The Effects of Concurrent Training on Molecular, Functional, and Clinical Outcomes in Breast Cancer Survivors: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
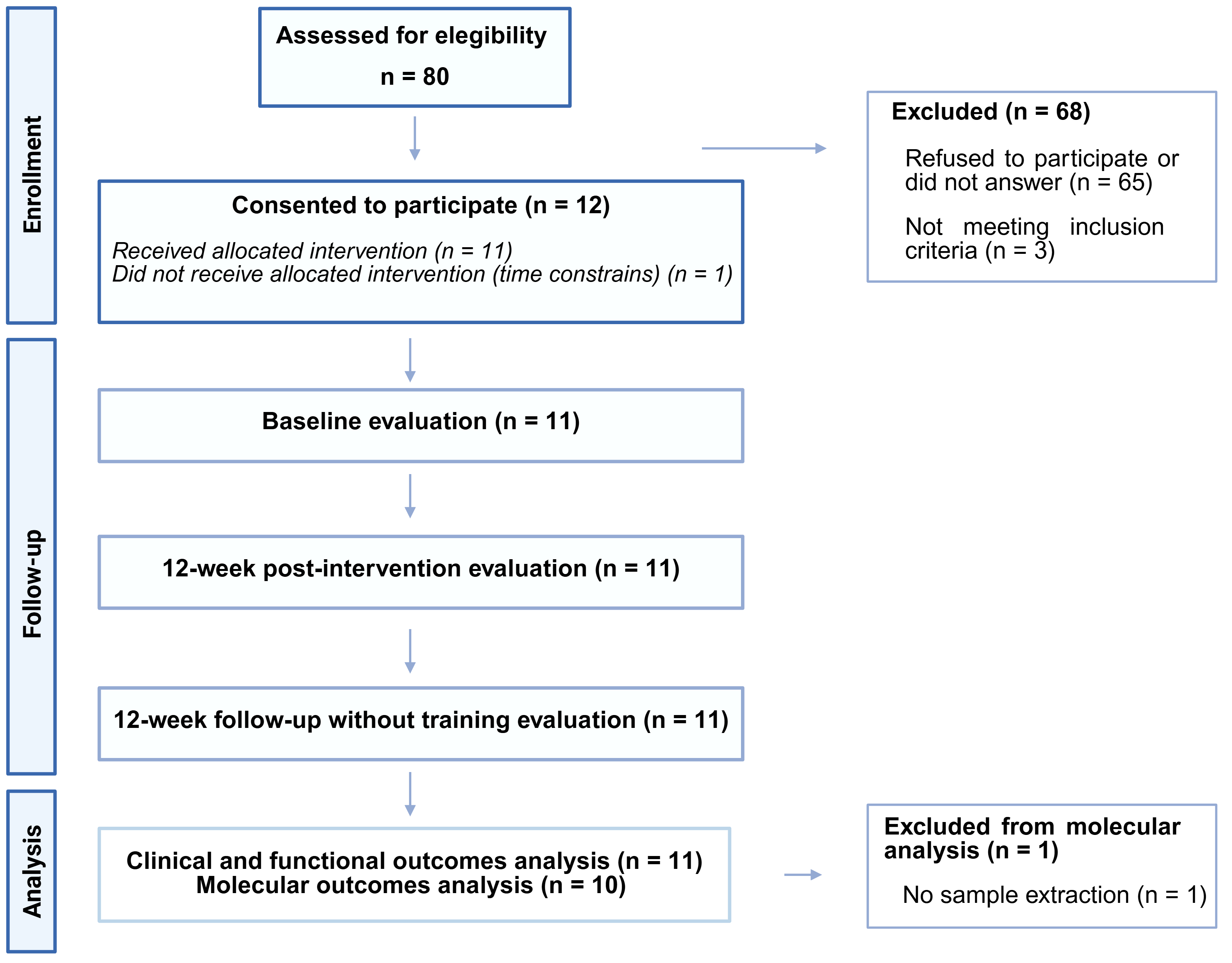
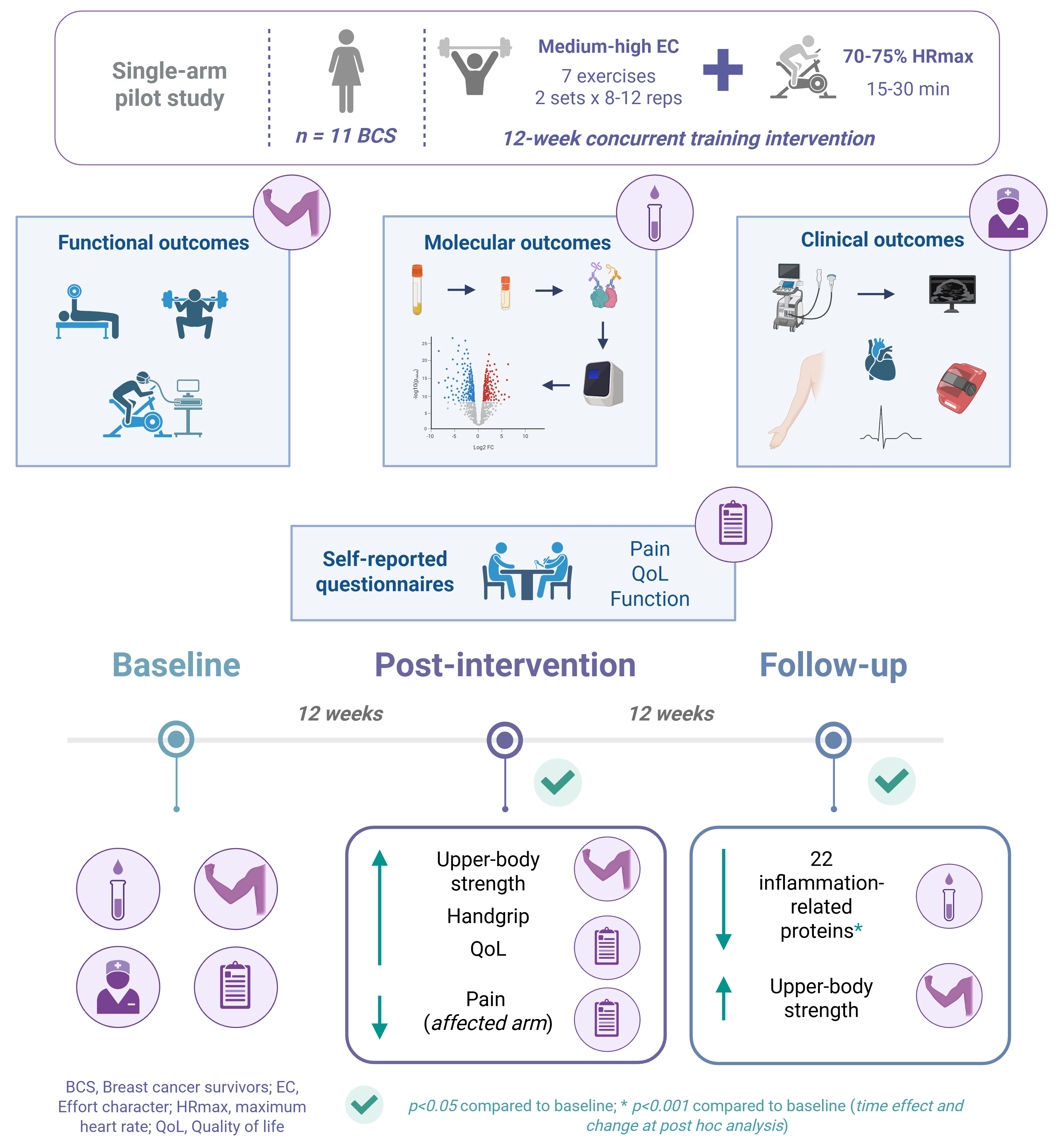
2.1. Study Design
2.2. Study Participants
2.3. Exercise Training Program and Bandage Application
2.4. Outcome Measurements
2.4.1. Primary Outcomes
Functional Outcomes
- •
- Upper- and lower-body strength
Molecular Outcomes
- •
- Inflammation-related proteins
2.4.2. Secondary Outcomes
Clinical Outcomes
- •
- Body mass index
- •
- Arm volume
- •
- Tissue thickness
- •
- Range of motion
- •
- Physical activity levels
- •
- Heart rate variability
Functional Outcomes
- •
- Cardiorespiratory fitness
- •
- Handgrip strength
Self-Reported Questionnaires
- •
- Pain
- •
- Quality of life
- •
- Upper-body function
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Adherence and Tolarance to Exercise Sessions
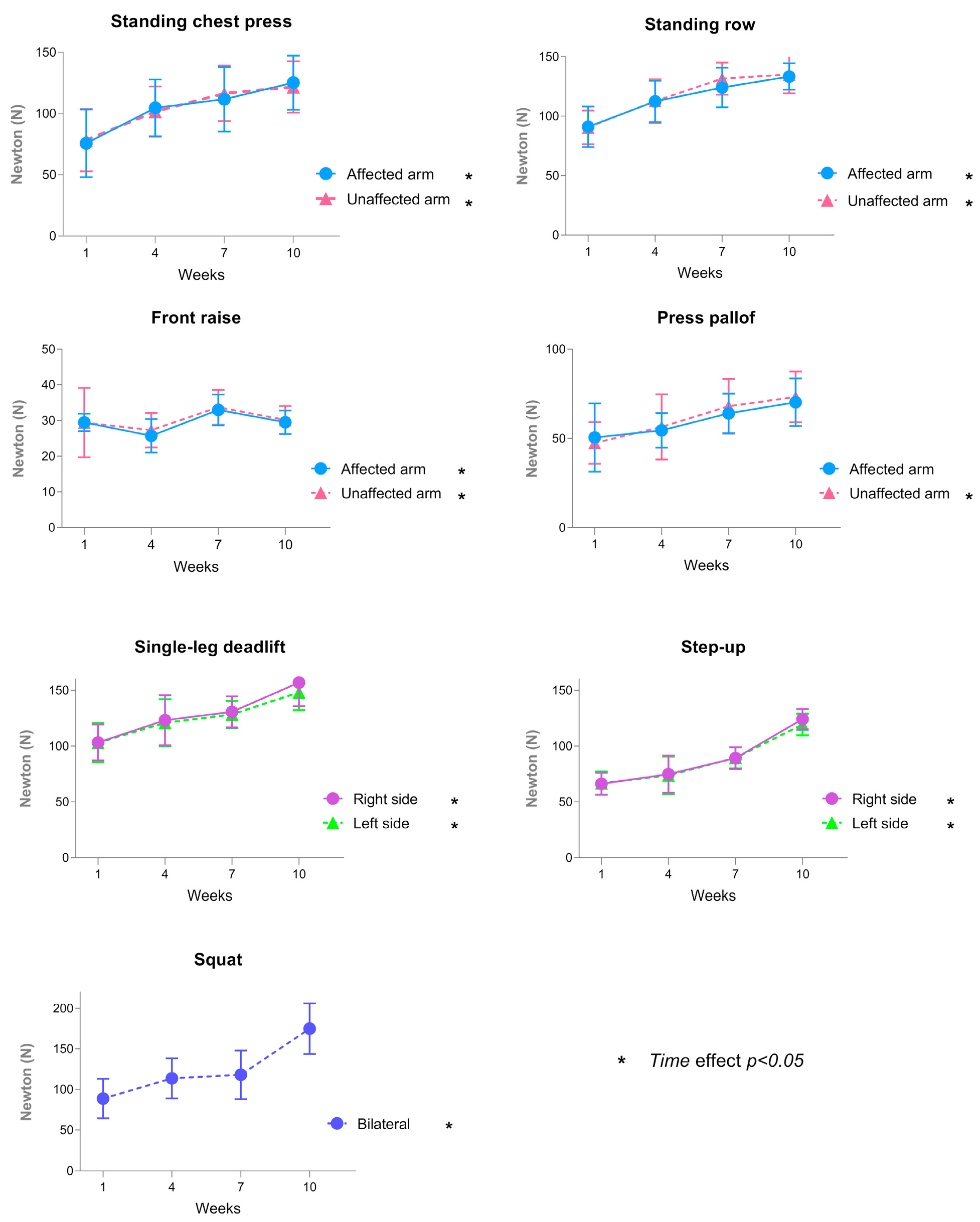
3.3. Results of Primary Outcomes
3.3.1. Functional Outcomes
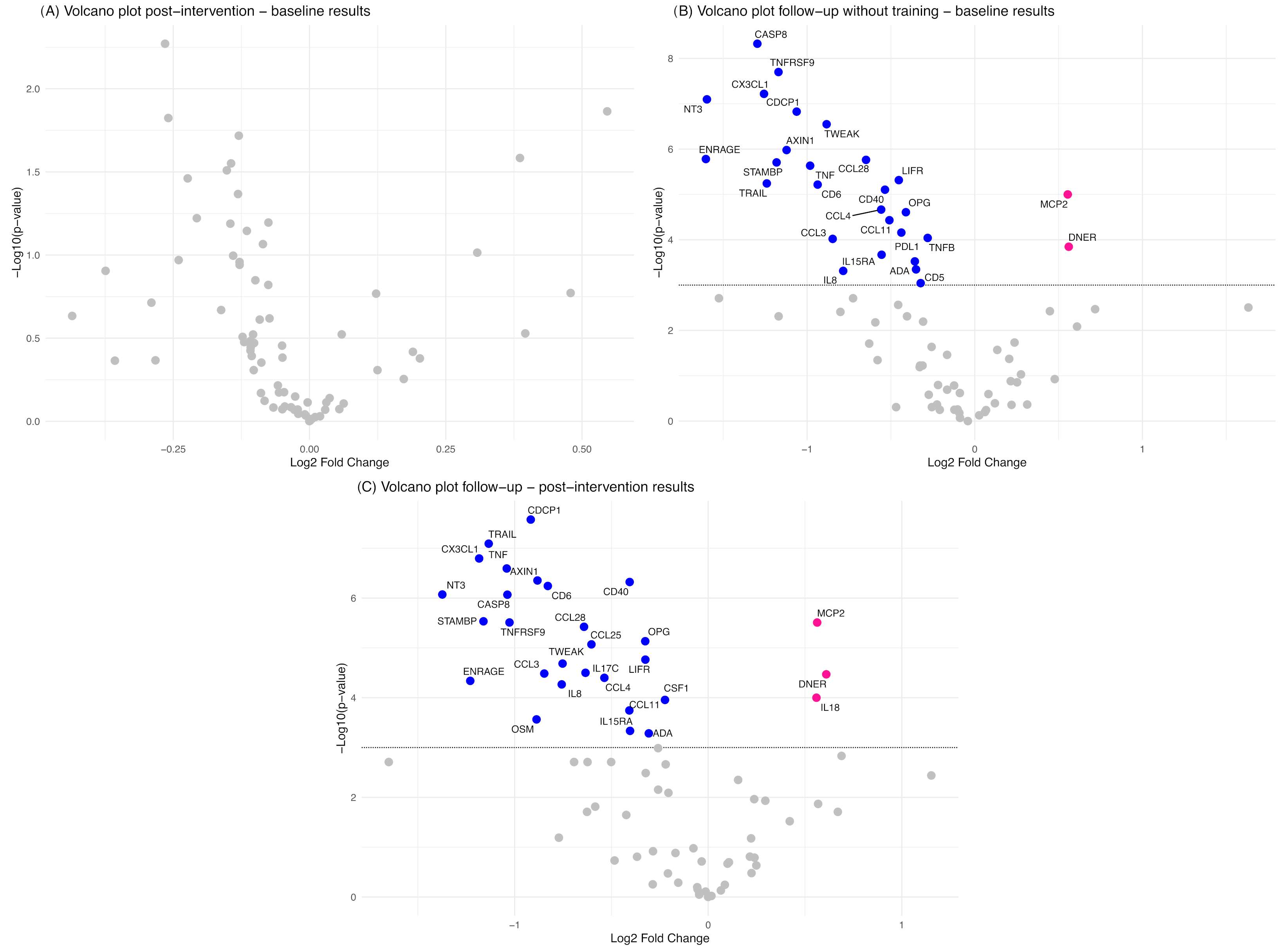
3.3.2. Molecular Outcomes
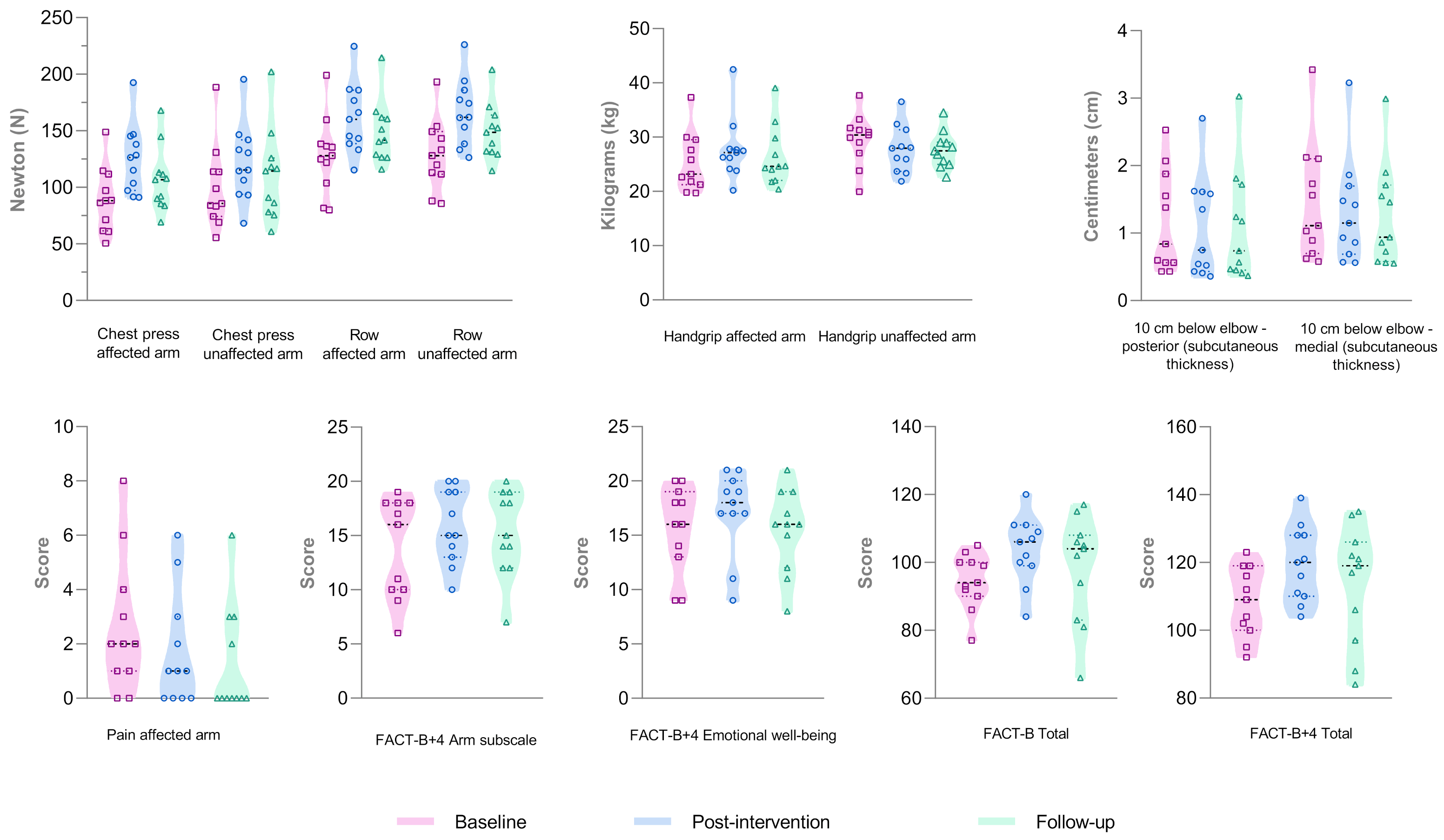
3.4. Results of Secondary Outcomes
3.4.1. Clinical Outcomes
3.4.2. Functional Outcomes
3.4.3. Self-Reported Questionnaires
3.5. Correlation Between Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | Adenosine deaminase |
| Akt | Protein kinase-B |
| ALND | Axillary lymph node dissection |
| ANOVA | Analysis of variance |
| AXIN1 | Axin-1 |
| BC | Breast cancer |
| BCRL | Breast cancer-related lymphedema |
| BMI | Body mass index |
| CASP-8 | Caspase-8 |
| CCL3 | C-C motif chemokine 3 |
| CCL4 | C-C motif chemokine 4 |
| CCL11 | Eotaxin |
| CCL25 | C-C motif chemokine 25 |
| CCL28 | C-C motif chemokine 28 |
| CDT | Complex decongestive therapy |
| CD5 | T-cell surface glycoprotein CD5 |
| CD6 | T-cell surface glycoprotein CD6 isoform |
| CD40 | CD40L receptor |
| CDCP1 | CUB domain-containing protein 1 |
| C3XCL1 | Fractalkine |
| CRF | Cardiorespiratory fitness |
| CRP | C-reactive protein |
| CSF-1 | Macrophage colony-stimulating factor 1 |
| DASH | Disabilities of the arm, shoulder, and hand |
| DNER | Delta and notch-like epidermal growth factor-related receptor |
| EC | Effort character |
| EN-RAGE | Protein S100-A12 |
| FACT-B+4 | Functional Assessment of Cancer Therapy-Breast |
| HF | High frequency |
| HR | Heart rate |
| HRV | Heart rate variability |
| IGF-1 | Insulin-like growth factor 1 |
| IL | Interleukin |
| ISL | International Society of Lymphology |
| IL-15RA | IL-15 receptor subunit alpha |
| LF | Low frequency |
| LIF-R | Leukemia inhibitory factor receptor |
| LOD | Limit of detection |
| MCP-2 | Monocyte chemotactic protein 2 |
| mTOR | Mammalian target of rapamycin |
| MVIC | Maximal voluntary isometric contraction |
| MVPA | Moderate-to-vigorous physical activity |
| NF-κB | Nuclear factor-κB |
| NRS | Numeric rating scale |
| NT-3 | Neurotrophin-3 |
| OPG | Osteoprotegerin |
| OSM | Oncostatin M |
| PD-L1 | Programmed cell death 1 ligand 1 |
| PEA | Proximity extension assay |
| PI3K | Phosphatidylinositol 3-kinase |
| QoL | Quality of life |
| RMSSD | Root mean square of successive differences |
| ROM | Range of motion |
| RPE | R rate of perceived exertion rate of perceived exertion |
| SD | Standard deviation |
| SDNN | Standard deviation of normal-to-normal intervals |
| SLNB | Sentinel lymph node biopsy |
| STAMBP | STAM-binding protein |
| TNF | Tumor necrosis factor |
| TNFB | TNF-beta |
| TNFRSF9 | TNF receptor superfamily member 9 |
| VO2peak | Peak oxygen consumption |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Heins, M.J.; de Ligt, K.M.; Verloop, J.; Siesling, S.; Korevaar, J.C.; PSCCR group. Adverse Health Effects after Breast Cancer up to 14 Years after Diagnosis. Breast 2022, 61, 22–28. [Google Scholar] [CrossRef]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-Term Effects of Breast Cancer Surgery, Treatment, and Survivor Care. J. Midwifery Womens Health 2019, 64, 713–724. [Google Scholar] [CrossRef]
- Neil-Sztramko, S.E.; Kirkham, A.A.; Hung, S.H.; Niksirat, N.; Nishikawa, K.; Campbell, K.L. Aerobic Capacity and Upper Limb Strength Are Reduced in Women Diagnosed with Breast Cancer: A Systematic Review. J. Physiother. 2014, 60, 189–200. [Google Scholar] [CrossRef]
- Valiyaveettil, D.; Joseph, D.; Malik, M. Cardiotoxicity in Breast Cancer Treatment: Causes and Mitigation. Cancer Treat. Res. Commun. 2023, 37, 100760. [Google Scholar] [CrossRef]
- Rockson, S.G.; Keeley, V.; Kilbreath, S.; Szuba, A.; Towers, A. Cancer-Associated Secondary Lymphoedema. Nat. Rev. Dis. Primers 2019, 5, 22. [Google Scholar] [CrossRef]
- Naoum, G.E.; Roberts, S.; Brunelle, C.L.; Shui, A.M.; Salama, L.; Daniell, K.; Gillespie, T.; Bucci, L.; Smith, B.L.; Ho, A.Y.; et al. Quantifying the Impact of Axillary Surgery and Nodal Irradiation on Breast Cancer-Related Lymphedema and Local Tumor Control: Long-Term Results From a Prospective Screening Trial. J. Clin. Oncol. 2020, 38, 3430–3438. [Google Scholar] [CrossRef]
- Brunelle, C.L.; Jackson, K.; Shallwani, S.M.; Hunley, J.H.; Kennedy, A.; Fench, S.; Hill, A.; Paskett, E.D.; Rush, K.; Thiadens, S.R.J.; et al. Evidence-Based Recommendations Regarding Risk Reduction Practices for People at Risk of or with Breast Cancer-Related Lymphedema: Consensus from an Expert Panel. Med. Oncol. 2024, 41, 298. [Google Scholar] [CrossRef]
- Brunelle, C.L.; Taghian, A.G. Breast Cancer-Related Lymphedema: Risk Stratification and a Continued Call for Screening. JCO Oncol. Pract. 2023, 19, 1081–1083. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; Brunelle, C.L.; Taghian, A. Breast Cancer-Related Lymphedema: Risk Factors, Screening, Management, and the Impact of Locoregional Treatment. J. Clin. Oncol. 2020, 38, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A.; et al. Elevated Biomarkers of Inflammation Are Associated with Reduced Survival among Breast Cancer Patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Cathcart-Rake, E.J.; Ruddy, K.J. Evidence-Based Guidance for Breast Cancer Survivorship. Hematol. Oncol. Clin. N. Am. 2023, 37, 225–243. [Google Scholar] [CrossRef]
- Joaquim, A.; Leão, I.; Antunes, P.; Capela, A.; Viamonte, S.; Alves, A.J.; Helguero, L.A.; Macedo, A. Impact of Physical Exercise Programs in Breast Cancer Survivors on Health-Related Quality of Life, Physical Fitness, and Body Composition: Evidence from Systematic Reviews and Meta-Analyses. Front. Oncol. 2022, 12, 955505. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Madeira, R.; Esteves, D.; Maia, A.; Alves, A.R.; Marques, D.L.; Neiva, H.P. Efficacy of Concurrent Training in Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Physical, Psychological, and Biomarker Variables. Healthcare 2024, 13, 33. [Google Scholar] [CrossRef]
- Tan, L.; Mei, J.; Tang, R.; Huang, D.; Qi, K.; Ossowski, Z.; Wang, X. Can Exercise as a Complementary Technique Manage Inflammatory Markers in Women with Breast Cancer Who Are Overweight and Obese? A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2025, 88, 103119. [Google Scholar] [CrossRef]
- Bettariga, F.; Taaffe, D.R.; Borsati, A.; Avancini, A.; Pilotto, S.; Lazzarini, S.G.; Lopez, P.; Maestroni, L.; Crainich, U.; Campbell, J.P.; et al. Effects of Exercise on Inflammation in Female Survivors of Nonmetastatic Breast Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2025, djaf062. [Google Scholar] [CrossRef]
- Abbasi, F.; Pourjalali, H.; do Nascimento, I.J.B.; Zargarzadeh, N.; Mousavi, S.M.; Eslami, R.; Milajerdi, A. The Effects of Exercise Training on Inflammatory Biomarkers in Patients with Breast Cancer: A Systematic Review and Meta-Analysis. Cytokine 2022, 149, 155712. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, N.; Ding, M.; Yuan, K. Effects of Exercise on Inflammatory Factors and IGF System in Breast Cancer Survivors: A Meta-Analysis. BMC Womens Health 2022, 22, 507. [Google Scholar] [CrossRef]
- Meneses-Echávez, J.F.; Correa-Bautista, J.E.; González-Jiménez, E.; Schmidt Río-Valle, J.; Elkins, M.R.; Lobelo, F.; Ramírez-Vélez, R. The Effect of Exercise Training on Mediators of Inflammation in Breast Cancer Survivors: A Systematic Review with Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Singh, B.; Hayes, S.; Peake, J.M.; Galvão, D.A.; Taaffe, D.R.; Spry, N.; Nosaka, K.; Cornish, B.; Schmitz, K.H.; et al. Acute Inflammatory Response to Low-, Moderate-, and High-Load Resistance Exercise in Women with Breast Cancer-Related Lymphedema. Integr. Cancer Ther. 2016, 15, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Hasenoehrl, T.; Palma, S.; Ramazanova, D.; Kölbl, H.; Dorner, T.E.; Keilani, M.; Crevenna, R. Resistance Exercise and Breast Cancer–Related Lymphedema—A Systematic Review Update and Meta-Analysis. Support. Care Cancer 2020, 28, 3593–3603. [Google Scholar] [CrossRef] [PubMed]
- Hasenoehrl, T.; Keilani, M.; Palma, S.; Crevenna, R. Resistance Exercise and Breast Cancer Related Lymphedema–a Systematic Review Update. Disabil. Rehabil. 2020, 42, 26–35. [Google Scholar] [CrossRef]
- Executive Committee of the International Society of Lymphology. The Diagnosis and Treatment of Peripheral Lymphedema: 2023 Consensus Document of The International Society of Lymphology. Lymphology 2023, 56, 133–151. [Google Scholar]
- Maroto-Izquierdo, S.; López-Ortiz, S.; Peñín-Grandes, S.; Santos-Lozano, A. Repetitions in Reserve: An Emerging Method for Strength Exercise Prescription in Special Populations. Strength. Cond. J. 2024, 47, 317–327. [Google Scholar] [CrossRef]
- Feito, Y.; Fountaine, C. General principles of exercise prescription. In ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Liguori, G., Feito, Y., Fountaine, C., Roy, B.A., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2021. [Google Scholar]
- Morishita, S.; Tsubaki, A.; Takabayashi, T.; Fu, J.B. Relationship between the Rating of Perceived Exertion Scale and the Load Intensity of Resistance Training. Strength. Cond. J. 2018, 40, 94–109. [Google Scholar] [CrossRef]
- Bünzen, C.; Knuth, J.; Bucher, M.; Weisser, B.; Schmidt, T. CORE-CERT Items as a Minimal Requirement for Replicability of Exercise Interventions: Results From Application to Exercise Studies for Breast Cancer Patients. J. Strength. Cond. Res. 2023, 37, e346–e360. [Google Scholar] [CrossRef]
- Damstra, R.J.; Partsch, H. Compression Therapy in Breast Cancer-Related Lymphedema: A Randomized, Controlled Comparative Study of Relation between Volume and Interface Pressure Changes. J. Vasc. Surg. 2009, 49, 1256–1263. [Google Scholar] [CrossRef]
- Torres-Lacomba, M.; Navarro-Brazález, B.; Prieto-Gómez, V.; Ferrandez, J.C.; Bouchet, J.Y.; Romay-Barrero, H. Effectiveness of Four Types of Bandages and Kinesio-Tape for Treating Breast-Cancer-Related Lymphoedema: A Randomized, Single-Blind, Clinical Trial. Clin. Rehabil. 2020, 34, 1230–1241. [Google Scholar] [CrossRef]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Björkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Dickens, E.R.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef] [PubMed]
- Haslam, D.E.; Li, J.; Dillon, S.T.; Gu, X.; Cao, Y.; Zeleznik, O.A.; Sasamoto, N.; Zhang, X.; Eliassen, A.H.; Liang, L.; et al. Stability and Reproducibility of Proteomic Profiles in Epidemiological Studies: Comparing the Olink and SOMAscan Platforms. Proteomics 2022, 22, e2100170. [Google Scholar] [CrossRef] [PubMed]
- Dylke, E. Measurement of Breast Cancer-Related Lymphoedema. J. Physiother. 2022, 68, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Bok, S.K.; Jeon, Y.; Hwang, P.S. Ultrasonographic Evaluation of the Effects of Progressive Resistive Exercise in Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2016, 14, 18–24. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Marín, P.J.; Torres-Luque, G.; Ruiz, J.R.; Lucía, A.; Garatachea, N. Technical Variability of the GT3X Accelerometer. Med. Eng. Phys. 2012, 34, 787–790. [Google Scholar] [CrossRef]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of Accelerometer Wear and Nonwear Time Classification Algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. Accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Lavín-Pérez, A.M.; Collado-Mateo, D.; Hinojo González, C.; de Juan Ferré, A.; Ruisánchez Villar, C.; Mayo, X.; Jiménez, A. High-Intensity Exercise Prescription Guided by Heart Rate Variability in Breast Cancer Patients: A Study Protocol for a Randomized Controlled Trial. BMC Sports Sci. Med. Rehabil. 2023, 15, 28. [Google Scholar] [CrossRef]
- Klassen, O.; Schmidt, M.E.; Scharhag-Rosenberger, F.; Sorkin, M.; Ulrich, C.M.; Schneeweiss, A.; Potthoff, K.; Steindorf, K.; Wiskemann, J. Cardiorespiratory Fitness in Breast Cancer Patients Undergoing Adjuvant Therapy. Acta Oncol. 2014, 53, 1356–1365. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Díaz-Rodríguez, L.; Fernández-De-Las-Peñas, C.; Ruiz, J.R.; Arroyo-Morales, M. The Handgrip Strength Test as a Measure of Function in Breast Cancer Survivors: Relationship to Cancer-Related Symptoms and Physical and Physiologic Parameters. Am. J. Phys. Med. Rehabil. 2012, 91, 774–782. [Google Scholar] [CrossRef]
- Ammitzbøll, G.; Johansen, C.; Lanng, C.; Andersen, E.W.; Kroman, N.; Zerahn, B.; Hyldegaard, O.; Wittenkamp, M.C.; Dalton, S.O. Progressive Resistance Training to Prevent Arm Lymphedema in the First Year after Breast Cancer Surgery: Results of a Randomized Controlled Trial. Cancer 2019, 125, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Belmonte Martínez, R.; Garin Boronat, O.; Segura Badía, M.; Sanz Latiesas, J.; Marco Navarro, E.; Ferrer Fores, M. Functional Assessment of Cancer Therapy Questionnaire for Breast Cancer (FACT-B+4). Spanish Version Validation. Med. Clin. 2011, 137, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Teresa Hervás, M.; Navarro Collado, M.J.; Peiró, S.; Rodrigo Pérez, J.L.; López Matéu, P.; Martínez Tello, I. Versión Española Del Cuestionario DASH. Adaptación Transcultural, Fiabilidad, Validez y Sensibilidad a Los Cambios. Med. Clin. 2006, 127, 441–447. [Google Scholar] [CrossRef]
- Julious, S.A. Sample Size of 12 per Group Rule of Thumb for a Pilot Study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical Notes for Clinical Researchers: Sample Size Calculation 3. Comparison of Several Means Using One-Way ANOVA. Restor. Dent. Endod. 2016, 41, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.; Tomczak, E. The Need to Report Effect Size Estimates Revisited. An Overview of Some Recommended Measures of Effect Size. Trends Sport. Sci. 2014, 1, 19–25. [Google Scholar]
- Pérez-Bilbao, T.; García-González, D.; Martos-Bermúdez, Á.; Nieto, S.; Del Campo, T.; Pérez-Ruiz, M.; San Juan, A.F. Effects of an Eight-Week Concurrent Training Program with Different Effort Character over Physical Fitness, Health-Related Quality of Life and Lipid Profile among Hospital Workers: Preliminary Results. Int. J. Environ. Res. Public Health 2021, 18, 9328. [Google Scholar] [CrossRef]
- Mills, R.C. Breast Cancer Survivors, Common Markers of Inflammation, and Exercise: A Narrative Review. Breast Cancer 2017, 11, 1178223417743976. [Google Scholar] [CrossRef]
- Cormie, P.; Galvão, D.A.; Spry, N.; Newton, R.U. Neither Heavy nor Light Load Resistance Exercise Acutely Exacerbates Lymphedema in Breast Cancer Survivor. Integr. Cancer Ther. 2013, 12, 423–432. [Google Scholar] [CrossRef]
- Jeucken, K.C.M.; Koning, J.J.; Mebius, R.E.; Tas, S.W. The Role of Endothelial Cells and TNF-Receptor Superfamily Members in Lymphoid Organogenesis and Function During Health and Inflammation. Front. Immunol. 2019, 10, 2700. [Google Scholar] [CrossRef]
- Croft, M.; Duan, W.; Choi, H.; Eun, S.Y.; Madireddi, S.; Mehta, A. TNF Superfamily in Inflammatory Disease: Translating Basic Insights. Trends Immunol. 2012, 33, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Palomino, D.C.T.; Marti, L.C. Chemokines and Immunity. Einstein 2015, 13, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Deng, L.; Wang, B.-Z. CCL28 Chemokine: An Anchoring Point Bridging Innate and Adaptive Immunity. Int. Immunopharmacol. 2017, 51, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Vinader, V.; Afarinkia, K. A Beginner’s Guide To Chemokines. Future Med. Chem. 2012, 4, 845–852. [Google Scholar] [CrossRef]
- Dranoff, G. Cytokines in Cancer Pathogenesis and Cancer Therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Reiss, K.A.; Forde, P.M.; Brahmer, J.R. Harnessing the Power of the Immune System via Blockade of PD-1 and PD-L1: A Promising New Anticancer Strategy. Immunotherapy 2014, 6, 459–475. [Google Scholar] [CrossRef]
- Consuegra-Fernández, M.; Lin, F.; Fox, D.A.; Lozano, F. Clinical and Experimental Evidence for Targeting CD6 in Immune-Based Disorders. Autoimmun. Rev. 2018, 17, 493–503. [Google Scholar] [CrossRef]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef]
- Turdo, F.; Bianchi, F.; Gasparini, P.; Sandri, M.; Sasso, M.; De Cecco, L.; Forte, L.; Casalini, P.; Aiello, P.; Sfondrini, L.; et al. CDCP1 Is a Novel Marker of the Most Aggressive Human Triple-Negative Breast Cancers. Oncotarget 2016, 7, 69649–69665. [Google Scholar] [CrossRef]
- Xiang, C.; Li, H.; Tang, W. Targeting CSF-1R Represents an Effective Strategy in Modulating Inflammatory Diseases. Pharmacol. Res. 2023, 187, 106566. [Google Scholar] [CrossRef]
- Qiu, L.; Sun, Y.; Ning, H.; Chen, G.; Zhao, W.; Gao, Y. The Scaffold Protein AXIN1: Gene Ontology, Signal Network, and Physiological Function. Cell Commun. Signal 2024, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, C.; Liao, Y.; Zhou, M.; Xu, W.; Zou, Z. Caspase-8 in Inflammatory Diseases: A Potential Therapeutic Target. Cell Mol. Biol. Lett. 2024, 29, 130. [Google Scholar] [CrossRef] [PubMed]
- Meijer, B.; Gearry, R.B.; Day, A.S. The Role of S100a12 as a Systemic Marker of Inflammation. Int. J. Inflam. 2012, 2012, 907078. [Google Scholar] [CrossRef]
- Viswanadhapalli, S.; Dileep, K.V.; Zhang, K.Y.J.; Nair, H.B.; Vadlamudi, R.K. Targeting LIF/LIFR Signaling in Cancer. Genes. Dis. 2022, 9, 973–980. [Google Scholar] [CrossRef]
- Jin, W. Roles of TrKC Signaling in the Regulation of Tumorigenicity and Metastasis of Cancer. Cancers 2020, 12, 147. [Google Scholar] [CrossRef]
- Dundar, M.S.; Belanova, I.; Bonetti, G.; Gelanova, V.; Kozacikova, R.; Veselenyiova, D.; Macchia, A.; Micheletti, C.; Medori, C.; Donato, K.; et al. Genetic Variants in Genes Correlated to the PI3K/AKT Pathway: The Role of ARAP3, CDH5, KIF11 and RELN in Primary Lymphedema. Lymphology 2023, 56, 152–159. [Google Scholar]
- Yang, Q.; Yan, D.; Zou, C.; Xue, Q.; Lin, S.; Huang, Q.; Li, X.; Tang, D.; Chen, X.; Liu, J. The Deubiquitinating Enzyme STAMBP Is a Newly Discovered Driver of Triple-Negative Breast Cancer Progression That Maintains RAI14 Protein Stability. Exp. Mol. Med. 2022, 54, 2047–2059. [Google Scholar] [CrossRef]
- Kim, S.H.; Song, Y.K.; Han, J.; Ko, Y.H.; Lee, H.; Kang, M.J.; Park, H.; Lee, H.; Kim, S. Pro-Inflammatory Cytokine Levels and Cancer-Related Fatigue in Breast Cancer Survivors: Effects of an Exercise Adherence Program. J. Breast Cancer 2020, 23, 205–217. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Parmentier, J.H.; Sami, N.; Lee, K.; Spicer, D.; Mack, W.J.; Sattler, F.; Mittelman, S.D. Adipose Tissue Inflammation in Breast Cancer Survivors: Effects of a 16-Week Combined Aerobic and Resistance Exercise Training Intervention. Breast Cancer Res. Treat. 2018, 168, 147–157. [Google Scholar] [CrossRef]
- Monazzami, A.; Momenpur, R.; Alipour, E.; Yari, K.; Payandeh, M. Effects of Eight-Week Combined Resistance and Endurance Training on Salivary Interleukin-12, Tumor Necrosis Factor, Cortisol, and Testosterone Levels in Patients with Breast Cancer. Int. J. Cancer Manag. 2021, 14, e109039. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of Inflammation and Their Association with Muscle Strength and Mass: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Shirinyfard Pilehrood, K.; Askari, G.; Sharifi, M.; Kargarfard, M.; Saraf-Bank, S. Elevated Risk of Possible Sarcopenia and Weak Muscle Strength with Higher Dietary Inflammatory Index in Iranian Breast Cancer Survivors: A Cross-Sectional Study. BMC Nutr. 2025, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Klassen, O.; Schmidt, M.E.; Ulrich, C.M.; Schneeweiss, A.; Potthoff, K.; Steindorf, K.; Wiskemann, J. Muscle Strength in Breast Cancer Patients Receiving Different Treatment Regimes. J. Cachexia Sarcopenia Muscle 2017, 8, 305–316. [Google Scholar] [CrossRef]
- Paramanandam, V.S.; Roberts, D. Weight Training Is Not Harmful for Women with Breast Cancer-Related Lymphoedema: A Systematic Review. J. Physiother. 2014, 60, 136–143. [Google Scholar] [CrossRef]
- Cešeiko, R.; Thomsen, S.N.; Tomsone, S.; Eglītis, J.; Vētra, A.; Srebnijs, A.; Timofejevs, M.; Purmalis, E.; Wang, E. Heavy Resistance Training in Breast Cancer Patients Undergoing Adjuvant Therapy. Med. Sci. Sports Exerc. 2020, 52, 1239–1247. [Google Scholar] [CrossRef]
- Rogers, B.H.; Brown, J.C.; Gater, D.R.; Schmitz, K.H. Association Between Maximal Bench Press Strength and Isometric Handgrip Strength Among Breast Cancer Survivors. Arch. Phys. Med. Rehabil. 2017, 98, 264–269. [Google Scholar] [CrossRef]
- Vaishya, R.; Misra, A.; Vaish, A.; Ursino, N.; D’Ambrosi, R. Hand Grip Strength as a Proposed New Vital Sign of Health: A Narrative Review of Evidences. J. Health Popul. Nutr. 2024, 43, 7. [Google Scholar] [CrossRef]
- Fuentes-Abolafio, I.J.; Roldán-Jiménez, C.; Campos, M.I.; Pajares-Hachero, B.I.; Alba-Conejo, E.; Cuesta-Vargas, A. Forearm Muscle Activity During the Handgrip Test in Breast Cancer Survivors: A Cross-Sectional Study. Clin. Breast Cancer 2023, 23, e175–e181. [Google Scholar] [CrossRef]
- Williamson, A.; Hoggart, B. Pain: A Review of Three Commonly Used Pain Rating Scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef]
- Steindorf, K.; Schmidt, M.E.; Klassen, O.; Ulrich, C.M.; Oelmann, J.; Habermann, N.; Beckhove, P.; Owen, R.; Debus, J.; Wiskemann, J.; et al. Randomized, Controlled Trial of Resistance Training in Breast Cancer Patients Receiving Adjuvant Radiotherapy: Results on Cancer-Related Fatigue and Quality of Life. Ann. Oncol. 2014, 25, 2237–2243. [Google Scholar] [CrossRef]
- Luan, B.; Li, Z.; Yang, Q.; Xu, Z.; Chen, Y.; Wang, M.; Chen, W.; Ge, F. The Effects of ACSM-Based Exercise on Breast Cancer-Related Lymphoedema: A Systematic Review and Meta-Analysis. Front. Physiol. 2024, 15, 1413764. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.G.; Toyserkani, N.M.; Hansen, F.G.; Bygum, A.; Sørensen, J.A. The Impact of Lymphedema on Health-Related Quality of Life up to 10 Years after Breast Cancer Treatment. NPJ Breast Cancer 2021, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Marchica, P.; D’Arpa, S.; Magno, S.; Rossi, C.; Forcina, L.; Capizzi, V.; Oieni, S.; Amato, C.; Piazza, D.; Gebbia, V. Integrated Treatment of Breast Cancer-Related Lymphedema: A Descriptive Review of the State of the Art. Anticancer. Res. 2021, 41, 3233–3246. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Figarska, S.; Sundström, J.; Fall, T.; Ärnlöv, J.; Ingelsson, E. Changes in Proteomic Profiles Are Related to Changes in BMI and Fat Distribution During 10 Years of Aging. Obesity 2020, 28, 178–186. [Google Scholar] [CrossRef]
- Hill, E.B.; Siebert, J.C.; Yazza, D.N.; Ostendorf, D.M.; Bing, K.; Wayland, L.; Scorsone, J.J.; Bessesen, D.H.; MacLean, P.S.; Melanson, E.L.; et al. Proteomics, Dietary Intake, and Changes in Cardiometabolic Health within a Behavioral Weight-Loss Intervention: A Pilot Study. Obesity 2022, 30, 2134–2145. [Google Scholar] [CrossRef]
- Arias-Crespo, M.; García-Fernández, R.; Calvo-Ayuso, N.; Martín-Vázquez, C.; de Fátima da Silva Vieira Martins, M.; Quiroga-Sánchez, E. Impact of Physical Exercise on Breast Cancer-Related Lymphedema and Non-Invasive Measurement Tools: A Systematic Review. Cancers 2025, 17, 333. [Google Scholar] [CrossRef]

| Breast Cancer Survivors (n = 11) | ||
|---|---|---|
| Age—years (mean ± SD) | 53.00 ± 7.17 | |
| Weight—kg (mean ± SD) | 65.65 ± 12.59 | |
| Height—m (mean ± SD) | 1.61 ± 0.06 | |
| BMI—kg·m−2 (mean ± SD) | 25.42 ± 4.31 | |
| Time since cancer diagnosis—years (mean ± SD) | 6.83 ± 4.56 | |
| Affected arm | Right—n (%) | 5 (45.45) |
| Left—n (%) | 6 (54.55) | |
| Breast surgery—n (%) | 11 (100) | |
| Tumorectomy—n (%) | 5 (45.45) | |
| Tumorectomy + simple mastectomy—n (%) | 1 (9.09) | |
| Radical mastectomy—n (%) | 3 (27.27) | |
| Modified radical mastectomy—n (%) | 2 (18.18) | |
| Axillary surgery—n (%) | 11 (100) | |
| SLNB—n (%) | 2 (18.18) | |
| ALND—n (%) | 8 (72.73) | |
| SLNB + ALND—n (%) | 1 (9.09) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Chico, C.; López-Ortiz, S.; Santiago-Pescador, S.; Guillén-Rogel, P.; Peñín-Grandes, S.; Musso-Daury, L.; Iruzubieta-Barragán, F.J.; Pinto-Fraga, J.; Maroto-Izquierdo, S.; del Río Solá, L.; et al. The Effects of Concurrent Training on Molecular, Functional, and Clinical Outcomes in Breast Cancer Survivors: A Pilot Study. Cancers 2025, 17, 1967. https://doi.org/10.3390/cancers17121967
García-Chico C, López-Ortiz S, Santiago-Pescador S, Guillén-Rogel P, Peñín-Grandes S, Musso-Daury L, Iruzubieta-Barragán FJ, Pinto-Fraga J, Maroto-Izquierdo S, del Río Solá L, et al. The Effects of Concurrent Training on Molecular, Functional, and Clinical Outcomes in Breast Cancer Survivors: A Pilot Study. Cancers. 2025; 17(12):1967. https://doi.org/10.3390/cancers17121967
Chicago/Turabian StyleGarcía-Chico, Celia, Susana López-Ortiz, Salvador Santiago-Pescador, Paloma Guillén-Rogel, Saúl Peñín-Grandes, Lisa Musso-Daury, Francisco Javier Iruzubieta-Barragán, José Pinto-Fraga, Sergio Maroto-Izquierdo, Lourdes del Río Solá, and et al. 2025. "The Effects of Concurrent Training on Molecular, Functional, and Clinical Outcomes in Breast Cancer Survivors: A Pilot Study" Cancers 17, no. 12: 1967. https://doi.org/10.3390/cancers17121967
APA StyleGarcía-Chico, C., López-Ortiz, S., Santiago-Pescador, S., Guillén-Rogel, P., Peñín-Grandes, S., Musso-Daury, L., Iruzubieta-Barragán, F. J., Pinto-Fraga, J., Maroto-Izquierdo, S., del Río Solá, L., & Santos-Lozano, A. (2025). The Effects of Concurrent Training on Molecular, Functional, and Clinical Outcomes in Breast Cancer Survivors: A Pilot Study. Cancers, 17(12), 1967. https://doi.org/10.3390/cancers17121967









