP/TAZ in cell reprogramming. Differentiation 2021, 122, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jafarinia, H.; Khalilimeybodi, A.; Barrasa-Fano, J.; Fraley, S.I.; Rangamani, P.; Carlier, A. Insights gained from computational modeling of YAP/TAZ signaling for cellular mechanotransduction. NPJ Syst. Biol. Appl. 2024, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- Sonnenschein, C.; Soto, A.M. The Society of Cells: Cancer and Control of Cell Proliferation; Springer: New York, NY, USA, 1999. [Google Scholar]
- Soto, A.M.; Sonnenschein, C. The tissue organization field theory of cancer: A testable replacement for the somatic mutation theory. Bioessays. 2011, 33, 332–340. [Google Scholar] [CrossRef]
- Jiang, S.; Li, T.; Yang, Z.; Yi, W.; Di, S.; Sun, Y.; Wang, D.; Yang, Y. AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res. Rev. 2017, 38, 18–27. [Google Scholar] [CrossRef]
- Baker, S.G. Rethinking carcinogenesis: The detached pericyte hypothesis. Med. Hypotheses 2020, 144, 110056. [Google Scholar] [CrossRef]
- Baker, S.G. The detached pericyte hypothesis: A novel explanation for many puzzling aspects of tumorigenesis. Organisms. J. Biol. Sci. 2018, 2, 25–41. [Google Scholar]
- Birbrair, A.; Zhang, T.; Files, D.C.; Mannava, S.; Smith, T.; Wang, Z.M.; Messi, M.L.; Mintz, A.; Delbono, O. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res. Ther. 2014, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Kubiak, J.Z.; Li, X.C.; Ghobrial, R.M. Pericytes, microvasular dysfunction, and chronic rejection. Transplantation 2015, 99, 658–667. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Lin, S.L.; Kobayashi, A.; Hudson, T.E.; Nowlin, B.T.; Bonventre, J.V.; Valerius, M.T.; McMahon, A.P.; Duffield, J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 2010, 176, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Yianni, V.; Sharpe, P.T. Perivascular-derived mesenchymal stem cells. J. Dent. Res. 2019, 98, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tang, Y.J.; Wei, Q.; Hirata, M.; Weng, A.; Han, I.; Alman, B.A. Mesenchymal tumors can derive from Ng2/Cspg4-expressing pericytes with β-Catenin modulating the neoplastic phenotype. Cell Rep. 2016, 16, 917–927. [Google Scholar] [CrossRef]
- Boone, C.W.; Jacobs, J.B. Sarcomas routinely produced from putatively nontumorigenic Balb/3T3 and C3H/10T1/2 cells by subcutaneous inoculation attached to plastic platelets. J. Supramol. Struct. 1976, 5, 131–137. [Google Scholar] [CrossRef]
- Takebayashi, T.; Horii, T.; Denno, H.; Nakamachi, N.; Otomo, K.; Kitamura, S.; Miyamoto, K.; Horiuchi, T.; Ohta, Y. Human mesenchymal stem cells differentiate to epithelial cells when cultured on thick collagen gel. Bio-Med. Mater. Eng. 2013, 23, 143–153. [Google Scholar] [CrossRef]
- Donnelly, J.M.; Engevik, A.; Feng, R.; Xiao, C.; Boivin, G.P.; Li, J.; Houghton, J.; Zavros, Y. Mesenchymal stem cells induce epithelial proliferation within the inflamed stomach. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 306, G1075–G1088. [Google Scholar] [CrossRef][Green Version]
- Aractingi, S.; Kanitakis, J.; Euvrard, S.; Le Danff, C.; Peguillet, I.; Khosrotehrani, K.; Lantz, O.; Carosella, E.D. Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res. 2005, 65, 1755–1760. [Google Scholar] [CrossRef]
- Kaufman, C.K.; Mosimann, C.; Fan, Z.P.; Yang, S.; Thomas, A.J.; Ablain, J.; Zon, L.I. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science 2016, 351, aad2197. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, J.; Li, L.; Liao, S.; He, J.; Zhou, S.; Zhou, Y. Pericytes in the tumor microenvironment. Cancer Lett. 2023, 556, 216074. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ding, J.; Ma, Z.; Sun, R.; Seoane, J.A.; Scott Shaffer, J.; Curtis, C. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 2019, 51, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Murgai, M.; Ju, W.; Eason, M.; Kline, J.; Beury, D.W.; Kaczanowska, S.; Kaplan, R.N. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat. Med. 2017, 23, 1176–1190. [Google Scholar] [CrossRef]
- Wang, X.; Balaji, S.; Steen, E.H.; Blum, A.J.; Li, H.; Chan, C.K.; Keswani, S.G. High-molecular weight hyaluronan attenuates tubulointerstitial scarring in kidney injury. JCI Insight 2020, 5, e136345. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Seluanov, A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013, 499, 346–349. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, X.; Lu, J.Y.; Boit, K.; Ablaeva, J.; Zakusilo, F.T.; Gorbunova, V. Increased hyaluronan by naked mole-rat Has2 improves healthspan in mice. Nature 2023, 621, 196–205. [Google Scholar] [CrossRef]
- Heckman-Stoddard, B.M.; DeCensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017, 60, 1639–1647. [Google Scholar] [CrossRef]
- Shankaraiah, R.C.; Callegari, E.; Guerriero, P.; Rimessi, A.; Pinton, P.; Gramantieri, L.; Silini, E.M.; Sabbioni, S.; Negrini, M. Metformin prevents liver tumourigenesis by attenuating fibrosis in a transgenic mouse model of hepatocellular carcinoma. Oncogene 2019, 38, 7035–7045. [Google Scholar] [CrossRef]
- Tajima, K.; Nakamura, A.; Shirakawa, J.; Togashi, Y.; Orime, K.; Sato, K.; Terauchi, Y. Metformin prevents liver tumorigenesis induced by high-fat diet in C57Bl/6 mice. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E987–E998. [Google Scholar] [CrossRef]
- Cheng, D.; Xu, Q.; Wang, Y.; Li, G.; Sun, W.; Ma, D.; Ni, C. Metformin attenuates silica-induced pulmonary fibrosis via AMPK signaling. J. Transl. Med. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Rangarajan, S.; Bone, N.B.; Zmijewska, A.A.; Jiang, S.; Park, D.W.; Bernard, K.; Zmijewski, J.W. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med. 2018, 24, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Heyens, L.J.M.; Busschots, D.; Koek, G.H.; Robaeys, G.; Francque, S. Liver fibrosis in non-alcoholic fatty liver disease: From liver biopsy to non-invasive biomarkers in diagnosis and treatment. Front. Med. 2021, 8, 615978. [Google Scholar] [CrossRef]
- Munsterman, I.D.; Kendall, T.J.; Khelil, N.; Popa, M.; Lomme, R.; Drenth, J.-H.; Tjwa, E.-L. Extracellular matrix components indicate remodelling activity in different fibrosis stages of human non-alcoholic fatty liver disease. Histopathology 2018, 73, 612–621. [Google Scholar] [CrossRef]
- Baker, S.G.; Kramer, B.S.; Corle, D. The fallacy of enrolling only high-risk subjects in cancer prevention trials: Can we afford a “free lunch”? BMC Med. Res. Method 2004, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Heckman-Stoddard, B.; Dabelea, D.; Gadde, K.M.; Ehrmann, D.; Ford, L.; Temprosa, M. Effect of metformin and lifestyle interventions on mortality in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2021, 44, 2775–2782. [Google Scholar] [CrossRef]
- Dickerman, B.A.; García-Albéniz, X.; Logan, R.W.; Denaxas, S.; Hernán, M.A. Evaluating metformin strategies for cancer prevention: A target trial emulation using electronic health records. Epidemiology 2023, 34, 690–699. [Google Scholar] [CrossRef]
- Huang, Y.; Kyriakides, T.R. The role of extracellular matrix in the pathophysiology of diabetic wounds. Matrix Biol. Plus 2020, 6–7, 100037. [Google Scholar] [CrossRef] [PubMed]
- Higurashi, T.; Hosono, K.; Takahashi, H.; Komiya, Y.; Umezawa, S.; Sakai, E.; Nakajima, A. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind; placebo-controlled; randomised phase 3 trial. Lancet Oncol. 2016, 17, 475–483. [Google Scholar] [CrossRef]
- Hosono, K.; Endo, H.; Takahashi, H.; Sugiyama, M.; Sakai, E.; Uchiyama, T.; Nakajima, A. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev. Res. 2010, 3, 1077–1083. [Google Scholar] [CrossRef]
- Baker, S.G.; Lindeman, K.S. Multiple discoveries in causal inference: LATE for the party. Chance 2024, 37, 21–25. [Google Scholar] [CrossRef]
- Baker, S.G.; Lindeman, K.S. Local average treatment effects with binary outcomes. Am. J. Epidemiol. 2024, kwae428. [Google Scholar] [CrossRef]
- Cotangco, K.R.; Liao, C.I.; Eakin, C.M.; Chan, A.; Cohen, J.; Kapp, D.S.; Chan, J.K. Trends in incidence of cancers associated with obesity and other modifiable risk factors among women, 2001–2018. Prev. Chronic Dis. 2023, 20, E21. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef] [PubMed]
- DeBari, M.K.; Abbott, R.D. Adipose tissue fibrosis: Mechanisms, models, and importance. Int. J. Mol. Sci. 2020, 21, 6030. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.C.; Rabhi, N.; Orofino, J.; Gamini, R.; Perissi, V.; Vernochet, C.; Farmer, S.R. The adipocyte acquires a fibroblast-like transcriptional signature in response to a high fat diet. Sci. Rep. 2020, 10, 2380. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.R.; Bhardwaj, P.; Choi, S.; Gonzalez, J.; Andresen Eguiluz, R.C.; Wang, K.; Fischbach, C. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl. Med. 2015, 7, 301ra130. [Google Scholar] [CrossRef]
- Baker, S.G. Evaluating risk prediction with data collection costs: Novel estimation of test tradeoff curves. Med. Decis. Mak. 2024, 44, 53–63. [Google Scholar] [CrossRef]
- Lee, J.S.; Sinn, D.H.; Park, S.Y.; Shin, H.J.; Lee, H.W.; Kim, B.K.; Kim, S.U. Liver stiffness-based risk prediction model for hepatocellular carcinoma in patients with Nonalcoholic Fatty Liver Disease. Cancers 2021, 13, 4567. [Google Scholar] [CrossRef]
- Tian, C.; Ye, C.; Guo, H.; Lu, K.; Yang, J.; Wang, X.; Song, C. Liver elastography-based risk score for predicting hepatocellular carcinoma risk. JNCI: J. Natl. Cancer Inst. 2025, 117, 761–771. [Google Scholar] [CrossRef]
- Loosen, S.H.; Kostev, K.; Demir, M.; Luedde, M.; Keitel, V.; Luedde, T.; Roderburg, C. An elevated FIB-4 score is associated with an increased incidence of liver cancer: A longitudinal analysis among 248, 224 outpatients in Germany. Eur. J. Cancer 2022, 168, 41–50. [Google Scholar] [CrossRef]
- Baker, S.G. Re: Combined associations of genetic and environmental risk factors: Implications for prevention of breast cancer. J. Natl. Cancer Inst. 2015, 107, djv127. [Google Scholar] [CrossRef][Green Version]
- Corte, M.D.; González, L.O.; Junquera, S.; Bongera, M.; Allende, M.T.; Vizoso, F.J. Analysis of the expression of hyaluronan in intraductal and invasive carcinomas of the breast. J. Cancer Res. Clin. Oncol. 2010, 136, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Frezzetti, D.; De Luca, A.; Normanno, N. Extracellular matrix proteins as circulating biomarkers for the diagnosis of non-small cell lung cancer patients. J. Thorac. Dis. 2019, 11, S1252. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.G.; Etzioni, R. Prediagnostic evaluation of multicancer detection tests: Design and analysis considerations. J. Natl. Cancer Inst. 2024, 116, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.G.; Lassere, M.-D.; Lo, W.P. Surrogate endpoint metaregression: Useful statistics for regulators and trialists. J. Clin. Epidemiol. 2024, 175, 111508. [Google Scholar] [CrossRef]
- Dixon-Zegeye, M.; Shaw, R.; Collins, L.; Perez-Smith, K.; Ooms, A.; Qiao, M.; Blagden, S.P. Cancer precision-prevention trial of metformin in adults with Li Fraumeni syndrome (MILI) undergoing yearly MRI surveillance: A randomised controlled trial protocol. Trials 2024, 25, 103. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, J.; Zhao, M.; Xie, T.X.; Tanaka, N.; Sano, D.; Myers, J.N. Gain-of-function mutant p53 promotes cell growth and cancer cell metabolism via inhibition of AMPK activation. Mol. Cell 2014, 54, 960–974. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Yao, Y. Targeting fibrosis; mechanisms and clinical trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.; Inoue, Y.; Brown, K.K. Nintedanib in progressive fibrosing interstitial lung diseases. New Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Aimo, A.; Spitaleri, G.; Nieri, D.; Tavanti, L.M.; Meschi, C.; Panichella, G.; Emdin, M. Pirfenidone for idiopathic pulmonary fibrosis and beyond. Card. Fail. Rev. 2022, 8, e12. [Google Scholar] [CrossRef]
- Shi, B.; Wang, W.; Korman, B.; Kai, L.; Wang, Q.; Wei, J.; Varga, J. Targeting CD38-dependent NAD+ metabolism to mitigate multiple organ fibrosis. Iscience 2020, 24, 101902. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xie, N.; Banerjee, S.; Dey, T.; Liu, R.M.; Antony, V.B.; Liu, G. CD38 mediates lung fibrosis by promoting alveolar epithelial cell aging. Am. J. Respir. Crit. Care Med. 2022, 206, 459–475. [Google Scholar] [CrossRef]
- Salama, Z.A.; Sadek, A.; Abdelhady, A.M.; Darweesh, S.K.; Morsy, S.A.; Esmat, G. Losartan may inhibit the progression of liver fibrosis in chronic HCV patients. Hepatobiliary Surg. Nutr. 2016, 5, 249. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.G. Maximum likelihood estimation with missing outcomes: From simplicity to complexity. Stat. Med. 2019, 38, 4453–4474. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Jiang, K.; Werner, J.; Bazhin, A.V.; D’Haese, J.G. The role of stellate cells in pancreatic ductal adenocarcinoma: Targeting perspectives. Front. Oncol. 2021, 10, 621937. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.; Taguchi, T.; Tombline, G.; Paige, V.; Janelsins, M.; Gilmore, N.; Seluanov, A.; Gorbunova, V. Hyaluronidase inhibitor delphinidin inhibits cancer metastasis. Sci. Rep. 2024, 14, 14958. [Google Scholar] [CrossRef]
- Im, N.K.; Jang, W.J.; Jeong, C.H.; Jeong, G.S. Delphinidin suppresses PMA-induced MMP-9 expression by blocking the NF-κB activation through MAPK signaling pathways in MCF-7 human breast carcinoma cells. J. Med. Food 2014, 17, 855–861. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, L.; Qiang, C.; Chen, C.; Shen, Z.; Ding, F.; Cui, X. The role of matrix metalloproteinase 9 in fibrosis diseases and its molecular mechanisms. Biomed. Pharmacother. 2024, 171, 116116. [Google Scholar] [CrossRef]
- Cho, J.S.; Kang, J.H.; Shin, J.M.; Park, I.H.; Lee, H.M. Inhibitory Effect of Delphinidin on Extracellular Matrix Production via the MAPK/NF-κB Pathway in Nasal Polyp-Derived Fibroblasts. Allergy Asthma Immunol. Res. 2015, 7, 276–282. [Google Scholar] [CrossRef]
- Alvarado, J.; Schoenlau, F.; Leschot, A.; Salgad, A.M.; Portales, P.V. Delphinol® standardized maqui berry extract significantly lowers blood glucose and improves blood lipid profile in prediabetic individuals in three-month clinical trial. Panminerva Med. 2016, 58, 1–6. [Google Scholar] [PubMed]

| Carcinogen Applied to Stroma | Carcinogen Applied to Re-Inserted Epithelial Tissue | Number of Mice | Fraction with Tumors (95% CI) |
|---|---|---|---|
| No | No | 6 | 0.00 (0.00, 0.39) |
| Yes | 10 | 0.00 (0.00, 0.29) | |
| Yes | No | 13 | 0.77 (0.50, 0.92) |
| Yes | 8 | 0.75 (0.41, 0.93) |
| Experimental Groups | Number of Mice | Fraction with Tumors (95% CI) |
|---|---|---|
| BRCA1 Mammary cells | 12 | 0.33 (0.14, 0.61) |
| BRCA1 Mammary cells + fibroblasts | 12 | 0.67 (0.39, 0.86) |
| BRCA1 Mammary cells + fibroblasts + MMP3 | 12 | 1.00 (0.76, 1.00) |
| Experiment | Experimental Groups | Number of Mice | Fraction with Tumors (95% CI) |
|---|---|---|---|
| 1 | Control | 16 | 0.69 (0.44, 0.86) |
| Metformin at start | 17 | 0.29 (0.13, 0.53) | |
| 2 | Control | 4 | 0.75 (0.30, 0.95) |
| Metformin delay | 6 | 0.83 (0.44, 0.97) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, S.G.; Sauter, E.R. The Role of the Extracellular Matrix in Cancer Prevention. Cancers 2025, 17, 1491. https://doi.org/10.3390/cancers17091491
Baker SG, Sauter ER. The Role of the Extracellular Matrix in Cancer Prevention. Cancers. 2025; 17(9):1491. https://doi.org/10.3390/cancers17091491
Chicago/Turabian StyleBaker, Stuart G., and Edward R. Sauter. 2025. "The Role of the Extracellular Matrix in Cancer Prevention" Cancers 17, no. 9: 1491. https://doi.org/10.3390/cancers17091491
APA StyleBaker, S. G., & Sauter, E. R. (2025). The Role of the Extracellular Matrix in Cancer Prevention. Cancers, 17(9), 1491. https://doi.org/10.3390/cancers17091491







