Surgical Management of Ipsilateral Breast Cancer Recurrence After Conservative Mastectomy and Prepectoral Breast Reconstruction: Exploring the Role of Wide Local Excision
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Patient and Data Collection
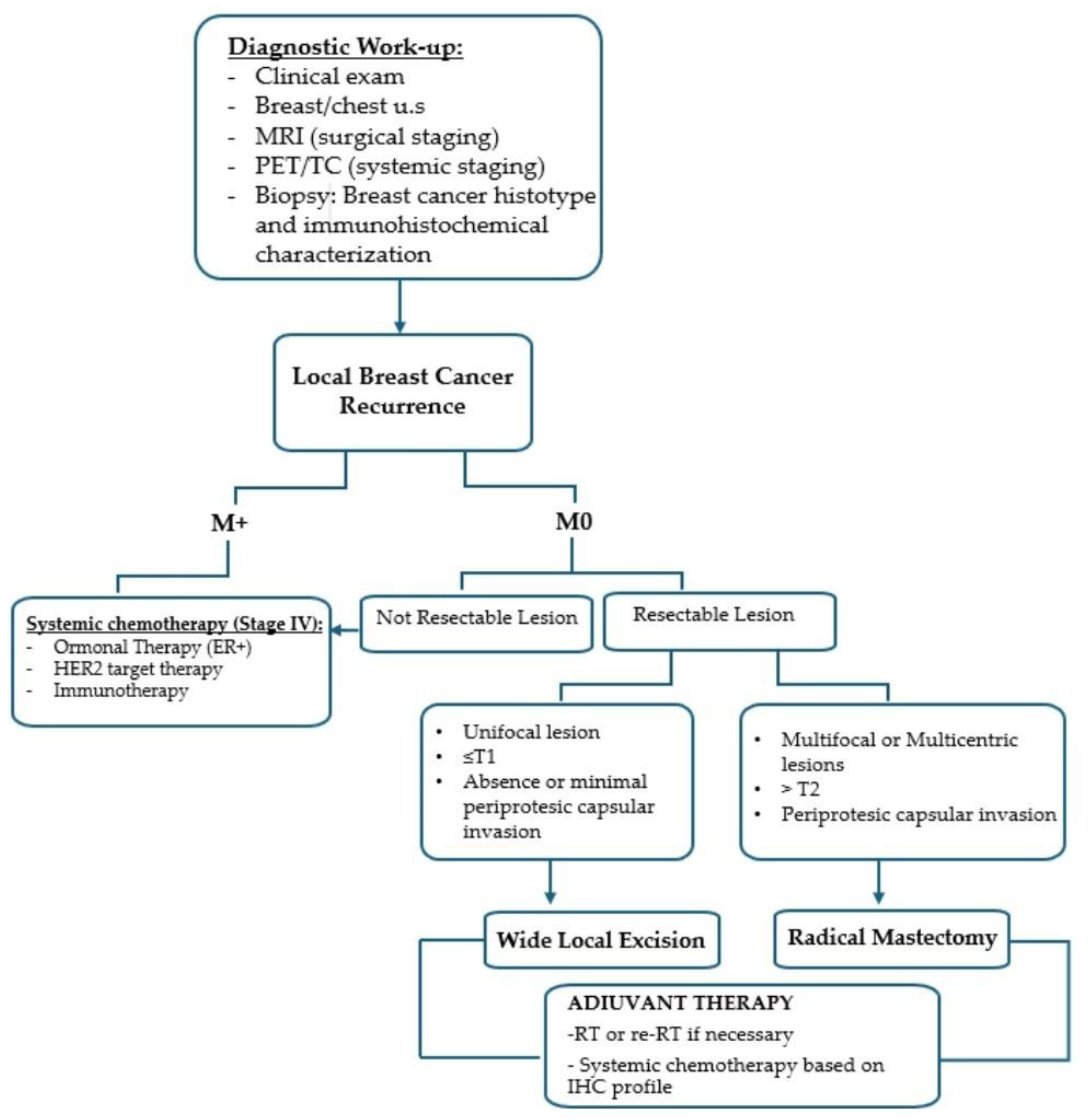
2.3. Management Algorithm
- •
- WLE was considered in cases of unifocal recurrence (≤T1) without or with minimal periprosthetic capsule invasion.
- •
- RM was indicated for multifocal/multicentric disease, tumor size > T2, or clear involvement of the periprosthetic capsule.
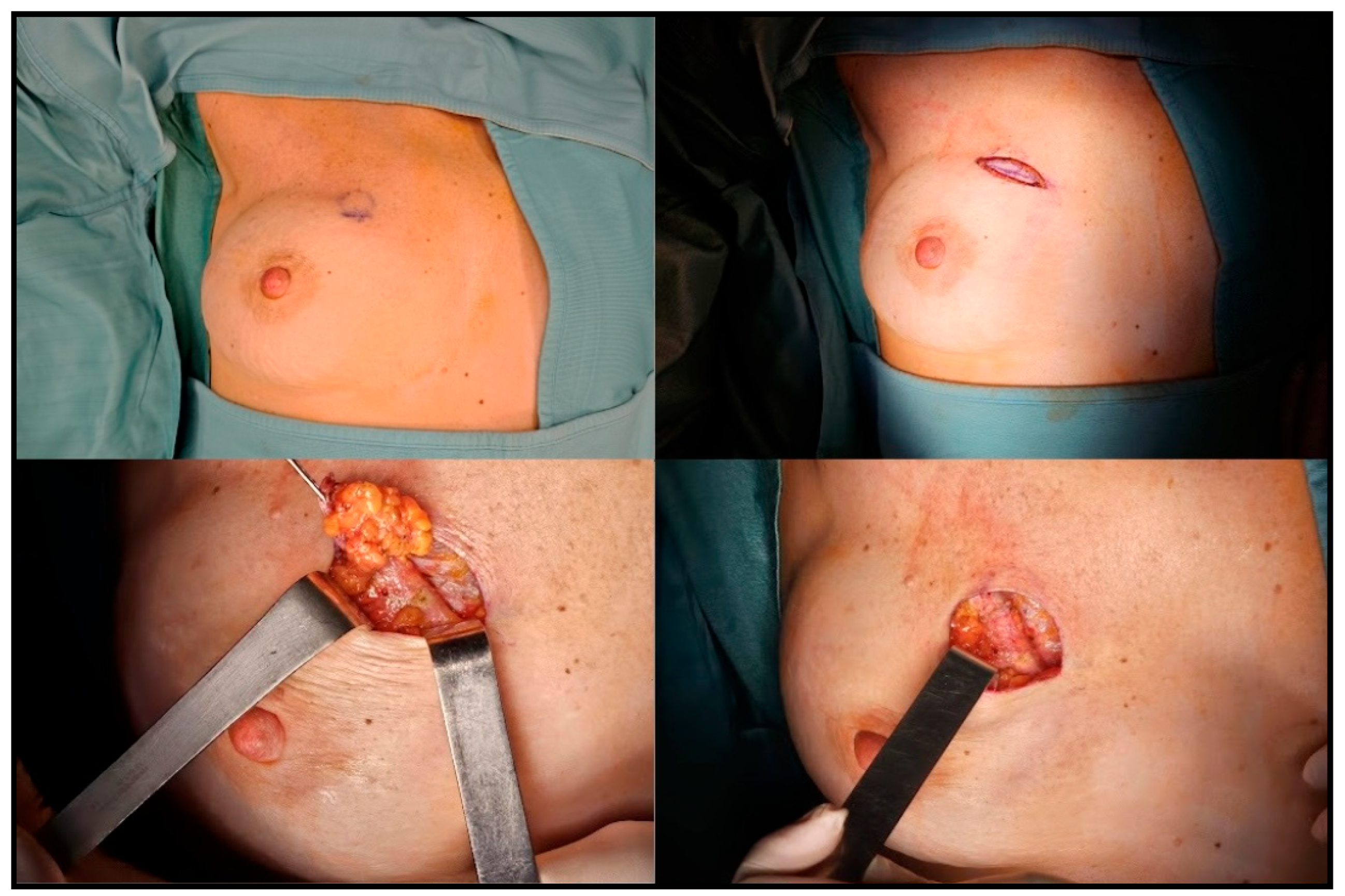
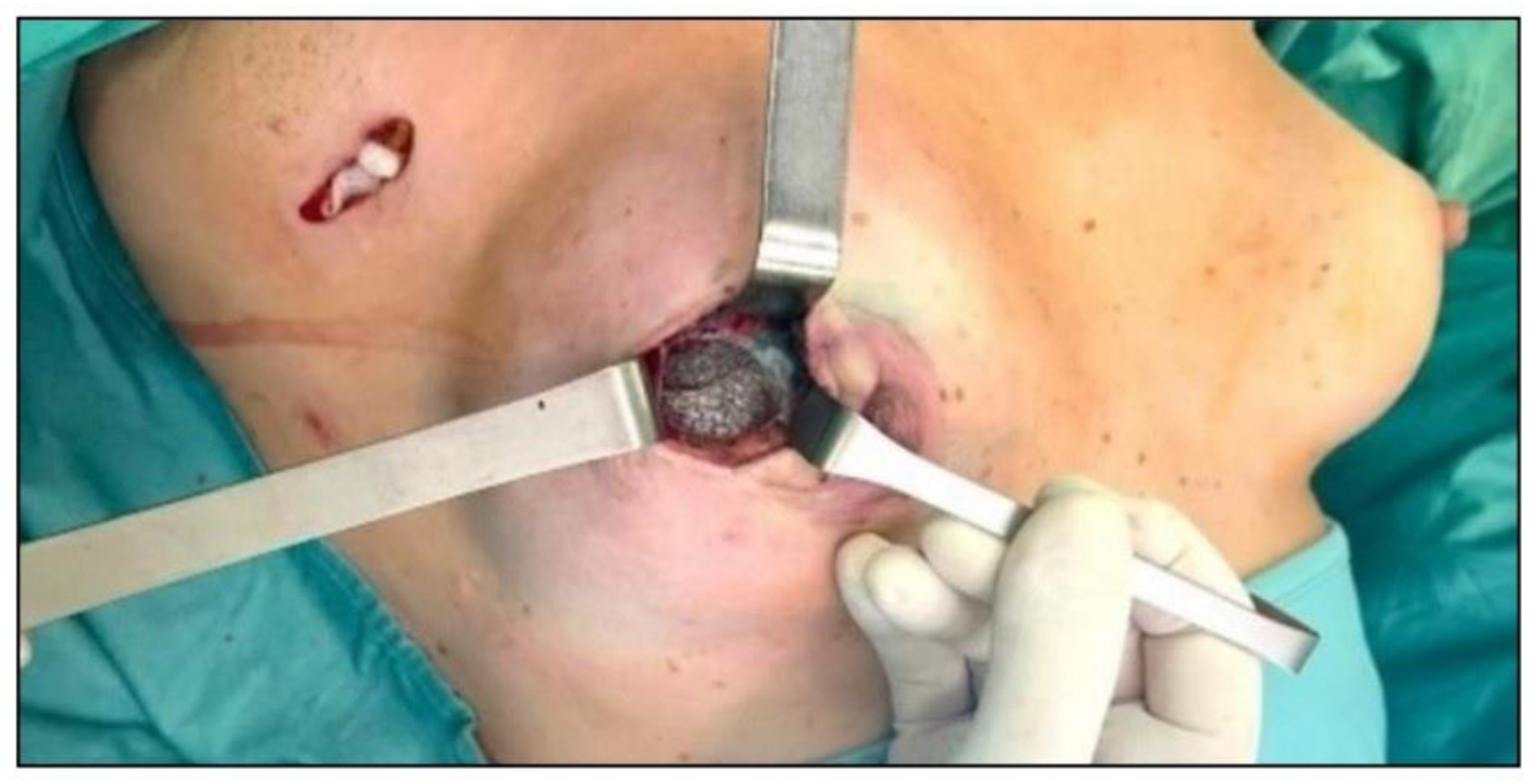
2.4. Surgical Treatment
- •
- •
- Radical mastectomy (RM) involved removal of the skin overlying the recurrence, the entire implant, and the surrounding capsule.
2.5. Adjuvant Therapy
3. Results
3.1. Pathological Characteristics
3.2. Surgical Technique
3.3. Adjuvant Treatment and Oncological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBBR | Implant-based breast reconstruction |
| IBCR | Ipsilateral breast cancer recurrence |
| WLE | Wide local excision |
| RM | Radical mastectomy |
| NSM | Nipple-sparing mastectomy |
| SSM | Skin-sparing mastectomy |
| DFS | Disease-free survival |
| OS | Overall survival |
References
- Nava, M.B.; Catanuto, G.; Pennati, A.; Garganese, G.; Spano, A. Conservative mastectomies. Aesthetic Plast. Surg. 2009, 33, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.; Harless, C.; Jacobson, S.R. Revisiting an Old Place: Single-Surgeon Experience on Post-Mastectomy Subcutaneous Implant-Based Breast Reconstruction. Breast J. 2017, 23, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Houvenaeghel, G.; Bannier, M.; Bouteille, C.; Tallet, C.; Sabiani, L.; Charavil, A.; Bertrand, A.; Van Troy, A.; Buttarelli, M.; Teyssandier, C.; et al. Postoperative Outcomes of Pre-Pectoral Versus Sub-Pectoral Implant Immediate Breast Reconstruction. Cancers 2024, 16, 1129. [Google Scholar] [CrossRef] [PubMed]
- Casella, D.; Kaciulyte, J.; Lo Torto, F.; Mori, F.L.; Barellini, L.; Fausto, A.; Fanelli, B.; Greco, M.; Ribuffo, D.; Marcasciano, M. “To Pre or Not to Pre”: Introduction of a Prepectoral Breast Reconstruction Assessment Score to Help Surgeons Solving the Decision-Making Dilemma. Retrospective Results of a Multicenter Experience. Plast. Reconstr. Surg. 2021, 147, 1278–1286. [Google Scholar] [CrossRef]
- Zingaretti, N.; Guarneri, G.F.; De Biasio, F.; Shoeib, M.A.; Parodi, P.C. The Use of Meshed Dermal Autograft in Breast Reconstruction. Aesthetic Plast. Surg. 2018, 42, 1704–1706. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.R.; Tandon, V.J.; Bertrand, A.A.; MacEachern, M.; Goldberg, M.; Salibian, A.; Pusic, A.L.; Festekjian, J.H.; Wilkins, E.G. Review of Outcomes in Prepectoral Prosthetic Breast Reconstruction with and without Surgical Mesh Assistance. Plast. Reconstr. Surg. 2021, 147, 305–315. [Google Scholar] [CrossRef]
- Nahabedian, M.Y. What Are the Long-Term Aesthetic Issues in Prepectoral Breast Reconstruction? Aesthetic Surg. J. 2020, 40, S29–S37. [Google Scholar] [CrossRef]
- King, C.A.; Bartholomew, A.J.; Sosin, M.; Avila, A.; Famiglietti, A.L.; Dekker, P.K.; Perez-Alvarez, I.M.; Song, D.H.; Fan, K.L.; Tousimis, E.A. A critical appraisal of late complications of prepectoral versus subpectoral breast reconstruction following nipple-sparing mastectomy. Ann. Surg. Oncol. 2021, 28, 9150–9158. [Google Scholar] [CrossRef]
- Franceschini, G.; Sanchez, A.M.; Di Leone, A.; Magno, S.; Moschella, F.; Accetta, C.; Natale, M.; Di Giorgio, D.; Scaldaferri, A.; D’Archi, S.; et al. Update on the surgical management of breast cancer. Ann. Ital. Chir. 2015, 86, 89–99. [Google Scholar] [PubMed]
- La Padula, S.; Pensato, R.; Al-Amer, R.; Hersant, B.; Meningaud, J.P.; Noel, W.; D’Andrea, F.; Rocco, N. Three Pedicle-Based Nipple-Sparing Skin-Reducing Mastectomy Combined with Prepectoral Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. 2024, 154, 430e–441e. [Google Scholar] [CrossRef] [PubMed]
- Rocco, N.; Catanuto, G.F.; Accardo, G.; Velotti, N.; Chiodini, P.; Cinquini, M.; Privitera, F.; Rispoli, C.; Nava, M.B. Implants versus autologous tissue flaps for breast reconstruction following mastectomy. Cochrane Database Syst. Rev. 2024, 10, CD013821. [Google Scholar] [CrossRef]
- Zingaretti, N.; Galvano, F.; Vittorini, P.; De Francesco, F.; Almesberger, D.; Riccio, M.; Vaienti, L.; Parodi, P.C. Smooth Prosthesis: Our Experience and Current State of Art in the Use of Smooth Sub-muscular Silicone Gel Breast Implants. Aesthetic Plast. Surg. 2019, 43, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Martorana, F.; Karakatsanis, A.; Caruso, F.; Caruso, M.; Castiglione, G.; Di Grazia, A.; Pane, F.; Rizzo, A.; Vigneri, P.; et al. Predictors of mastectomy in breast cancer patients with complete remission of primary tumor after neoadjuvant therapy: A retrospective study. Eur. J. Surg. Oncol. 2024, 50, 108732. [Google Scholar] [CrossRef] [PubMed]
- Zingaretti, N.; Piana, M.; Battellino, L.; Galvano, F.; De Francesco, F.; Riccio, M.; Beorchia, Y.; Castriotta, L.; Parodi, P.C. Pre-pectoral Breast Reconstruction: Surgical and Patient-Reported Outcomes of Two-Stages vs Single-Stage Implant-Based Breast Reconstruction. Aesthetic Plast. Surg. 2024, 48, 1759–1772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujihara, M.; Yamasaki, R.; Ito, M.; Shien, T.; Maeda, R.; Kin, T.; Ueno, A.; Kajiwara, Y.; Kawasaki, K.; Ichimura, K.; et al. Risk factors of local recurrence following implant-based breast reconstruction in breast cancer patients. BMC Women’s Health 2021, 21, 147. [Google Scholar] [CrossRef]
- Robertson, S.A.; Cutress, R.I. Mastectomy skin flap thickness. Eur. J. Surg. Oncol. 2018, 44, 1118. [Google Scholar] [CrossRef] [PubMed]
- Catanuto, G.; Gentile, D.; Martorana, F.; Tomatis, M.; Ponti, A.; Marotti, L.; Aristei, C.; Cardoso, M.J.; Cheung, K.L.; Curigliano, G.; et al. Clinico-pathological features predicting indication to mastectomy in breast cancer patients achieving complete response after neoadjuvant therapy: A retrospective analysis of the EUSOMA database. Eur. J. Surg. Oncol. 2025, 51, 109643. [Google Scholar] [CrossRef] [PubMed]
- Barone Adesi, L.; Salgarello, M.; Di Leone, A.; Visconti, G.; Conti, M.; Belli, P.; Scardina, L.; Tarantino, G.; Franceschini, G. Personalizing Breast Cancer Surgery: Harnessing the Power of ROME (Radiological and Oncoplastic Multidisciplinary Evaluation). J. Pers. Med. 2025, 15, 114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostapenko, E.; Nixdorf, L.; Devyatko, Y.; Exner, R.; Wimmer, K.; Fitzal, F. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Systemic Review and Meta-analysis. Ann. Surg. Oncol. 2023, 30, 126–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Yu, L.; Huang, M.; Huang, Y.; Li, C.; Liang, Y.; Liang, W.; Qin, T. Comparative complications of prepectoral versus subpectoral breast reconstruction in patients with breast cancer: A meta-analysis. Front. Oncol. 2024, 14, 1439293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, L.; Su, Y.; Xiu, B.; Huang, X.; Chi, W.; Hou, J.; Zhang, Y.; Tian, J.; Wang, J.; Wu, J. Comparison of prepectoral and subpectoral breast reconstruction after mastectomies: A systematic review and meta analysis. Eur. J. Surg. Oncol. 2019, 45, 1542–1550. [Google Scholar] [CrossRef]
- Scardina, L.; Di Leone, A.; Sanchez, A.M.; Accetta, C.; Barone Adesi, L.; Biondi, E.; Carnassale, B.; D’Archi, S.; De Lauretis, F.; Di Guglielmo, E.; et al. Oncological Safety of Prepectoral Implant-Based Breast Reconstruction After Conservative Mastectomy: Insights from 842 Consecutive Breast Cancer Patients. Cancers 2025, 17, 925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vidya, R.; Berna, G.; Sbitany, H.; Nahabedian, M.; Becker, H.; Reitsamer, R.; Rancati, A.; Macmillan, D.; Cawthorn, S. Prepectoral implant-based breast reconstruction: A joint consensus guide from UK, European and USA breast and plastic reconstructive surgeons. Ecancermedicalscience 2019, 13, 927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scardina, L.; Franceschini, G. Redefining conservative mastectomy: The evolution of surgical techniques. Front. Oncol. 2025, 15, 1575095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Leone, A.; Franco, A.; Terribile, D.A.; Magno, S.; Fabi, A.; Sanchez, A.M.; D’Archi, S.; Scardina, L.; Natale, M.; Mason, E.J.; et al. Level II Oncoplastic Surgery as an Alternative Option to Mastectomy with Immediate Breast Reconstruction in the Neoadjuvant Setting: A Multidisciplinary Single Center Experience. Cancers 2022, 14, 1275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scardina, L.; Di Leone, A.; Biondi, E.; Carnassale, B.; Sanchez, A.M.; D’Archi, S.; Franco, A.; Moschella, F.; Magno, S.; Terribile, D.; et al. Prepectoral vs. Submuscular Immediate Breast Reconstruction in Patients Undergoing Mastectomy after Neoadjuvant Chemotherapy: Our Early Experience. J. Pers. Med. 2022, 12, 1533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaidar-Person, O.; Vrou Offersen, B.; Hol, S.; Arenas, M.; Aristei, C.; Bourgier, C.; Cardoso, M.J.; Chua, B.; Coles, C.E.; Engberg Damsgaard, T.; et al. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother. Oncol. 2019, 137, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rauschecker, H.; Clarke, M.; Gatzemeier, W.; Recht, A. Systemic therapy for treating locoregional recurrence in women with breast cancer. Cochrane Database Syst. Rev. 2001, 2001, CD002195. [Google Scholar] [CrossRef]
- Wapnir, I.L.; Khan, A. Current Strategies for the Management of Locoregional Breast Cancer Recurrence. Oncology 2019, 33, 19–25. [Google Scholar] [PubMed]
- Deutschmann, C.; Singer, C.F.; Gschwantler-Kaulich, D.; Pfeiler, G.; Leser, C.; Baltzer, P.A.T.; Helbich, T.H.; Kraus, C.; Korbatits, R.; Marzogi, A.; et al. Residual fibroglandular breast tissue after mastectomy is associated with an increased risk of a local recurrence or a new primary breast cancer. BMC Cancer 2023, 23, 281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Kim, E.K.; Oh, J.Y.; Kwon, H.C.; Kim, S.H.; Kim, D.C.; Lee, M.; Cho, S.H.; Nam, K.J. US screening for detection of nonpalpable locoregional recurrence after mastectomy. Eur. J. Radiol. 2013, 82, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, B.J.; Park, G.E.; Kim, S.H. The Usefulness of Magnetic Resonance Imaging (MRI) for the Detection of Local Recurrence after Mastectomy with Reconstructive Surgery in Breast Cancer Patients. Diagnostics 2022, 12, 2203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Angelo, A.; Trombadori, C.M.L.; Caprini, F.; Lo Cicero, S.; Longo, V.; Ferrara, F.; Palma, S.; Conti, M.; Franco, A.; Scardina, L.; et al. Efficacy and Accuracy of Using Magnetic Seed for Preoperative Non-Palpable Breast Lesions Localization: Our Experience with Magseed. Curr. Oncol. 2022, 29, 8468–8474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, A.Y.; Hu, Z.I.; Mehrara, B.J.; Wilkins, E.G. Radiotherapy in the setting of breast reconstruction: Types, techniques, and timing. Lancet Oncol. 2017, 18, e742–e753. [Google Scholar] [CrossRef] [PubMed]
- Chagpar, A.; Langstein, H.N.; Kronowitz, S.J.; Singletary, S.E.; Ross, M.I.; Buchholz, T.A.; Hunt, K.K.; Kuerer, H.M. Treatment and outcome of patients with chest wall recurrence after mastectomy and breast reconstruction. Am. J. Surg. 2004, 187, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Wadasadawala, T.; Vadgaonkar, R.; Bajpai, J. Management of Isolated Locoregional Recurrences in Breast Cancer: A Review of Local and Systemic Modalities. Clin. Breast Cancer 2017, 17, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.A.; Ali, S.; Hunt, K.K. Multidisciplinary Management of Locoregional Recurrent Breast Cancer. J. Clin. Oncol. 2020, 38, 2321–2328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuo, S.H.; Huang, C.S.; Kuo, W.H.; Cheng, A.L.; Chang, K.J.; Chia-Hsien Cheng, J. Comprehensive locoregional treatment and systemic therapy for postmastectomy isolated locoregional recurrence. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Danish Breast Cancer Cooperative Group; Nielsen, H.M.; Overgaard, M.; Grau, C.; Jensen, A.R.; Overgaard, J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: Long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J. Clin. Oncol. 2006, 24, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Ragaz, J.; Olivotto, I.A.; Spinelli, J.J.; Phillips, N.; Jackson, S.M.; Wilson, K.S.; Knowling, M.A.; Coppin, C.M.; Weir, L.; Gelmon, K.; et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J. Natl. Cancer Inst. 2005, 97, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Han, H.H.; Kim, H.J.; Lee, J.; Chung, I.Y.; Kim, J.; Lee, S.; Han, J.; Eom, J.S.; Kim, S.B.; et al. Locoregional recurrence following nipple-sparing mastectomy with immediate breast reconstruction: Patterns and prognostic significance. Eur. J. Surg. Oncol. 2021, 47, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Masetti, R.; D’Ugo, D.; Palumbo, F.; D’Alba, P.; Mulè, A.; Costantini, M.; Belli, P.; Picciocchi, A. Synchronous bilateral Paget’s disease of the nipple associated with bilateral breast carcinoma. Breast J. 2005, 11, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Ishitobi, M.; Nagura, N.; Shimo, A.; Seki, H.; Ogiya, A.; Sakurai, T.; Seto, Y.; Oshiro, C.; Sasada, S.; et al. Classification of Local Recurrence After Nipple-Sparing Mastectomy Based on Location: The Features of Nipple-Areolar Recurrence Differ from Those of Other Local Recurrences. Ann. Surg. Oncol. 2023, 30, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Agresti, R.; Sandri, M.; Gennaro, M.; Bianchi, G.; Maugeri, I.; Rampa, M.; Capri, G.; Carcangiu, M.L.; Trecate, G.; Riggio, E.; et al. Evaluation of Local Oncologic Safety in Nipple-Areola Complex-sparing Mastectomy After Primary Chemotherapy: A Propensity Score-matched Study. Clin. Breast Cancer 2017, 17, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Moossdorff, M.; van Roozendaal, L.M.; Strobbe, L.J.; Aebi, S.; Cameron, D.A.; Dixon, J.M.; Giuliano, A.E.; Haffty, B.G.; Hickey, B.E.; Hudis, C.A.; et al. Maastricht Delphi consensus on event definitions for classification of recurrence in breast cancer research. J. Natl. Cancer Inst. 2014, 106, dju288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De La Cruz, L.; Moody, A.M.; Tappy, E.E.; Blankenship, S.A.; Hecht, E.M. Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple-Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann. Surg. Oncol. 2015, 22, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Golijanin, D.; Radovanović, Z.; Radovanović, D.; Đermanović, A.; Starčević, S.; Đermanović, M. Molecular subtype and risk of local recurrence after nipplesparing mastectomy for breast cancer. Oncol. Lett. 2024, 28, 389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galimberti, V.; Morigi, C.; Bagnardi, V.; Corso, G.; Vicini, E.; Fontana, S.K.R.; Naninato, P.; Ratini, S.; Magnoni, F.; Toesca, A.; et al. Oncological Outcomes of Nipple-Sparing Mastectomy: A Single-Center Experience of 1989 Patients. Ann. Surg. Oncol. 2018, 25, 3849–3857. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Byun, H.K.; Kim, J.W.; Kim, K.H.; Lee, J.; Cho, Y.; Lee, I.J.; Keum, K.C.; Suh, C.O.; Kim, Y.B. Three-dimensional analysis of patterns of locoregional recurrence after treatment in breast cancer patients: Validation of the ESTRO consensus guideline on target volume. Radiother. Oncol. 2017, 122, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Ki, Y.; Kim, W.; Nam, J.; Kim, D.; Park, J.; Kim, H.Y.; Jung, Y.J.; Choo, K.S.; Nam, K.J.; et al. Pattern of local recurrence after mastectomy and reconstruction in breast cancer patients: A systematic review. Gland. Surg. 2021, 10, 2037–2046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siponen, E.T.; Joensuu, H.; Leidenius, M.H. Local recurrence of breast cancer after mastectomy and modern multidisciplinary treatment. Acta Oncol. 2013, 52, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Agarwal, V.K.; Nayak, V.; Yogsrivas, R.; Gulia, A. Surgical Management of Locoregional Recurrence in Breast Cancer. Indian J. Surg. Oncol. 2021, 12, 616–623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Shi, X.; Li, J.; Wu, G. Prognosis of Surgical Treatment After Ipsilateral Breast Tumor Recurrence. J. Surg. Res. 2021, 258, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, O.D.; Botteri, E.; Sangalli, C.; Galimberti, V.; Porpiglia, M.; Agresti, R.; Luini, A.; Viale, G.; Cassano, E.; Peradze, N.; et al. Sentinel Lymph Node Biopsy vs No Axillary Surgery in Patients With Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes: The SOUND Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1557–1564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reimer, T.; Stachs, A.; Veselinovic, K.; Kühn, T.; Heil, J.; Polata, S.; Marmé, F.; Müller, T.; Hildebrandt, G.; Krug, D.; et al. Axillary Surgery in Breast Cancer—Primary Results of the INSEMA Trial. N. Engl. J. Med. 2025, 392, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- de Boniface, J.; Filtenborg Tvedskov, T.; Rydén, L.; Szulkin, R.; Reimer, T.; Kühn, T.; Kontos, M.; Gentilini, O.D.; Olofsson Bagge, R.; Sund, M.; et al. Omitting Axillary Dissection in Breast Cancer with Sentinel-Node Metastases. N. Engl. J. Med. 2024, 390, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
| Overall | WLE (Wild Local Excision) | RM (Re-Mastectomy) | |
|---|---|---|---|
| 12 | 9 (75%) | 3 (25%) | |
| Tumor Classification | |||
| Tis | 1 (8.3%) | 1 (11.1%) | 1 (33.3%) |
| T1 | 9 (75%) | 8 (88.9%) | 1 (33.3%) |
| T2 | 1 (8.3%) | 0 (0%) | 1 (33.3%) |
| T3 | 1 (8.3%) | 0 (0%) | 0 (0%) |
| Histologic Subtype | |||
| IDC | 10 (83.3%) | 7 (77.78%) | 2 (66.7%) |
| CDIS | 1 (8.3%) | 1(11.1%) | 0 (0%) |
| Other | 1 (8.3%) | 1 (11.1%) | 1 (33.3%) |
| ER status | |||
| Positive | 6 (50%) | 6 (66.7%) | 1 (33.3%) |
| Negative | 6 (50%) | 3 (33.3%) | 2 (66.7%) |
| HER2 status | |||
| Positive | 4 (33.4%) | 3 (33.3%) | 1 (33.3%) |
| Negative | 8 (66.6%) | 6 (66.7%) | 2 (66.7%) |
| Triple Negative | 2 (16.6%) | 1 (11.1%) | 1 (33.3%) |
| Multifocality | |||
| Yes | 1 (8.3%) | 3 (33.3%) | 1 (33.3%) |
| No | 11 (81.7%) | 6 (66.7%) | 2 (66.7%) |
| Axillary Surgery | |||
| Yes | 3 (25%) | 3 (33.3%) | 1 (33.3%) |
| No | 9 (75%) | 6 (66,7%) | 2 (66.7%) |
| Systemic Therapy | |||
| Yes | 2 (16.6%) | 1 (11.1%) | 1 (33.3%) |
| No | 10 (83.4%) | 8 (88.9%) | 2 (66.7%) |
| Implant | |||
| Original implant salvaged | 9 (75%) | 9 (100%) | 0 (0%) |
| Implant removed | 2 (16.6%) | 0 (0%) | 2 (66.7%) |
| Substitution | 1 (8.3%) | 0 (0%) | 1 (33.3%) |
| Capsule Procedure | |||
| None | 6 (50%) | 6 (66.7%) | 0 (0%) |
| Capsulectomy | 6 (50%) | 3 (33.3%) | 3 (100%) |
| Adjuvant Treatment | |||
| Chemotherapy | 5 (41.6%) | 4 (44.4%) | 1(33.3%) |
| Radiotherapy | 6 (50%) | 5 (55.6%) | 1(33.3%) |
| Hormonal Therapy | 6 (50%) | 5 (55.6%) | 1 (33.3%) |
| Immunotherapy | 2 (16.6%) | 2 (22.2%) | 0 (0%) |
| Same QQ as Primary Tumor | |||
| Yes | 6 (50%) | 4 (44.4%) | 2 (66.7%) |
| No | 6 (50%) | 5 (55.6%) | 1 (33.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scardina, L.; Petrazzuolo, E.; Accetta, C.; Carnassale, B.; D’Archi, S.; Di Leone, A.; Di Pumpo, A.; Di Guglielmo, E.; De Lauretis, F.; Franco, A.; et al. Surgical Management of Ipsilateral Breast Cancer Recurrence After Conservative Mastectomy and Prepectoral Breast Reconstruction: Exploring the Role of Wide Local Excision. Cancers 2025, 17, 2881. https://doi.org/10.3390/cancers17172881
Scardina L, Petrazzuolo E, Accetta C, Carnassale B, D’Archi S, Di Leone A, Di Pumpo A, Di Guglielmo E, De Lauretis F, Franco A, et al. Surgical Management of Ipsilateral Breast Cancer Recurrence After Conservative Mastectomy and Prepectoral Breast Reconstruction: Exploring the Role of Wide Local Excision. Cancers. 2025; 17(17):2881. https://doi.org/10.3390/cancers17172881
Chicago/Turabian StyleScardina, Lorenzo, Eleonora Petrazzuolo, Cristina Accetta, Beatrice Carnassale, Sabatino D’Archi, Alba Di Leone, Annasilvia Di Pumpo, Enrico Di Guglielmo, Flavia De Lauretis, Antonio Franco, and et al. 2025. "Surgical Management of Ipsilateral Breast Cancer Recurrence After Conservative Mastectomy and Prepectoral Breast Reconstruction: Exploring the Role of Wide Local Excision" Cancers 17, no. 17: 2881. https://doi.org/10.3390/cancers17172881
APA StyleScardina, L., Petrazzuolo, E., Accetta, C., Carnassale, B., D’Archi, S., Di Leone, A., Di Pumpo, A., Di Guglielmo, E., De Lauretis, F., Franco, A., Gagliardi, F., Magno, S., Moschella, F., Natale, M., Rianna, C., Sanchez, A. M., Silenzi, M., & Franceschini, G. (2025). Surgical Management of Ipsilateral Breast Cancer Recurrence After Conservative Mastectomy and Prepectoral Breast Reconstruction: Exploring the Role of Wide Local Excision. Cancers, 17(17), 2881. https://doi.org/10.3390/cancers17172881














