Evaluation of Tumor Budding and Poorly Defined Clusters as Histological Biomarkers in Squamous Cell Carcinomas of the Vulva †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
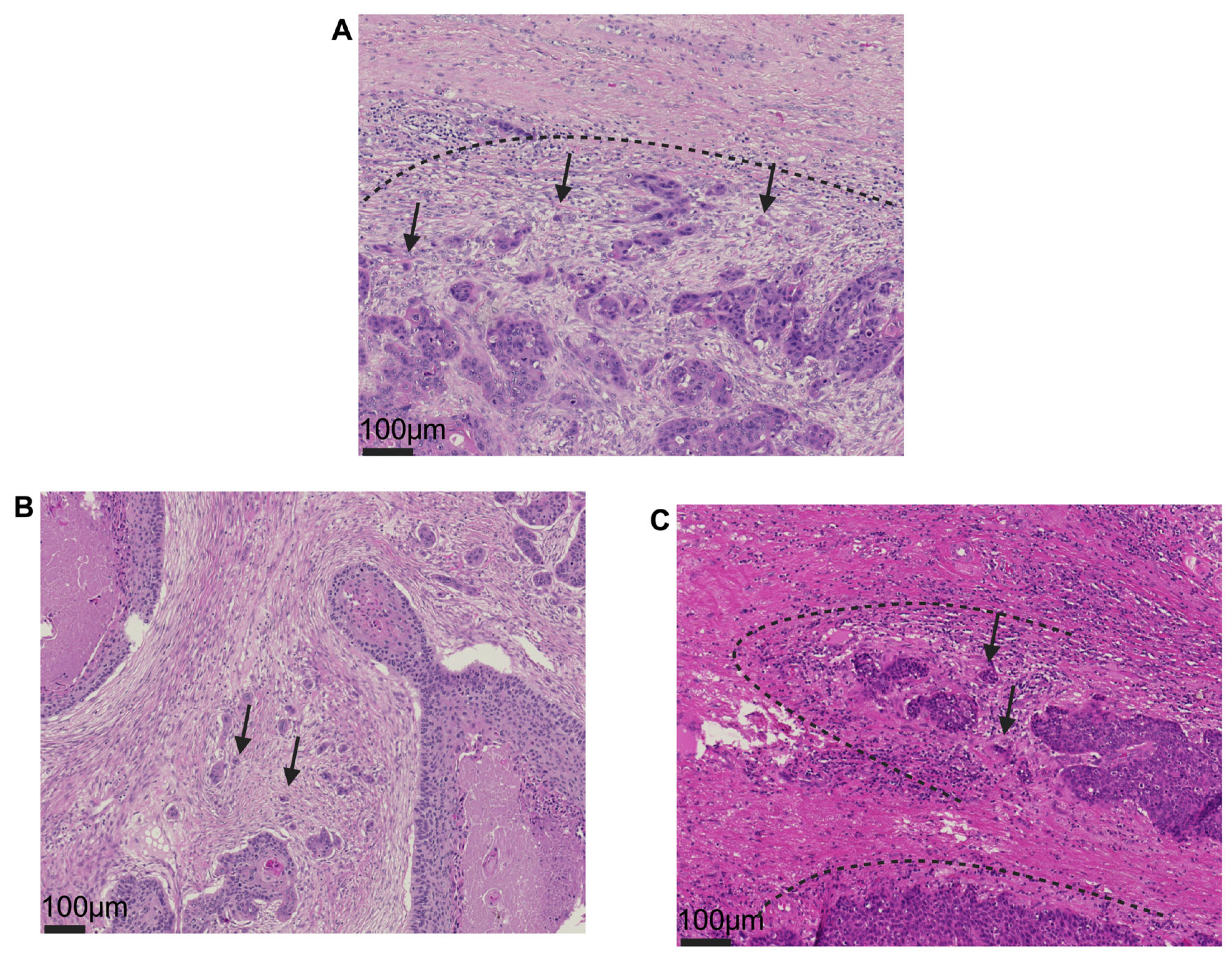
3. Results
3.1. Clinico-Pathologic Characteristics
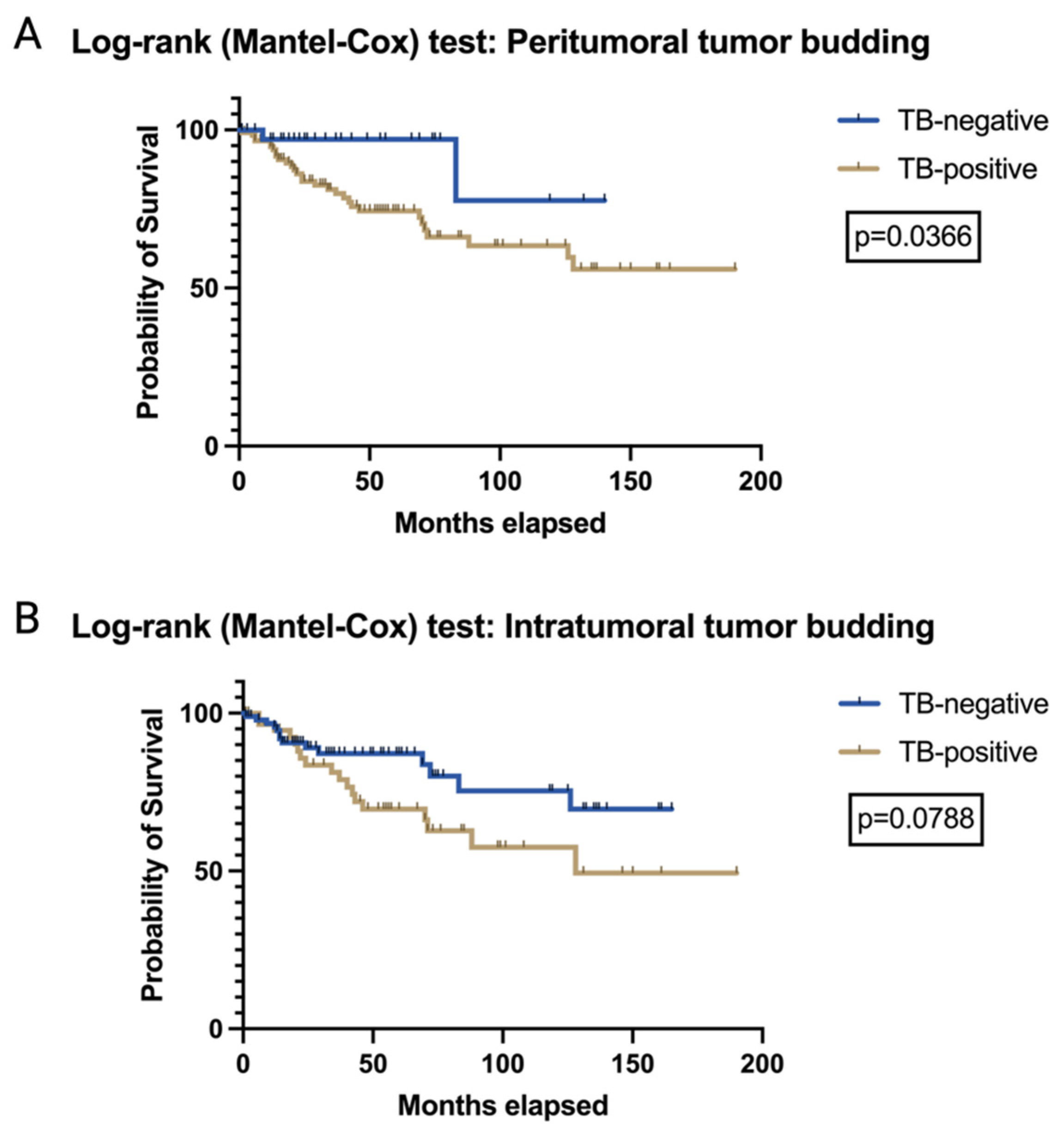
3.2. The Prognostic Relevance of TB and PDCs in VSCCs
3.3. Association of TB and PDCs with HPV Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Julia, C.J.; Hoang, L. A Review of Prognostic Factors in Squamous Cell Carcinoma of the Vulva: Evidence from the Last Decade. Semin. Diagn. Pathol. 2021, 38, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. Vulvar Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerová, D.; Creutzberg, C.L.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 1023–1043. [Google Scholar] [CrossRef] [PubMed]
- Schnürch, H.G.; Ackermann, S.; Alt, C.D.; Barinoff, J.; Böing, C.; Dannecker, C.; Gieseking, F.; Günthert, A.; Hantschmann, P.; Horn, L.C.; et al. Diagnosis, Therapy and Follow-up Care of Vulvar Cancer and its Precursors. Guideline of the DGGG and DKG (S2k-Level, AWMF Registry Number 015/059, November 2015. Geburtshilfe Und Frauenheilkd. 2016, 76, 1035–1049. [Google Scholar] [CrossRef]
- Thompson, E.F.; Hoang, L.; Höhn, A.K.; Palicelli, A.; Talia, K.L.; Tchrakian, N.; Senz, J.; Rusike, R.; Jordan, S.; Jamieson, A.; et al. Molecular Subclassification of Vulvar Squamous Cell Carcinoma: Reproducibility and Prognostic Significance of a Novel Surgical Technique. Int. J. Gynecol. Cancer 2022, 32, 977–985. [Google Scholar] [CrossRef]
- Carreras-Dieguez, N.; Guerrero, J.; Rodrigo-Calvo, M.T.; Ribera-Cortada, I.; Trias, I.; Jares, P.; López Del Campo, R.; Saco, A.; Munmany, M.; Marimon, L.; et al. Molecular Landscape of Vulvar Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 7069. [Google Scholar] [CrossRef]
- Corey, L.; Wallbillich, J.J.; Wu, S.; Farrell, A.; Hodges, K.; Xiu, J.; Nabhan, C.; Guastella, A.; Kheil, M.; Gogoi, R.; et al. The Genomic Landscape of Vulvar Squamous Cell Carcinoma. Int. J. Gynecol. Pathol. 2023, 42, 515–522. [Google Scholar] [CrossRef]
- Kortekaas, K.E.; Bastiaannet, E.; van Doorn, H.C.; de Vos van Steenwijk, P.J.; Ewing-Graham, P.C.; Creutzberg, C.L.; Akdeniz, K.; Nooij, L.S.; van der Burg, S.H.; Bosse, T.; et al. Vulvar Cancer Subclassification by HPV and P53 Status Results in Three Clinically Distinct Subtypes. Gynecol. Oncol. 2020, 159, 649–656. [Google Scholar] [CrossRef]
- Zare, S.Y.; Ciscato, A.; Fadare, O. Tumor Budding Activity Is an Independent Prognostic Factor in Squamous Cell Carcinoma of the Vulva. Hum. Pathol. 2022, 126, 77–86. [Google Scholar] [CrossRef]
- Trietsch, M.D.; Peters, A.A.W.; Gaarenstroom, K.N.; van Koningsbrugge, S.H.L.; ter Haar, N.T.; Osse, E.M.; Halbesma, N.; Fleuren, G.J. Spindle Cell Morphology Is Related to Poor Prognosis in Vulvar Squamous Cell Carcinoma. Br. J. Cancer 2013, 109, 2259–2265. [Google Scholar] [CrossRef]
- Dongre, H.N.; Elnour, R.; Tornaas, S.; Fromreide, S.; Thomsen, L.C.V.; Kolseth, I.B.M.; Nginamau, E.S.; Johannessen, A.C.; Vintermyr, O.K.; Costea, D.E.; et al. TP53 Mutation and Human Papilloma Virus Status as Independent Prognostic Factors in a Norwegian Cohort of Vulva Squamous Cell Carcinoma. Acta Obstet. Gynecol. Scand. 2024, 103, 165–175. [Google Scholar] [CrossRef]
- Togni, L.; Caponio, V.C.A.; Zerman, N.; Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Balercia, A.; Mascitti, M.; Santarelli, A. The Emerging Impact of Tumor Budding in Oral Squamous Cell Carcinoma: Main Issues and Clinical Relevance of a New Prognostic Marker. Cancers 2022, 14, 3571. [Google Scholar] [CrossRef] [PubMed]
- Ailia, M.J.; Thakur, N.; Chong, Y.; Yim, K. Tumor Budding in Gynecologic Cancer as a Marker for Poor Survival: A Systematic Review and Meta-Analysis of the Perspectives of Epithelial–Mesenchymal Transition. Cancers 2022, 14, 1431. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.-F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for Reporting Tumor Budding in Colorectal Cancer Based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Dourado, M.R.; Miwa, K.Y.M.; Hamada, G.B.; Paranaíba, L.M.R.; Sawazaki-Calone, Í.; Domingueti, C.B.; Ervolino de Oliveira, C.; Furlan, E.C.B.; Longo, B.C.; Almangush, A.; et al. Prognostication for Oral Squamous Cell Carcinoma Patients Based on the Tumour–Stroma Ratio and Tumour Budding. Histopathology 2020, 76, 906–918. [Google Scholar] [CrossRef]
- Dawson, H.; Lugli, A. Molecular and Pathogenetic Aspects of Tumor Budding in Colorectal Cancer. Front. Med. 2015, 2, 11. [Google Scholar] [CrossRef]
- Grigore, A.; Jolly, M.; Jia, D.; Farach-Carson, M.; Levine, H. Tumor Budding: The Name Is EMT. Partial EMT. J. Clin. Med. 2016, 5, 51. [Google Scholar] [CrossRef]
- Knebel, M.; Philipp Kühn, J.; Körner, S.; Braun, F.; Brust, L.; Wemmert, S.; Smola, S.; Ertz, M.; Wagner, M.; Schick, B.; et al. Optimizing Prognostic Assessment in High-Risk Head and Neck Squamous Cell Carcinomas: The Impact of Tumor Budding and a Novel Histomorphological Scoring System. Cancer Med. 2025, 14, e70685. [Google Scholar] [CrossRef]
- Stögbauer, F.; Beck, S.; Ourailidis, I.; Hess, J.; Poremba, C.; Lauterbach, M.; Wollenberg, B.; Buchberger, A.M.S.; Jesinghaus, M.; Schirmacher, P.; et al. Tumour Budding-Based Grading as Independent Prognostic Biomarker in HPV-Positive and HPV-Negative Head and Neck Cancer. Br. J. Cancer 2023, 128, 2295–2306. [Google Scholar] [CrossRef]
- Boxberg, M.; Jesinghaus, M.; Dorfner, C.; Mogler, C.; Drecoll, E.; Warth, A.; Steiger, K.; Bollwein, C.; Meyer, P.; Wolff, K.D.; et al. Tumour Budding Activity and Cell Nest Size Determine Patient Outcome in Oral Squamous Cell Carcinoma: Proposal for an Adjusted Grading System. Histopathology 2017, 70, 1125–1137. [Google Scholar] [CrossRef]
- Jesinghaus, M.; Strehl, J.; Boxberg, M.; Brühl, F.; Wenzel, A.; Konukiewitz, B.; Schlitter, A.M.; Steiger, K.; Warth, A.; Schnelzer, A.; et al. Introducing a Novel Highly Prognostic Grading Scheme Based on Tumour Budding and Cell Nest Size for Squamous Cell Carcinoma of the Uterine Cervix. J. Pathol. Clin. Res. 2018, 4, 93–102. [Google Scholar] [CrossRef]
- Zare, S.Y.; Aisagbonhi, O.; Hasteh, F.; Fadare, O. Independent Validation of Tumor Budding Activity and Cell Nest Size as Determinants of Patient Outcome in Squamous Cell Carcinoma of the Uterine Cervix. Am. J. Surg. Pathol. 2020, 44, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Otsuru, M.; Yanamoto, S.; Hasegawa, T.; Aizawa, H.; Kamata, T.; Yamakawa, N.; Kohgo, T.; Ito, A.; Noda, Y.; et al. Progression Level of Extracapsular Spread and Tumor Budding for Cervical Lymph Node Metastasis of OSCC. Clin. Oral. Investig. 2018, 22, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Morodomi, T.; Isomoto, H.; Shirouzu, K.; Kakegawa, K.; Irie, K.; Morimatsu, M. An Index for Estimating the Probability of Lymph Node Metastasis in Rectal Cancers. Lymph Node Metastasis and the Histopathology of Actively Invasive Regions of Cancer. Cancer 1989, 63, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, F.; Cao, W.; Wang, K.; He, J.; Wang, H.; Wang, Y. Prognostic Value of Poorly Differentiated Clusters in Invasive Breast Cancer. World J. Surg. Oncol. 2014, 12, 310. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kaizaki, Y.; Kogami, A.; Hara, T.; Sakai, Y.; Tsuchida, T. Prognostic Significance of Tumor Budding, Poorly Differentiated Cluster, and Desmoplastic Reaction in Endometrioid Endometrial Carcinomas. J. Obstet. Gynaecol. Res. 2021, 47, 3958–3967. [Google Scholar] [CrossRef]
- Lugli, A.; Vlajnic, T.; Giger, O.; Karamitopoulou, E.; Patsouris, E.S.; Peros, G.; Terracciano, L.M.; Zlobec, I. Intratumoral Budding as a Potential Parameter of Tumor Progression in Mismatch Repair–Proficient and Mismatch Repair–Deficient Colorectal Cancer Patients. Hum. Pathol. 2011, 42, 1833–1840. [Google Scholar] [CrossRef]
- Chong, G.O.; Jee-Young Park, N.; Han, H.S.; Cho, J.; Kim, M.-G.; Choi, Y.; Yeo, J.Y.; Lee, Y.H.; Hong, D.G.; Park, J.Y. Intratumoral Budding: A Novel Prognostic Biomarker for Tumor Recurrence and a Potential Predictor of Nodal Metastasis in Uterine Cervical Cancer. Eur. J. Surg. Oncol. 2021, 47, 3182–3187. [Google Scholar] [CrossRef]
- Miyazaki, M.; Aoki, M.; Okado, Y.; Koga, K.; Hamasaki, M.; Kiyomi, F.; Sakata, T.; Nakagawa, T.; Nabeshima, K. Poorly Differentiated Clusters Predict a Poor Prognosis for External Auditory Canal Carcinoma. Head Neck Pathol. 2019, 13, 198–207. [Google Scholar] [CrossRef]
- Moorman, A.M.; Vink, R.; Heijmans, H.J.; van der Palen, J.; Kouwenhoven, E.A. The Prognostic Value of Tumour-Stroma Ratio in Triple-Negative Breast Cancer. Eur. J. Surg. Oncol. 2012, 38, 307–313. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Liu, W.; Liu, X. Prognostic Significance of the Tumor-Stroma Ratio in Epithelial Ovarian Cancer. Biomed. Res. Int. 2015, 2015, 589301. [Google Scholar] [CrossRef]
- Alvi, M.A.; Wilson, R.H.; Salto-Tellez, M. Rare Cancers: The Greatest Inequality in Cancer Research and Oncology Treatment. Br. J. Cancer 2017, 117, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.; Sahm, F.; Aldape, K. DNA Methylation Profiling as a Model for Discovery and Precision Diagnostics in Neuro-Oncology. Neuro Oncol. 2021, 23, S16–S29. [Google Scholar] [CrossRef] [PubMed]
- Klamminger, G.G.; Bitterlich, A.; Nigdelis, M.P.; Hamoud, B.H.; Solomayer, E.F.; Wagner, M. Comparison of Different Histomorphological Grading Systems in Vulvar Squamous Cell Carcinoma. Arch. Gynecol. Obstet. 2024, 310, 3091–3097. [Google Scholar] [CrossRef]
- Schwab, R.; Schiestl, L.J.; Cascant Ortolano, L.; Klecker, P.H.; Schmidt, M.W.; Almstedt, K.; Heimes, A.-S.; Brenner, W.; Stewen, K.; Schmidt, M.; et al. Efficacy of Pembrolizumab in Advanced Cancer of the Vulva: A Systematic Review and Single-Arm Meta-Analysis. Front. Oncol. 2024, 14, 1352975. [Google Scholar] [CrossRef]
- Amant, F.; van Velzen, A.F.; Reyners, A.; Zijlmans, H.; Schaake, E.E.; Nooij, L. Primary Chemoradiation versus Neoadjuvant Chemotherapy Followed by Surgery as Treatment Strategy for Locally Advanced Vulvar Carcinoma (VULCANize2). Int. J. Gynecol. Cancer 2024, 34, 1639–1642. [Google Scholar] [CrossRef]
- Kuhn, T.M.; Ahmad, S.; Recio, F.O.; Awada, A.; McKenzie, N.D.; Kendrick, J.E.; Keller, A.; Holloway, R.W. Neoadjuvant Chemotherapy with Bevacizumab for Locally Advanced Vulvar Cancer. Int. J. Gynecol. Cancer 2024, 34, 977–984. [Google Scholar] [CrossRef]
- Mazzotta, M.; Pizzuti, L.; Krasniqi, E.; Di Lisa, F.S.; Cappuzzo, F.; Landi, L.; Sergi, D.; Pelle, F.; Cappelli, S.; Botti, C.; et al. Role of Chemotherapy in Vulvar Cancers: Time to Rethink Standard of Care? Cancers 2021, 13, 4061. [Google Scholar] [CrossRef]
- Klamminger, G.G.; Bitterlich, A.; Haj Hamoud, B.; Solomayer, E.F.; Ertz, M.; Hasenburg, A.; Wagner, M.; Nigdelis, M. Evaluation of Tumor Budding, Poorly Defined Clusters, and Tumor-Stroma Ratio as Histological Biomarkers in Squamous Cell Carcinoma of the Vulva. Int. J. Gynecol. Cancer 2025, 35, 100419. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klamminger, G.G.; Bitterlich, A.; Haj Hamoud, B.; Solomayer, E.-F.; Ertz, M.; Schnöder, L.; Holleczek, B.; Brenner, W.; Hasenburg, A.; Wagner, M.; et al. Evaluation of Tumor Budding and Poorly Defined Clusters as Histological Biomarkers in Squamous Cell Carcinomas of the Vulva. Cancers 2025, 17, 1718. https://doi.org/10.3390/cancers17101718
Klamminger GG, Bitterlich A, Haj Hamoud B, Solomayer E-F, Ertz M, Schnöder L, Holleczek B, Brenner W, Hasenburg A, Wagner M, et al. Evaluation of Tumor Budding and Poorly Defined Clusters as Histological Biomarkers in Squamous Cell Carcinomas of the Vulva. Cancers. 2025; 17(10):1718. https://doi.org/10.3390/cancers17101718
Chicago/Turabian StyleKlamminger, Gilbert Georg, Annick Bitterlich, Bashar Haj Hamoud, Erich-Franz Solomayer, Martin Ertz, Laura Schnöder, Bernd Holleczek, Walburgis Brenner, Annette Hasenburg, Mathias Wagner, and et al. 2025. "Evaluation of Tumor Budding and Poorly Defined Clusters as Histological Biomarkers in Squamous Cell Carcinomas of the Vulva" Cancers 17, no. 10: 1718. https://doi.org/10.3390/cancers17101718
APA StyleKlamminger, G. G., Bitterlich, A., Haj Hamoud, B., Solomayer, E.-F., Ertz, M., Schnöder, L., Holleczek, B., Brenner, W., Hasenburg, A., Wagner, M., & Nigdelis, M. P. (2025). Evaluation of Tumor Budding and Poorly Defined Clusters as Histological Biomarkers in Squamous Cell Carcinomas of the Vulva. Cancers, 17(10), 1718. https://doi.org/10.3390/cancers17101718






