Expression Characteristics of 3-Marker Panel (PAX2, PTEN, and β-Catenin) in Benign Interval and Secretory Endometrium and Secretory Endometrial Precancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Immunohistochemistry
2.3. Interpretation of 3-Marker Panel
2.4. Statistical Analysis
3. Results
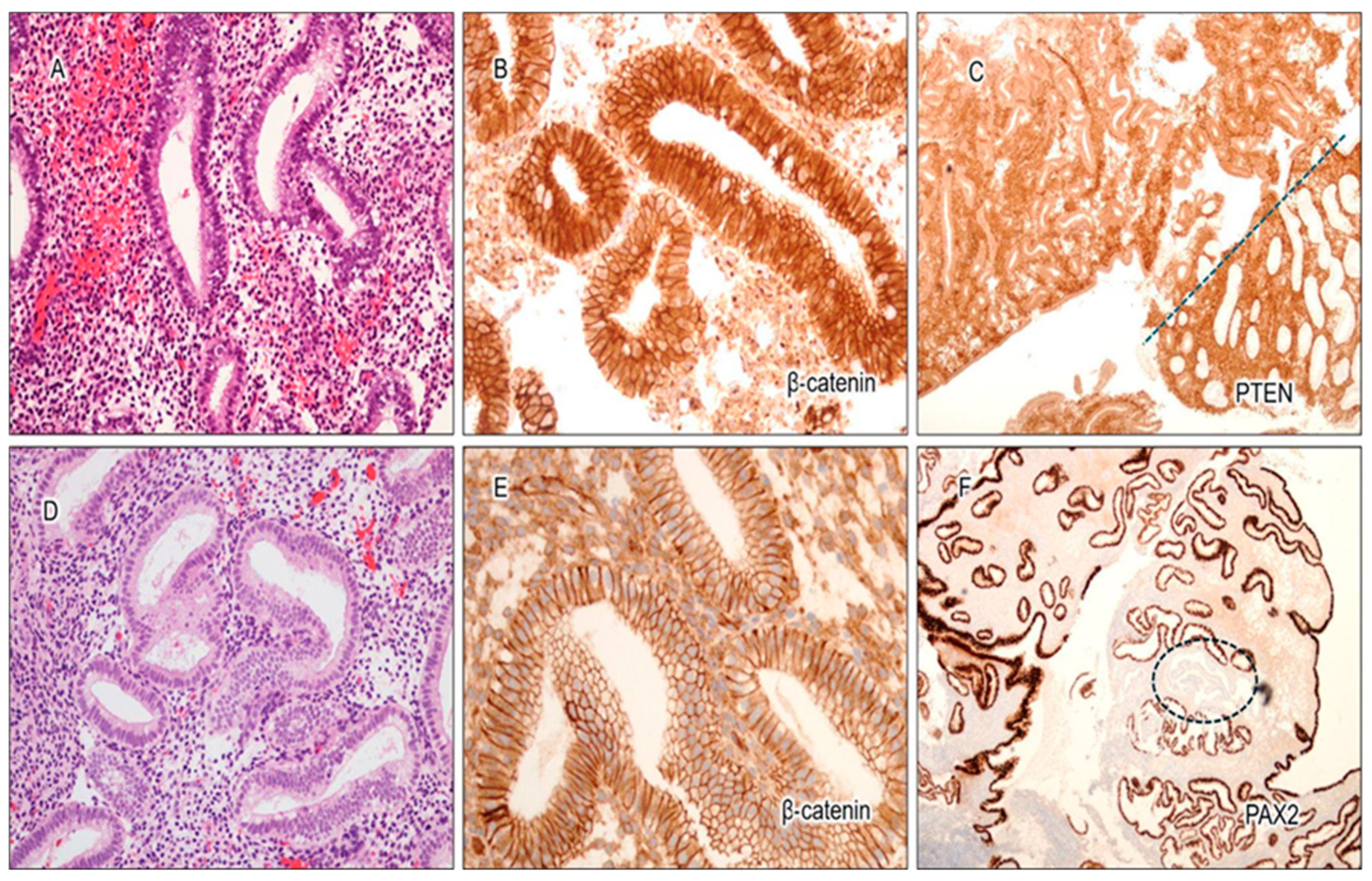
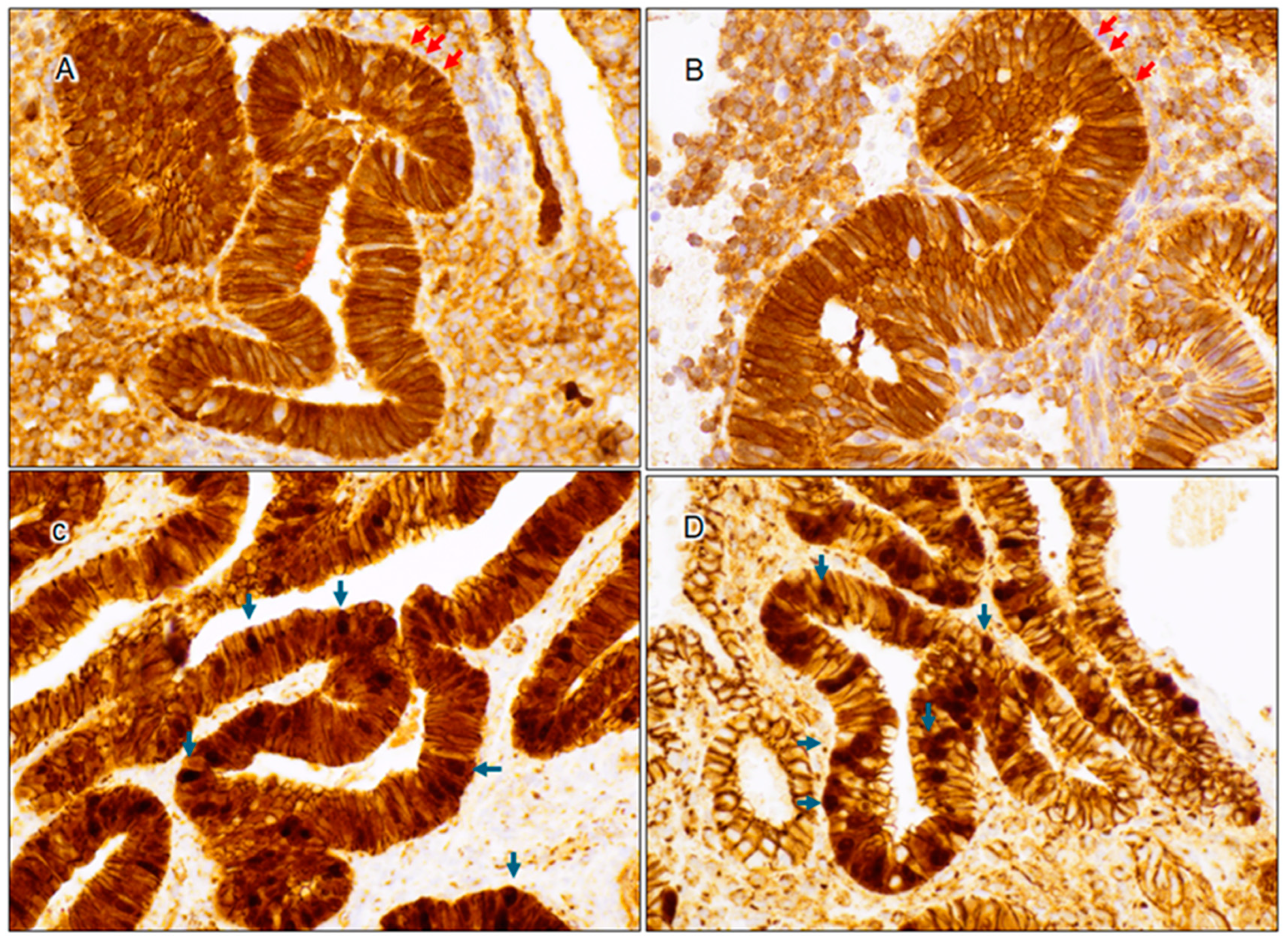
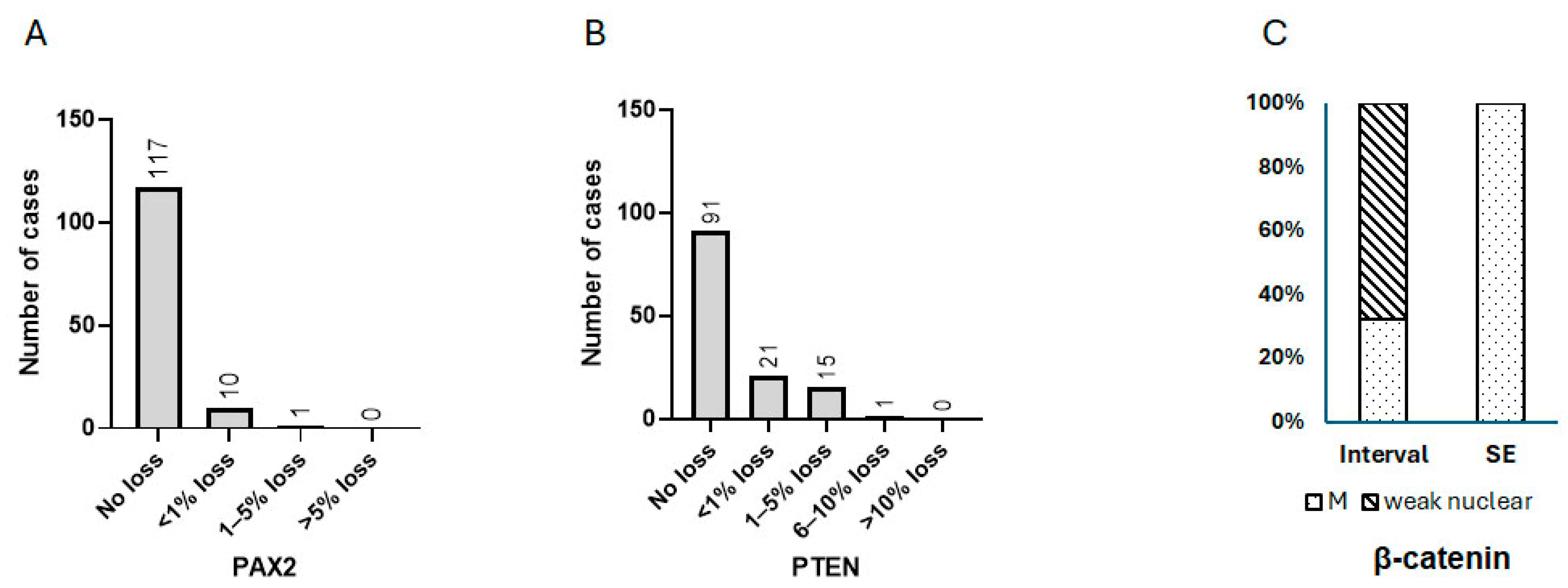
3.1. Expression Characteristics of PAX2, PTEN, and β-Catenin in Benign Interval and Secretory Endometrium
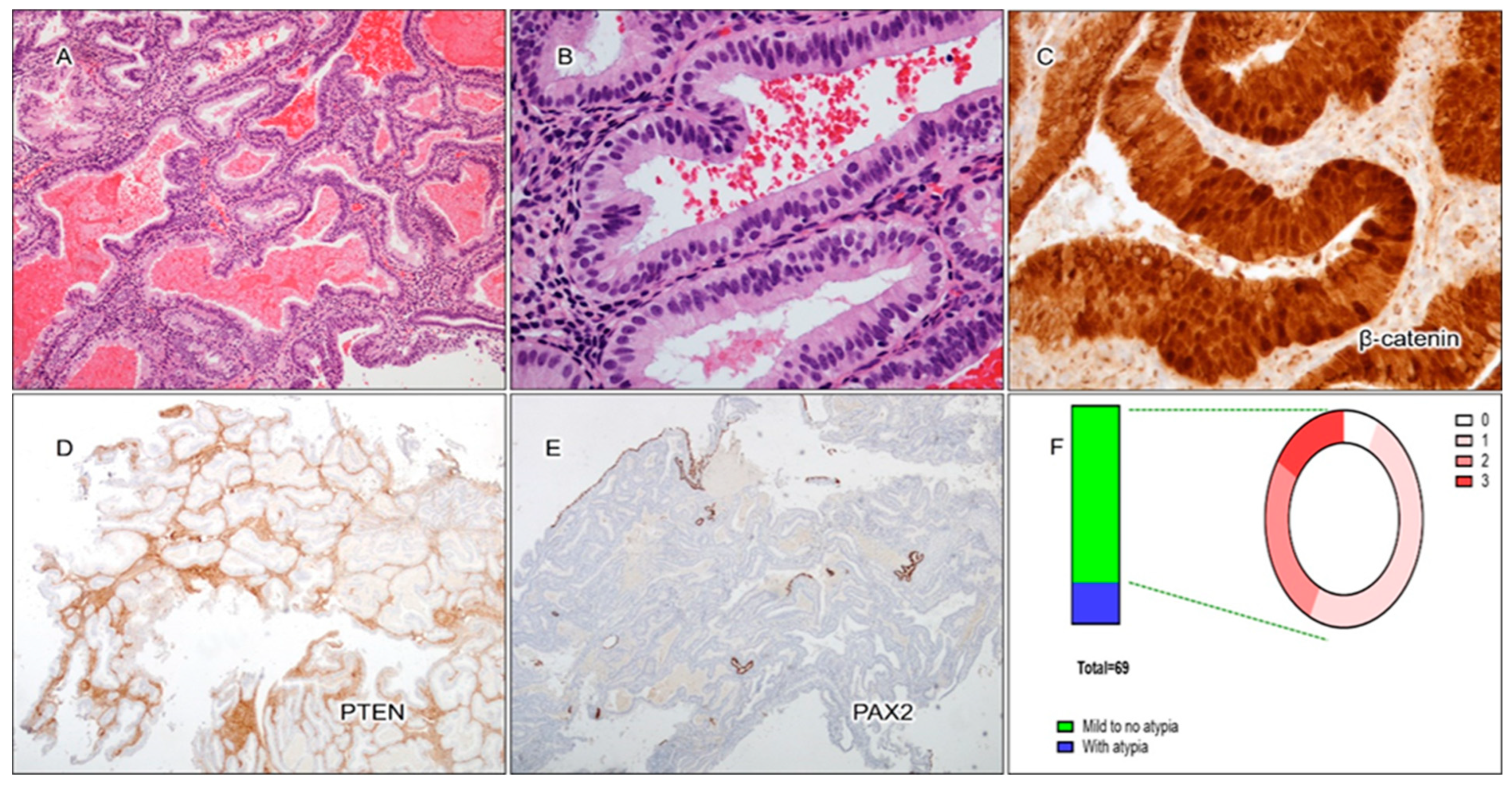
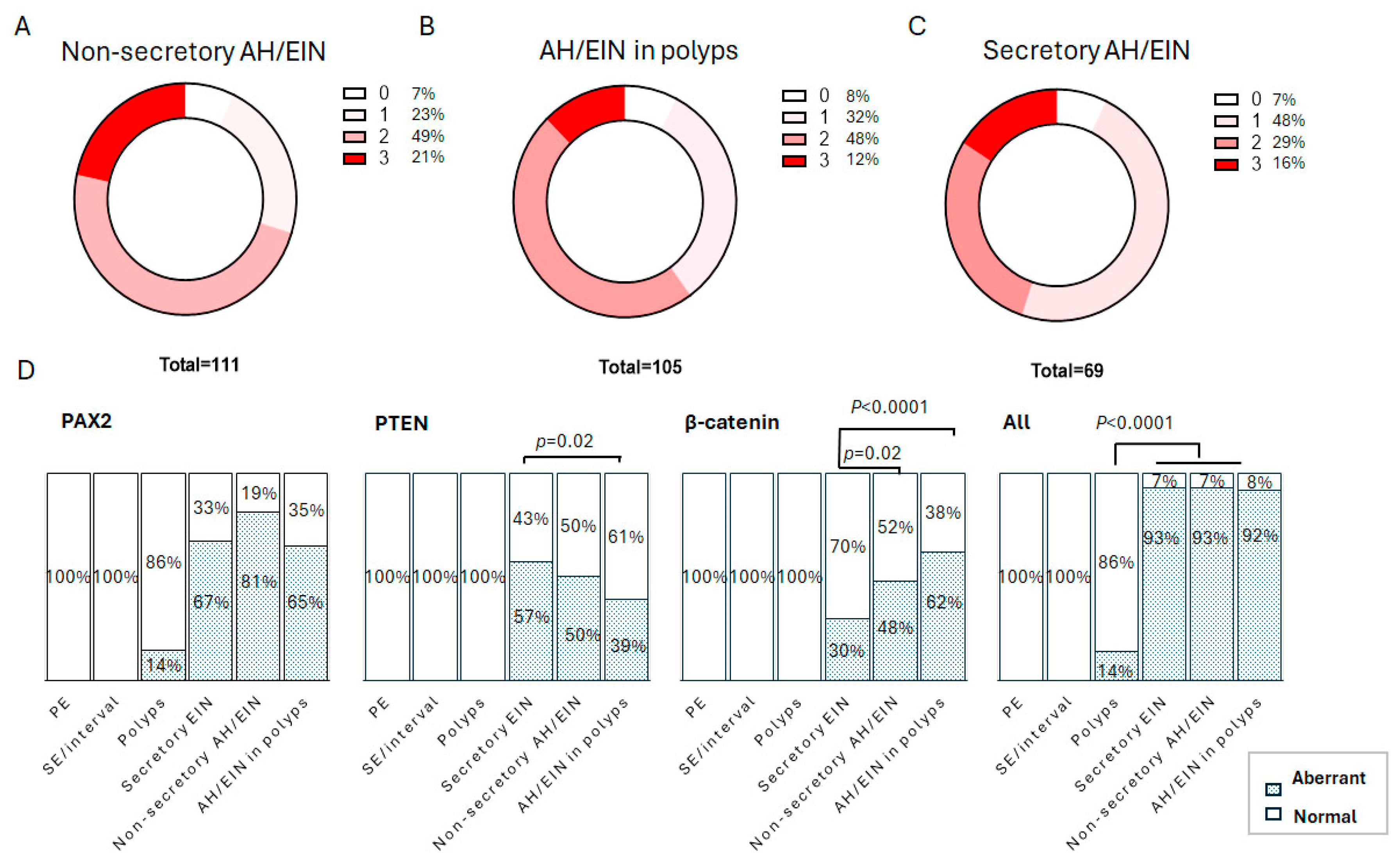
3.2. Cytological Analysis and Analysis of PAX2, PTEN, and β-Catenin in Secretory AH/EIN
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Constantine, G.D.; Kessler, G.; Graham, S.; Goldstein, S.R. Increased Incidence of Endometrial Cancer Following the Women’s Health Initiative: An Assessment of Risk Factors. J. Womens Health 2019, 28, 237–243. [Google Scholar] [CrossRef]
- World Health Organization. WHO Classification of Tumours of Female Genital Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 4. [Google Scholar]
- Niu, S.; Molberg, K.; Castrillon, D.H.; Lucas, E.; Chen, H. Biomarkers in the Diagnosis of Endometrial Precancers. Molecular Characteristics, Candidate Immunohistochemical Markers, and Promising Results of Three-Marker Panel: Current Status and Future Directions. Cancers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Villacorte, M.; Suzuki, K.; Hirasawa, A.; Ohkawa, Y.; Suyama, M.; Maruyama, T.; Aoki, D.; Ogino, Y.; Miyagawa, S.; Terabayashi, T.; et al. beta-Catenin signaling regulates Foxa2 expression during endometrial hyperplasia formation. Oncogene 2013, 32, 3477–3482. [Google Scholar] [CrossRef]
- Morin, P.J. beta-catenin signaling and cancer. Bioessays 1999, 21, 1021–1030. [Google Scholar] [CrossRef]
- Aguilar, M.; Chen, H.; Rivera-Colon, G.; Niu, S.; Carrick, K.; Gwin, K.; Cuevas, I.C.; Sahoo, S.S.; Li, H.D.; Zhang, S.; et al. Reliable Identification of Endometrial Precancers Through Combined Pax2, β-Catenin, and Pten Immunohistochemistry. Am. J. Surg. Pathol. 2022, 46, 404–414. [Google Scholar] [CrossRef]
- Monte, N.M.; Webster, K.A.; Neuberg, D.; Dressler, G.R.; Mutter, G.L. Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer. Cancer Res. 2010, 70, 6225–6232. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Liang, J.; Shi, B.; Wu, G.; Zhang, Y.; Wang, D.; Li, R.; Yi, X.; Zhang, H.; et al. Hypomethylation-linked activation of PAX2 mediates tamoxifen-stimulated endometrial carcinogenesis. Nature 2005, 438, 981–987. [Google Scholar] [CrossRef]
- Moreno-Bueno, G.; Hardisson, D.; Sarrio, D.; Sanchez, C.; Cassia, R.; Prat, J.; Herman, J.G.; Esteller, M.; Matias-Guiu, X.; Palacios, J. Abnormalities of E- and P-cadherin and catenin (beta-, gamma-catenin, and p120ctn) expression in endometrial cancer and endometrial atypical hyperplasia. J. Pathol. 2003, 199, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Cargile, C.B.; Blazes, M.S.; van Rees, B.; Kurman, R.J.; Ellenson, L.H. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998, 58, 3254–3258. [Google Scholar]
- Maxwell, G.L.; Risinger, J.I.; Gumbs, C.; Shaw, H.; Bentley, R.C.; Barrett, J.C.; Berchuck, A.; Futreal, P.A. Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Res. 1998, 58, 2500–2503. [Google Scholar] [PubMed]
- Duggan, B.D.; Felix, J.C.; Muderspach, L.I.; Tsao, J.L.; Shibata, D.K. Early mutational activation of the c-Ki-ras oncogene in endometrial carcinoma. Cancer Res. 1994, 54, 1604–1607. [Google Scholar]
- Mutter, G.L.; Wada, H.; Faquin, W.C.; Enomoto, T. K-ras mutations appear in the premalignant phase of both microsatellite stable and unstable endometrial carcinogenesis. Mol. Pathol. 1999, 52, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Nishii, H.; Takahashi, H.; Tada, A.; Furusato, M.; Terashima, Y.; Siegal, G.P.; Parker, S.L.; Kohler, M.F.; Berchuck, A.; et al. Mutation of the Ki-ras protooncogene in human endometrial hyperplasia and carcinoma. Cancer Res. 1993, 53, 1906–1910. [Google Scholar]
- Werner, H.M.; Berg, A.; Wik, E.; Birkeland, E.; Krakstad, C.; Kusonmano, K.; Petersen, K.; Kalland, K.H.; Oyan, A.M.; Akslen, L.A.; et al. ARID1A loss is prevalent in endometrial hyperplasia with atypia and low-grade endometrioid carcinomas. Mod. Pathol. 2013, 26, 428–434. [Google Scholar] [CrossRef]
- Patricio, P.; Ramalho-Carvalho, J.; Costa-Pinheiro, P.; Almeida, M.; Barros-Silva, J.D.; Vieira, J.; Dias, P.C.; Lobo, F.; Oliveira, J.; Teixeira, M.R.; et al. Deregulation of PAX2 expression in renal cell tumours: Mechanisms and potential use in differential diagnosis. J. Cell. Mol. Med. 2013, 17, 1048–1058. [Google Scholar] [CrossRef]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef]
- Lucas, E.; Niu, S.; Aguilar, M.; Molberg, K.; Carrick, K.; Rivera-Colon, G.; Gwin, K.; Wang, Y.; Zheng, W.; Castrillon, D.H.; et al. Utility of a PAX2, PTEN, and β-catenin Panel in the Diagnosis of Atypical Hyperplasia/Endometrioid Intraepithelial Neoplasia in Endometrial Polyps. Am. J. Surg. Pathol. 2023, 47, 1019–1026. [Google Scholar] [CrossRef]
- Wyvekens, N.; Mutter, G.L.; Nucci, M.R.; Kolin, D.L.; Parra-Herran, C. Lesions sub-diagnostic of endometrioid intra-epithelial neoplasia/atypical hyperplasia: Value of morphology and immunohistochemistry in predicting neoplastic outcome. Histopathology 2024, 85, 579–589. [Google Scholar] [CrossRef]
- Chen, H.; Lucas, E.; Strickland, A.L.; Carrick, K.; Gwin, K.; Castrillon, D.H.; Rivera-Colon, G.; Niu, S.; Molberg, K.H.; Zheng, W. Specific Biomarker Expression Patterns in the Diagnosis of Residual and Recurrent Endometrial Precancers After Progestin Treatment: A Longitudinal Study. Am. J. Surg. Pathol. 2020, 44, 1429–1439. [Google Scholar] [CrossRef]
- Mutter, G.L.; Ince, T.A.; Baak, J.P.; Kust, G.A.; Zhou, X.P.; Eng, C. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res. 2001, 61, 4311–4314. [Google Scholar] [PubMed]
- Ronsini, C.; Iavarone, I.; Vastarella, M.G.; Della Corte, L.; Andreoli, G.; Bifulco, G.; Cobellis, L.; De Franciscis, P. SIR-EN—New Biomarker for Identifying Patients at Risk of Endometrial Carcinoma in Abnormal Uterine Bleeding at Menopause. Cancers 2024, 16, 3567. [Google Scholar] [CrossRef]
- Vizza, E.; Corrado, G.; De Angeli, M.; Carosi, M.; Mancini, E.; Baiocco, E.; Chiofalo, B.; Patrizi, L.; Zampa, A.; Piaggio, G.; et al. Serum DNA Integrity Index as a Potential Molecular Biomarker in Endometrial Cancer. J. Exp. Clin. Cancer Res. 2018, 37, 1–9. [Google Scholar] [CrossRef]
- Cicchillitti, L.; Corrado, G.; De Angeli, M.; Mancini, E.; Baiocco, E.; Patrizi, L.; Zampa, A.; Merola, R.; Martayan, R.; Conti, L.; et al. Circulating Cell-Free DNA Content as Blood Based Biomarker in Endometrial Cancer. Oncotarget 2017, 8, 115230. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.L.; Gorsia, D.N.; Collins, A.; Sandhu, P.; Foreman, N.; Gore, A.; Wood, J.; Kent, C.; Silcock, L.; Guttery, D.S. Utility of Circulating Tumor DNA for Detection and Monitoring of Endometrial Cancer Recurrence and Progression. Cancers 2020, 12, 2231. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Tsuda, H.; Nishimura, S.; Nomura, H.; Kataoka, F.; Chiyoda, T.; Tanaka, K.; Iguchi, Y.; Susumu, N.; Aoki, D. Role of Circulating Free Alu DNA in Endometrial Cancer. Int. J. Gynecol. Cancer 2012, 22, 82–86. [Google Scholar] [CrossRef]
- Cisneros-Villanueva, M.; Hidalgo-Pérez, L.; Rios-Romero, M.; Cedro-Tanda, A.; Ruiz-Villavicencio, C.A.; Page, K.; Hastings, R.; Fernandez-Garcia, D.; Allsopp, R.; Fonseca-Montaño, M.A.; et al. Cell-free DNA analysis in current cancer clinical trials: A review. Br. J. Cancer 2022, 126, 391–400. [Google Scholar] [CrossRef]

| Markers | Results | No/Mild Nuclear Atypia | With Nuclear Atypia | Total |
|---|---|---|---|---|
| PAX2 | Intact | 19 (34%) | 4 (31%) | 23 (33%) |
| Loss | 37 (66%) | 9 (69%) | 46 (67%) | |
| PTEN | Intact | 26 (46%) | 4 (31%) | 30 (43%) |
| Loss | 30 (54%) | 9 (69%) | 39 (57%) | |
| β-catenin | Membranous | 38 (68%) | 10 (77%) | 48 (70%) |
| Nuclear | 18 (32%) | 3 (23%) | 21 (30%) | |
| Aberrant | All 3 | 9 (16%) | 2 (15%) | 11 (16%) |
| PAX2 + PTEN | 9 (16%) | 4 (31%) | 13 (19%) | |
| PAX2 + β-catenin | 5 (9%) | 1 (8%) | 6 (9%) | |
| PTEN + β-catenin | 1 (2%) | 0 (0%) | 1 (1%) | |
| All normal | 4 (7%) | 1 (8%) | 5 (7%) | |
| Total | 56 | 13 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, S.; Molberg, K.; Chen, J.; Conrad, L.; Lucas, E.; Chen, H. Expression Characteristics of 3-Marker Panel (PAX2, PTEN, and β-Catenin) in Benign Interval and Secretory Endometrium and Secretory Endometrial Precancer. Cancers 2025, 17, 1495. https://doi.org/10.3390/cancers17091495
Niu S, Molberg K, Chen J, Conrad L, Lucas E, Chen H. Expression Characteristics of 3-Marker Panel (PAX2, PTEN, and β-Catenin) in Benign Interval and Secretory Endometrium and Secretory Endometrial Precancer. Cancers. 2025; 17(9):1495. https://doi.org/10.3390/cancers17091495
Chicago/Turabian StyleNiu, Shuang, Kyle Molberg, Jackson Chen, Lesley Conrad, Elena Lucas, and Hao Chen. 2025. "Expression Characteristics of 3-Marker Panel (PAX2, PTEN, and β-Catenin) in Benign Interval and Secretory Endometrium and Secretory Endometrial Precancer" Cancers 17, no. 9: 1495. https://doi.org/10.3390/cancers17091495
APA StyleNiu, S., Molberg, K., Chen, J., Conrad, L., Lucas, E., & Chen, H. (2025). Expression Characteristics of 3-Marker Panel (PAX2, PTEN, and β-Catenin) in Benign Interval and Secretory Endometrium and Secretory Endometrial Precancer. Cancers, 17(9), 1495. https://doi.org/10.3390/cancers17091495






