Full-Thickness Chest Wall Resection and Reconstruction for Locally Invasive Phyllodes Tumors: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Protocol and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
- -
- Population: patients diagnosed with locally invasive PT (primary or recurrent) confirmed by histopathology, with evidence of chest wall invasion on imaging or intra-operative findings.
- -
- Intervention: FTCWR, defined as resection involving at least one rib and/or part of the sternum with overlying soft tissue.
- -
- Comparators: not applicable.
- -
- Outcomes: studies reporting at least one of the following: post-operative complications (immediate, early, or late), length of hospital stay, in-hospital mortality, duration of follow-up, LR, distant recurrence, survival or mortality outcomes, patient-reported outcomes, or quality of life (QoL).
2.4. Data Extraction and Synthesis
2.5. Quality Assessment of Included Studies
3. Results
3.1. Literature Retrieval
3.2. Quality Assessment
3.3. Patient and Tumor Characteristics Based on Included Studies
3.4. Radiological Investigations
3.5. Histological Investigations
3.6. Operative Details of Full-Thickness Chest Wall Resection (FTCWR)
3.7. Reconstructive Techniques
3.8. Post-Operative Outcomes and Complications
3.9. Oncologic Outcomes, Patient-Reported Outcomes, and QoL
4. Discussion
Strengths and Limitations of Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dyer, N.H.; Bridger, J.E.; Taylor, R.S. Cystosarcoma phylloides. J. Br. Surg. 1966, 53, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Ellis, I.O. Myoepithelial and epithelial–myoepithelial, mesenchymal and fibroepithelial breast lesions: Updates from the WHO Classification of Tumours of the Breast 2012. J. Clin. Pathol. 2013, 66, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Rosen, P.P.; Oberman, H.A. Tumors of the Mammary Gland; Armed Forces Institute of Pathology: Washington, DC, USA, 1993. [Google Scholar]
- Tan, B.Y.; Acs, G.; Apple, S.K.; Badve, S.; Bleiweiss, I.J.; Brogi, E.; Calvo, J.P.; Dabbs, D.J.; O Ellis, I.; Eusebi, V.; et al. Phyllodes tumours of the breast: A consensus review. Histopathology 2016, 68, 5–21. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, Q.; Jiang, Y.; Li, J.; Wang, H.; Zhang, J.; You, S.; Liu, H. Local Recurrent Phyllodes Tumors of the Breast: Clinical and Sonographic Features. J. Ultrasound Med. 2015, 34, 1631–1638. [Google Scholar] [CrossRef]
- NCCN Guidelines Breast Cancer Version 6.2024. Published Online 11 November 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 12 November 2024).
- Belkacémi, Y.; Bousquet, G.; Marsiglia, H.; Ray-Coquard, I.; Magné, N.; Malard, Y.; Lacroix, M.; Gutierrez, C.; Senkus, E.; Christie, D.; et al. Phyllodes Tumor of the Breast. Int. J. Radiat. Oncol. 2008, 70, 492–500. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Sun, X.; Yang, Z.; Chen, X.; Shao, Z.; Yu, X.; Guo, X. Prognostic factors in breast phyllodes tumors: A nomogram based on a retrospective cohort study of 404 patients. Cancer Med. 2018, 7, 1030–1042. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Y.; Zhu, L.; Cartwright, P.; Song, E.; Jacobs, L.; Chen, K. Local Recurrence of Benign, Borderline, and Malignant Phyllodes Tumors of the Breast: A Systematic Review and Meta-analysis. Ann. Surg. Oncol 2019, 26, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Chen, K.; Zeng, J.; Bi, Z.; Guo, M.; Chen, Y.; Yao, Y.; Wu, W.; Liang, S.; Nie, Y. Adjuvant radiotherapy and chemotherapy for patients with breast phyllodes tumors: A systematic review and meta-analysis. BMC Cancer 2019, 19, 372. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, K. Radiation therapy for malignant phyllodes tumor of the breast: An analysis of SEER data. Breast 2017, 32, 26–32. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024). Cochrane. 2024. Available online: http://www.training.cochrane.org/handbook (accessed on 10 March 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software. Available online: http://www.covidence.org (accessed on 10 March 2025).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. 2011. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 March 2025).
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evi-Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, J. AACODS Checklist. 2010. Available online: http://dspace.flinders.edu.au/dspace/ (accessed on 10 March 2025).
- Anile, M.; Venuta, F.; Rendina, E.A.; Coloni, G.F. Huge Cystrosarcoma Phyllodes Invading the Chest Wall. Asian Cardiovas. Thora. Ann. 2007, 15, 359. [Google Scholar] [CrossRef]
- Awwal, R.; Shashi, S.A.; Khondokar, M.S.; Khundkar, S.H. Management of recurrent phyllodes with full thickness chest wall resection. Bangladesh J. Plast. Surg. 2010, 1, 9–13. [Google Scholar] [CrossRef]
- Goel, A.; Insa, R.; Gaur, M.K.; Garg, P.K. Palliative Surgery for Metastatic Fungating Phyllodes Tumors: A Series of Two Cases. Perm. J. 2018, 22, 17–100. [Google Scholar] [CrossRef]
- Chaudhry, I.U.; Asban, A.; Mahboub, T.; Arini, A. Recurrent phyllodes sarcoma of breast with complete chest wall invasion, a multidisciplinary approach for radical resection. BMJ Case Rep. 2013, 2013, bcr2012008110. [Google Scholar] [CrossRef]
- Ito, T.; Ito, K.-I.; Okada, T.; Murayama, K.; Hanamura, T.; Kanai, T.; Maeno, K.; Mochizuki, Y.; Kondo, R.; Amano, J.; et al. Full-thickness chest-wall resection followed by thorax reconstruction for recurrent malignant phyllodes tumor. Int. J. Clin. Oncol. 2011, 16, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Puri, G.; Kataria, K.; Jayaram, J. Complex chest wall reconstruction after excision of malignant phyllodes tumour. BMJ Case Rep. 2022, 15, e247067. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüven, A. Multidisciplinary Approach to Giant Malignant Recurrent Phyllodes Tumor of Breast: A Case Report and Review of the Literature. Acta Medica 2017, 48, 18–24. [Google Scholar]
- Fang, C.L.; Hsu, C.H.; Tu, C.W. Malignant Phyllodes Tumor Recurrence in the Pleural Cavity via the Deep Inferior Epigastric Perforator Flap and Internal Mammary Vessel Bundle: A Case Report. Ann. Plast. Surg. 2019, 82, 618–621. [Google Scholar] [CrossRef]
- Mindikogˇlu, A.N.; Aktan, K. Recurrent cystosarcoma phylloides of breast: Extensive full-thickness excision of chest wall with immediate repair using steel mesh and a latissimus dorsi myocutaneous flap. Br. J. Plast. Surg. 1983, 36, 519–521. [Google Scholar] [CrossRef]
- Murthy, K.P.; Chakravarthy, R.P. Malignant phyllodes tumor with chondrosarcomatous differentiation: Radiological-pathological correlation. J. Clin. Imaging Sci. 2014, 4, 52. [Google Scholar] [CrossRef]
- Nagasaka, S.; Yamasaki, Y.; Kuwata, K.; Ohno, K.; Sakaguchi, T. A case of giant malignant phyllodes tumor of the breast performed extended radical mastectomy with wide excision of the thoracic wall. J. Jpn. Pr. Surg. Soc. 1996, 57, 61–66. [Google Scholar] [CrossRef][Green Version]
- Balachandran, N.R.; Abdullah, N.; Ismail, M.I.; Wong, Y.P.; Azmi, M.I. Recurrent and transformation of borderline to malignant phyllodes tumour with osteoid differentiation: A case report and literature review. Front. Oncol. 2024, 14, 1377074. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.D.; Terra, R.M.; Fernandez, A.; Rawet, V.; Jatene, F.B. Full-thickness chest wall resection for recurrent breast phyllodes tumor. Ann. Thorac. Surg. 2007, 83, 2196–2197. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, A.; Farooq, M. Resection and reconstruction following recurrent malignant phyllodes-Case report and review of literature. Ann. Med. Surg. 2017, 16, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Schizas, N.; Lazopoulos, A.; Rallis, T.; Paliouras, D.; Barbetakis, N. Relapsing phyllodes cystosarcoma and chest wall invasion with long-term survival: Is aggressive surgery worthwhile? Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 447–450. [Google Scholar] [CrossRef]
- Suan, E.A.; Tabilog, M.; Kangleon, R.; Ranile, H.; Senerpida, A. Malignant Phyllodes Tumor in the Male: A Case Report. Philipp. J. Surg. Spec. 2023, 78, 26–30. [Google Scholar] [CrossRef]
- Tan, B.K.; Tan, P.; Wong, C.H.; Koong, H.N. Chest wall reconstruction using a combined musculocutaneous anterolateral-anteromedial thigh flap. Indian J. Plast. Surg. 2010, 43, 88. [Google Scholar] [CrossRef]
- Boonipat, T.; Ji, L.; Manrique, O.J.; Chen, H.C. Combined bipedicled latissimus dorsi and groin flap for anterior chest wall reconstruction. BMJ Case Rep. 2019, 12, e227372. [Google Scholar] [CrossRef]
- Girotti, P.N.C.; Bianchi, F. Chest wall reconstruction, prosthesis and allografts: A narrative review. J. Thorac. Dis. 2023, 15, 7077–7087. [Google Scholar] [CrossRef]
- Kapiris, I.; Nasiri, N.; A’Hern, R.; Healy, V.; Gui, G.P.H. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur. J. Surg. Oncol. EJSO 2001, 27, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Tsujii, M.; Hagi, T.; Nakamura, T.; Kita, K.; Shimamoto, A.; Kataoka, T.; Takao, M.; Sudo, A. Full-thickness chest wall resection for malignant chest wall tumors and postoperative problems. Front. Oncol. 2023, 13, 1104536. [Google Scholar] [CrossRef] [PubMed]
- Ramakant, P.; Chakravarthy, S.; Cherian, J.; Abraham, D.; Paul, M. Challenges in management of phyllodes tumors of the breast: A retrospective analysis of 150 patients. Indian J. Cancer 2013, 50, 345. [Google Scholar] [CrossRef]
- Yu, C.Y.; Huang, T.W.; Tam, K.W. Management of phyllodes tumor: A systematic review and meta-analysis of real-world evidence. Int. J. Surg. 2022, 107, 106969. [Google Scholar] [CrossRef]
- Eng, J.; Sabanathan, S.; Mearns, A. Chest wall reconstruction after resection of primary malignant chest wall tumours. Eur. J. Cardio-Thorac. Surg. 1990, 4, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Mansour, K.A.; Thourani, V.H.; Losken, A.; Reeves, J.G.; Miller Jr, J.I.; Carlson, G.W.; Jones, G.E. Chest wall resections and reconstruction: A 25-year experience. Ann. Thorac. Surg. 2002, 73, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; Brandolini, J.; Pardolesi, A.; Argnani, D.; Mengozzi, M.; Dell’amore, A.; Solli, P. Materials and techniques in chest wall reconstruction: A review. J. Vis. Surg. 2017, 3, 95. [Google Scholar] [CrossRef]
- Nandi, S.; Chhebbi, M.; Saini, S. Comprehensive Analysis of Chest Wall Resection: Indications, Reconstruction, and Results: A Systematic Review. J. Clin. Diagn. Res. 2024, 18, XE1–XE6. [Google Scholar] [CrossRef]
- Ong, K.; Ong, C.S.; Chua, Y.C.; Fazuludeen, A.A.; Ahmed, A.D.B. The painless combination of anatomically contoured titanium plates and porcine dermal collagen patch for chest wall reconstruction. J. Thorac. Dis. 2018, 10, 2890–2897. [Google Scholar] [CrossRef]
- Ng, C.S.; Ho, A.M.; Lau, R.W.; Wong, R.H. Chest wall reconstruction with MatrixRib system: Avoiding pitfalls. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 402–403. [Google Scholar] [CrossRef]
- Azoury, S.C.; Grimm, J.C.; Tuffaha, S.H.; Broyles, J.M.; Fischer, A.C.; Yang, S.C.; Tufaro, A.P. Chest Wall Reconstruction: Evolution Over a Decade and Experience with a Novel Technique for Complex Defects. Ann. Plast. Surg. 2016, 76, 231–237. [Google Scholar] [CrossRef]
- Kang, J.; Tian, Y.; Zheng, J.; Lu, D.; Cai, K.; Wang, L.; Li, D. Functional design and biomechanical evaluation of 3D printing PEEK flexible implant for chest wall reconstruction. Comput. Methods Programs Biomed. 2022, 225, 107105. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Q.; Huang, F.G.; Zhao, Y.F.; Qin, T.W.; Li, X.Q.; Liu, C.; Li, L.J.; Yang, Z.M. Tissue-Engineered Ribs for Chest Wall Reconstruction: A Case with 12-Year Follow-Up. Regen. Med. 2014, 9, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Weyant, M.J.; Bains, M.S.; Venkatraman, E.; Downey, R.J.; Park, B.J.; Flores, R.M.; Rizk, N.; Rusch, V.W. Results of Chest Wall Resection and Reconstruction With and Without Rigid Prosthesis. Ann. Thorac. Surg. 2006, 81, 279–285. [Google Scholar] [CrossRef]
- Zacha, S.; Jarosz, K.; Kokot, K.; Biłas, J.; Skonieczna-Żydecka, K.; Gerus, S.; Kojder, K.; Biernawska, J. Benefits of the Erector Spinae Plane Block before Cryoanalgesia in Children Undergoing Surgery for Funnel Chest Deformity. J. Pers. Med. 2023, 13, 1696. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.; Jacques, V.; Abdelnour-Berchtold, E.; Krueger, T.; Perentes, J.Y.; Zellweger, M.; Gonzalez, M. Enhanced recovery after chest wall resection and reconstruction: A clinical practice review. J. Thorac. Dis. 2024, 16, 2604–2612. [Google Scholar] [CrossRef]
- Dhillon, G.; Buddhavarapu, V.S.; Grewal, H.; Munjal, R.; Verma, R.K.; Surani, S.; Kashyap, R. Evidence-based Practice Interventions for Reducing Postoperative Pulmonary Complications: A Narrative Review. Open Respir. Med. J. 2023, 17, e18743064271499. [Google Scholar] [CrossRef]
- Sparreboom, C.L.; Hop, M.J.; Mazaheri, M.; Rothbarth, J.; Maat, A.P.; Corten, E.M.; Mureau, M.A. Surgical Outcomes after Full Thickness Chest Wall Resection Followed by Immediate Reconstruction: A 7-Year Observational Study of 42 Cases. JPRAS Open 2024, 41, 14–24. [Google Scholar] [CrossRef]

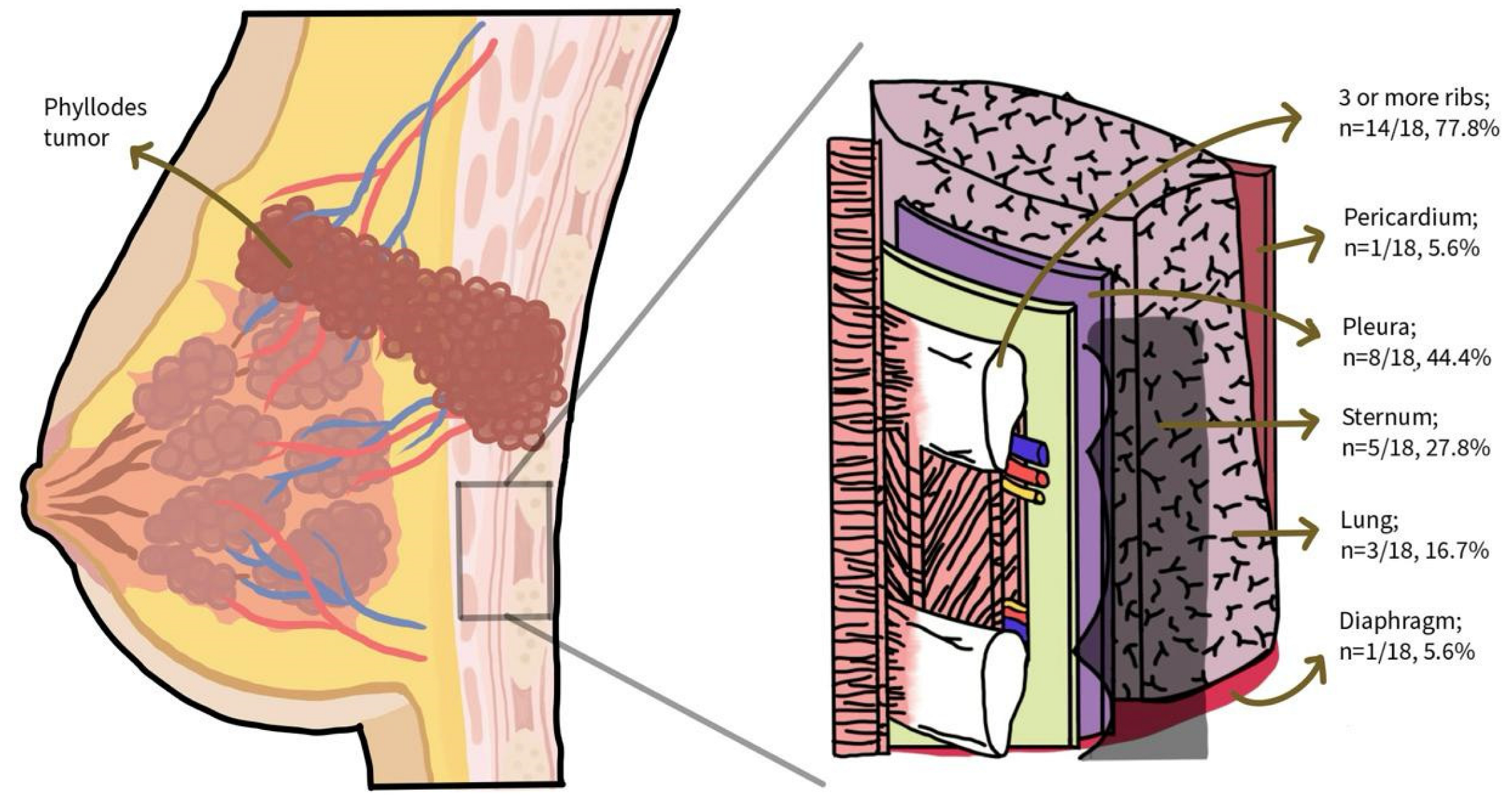
| Study (First Author, Year, Country) | Age/Gender | Subtype | Tumor Status | Tumor Size (cm) | Chest Wall Invasion (Imaging Modality) | Type of Surgery | Post-Op Complications | Recurrence (Time, Site) | Mortality | Patient-Reported Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Anile, 2007, Italy [18] | 65/F | Malignant | NR | >5 | CT: chest wall invasion | FTCWR | NR | Distant 1 (48 mo DFS) | Yes | NR |
| Awwal, 2010, Bangladesh [19] | 50/F | Malignant | Recurrent | >5 | NR | En bloc FTCWR | NR | None | No | NR |
| Goel, 2018, India [20] | 28/F | Malignant | Recurrent | 15 | CT: chest wall invasion + metastatic lung nodules | En bloc FTCWR | NR | None, but had progression of metastatic lung nodules | Yes (6 mo) | HDRS improved; 24 to 3 |
| Chaudhry, 2013, Saudi Arabia [21] | 30/F | Malignant | Recurrent | 15 | CT: chest wall invasion | En bloc FTCWR | NIL | None | No | NR |
| Ito, 2011, Japan [22] | 39/F | Malignant | Recurrent | 14 | CT: muscle, sternum, and rib invasion + metastatic lung nodule | En bloc FTCWR | NIL | None | No | NR |
| Gupta, 2022, India [23] | NR | Malignant | Recurrent | 18.5 | CT: rib and costal cartilage invasion | 2-staged FTCWR | Pneumonia | None | Yes (2 mo DFS) | NR |
| Küçükgüven, 2017, Turkey [24] | 38/F | Malignant | Recurrent | 11.7 | US, CT, MRI: muscle invasion Bone scintigraphy: costochondral joint invasion | En bloc FTCWR | Pleural effusion | None | No | NR |
| Fang, 2019, Taiwan [25] | 53/F | Malignant | Recurrent | >5 | CT: DIEP flap and pleural invasion | En bloc FTCWR | NR | Distant (1 mo DFS) | Yes (3 mo) | NR |
| Mindikogˇlu, 1983, Turkey [26] | 18/F | Malignant | Recurrent | 25 | CT: rib erosion | En bloc FTCWR | Paradoxical chest motion | None | No | NR |
| Murthy, 2014, India [27] | 63/F | Malignant | NR | 16 | CT: muscle and rib invasion | En bloc FTCWR | NR | None | No | NR |
| Nagasaka, 1996, Japan [28] | 45/F | Malignant | Primary | 30 | CT, MRI: chest wall and sternal invasion | En bloc FTCWR | Wound and mesh infection | None | No | NR |
| Balachandran, 2024, Malaysia [29] | 20/F | Malignant | Recurrent | 8.5 | CT: muscle, ribs, and pleural invasion | En bloc FTCWR | NR | Distant (2 mo DFS) | Yes (5 mo) | NR |
| Neto, 2007, Brazil [30] | 43/F | Low-grade | Recurrent | 7 | CT: chest wall invasion | En bloc FTCWR | NR | None | No | NR |
| Rajesh, 2017, India [31] | 27/F | Malignant | Recurrent | 18 | MRI: Muscle and ribs invasion | En bloc FTCWR | NIL | None | No | Emotional respite |
| Schizas, 2021, Greece [32] | 46/F | Malignant | Recurrent | 26 | CT, MRI: chest wall, costal cartilage invasion | En bloc FTCWR | Wound dehiscence and mesh contamination | Local (3 yr DFS) | No | Good QoL 2 |
| Suan, 2023, Philippines [33] | 45/M | Malignant | Primary | 17.5 | CT: chest wall, pleural, costal cartilage invasion | En bloc FTCWR | Pain, delayed lung expansion, poor healing of skin graft | None | No | NR |
| Tan, 2010, Singapore [34] | 52/F | Malignant | Recurrent | 12 | NR | En bloc FTCWR | Knee weakness | Distant (12 mo DFS) | Yes (12 mo) | NR |
| Boonipat, 2019, USA [35] | 43/F | Malignant | Recurrent | 38 | CT: rib invasion | En bloc FTCWR | Pain, poor healing of skin graft | None | No | Happy with recovery |
| First Author, Year | No. of Ribs Resected | Sternum Resected | Pleura Resected | Pericardium Resected | Diaphragm Resected | Lung Resected | Margins | Reconstruction Method | Surgical Team |
|---|---|---|---|---|---|---|---|---|---|
| Anile, 2007 [18] | NR | NR | NR | NR | NR | NR | NR | Marlex mesh + LD flap | Thoracic surgeon |
| Awwal, 2010 [19] | NR | No | No | No | No | No | NR | Prolene mesh + LD flap | Plastic surgeon |
| Goel, 2018 [20] | NR | No | No | No | No | No | NR | LD flap | Multidisciplinary, including surgical oncologist |
| Chaudhry, 2013 [21] | ≥3 | Yes | No | Pericardial fat | No | No | Negative margins | PMMA cement marlex mesh sand-witch + LD flap | Breast, thoracic, plastic surgeons |
| Ito, 2011 [22] | 4 | Yes | No | No | No | Yes | 2 cm negative margins | Composix mesh + LD flap | Breast, respiratory, plastic surgeons |
| Gupta, 2022 [23] | 3 (Left side), 6 (Right side) | Yes | Yes | No | Yes | Yes | Negative margins | Polypropylene mesh + LD (Left) and greater omentum (Right) flap | Multidisciplinary (unspecified) |
| Küçükgüven, 2017 [24] | 3 | No | No | No | No | No | 2 cm negative margins | Gore-Tex (2 mm) DualMesh + TRAM flap | Thoracic, plastic surgeons |
| Fang, 2019 [25] | 3 | No | Yes | No | No | No | 2 cm negative margins | ALT flap | Thoracic, plastic surgeons |
| Mindikogˇlu, 1983 [26] | 4 | No | Yes | Yes | No | Yes | NR | Stainless steel mesh + LD flap | Thoracic, plastic surgeons |
| Murthy, 2014 [27] | 3 | No | No | No | No | No | NR | NR | NR |
| Nagasaka, 1996 [28] | 3 | Yes | No | No | No | No | NR | Marlex mesh + TRAM flap | |
| Balachandran, 2024 [29] | 3 | No | Yes | No | No | No | Close margin (3 mm) | Prolene mesh + MatrixRIB + LD flap | Breast, cardiothoracic surgeons |
| Neto, 2007 [30] | NR | No | No | No | No | No | 4 cm negative margins | Composite mesh + PMMA cement + LD flap | Thoracic surgeon |
| Rajesh, 2017 [31] | 4 | Yes | Yes | No | No | No | 1.8 cm negative margins | PTFE mesh + rotating cutaneous full-thickness flap | Surgical oncologist |
| Schizas, 2021 [32] | 4 | No | Yes | No | No | No | Negative margins | Polypropylene mesh + LD flap | Thoracic surgeon |
| Suan, 2023 [33] | 5 | No | No | No | No | No | Negative margins | Mesh + TRAM flap | Thoracic, plastic surgeons |
| Tan, 2010 [34] | 6 | No | Yes | No | No | No | NR | PMMA cement prolene mesh sand-witch + ALT and AMT flap | Thoracic, plastic surgeons |
| Boonipat, 2019 [35] | 3 | No | Yes | No | No | No | NR | Autologous rib + bipedicled groin and LD flap | Thoracic, plastic, general surgeons |
| Tumour Subtype | Age, Gender | Nature of Tumor (Before FTCWR) | Tumor Size (cm) | En bloc or Separately Resected | Margin Status | Margin Width (mm) | No. Ribs Resected | Pleural Involvement | Adjuvant Therapy |
|---|---|---|---|---|---|---|---|---|---|
| Malignant | 46F | Fourth recurrence | 18 | En bloc | Negative | NR | 4 | Yes | NA |
| Malignant | 65F | NR | >5 | NR | NR | NR | NR | NR | NR |
| Malignant | 53F | Second recurrence | >5 | En bloc | Negative | 20 | 3 | Yes | Radiotherapy |
| Malignant | 20F | Second recurrence | 8.5 | En bloc | Negative | 3 | 3 | Yes | NA |
| Malignant | 52F | Second recurrence | 12 | En bloc | NR | NR | 6 | Yes | Radiotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.S.; Goh, R.T.H.; Ang, B.W.N.; Lee, A.W.X.; Leong, E.K.F.; Leow, L.; Ng, Q.X.; Goh, S.S.N. Full-Thickness Chest Wall Resection and Reconstruction for Locally Invasive Phyllodes Tumors: A Systematic Review. Cancers 2025, 17, 1907. https://doi.org/10.3390/cancers17121907
Lim YS, Goh RTH, Ang BWN, Lee AWX, Leong EKF, Leow L, Ng QX, Goh SSN. Full-Thickness Chest Wall Resection and Reconstruction for Locally Invasive Phyllodes Tumors: A Systematic Review. Cancers. 2025; 17(12):1907. https://doi.org/10.3390/cancers17121907
Chicago/Turabian StyleLim, Yun Sun, Ryan Tsui Hon Goh, Breanna Wei Ning Ang, Ailica Wan Xin Lee, Eugene Kwong Fei Leong, Lowell Leow, Qin Xiang Ng, and Serene Si Ning Goh. 2025. "Full-Thickness Chest Wall Resection and Reconstruction for Locally Invasive Phyllodes Tumors: A Systematic Review" Cancers 17, no. 12: 1907. https://doi.org/10.3390/cancers17121907
APA StyleLim, Y. S., Goh, R. T. H., Ang, B. W. N., Lee, A. W. X., Leong, E. K. F., Leow, L., Ng, Q. X., & Goh, S. S. N. (2025). Full-Thickness Chest Wall Resection and Reconstruction for Locally Invasive Phyllodes Tumors: A Systematic Review. Cancers, 17(12), 1907. https://doi.org/10.3390/cancers17121907










