Clinical Outcomes of Cervical Adenocarcinoma In Situ According to Conservative or Demolitive Treatment: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
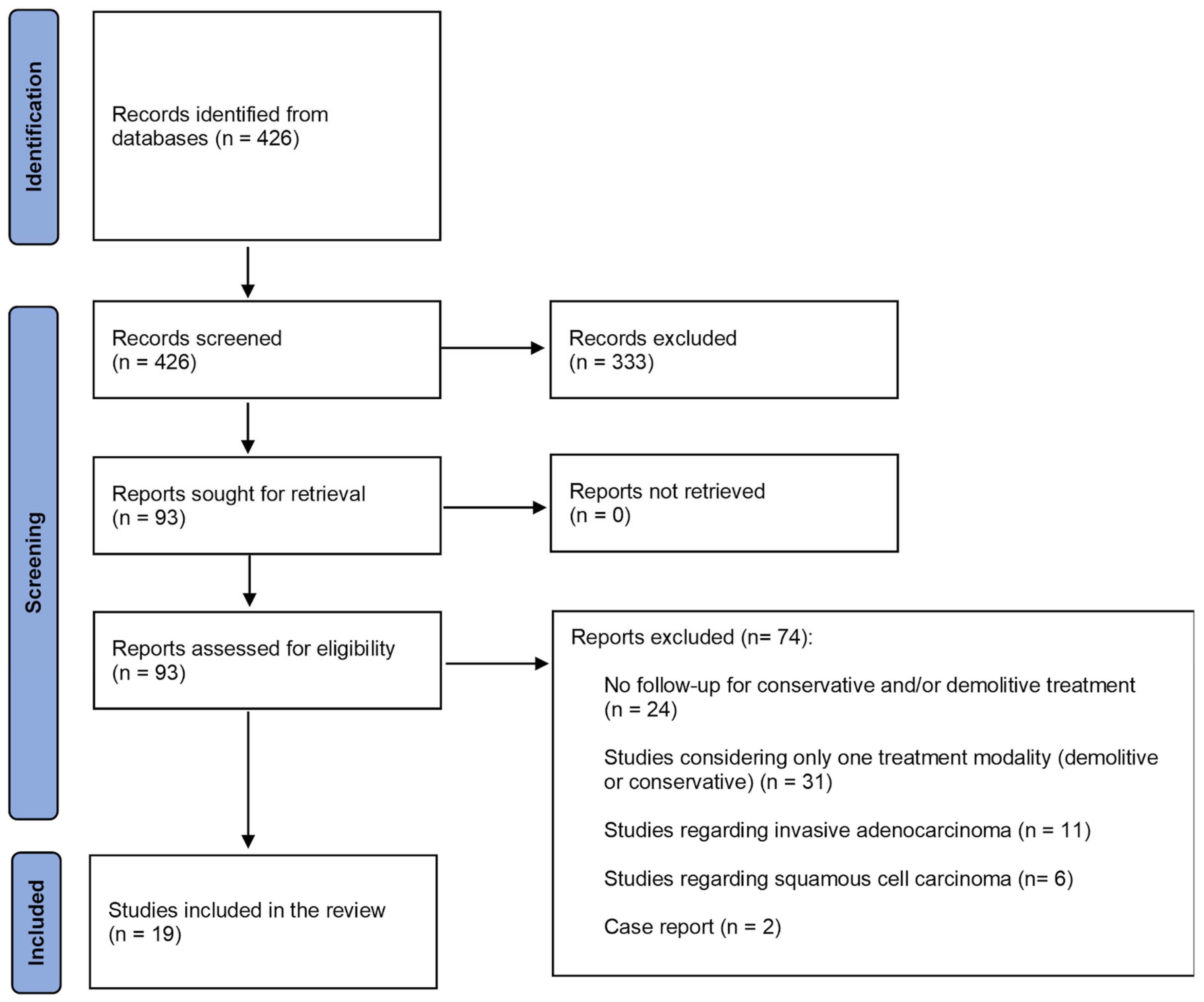
3.1. Study Selection, Study Characteristics, and Risk of Bias of Included Studies
3.2. Patient Characteristics
3.3. Synthesis of Results
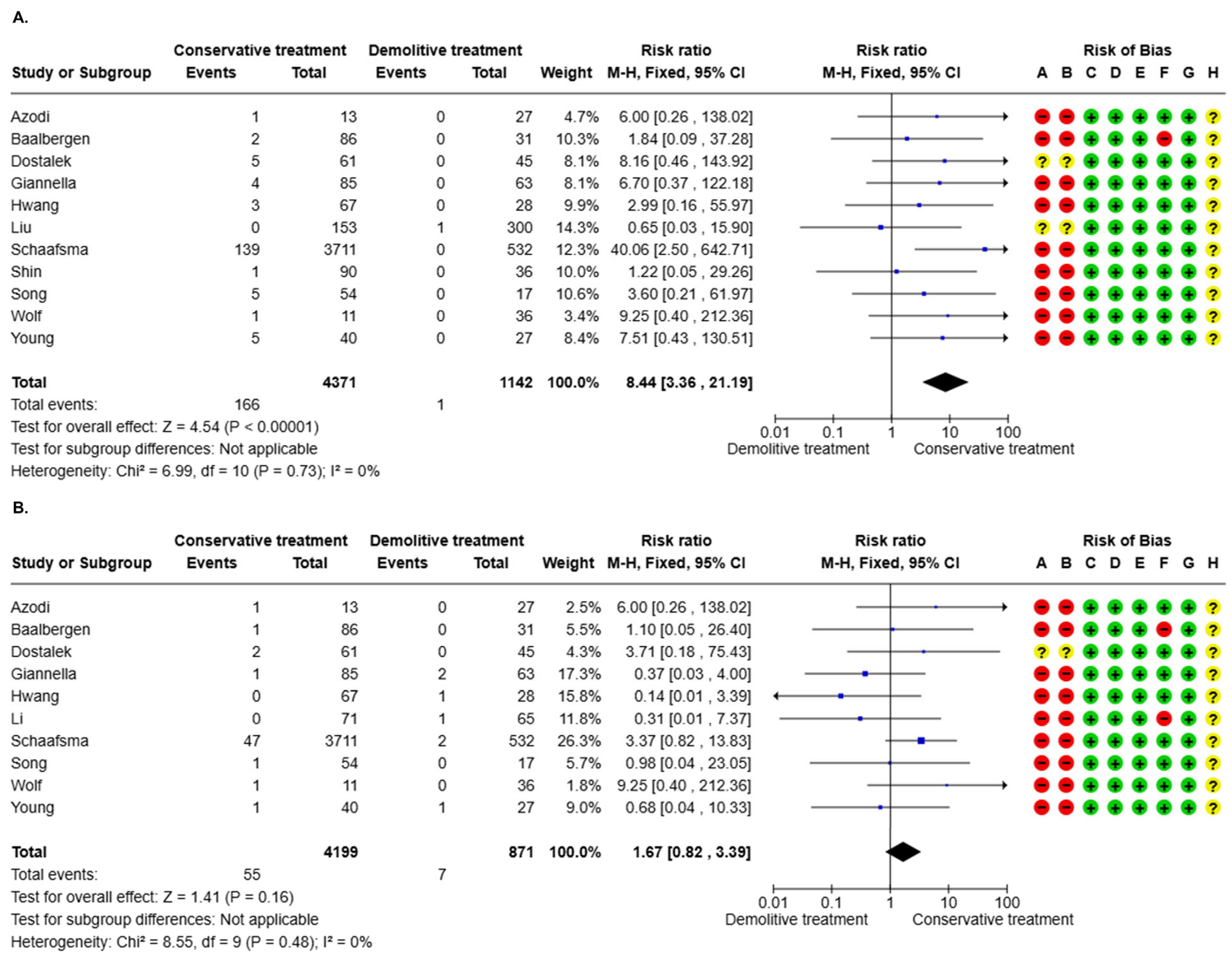
3.3.1. AIS Recurrence (16 Studies)
3.3.2. Invasive Recurrence (16 Studies)
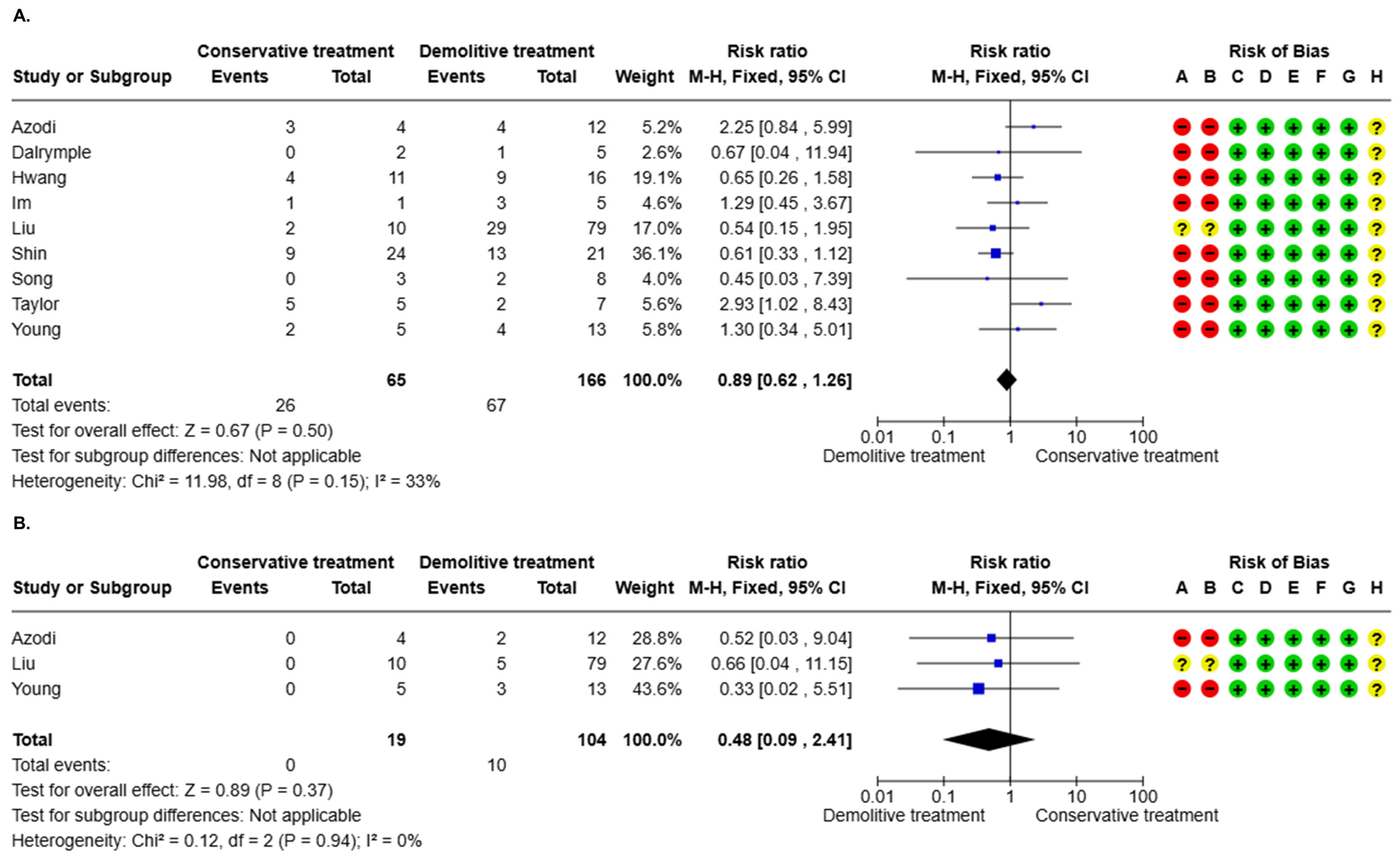
3.3.3. AIS Residual After Positive Margin (10 Studies)
3.3.4. Invasive Residual After Positive Margin (10 Studies)
3.4. Meta-Regression
4. Discussion
4.1. Summary of Main Results
4.2. Comparison with Existing Literature
4.3. Strengths and Limitations
4.4. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Adenocarcinoma In Situ |
| CI | Confidence Interval |
| CIN | Cervical Intraepithelial Neoplasia |
| GRADE | Grades of Recommendation, Assessment, Development and Evaluation |
| HPV | Human Papillomavirus |
| MOOSE | Meta-analyses Of Observational Studies in Epidemiology |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | The International Prospective Register of Systematic Reviews |
| RR | Risk Ratio |
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, 197–206. [Google Scholar] [CrossRef]
- Giannella, L.; Di Giuseppe, J.; Delli Carpini, G.; Grelloni, C.; Fichera, M.; Sartini, G.; Caimmi, S.; Natalini, L.; Ciavattini, A. HPV-Negative Adenocarcinomas of the Uterine Cervix: From Molecular Characterization to Clinical Implications. Int. J. Mol. Sci. 2022, 23, 15022. [Google Scholar] [CrossRef]
- Gadducci, A.; Guerrieri, M.E.; Cosio, S. Adenocarcinoma of the uterine cervix: Pathologic features, treatment options, clinical outcome and prognostic variables. Crit. Rev. Oncol./Hematol. 2019, 135, 103–114. [Google Scholar] [CrossRef]
- Loureiro, J.; Oliva, E. The spectrum of cervical glandular neoplasia and issues in differential diagnosis. Arch. Pathol. Lab. Med. 2014, 138, 453–483. [Google Scholar] [CrossRef]
- Salani, R.; Puri, I.; Bristow, R.E. Adenocarcinoma in situ of the uterine cervix: A metaanalysis of 1278 patients evaluating the predictive value of conization margin status. Am. J. Obstet. Gynecol. 2009, 200, 182.e1–182.e5. [Google Scholar] [CrossRef]
- Baldur-Felskov, B.; Munk, C.; Nielsen, T.S.; Dehlendorff, C.; Kirschner, B.; Junge, J.; Kjaer, S.K. Trends in the incidence of cervical cancer and severe precancerous lesions in Denmark, 1997–2012. Cancer Causes Control 2015, 26, 1105–1116. [Google Scholar] [CrossRef]
- Wang, M.; Huang, K.; Wong, M.C.S.; Zhang, X. Global Cervical Cancer Incidence by Histological Subtype and Implications for Screening Methods. J. Epidemiol. Glob. Health 2024, 14, 94–101. [Google Scholar] [CrossRef]
- Orumaa, M.; Leinonen, M.K.; Campbell, S.; Karlsson, M.; Jakobsson, M.; Nieminen, P.; Anttila, A. Recent increase in incidence of cervical precancerous lesions in Norway: Nationwide study from 1992 to 2016. Int. J. Cancer 2019, 145, 2629–2638. [Google Scholar] [CrossRef]
- Bray, F.; Carstensen, B.; Møller, H.; Zappa, M.; Zakelj, M.P.; Lawrence, G.; Hakama, M.; Kuque, I.; Smith, J.S.; Parkin, D.M. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2191–2199. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Tumours Editorial Board. Tumours of the uterine cervix: Adenocarcinomas. In Female Genital Tumours, 5th ed.; WHO Classification of Tumours Series; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 4, Available online: https://publications.iarc.fr/592 (accessed on 17 April 2025).
- Baalbergen, A.; Helmerhorst, T.J. Adenocarcinoma in situ of the uterine cervix—A systematic review. Int. J. Gynecol. Cancer 2014, 24, 1543–1548. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines: Updates Through 2023. J. Low. Genit. Tract Dis. 2024, 28, 3–6. [Google Scholar] [CrossRef]
- McGee, A.E.; Alibegashvili, T.; Elfgren, K.; Frey, B.; Grigore, M.; Heinonen, A.; Jach, R.; Jariene, K.; Kesic, V.; Küppers, V.; et al. European consensus statement on expert colposcopy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 290, 27–37. [Google Scholar] [CrossRef]
- Willows, K.; Selk, A.; Auclair, M.H.; Jim, B.; Jumah, N.; Nation, J.; Proctor, L.; Iazzi, M.; Bentley, J. 2023 Canadian Colposcopy Guideline: A Risk-Based Approach to Management and Surveillance of Cervical Dysplasia. Curr. Oncol. 2023, 30, 5738–5768. [Google Scholar] [CrossRef]
- Ciavattini, A.; Giannella, L.; Delli Carpini, G.; Tsiroglou, D.; Sopracordevole, F.; Chiossi, G.; Di Giuseppe, J. Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV). Adenocarcinoma in situ of the uterine cervix: Clinical practice guidelines from the Italian Society of Colposcopy and Cervical Pathology (SICPCV). Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 273–277. [Google Scholar] [CrossRef]
- Teoh, D.; Musa, F.; Salani, R.; Huh, W.; Jimenez, E. Diagnosis and Management of Adenocarcinoma In Situ: A Society of Gynecologic Oncology Evidence-Based Review and Recommendations. Obstet. Gynecol. 2020, 135, 869–878. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, 4919. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 6.5 (Updated August 2024); Cochrane: 2024. Available online: www.training.cochrane.org/handbook (accessed on 17 April 2025).
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations, Updated October 2013; The GRADE Working Group: 2013. Available online: https://www.guidelinedevelopment.org/handbook (accessed on 17 April 2025).
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]; McMaster University and Evidence Prime: Hamilton, ON, Canada. 2024. Available online: https://www.gradepro.org (accessed on 17 April 2025).
- Azodi, M.; Chambers, S.K.; Rutherford, T.J.; Kohorn, E.I.; Schwartz, P.E.; Chambers, J.T. Adenocarcinoma in situ of the cervix: Management and outcome. Gynecol. Oncol. 1999, 73, 348–353. [Google Scholar] [CrossRef]
- Baalbergen, A.; Molijn, A.C.; Quint, W.G.; Smedts, F.; Helmerhorst, T.J. Conservative Treatment Seems the Best Choice in Adenocarcinoma In Situ of the Cervix Uteri. J. Low. Genit. Tract Dis. 2015, 19, 239–243. [Google Scholar] [CrossRef]
- Bryson, P.; Stulberg, R.; Shepherd, L.; McLelland, K.; Jeffrey, J. Is electrosurgical loop excision with negative margins sufficient treatment for cervical ACIS? Gynecol. Oncol. 2004, 93, 465–468. [Google Scholar] [CrossRef]
- Dalrymple, C.; Valmadre, S.; Cook, A.; Atkinson, K.; Carter, J.; Houghton, C.R.; Russell, P. Cold knife versus laser cone biopsy for adenocarcinoma in situ of the cervix—A comparison of management and outcome. Int. J. Gynecol. Cancer 2008, 18, 116–120. [Google Scholar] [CrossRef]
- Dostalek, L.; Freitag, P.; Slovackova, M.; Zima, T.; Komarc, M.; Fricova, L.; Fucik, T.; Nemejcova, K.; Cibula, D.; Brynda, D.; et al. HPV testing as an effective triage strategy in the follow-up after fertility-sparing treatment for glandular lesions of the uterine cervix. Int. J. Gynecol. Cancer 2024, 34, 216–223. [Google Scholar] [CrossRef]
- Giannella, L.; Delli Carpini, G.; Di Giuseppe, J.; Bogani, G.; Sopracordevole, F.; Clemente, N.; Giorda, G.; De Vincenzo, R.P.; Evangelista, M.T.; Gardella, B.; et al. In Situ/Microinvasive Adenocarcinoma of the Uterine Cervix and HPV-Type Impact: Pathologic Features, Treatment Options, and Follow-Up Outcomes—Cervical Adenocarcinoma Study Group (CAS-Group). Cancers 2023, 15, 2876. [Google Scholar] [CrossRef]
- Hwang, D.M.; Lickrish, G.M.; Chapman, W.; Colgan, T.J. Long-term surveillance is required for all women treated for cervical adenocarcinoma in situ. J. Low. Genit. Tract Dis. 2004, 8, 125–131. [Google Scholar] [CrossRef]
- Im, D.D.; Duska, L.R.; Rosenshein, N.B. Adequacy of conization margins in adenocarcinoma in situ of the cervix as a predictor of residual disease. Gynecol. Oncol. 1995, 59, 179–182. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C. Long-term follow-up results from women with cervical adenocarcinoma in situ treated by conization: An experience from a large academic women’s hospital. J. Low. Genit. Tract Dis. 2013, 17, 452–458. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Wan, X.; Zou, J.; Zhu, Y.; Lv, W.; Shen, Y. Comparison of the safety between cervical conization and hysterectomy for patients with cervical adenocarcinoma in situ. J. Gynecol. Oncol. 2023, 34, e8. [Google Scholar] [CrossRef]
- Omnes, S.; Morice, P.; Camatte, S.; Rouzier, R.; Pautier, P.; Pomel, C.; Lhommé, C.; Haie-Meder, C.; Duvillard, P.; Castaigne, D. Modalités et limites du traitement conservateur des adénocarcinomes in situ du col utérin: Analyse de neuf cas et revue de la littérature [Modalities and limits of conservative treatment of adenocarcinoma in situ of the uterine cervix: Analysis of nine cases and review of the literature]. Gynécologie Obs. Fertil. 2003, 31, 912–919. [Google Scholar] [CrossRef]
- Schaafsma, M.; Schuurman, T.N.; Kootstra, P.; Issa, D.; Hermans, I.; Bleeker, M.C.G.; Zusterzeel, P.L.M.; Bekkers, R.L.M.; Siebers, A.G.; Mom, C.H.; et al. Nationwide cohort study on the risk of high-grade cervical dysplasia and carcinoma after conservative treatment or hysterectomy for adenocarcinoma in situ. Int. J. Cancer 2025, 156, 1203–1212. [Google Scholar] [CrossRef]
- Shin, C.H.; Schorge, J.O.; Lee, K.R.; Sheets, E.E. Conservative management of adenocarcinoma in situ of the cervix. Gynecol. Oncol. 2000, 79, 6–10. [Google Scholar] [CrossRef]
- Song, T.; Lee, Y.Y.; Choi, C.H.; Kim, T.J.; Lee, J.W.; Bae, D.S.; Kim, B.G. The effect of coexisting squamous cell lesions on prognosis in patients with cervical adenocarcinoma in situ. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 190, 26–30. [Google Scholar] [CrossRef]
- Tay, E.H.; Yew, W.S.; Ho, T.H. Management of adenocarcinoma in situ (ACIS) of the uterine cervix—A clinical dilemma. Singap. Med. J. 1999, 40, 36–39. [Google Scholar]
- Taylor, J.S.; Panico, V.; Caputo, T.; Gerber, D.; Gupta, D.; Pirog, E.; Holcomb, K. Clinical outcomes of patients with adenocarcinoma in situ of the cervix treated by conization. Eur. J. Gynaecol. Oncol. 2014, 35, 641–645. [Google Scholar]
- Wang, X.; Bi, Y.; Wu, H.; Wu, M.; Li, L. Oncologic and obstetric outcomes after conization for adenocarcinoma in situ or stage IA1 cervical cancer. Sci. Rep. 2020, 10, 19920. [Google Scholar] [CrossRef]
- Wolf, J.K.; Levenback, C.; Malpica, A.; Morris, M.; Burke, T.; Mitchell, M.F. Adenocarcinoma in situ of the cervix: Significance of cone biopsy margins. Obstet. Gynecol. 1996, 88, 82–86. [Google Scholar] [CrossRef]
- Young, J.L.; Jazaeri, A.A.; Lachance, J.A.; Stoler, M.H.; Irvin, W.P.; Rice, L.W.; Andersen, W.A.; Modesitt, S.C. Cervical adenocarcinoma in situ: The predictive value of conization margin status. Am. J. Obstet. Gynecol. 2007, 197, 195.e1–195.e8. [Google Scholar] [CrossRef]
- Costa, S.; Venturoli, S.; Negri, G.; Sideri, M.; Preti, M.; Pesaresi, M.; Falasca, A.; Barbieri, D.; Zerbini, M.; Santini, D.; et al. Factors predicting the outcome of conservatively treated adenocarcinoma in situ of the uterine cervix: An analysis of 166 cases. Gynecol. Oncol. 2012, 124, 490–495. [Google Scholar] [CrossRef]
- Soutter, W.P.; Sasieni, P.; Panoskaltsis, T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int. J. Cancer 2006, 118, 2048–2055. [Google Scholar] [CrossRef]
- Ghaem-Maghami, S.; Sagi, S.; Majeed, G.; Soutter, W.P. Incomplete excision of cervical intraepithelial neoplasia and risk of treatment failure: A meta-analysis. Lancet Oncol. 2007, 8, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Jentschke, M.; Kampers, J.; Becker, J.; Sibbertsen, P.; Hillemanns, P. Prophylactic HPV vaccination after conization: A systematic review and meta-analysis. Vaccine 2020, 38, 6402–6409. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Kalliala, I.; Bowden, S.J.; Athanasiou, A.; Paraskevaidi, M.; Paraskevaidis, E.; Dillner, J.; Nieminen, P.; Strander, B.; Sasieni, P.; et al. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis. BMJ 2022, 378, e070135. [Google Scholar] [CrossRef]
- Srisomboon, S.; Tantipalakorn, C.; Charoenkwan, K.; Srisomboon, J. Cervical screening results leading to detection of adenocarcinoma in situ of the uterine cervix. Asian Pac. J. Cancer Prev. 2019, 20, 377–382. [Google Scholar] [CrossRef]
- Giannella, L.; Delli Carpini, G.; Di Giuseppe, J.; Grelloni, C.; Bogani, G.; Dri, M.; Sopracordevole, F.; Clemente, N.; Giorda, G.; De Vincenzo, R.; et al. Long-Term Follow-Up Outcomes in Women with In Situ/Microinvasive Adenocarcinoma of the Uterine Cervix Undergoing Conservative Treatment—Cervical Adenocarcinoma Study Group Italian Society of Colposcopy and Cervico-Vaginal Pathology. Cancers 2024, 16, 1241. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.; Li, L. Comparison of cold-knife conization versus loop electrosurgical excision for cervical adenocarcinoma in situ (ACIS): A systematic review and meta-analysis. PLoS ONE 2017, 12, e0170587. [Google Scholar] [CrossRef]
- Follow-Up After Excisional Treatment for AIS. Available online: https://www.cancer.org.au/clinical-guidelines/cervical-cancer/cervical-cancer-screening/management-of-glandular-abnormalities/follow-up-after-excisional-treatment-for-ais (accessed on 17 April 2025).
| First Author | Year | Country | Study Design | Study Period |
|---|---|---|---|---|
| Azodi et al. [24] | 1999 | USA | Retrospective | 1988–1996 |
| Baalbergen et al. [25] | 2015 | Holland | Retrospective | 1989–2012 |
| Bryson et al. [26] | 2004 | Canada | Retrospective | 1990–2003 |
| Dalrymple et al. [27] | 2008 | Australia | Retrospective | - |
| Dostalek et al. [28] | 2023 | Czech Republic | Retrospective | 2002–2023 |
| Giannella et al. [29] | 2022 | Italy | Retrospective | 2012–2016 |
| Hwang et al. [30] | 2004 | Canada | Retrospective | 1980–2002 |
| Im et al. [31] | 1995 | USA | Retrospective | 1988–1994 |
| Li et al. [32] | 2013 | USA | Retrospective | 2000–2010 |
| Liu et al. [33] | 2022 | China | Retrospective | 2007–2021 |
| Omnes et al. [34] | 2003 | France | Retrospective | 1974–2000 |
| Schaafsma et al. [35] | 2025 | The Netherlands | Retrospective | 1990–2021 |
| Shin et al. [36] | 2000 | USA | Retrospective | 1987–1999 |
| Song et al. [37] | 2015 | Republic of Korea | Retrospective | 2000–2011 |
| Tay et al. [38] | 1999 | Singapore | Retrospective | 1991–1996 |
| Taylor et al. [39] | 2014 | USA | Retrospective | 1998–2011 |
| Wang et al. [40] | 2020 | China | Retrospective | 2002–2018 |
| Wolf et al. [41] | 1997 | USA | Retrospective | 1984–1993 |
| Young et al. [42] | 2007 | USA | Retrospective | 1998–2006 |
| First Author | Conservative Treatment | Demolitive Treatment |
|---|---|---|
| Azodi et al. [24] | CKC; LEEP; LC ± ECC | Piver ARH type I-III; VH; LAVH. |
| Baalbergen et al. [25] | CKC; LEEP; LC | Hysterectomy |
| Bryson et al. [26] | LEEP | Hysterectomy |
| Dalrymple et al. [27] | CKC; LC | LAVH; RH ± PLND |
| Dostalek et al. [28] | LEEP; CKC; SVT | Hysterectomy |
| Giannella et al. [29] | CKC; LEEP; LC | RH ± LPND |
| Hwang et al. [30] | CKC; LEEP; LC | RH |
| Im et al. [31] | CKC; LEEP; LC | EH; RH;VH |
| Li et al. [32] | Conization | Hysterectomy |
| Liu et al. [33] | CKC; LEEP | Hysterectomy |
| Omnes et al. [34] | CKC; LEEP; SVT | SH; RH |
| Schaafsma et al. [35] | CKC, LLETZ | Hysterectomy |
| Shin et al. [36] | CKC; LEEP | Hysterectomy |
| Song et al. [37] | LEEP | Hysterectomy |
| Tay et al. [38] | CKC; LLETZ; LC | Hysterectomy |
| Taylor et al. [39] | CKC; LEEP | Hysterectomy |
| Wang et al. [40] | CKC; ESC | Hysterectomy |
| Wolf et al. [41] | CKC; LEEP; LC | SH; RH |
| Young et al. [42] | CKC; LEEP; LC | Hysterectomy |
| Certainty Assessment | Summary of Findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (Studies) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty of Evidence | Study Event Rates (%) | Relative Effect (95% CI) | Anticipated Absolute Effects | ||
| With Demolitive Treatment | With Conservative Treatment | Risk with Demolitive Treatment | Risk Difference with Conservative Treatment | ||||||||
| AIS recurrence | |||||||||||
| 5513 (11 non-randomized studies) | serious a | not serious | not serious | serious b | none | Very Low a,b | 1/1142 (0.1%) | 166/4371 (3.8%) | RR 8.44 (3.36 to 21.19) | 1 per 1.000 | 7 more per 1.000 (from 2 more to 18 more) |
| Invasive recurrence | |||||||||||
| 5070 (10 non-randomized studies) | serious c | not serious | not serious | serious d | none | Very Low c,d | 7/871 (0.8%) | 55/4199 (1.3%) | RR 1.67 (0.82 to 3.39) | 8 per 1.000 | 5 more per 1.000 (from 1 fewer to 19 more) |
| AIS residual | |||||||||||
| 231 (9 non-randomized studies) | serious e | not serious | not serious | serious f | none | Very Low e,f | 67/166 (40.4%) | 26/65 (40.0%) | RR 0.89 (0.62 to 1.26) | 404 per 1.000 | 44 fewer per 1.000 (from 153 fewer to 105 more) |
| Invasive residual | |||||||||||
| 123 (3 non-randomized studies) | serious g | not serious | not serious | serious h | none | Very Low g,h | 10/104 (9.6%) | 0/19 (0.0%) | RR 0.48 (0.09 to 2.41) | 96 per 1.000 | 50 fewer per 1.000 (from 88 fewer to 136 more) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delli Carpini, G.; Cicoli, C.; Bernardi, M.; Di Giuseppe, J.; Giannella, L.; Ciavattini, A. Clinical Outcomes of Cervical Adenocarcinoma In Situ According to Conservative or Demolitive Treatment: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 1839. https://doi.org/10.3390/cancers17111839
Delli Carpini G, Cicoli C, Bernardi M, Di Giuseppe J, Giannella L, Ciavattini A. Clinical Outcomes of Cervical Adenocarcinoma In Situ According to Conservative or Demolitive Treatment: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(11):1839. https://doi.org/10.3390/cancers17111839
Chicago/Turabian StyleDelli Carpini, Giovanni, Camilla Cicoli, Marco Bernardi, Jacopo Di Giuseppe, Luca Giannella, and Andrea Ciavattini. 2025. "Clinical Outcomes of Cervical Adenocarcinoma In Situ According to Conservative or Demolitive Treatment: A Systematic Review and Meta-Analysis" Cancers 17, no. 11: 1839. https://doi.org/10.3390/cancers17111839
APA StyleDelli Carpini, G., Cicoli, C., Bernardi, M., Di Giuseppe, J., Giannella, L., & Ciavattini, A. (2025). Clinical Outcomes of Cervical Adenocarcinoma In Situ According to Conservative or Demolitive Treatment: A Systematic Review and Meta-Analysis. Cancers, 17(11), 1839. https://doi.org/10.3390/cancers17111839







