Mucin4 (MUC4) Antibody Labeled with an NIR Dye Brightly Targets Pancreatic Cancer Liver Metastases and Peritoneal Carcinomatosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell Culture
2.3. Xenograft Establishment of Pancreatic Cancer Cell Lines
2.4. Liver Orthotopic Implant Metastasis Model Establishment
2.5. Peritoneal Carcinomatosis Mouse Model Establishment
2.6. Antibody-Conjugate Preparation and Administration
2.7. Near-Infrared Imaging and Fluorescence Image Analysis
2.8. Biodistribution
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Targeting of Pancreatic Cancer Metastasis in a Liver Implant Model
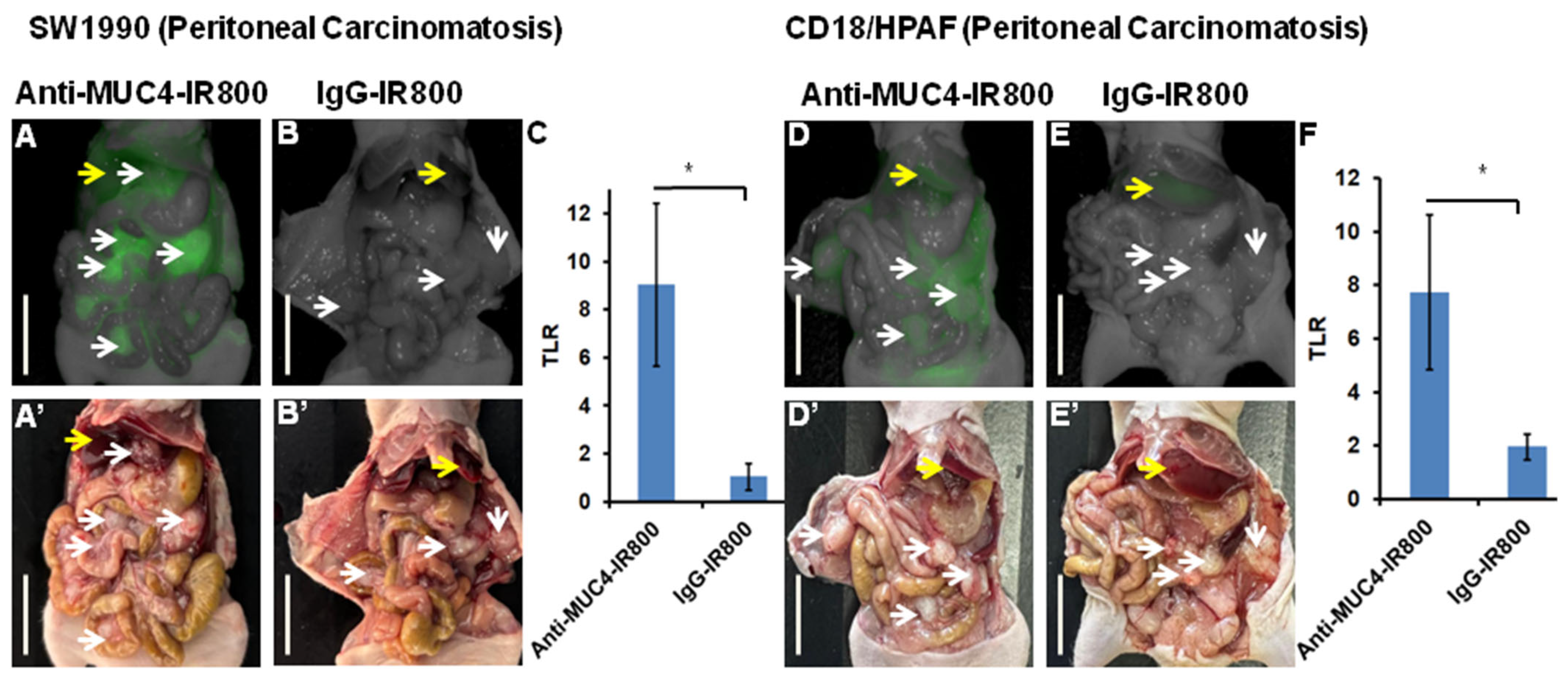
3.2. Targeting Pancreatic Cancer Metastasis in a Peritoneal Carcinomatosis Mouse Model
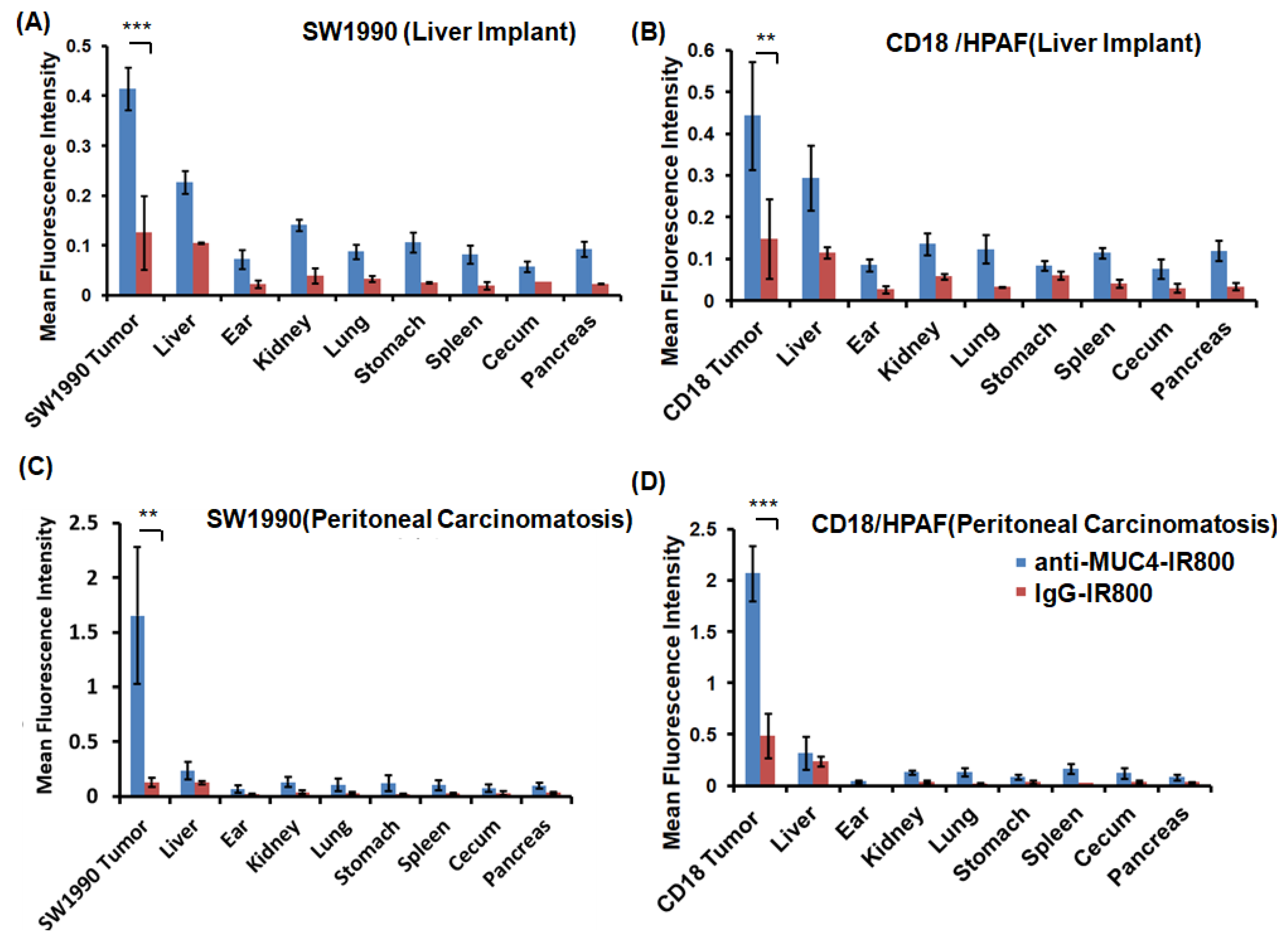
3.3. Flourescence Biodistribution of Anti-MUC4-IR800 and IgG-IR800
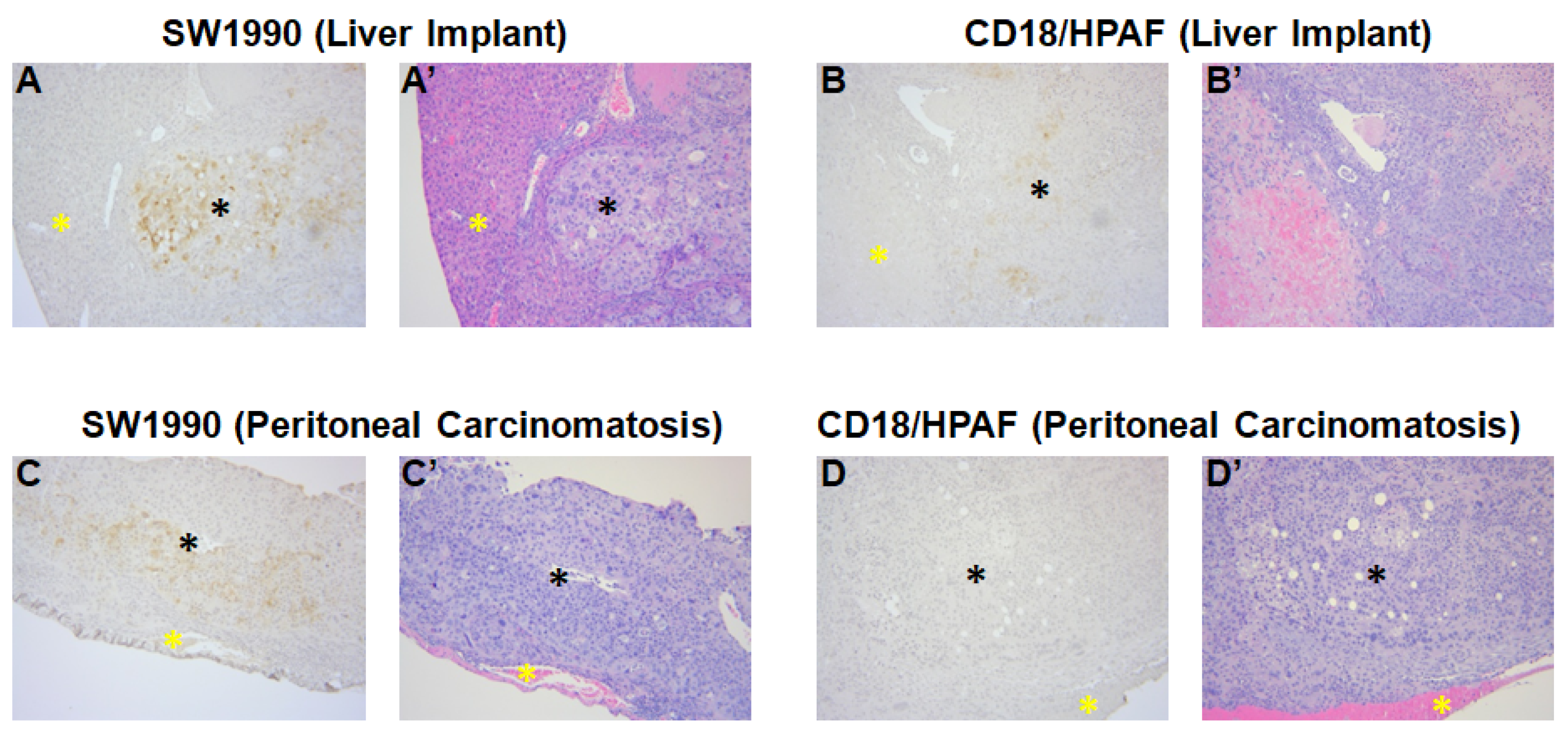
3.4. Confirmation of MUC4 Expression in Tumors by Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Ren, B.; Yang, G.; Wang, H.; Chen, G.; You, L.; Zhang, T.; Zhao, Y. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol. Life Sci. 2020, 77, 305–321. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Brody, J.R. Therapeutic Implications of Molecular Subtyping for Pancreatic Cancer. Oncology 2017, 31, 159–166, 168. [Google Scholar]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Jiang, F.; Lv, X.; Zhang, R.; Lu, A.; Zhang, G. A Mini-Review for Cancer Immunotherapy: Molecular Understanding of PD-1/PD-L1 Pathway & Translational Blockade of Immune Checkpoints. Int. J. Mol. Sci. 2016, 17, 1151. [Google Scholar] [CrossRef]
- van der Geest, L.G.M.; Lemmens, V.; de Hingh, I.; van Laarhoven, C.; Bollen, T.L.; Nio, C.Y.; van Eijck, C.H.J.; Busch, O.R.C.; Besselink, M.G. Nationwide outcomes in patients undergoing surgical exploration without resection for pancreatic cancer. Br. J. Surg. 2017, 104, 1568–1577. [Google Scholar] [CrossRef]
- Maire, F.; Cibot, J.O.; Compagne, C.; Hentic, O.; Hammel, P.; Muller, N.; Ponsot, P.; Levy, P.; Ruszniewski, P. Epidemiology of pancreatic cancer in France: Descriptive study from the French national hospital database. Eur. J. Gastroenterol. Hepatol. 2017, 29, 904–908. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; Cunningham, S.C.; Cameron, J.L.; Torrez, S.; Tapazoglou, N.; Herman, J.M.; Makary, M.A.; Eckhauser, F.; Wang, J.; Hirose, K.; et al. Palliative surgical management of patients with unresectable pancreatic adenocarcinoma: Trends and lessons learned from a large, single institution experience. J. Gastrointest. Surg. 2011, 15, 1917–1927. [Google Scholar] [CrossRef]
- Wiedmann, L.; De Angelis Rigotti, F.; Vaquero-Siguero, N.; Donato, E.; Espinet, E.; Moll, I.; Alsina-Sanchis, E.; Bohnenberger, H.; Fernandez-Florido, E.; Mülfarth, R.; et al. HAPLN1 potentiates peritoneal metastasis in pancreatic cancer. Nat. Commun. 2023, 14, 2353. [Google Scholar] [CrossRef]
- Avula, L.R.; Hagerty, B.; Alewine, C. Molecular mediators of peritoneal metastasis in pancreatic cancer. Cancer Metastasis Rev. 2020, 39, 1223–1243. [Google Scholar] [CrossRef]
- Hishinuma, S.; Ogata, Y.; Tomikawa, M.; Ozawa, I.; Hirabayashi, K.; Igarashi, S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J. Gastrointest. Surg. 2006, 10, 511–518. [Google Scholar] [CrossRef]
- Morizane, C.; Okusaka, T.; Morita, S.; Tanaka, K.; Ueno, H.; Kondo, S.; Ikeda, M.; Nakachi, K.; Mitsunaga, S. Construction and validation of a prognostic index for patients with metastatic pancreatic adenocarcinoma. Pancreas 2011, 40, 415–421. [Google Scholar] [CrossRef]
- Tran Cao, H.S.; Kaushal, S.; Menen, R.S.; Metildi, C.A.; Lee, C.; Snyder, C.S.; Talamini, M.A.; Hoffman, R.M.; Bouvet, M. Submillimeter-resolution fluorescence laparoscopy of pancreatic cancer in a carcinomatosis mouse model visualizes metastases not seen with standard laparoscopy. J. Laparoendosc. Adv. Surg. Tech. A 2011, 21, 485–489. [Google Scholar] [CrossRef]
- Tran Cao, H.S.; Kaushal, S.; Metildi, C.A.; Menen, R.S.; Lee, C.; Snyder, C.S.; Messer, K.; Pu, M.; Luiken, G.A.; Talamini, M.A.; et al. Tumor-specific fluorescence antibody imaging enables accurate staging laparoscopy in an orthotopic model of pancreatic cancer. Hepatogastroenterology 2012, 59, 1994–1999. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Hansson, G.C. Membrane mucins of the intestine at a glance. J. Cell Sci. 2020, 133, jcs240929. [Google Scholar] [CrossRef]
- Wang, S.; You, L.; Dai, M.; Zhao, Y. Mucins in pancreatic cancer: A well-established but promising family for diagnosis, prognosis and therapy. J. Cell Mol. Med. 2020, 24, 10279–10289. [Google Scholar] [CrossRef]
- Hollingsworth, M.A.; Strawhecker, J.M.; Caffrey, T.C.; Mack, D.R. Expression of MUC1, MUC2, MUC3 and MUC4 mucin mRNAs in human pancreatic and intestinal tumor cell lines. Int. J. Cancer 1994, 57, 198–203. [Google Scholar] [CrossRef]
- Swartz, M.J.; Batra, S.K.; Varshney, G.C.; Hollingsworth, M.A.; Yeo, C.J.; Cameron, J.L.; Wilentz, R.E.; Hruban, R.H.; Argani, P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am. J. Clin. Pathol. 2002, 117, 791–796. [Google Scholar] [CrossRef]
- Moniaux, N.; Chaturvedi, P.; Varshney, G.C.; Meza, J.L.; Rodriguez-Sierra, J.F.; Aubert, J.P.; Batra, S.K. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br. J. Cancer 2007, 97, 345–357. [Google Scholar] [CrossRef]
- Jaiswal, S.; Cox, K.E.; Amirfakhri, S.; Din Parast Saleh, A.; Kobayashi, K.; Lwin, T.M.; Talib, S.; Aithal, A.; Mallya, K.; Jain, M.; et al. Targeting Human Pancreatic Cancer with a Fluorophore-Conjugated Mucin 4 (MUC4) Antibody: Initial Characterization in Orthotopic Cell Line Mouse Models. J. Clin. Med. 2024, 13, 6211. [Google Scholar] [CrossRef]
- Kyriazis, A.P.; McCombs, W.B., 3rd; Sandberg, A.A.; Kyriazis, A.A.; Sloane, N.H.; Lepera, R. Establishment and characterization of human pancreatic adenocarcinoma cell line SW-1990 in tissue culture and the nude mouse. Cancer Res. 1983, 43, 4393–4401. [Google Scholar]
- Metzgar, R.S.; Gaillard, M.T.; Levine, S.J.; Tuck, F.L.; Bossen, E.H.; Borowitz, M.J. Antigens of human pancreatic adenocarcinoma cells defined by murine monoclonal antibodies. Cancer Res. 1982, 42, 601–608. [Google Scholar]
- Lee, K.H.; Cox, K.E.; Amirfakhri, S.; Jaiswal, S.; Liu, S.; Hosseini, M.; Lwin, T.M.; Yazaki, P.J.; Hoffman, R.M.; Bouvet, M. Accurate Co-Localization of Luciferase Expression and Fluorescent Anti-CEA Antibody Targeting of Liver Metastases in an Orthotopic Mouse Model of Colon Cancer. Cancers 2024, 16, 3341. [Google Scholar] [CrossRef]
- Orosco, R.K.; Tapia, V.J.; Califano, J.A.; Clary, B.; Cohen, E.E.W.; Kane, C.; Lippman, S.M.; Messer, K.; Molinolo, A.; Murphy, J.D.; et al. Positive Surgical Margins in the 10 Most Common Solid Cancers. Sci. Rep. 2018, 8, 5686. [Google Scholar] [CrossRef]
- Pogue, B.W.; Rosenthal, E.L. Review of successful pathways for regulatory approvals in open-field fluorescence-guided surgery. J. Biomed. Opt. 2021, 26, 030901. [Google Scholar] [CrossRef]
- Van Keulen, S.; Hom, M.; White, H.; Rosenthal, E.L.; Baik, F.M. The Evolution of Fluorescence-Guided Surgery. Mol. Imaging Biol. 2023, 25, 36–45. [Google Scholar] [CrossRef]
- Bailey, L.; Stone, L.D.; Gonzalez, M.L.; Thomas, C.M.; Jeyarajan, H.; Warram, J.M.; Panuganti, B. Panitumumab-IRDye800 Improves Laryngeal Tumor Mapping During Transoral Laser Microsurgery. Laryngoscope 2024, 134, 1837–1841. [Google Scholar] [CrossRef]
- Lu, G.; van den Berg, N.S.; Martin, B.A.; Nishio, N.; Hart, Z.P.; van Keulen, S.; Fakurnejad, S.; Chirita, S.U.; Raymundo, R.C.; Yi, G.; et al. Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: A phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol. Hepatol. 2020, 5, 753–764. [Google Scholar] [CrossRef]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; van den Berg, N.S.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J. Neurooncol. 2018, 139, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Warram, J.M.; de Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, G. Fluorescence-guided craniotomy of glioblastoma using panitumumab-IRDye800. Neurosurg. Focus. Video 2022, 6, V9. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, M.; Framery, B.; Boonstra, M.C.; Garambois, V.; Quenet, F.; Dumas, K.; Scherninski, F.; Cailler, F.; Vahrmeijer, A.L.; Pelegrin, A. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg. Oncol. 2017, 26, 153–162. [Google Scholar] [CrossRef]
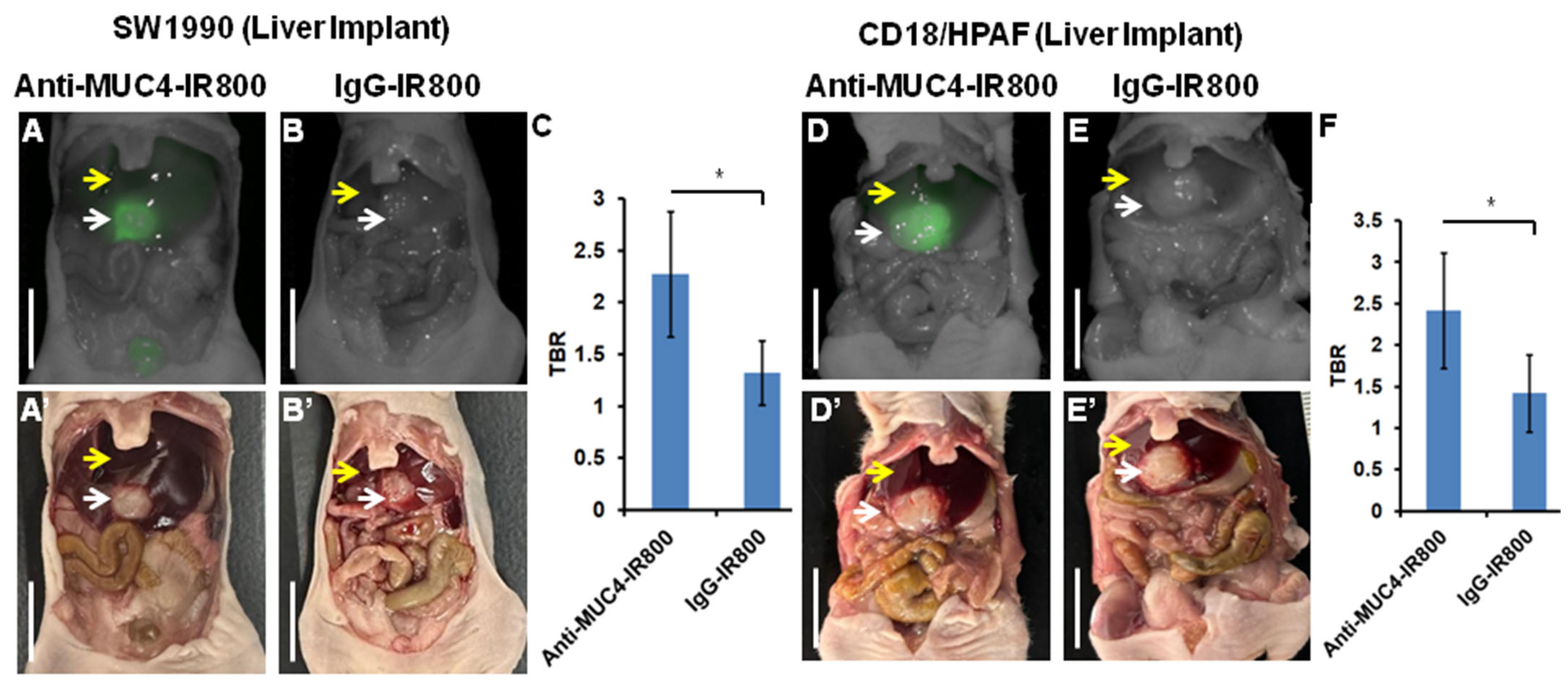
| Mouse | SW1990 Tumor (mFI) | Liver (mFI) | SW1990 Tumor/Liver (TBR) | CD18/HPAF Tumor (mFI) | Liver (mFI) | CD18/HPAF Tumor/Liver (TBR) |
|---|---|---|---|---|---|---|
| 1 | 0.601 | 0.198 | 3.035 | 0.359 | 0.171 | 2.099 |
| 2 | 0.5 | 0.214 | 2.336 | 0.878 | 0.318 | 2.761 |
| 3 | 0.447 | 0.207 | 2.159 | 0.734 | 0.203 | 3.615 |
| 4 | 0.455 | 0.291 | 1.563 | 0.611 | 0.325 | 1.880 |
| 5 | 0.357 | 0.326 | 1.095 | 0.544 | 0.313 | 1.738 |
| Average (±SD) | 0.50075 (±0.070) | 0.2275 (±0.042) | 2.273 (±0.605) | 0.6252 (±0.175) | 0.266 (±0.065) | 2.418 (±0.693) |
| p-value | 0.0007 | 0.04 | 0.012 | 0.03 |
| Mouse | SW1990 Tumor (mFI) (Total) | Liver (mFI) | SW1990 Tumor/Liver (TLR) | CD18/HPAF Tumor (mFI) (Total) | Liver (mFI) | CD18/HPAF Tumor/Liver (TLR) |
|---|---|---|---|---|---|---|
| 1 | 2.404 | 0.171 | 14.058 | 1.959 | 0.16 | 12.243 |
| 2 | 1.752 | 0.181 | 9.679 | 2.454 | 0.587 | 4.180 |
| 3 | 0.871 | 0.113 | 7.707 | 2.144 | 0.300 | 7.146 |
| 4 | 1.586 | 0.334 | 4.748 | 1.718 | 0.233 | 7.373 |
| Average (±SD) | 1.653 (±0.545) | 0.334 (0.0817) | 9.048 (±3.383) | 2.068 (± 0.268) | 0.320 (± 0.161) | 7.736 (±2.891) |
| p-value | 0.007 | 0.02 | 0.0003 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, S.; Amirfakhri, S.; Bravo, J.; Kobayashi, K.; Aithal, A.; Talib, S.; Mallya, K.; Jain, M.; Mohs, A.M.; Hoffman, R.M.; et al. Mucin4 (MUC4) Antibody Labeled with an NIR Dye Brightly Targets Pancreatic Cancer Liver Metastases and Peritoneal Carcinomatosis. Cancers 2025, 17, 2031. https://doi.org/10.3390/cancers17122031
Jaiswal S, Amirfakhri S, Bravo J, Kobayashi K, Aithal A, Talib S, Mallya K, Jain M, Mohs AM, Hoffman RM, et al. Mucin4 (MUC4) Antibody Labeled with an NIR Dye Brightly Targets Pancreatic Cancer Liver Metastases and Peritoneal Carcinomatosis. Cancers. 2025; 17(12):2031. https://doi.org/10.3390/cancers17122031
Chicago/Turabian StyleJaiswal, Sunidhi, Siamak Amirfakhri, Javier Bravo, Keita Kobayashi, Abhijit Aithal, Sumbal Talib, Kavita Mallya, Maneesh Jain, Aaron M. Mohs, Robert M. Hoffman, and et al. 2025. "Mucin4 (MUC4) Antibody Labeled with an NIR Dye Brightly Targets Pancreatic Cancer Liver Metastases and Peritoneal Carcinomatosis" Cancers 17, no. 12: 2031. https://doi.org/10.3390/cancers17122031
APA StyleJaiswal, S., Amirfakhri, S., Bravo, J., Kobayashi, K., Aithal, A., Talib, S., Mallya, K., Jain, M., Mohs, A. M., Hoffman, R. M., Batra, S. K., & Bouvet, M. (2025). Mucin4 (MUC4) Antibody Labeled with an NIR Dye Brightly Targets Pancreatic Cancer Liver Metastases and Peritoneal Carcinomatosis. Cancers, 17(12), 2031. https://doi.org/10.3390/cancers17122031







