Protein Biomarkers Enable Sensitive and Specific Cervical Intraepithelial Neoplasia (CIN) II/III+ Detection: One Step Closer to Universal Cervical Cancer Screening
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Comparison of Buffer Efficiency in Cell Lysis and Protein Recovery from Cultured Cervical Cells
2.3. Protein Extraction from Clinical Cervical Swab Samples
2.4. Biomarker Detection and Quantification Using ELISAs
2.5. Biomarker Detection Using ICC
2.6. IHC on Cervical TMAs
2.7. Statistical Analyses
3. Results
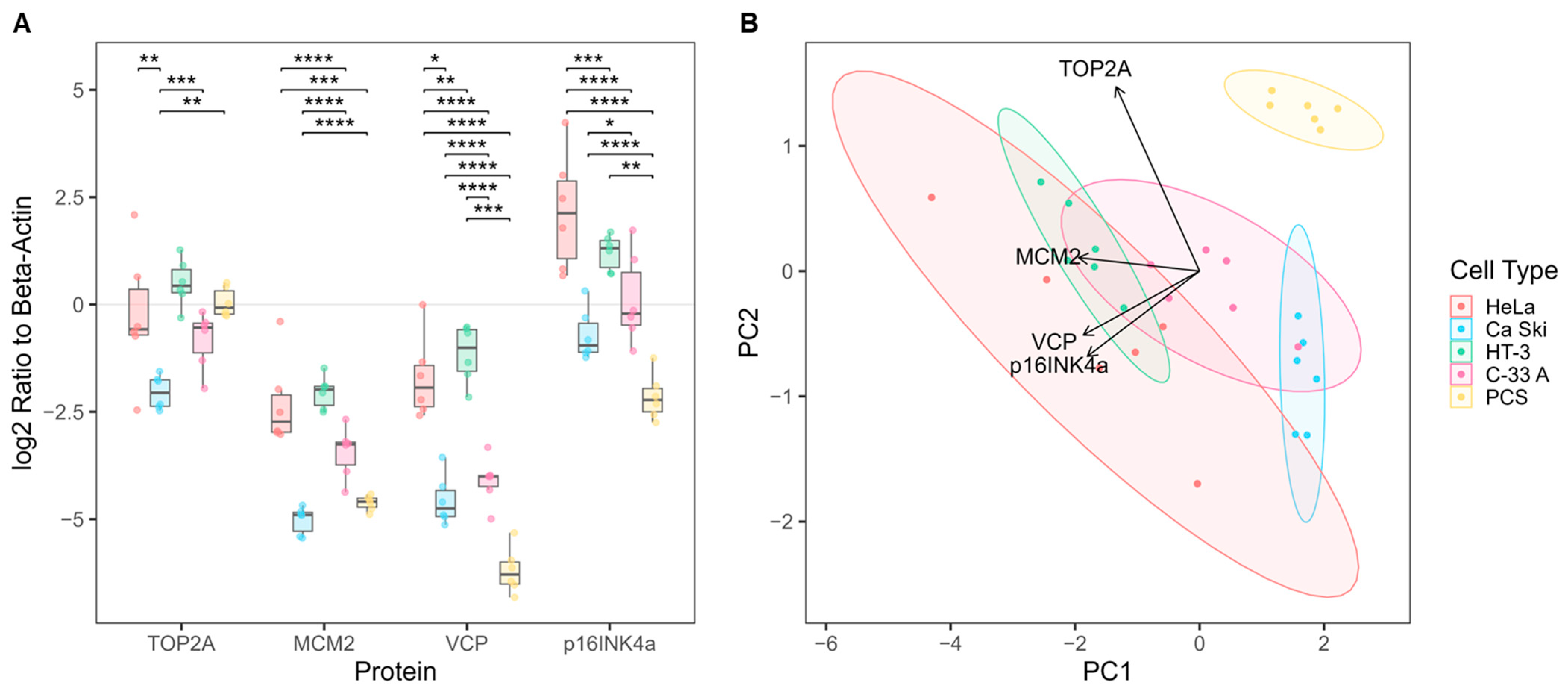
3.1. Comparative Analysis of Protein Yield from CC Cell Lines and PCS Cells Using Different Lysis Buffers
3.2. Biomarker Expression in Cultured Cervical Cells
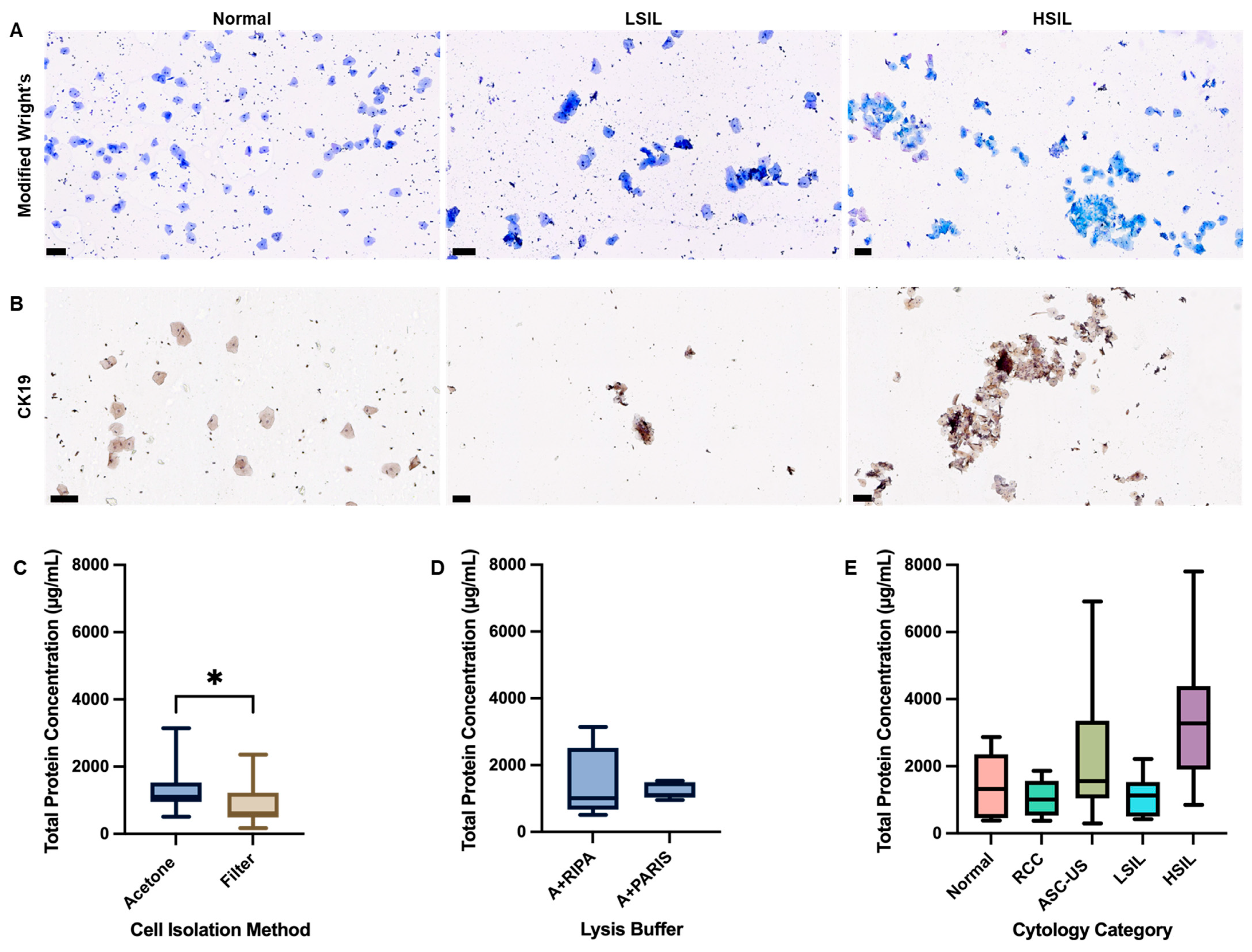
3.3. Optimization and Clinical Translation of Total Protein Extraction from Clinical Cervical Swab Samples
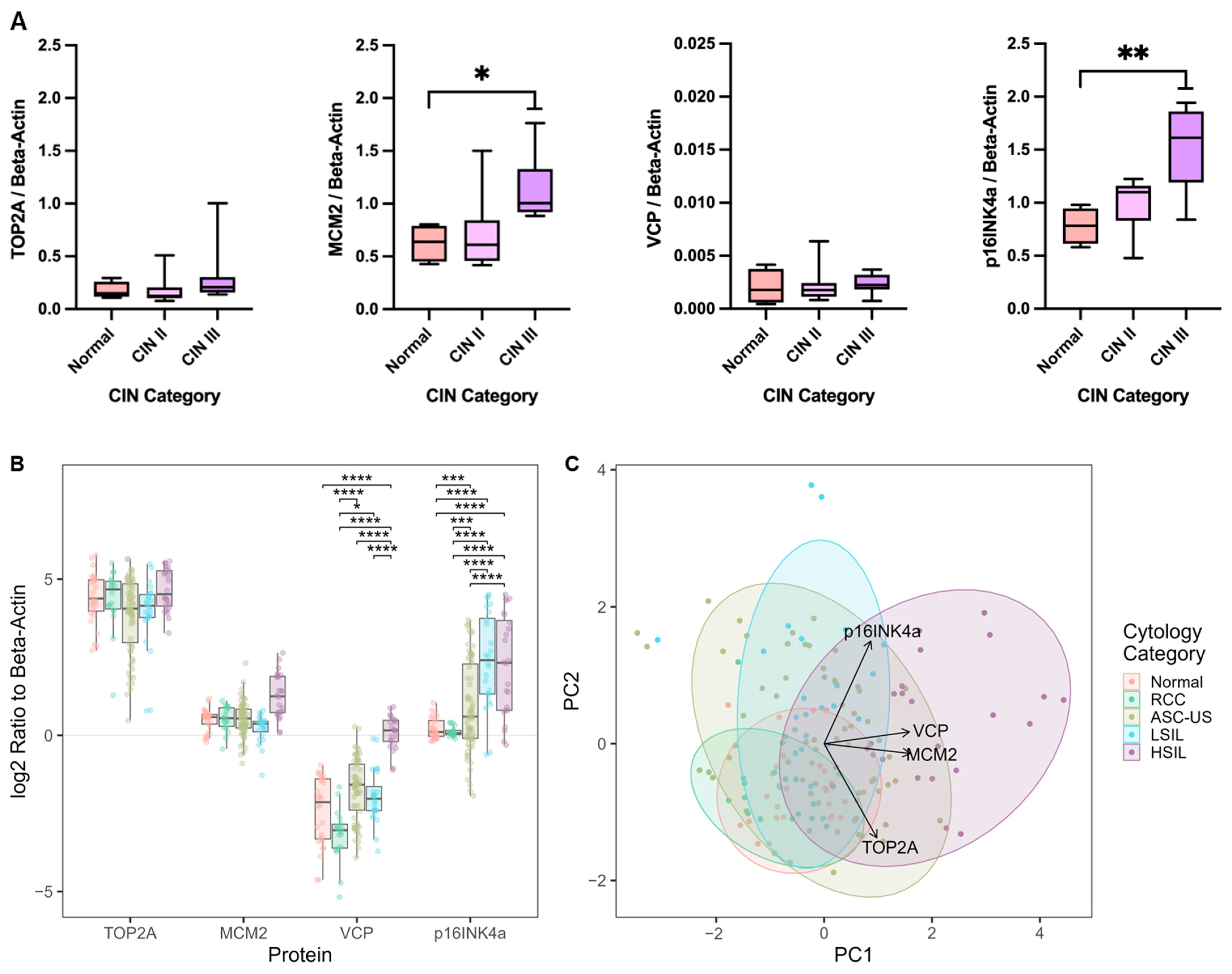
3.4. Biomarker Expression in Clinical Cervical Swab Samples
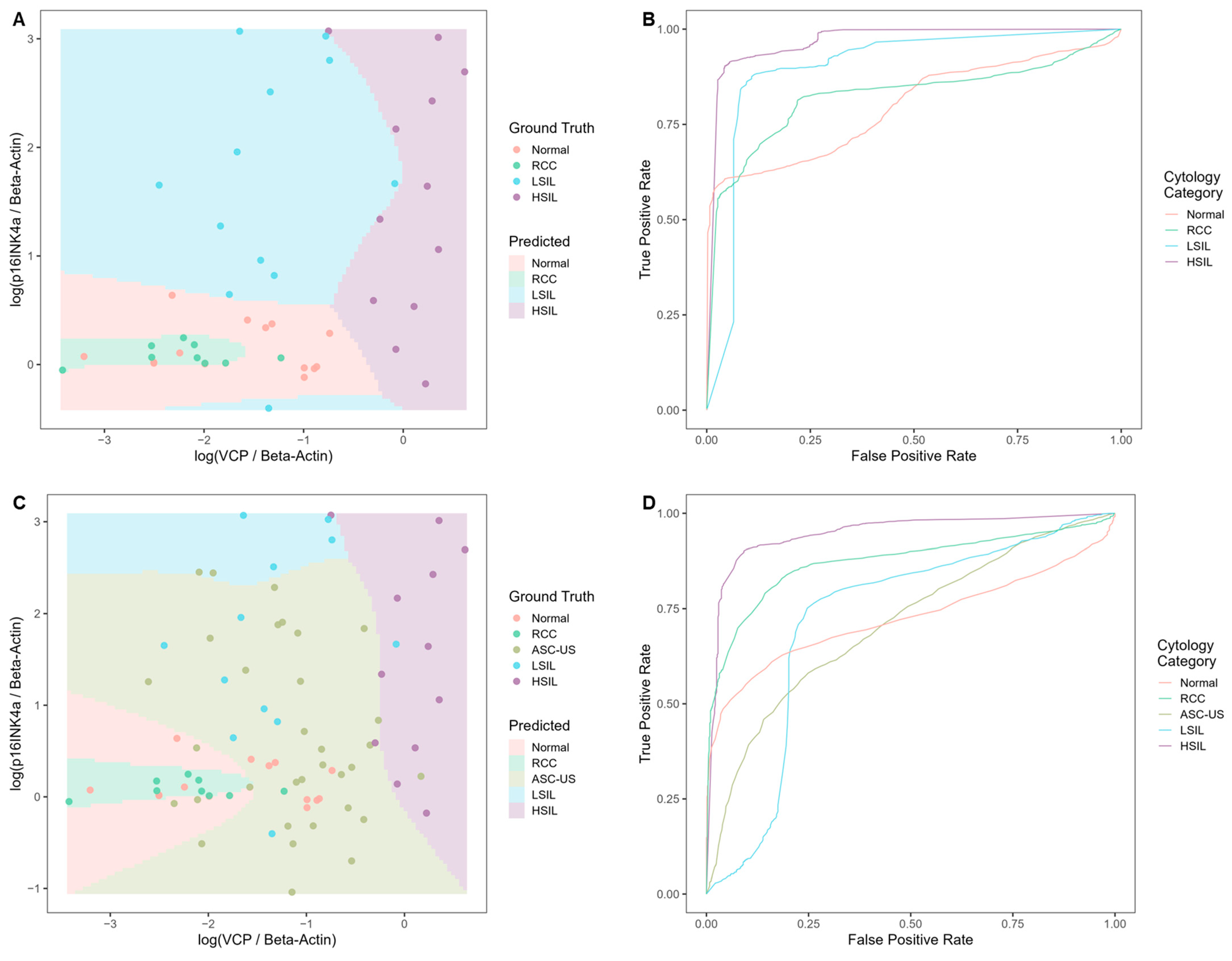
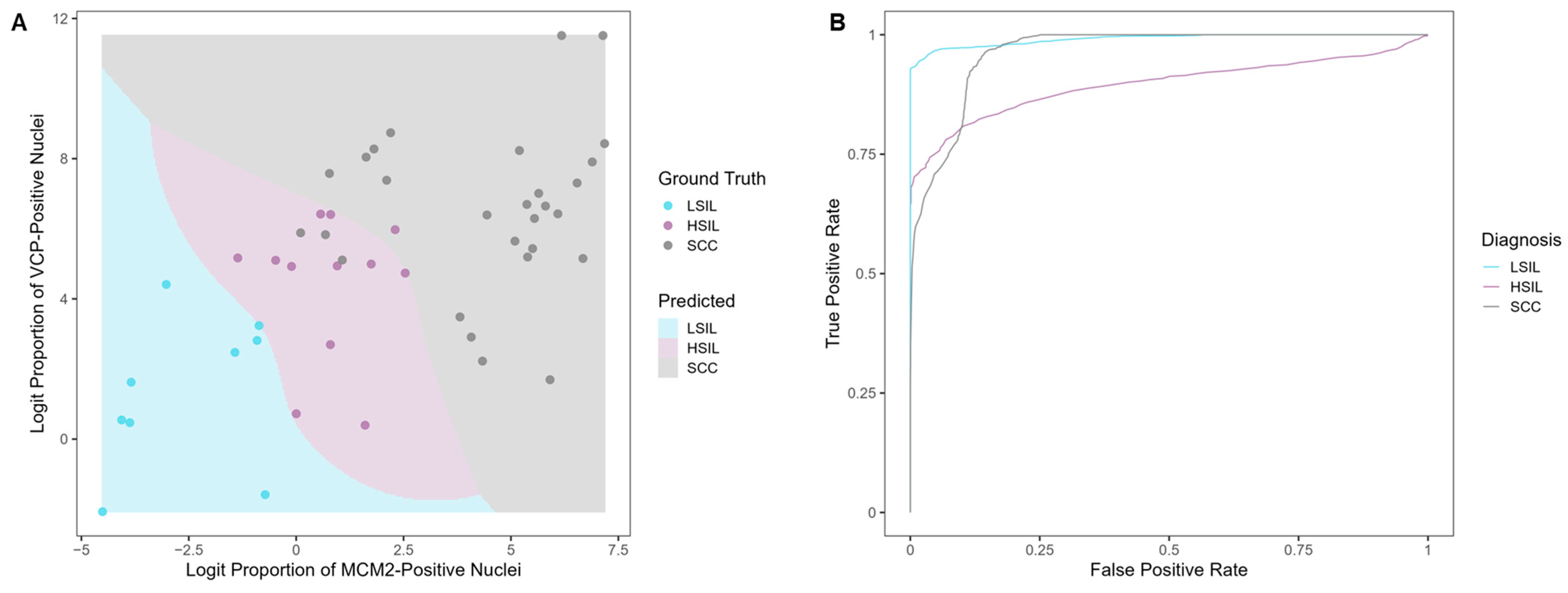
Evaluating the Classification Potential of the Four Biomarkers in Lysates of Clinical Cervical Swab Specimens
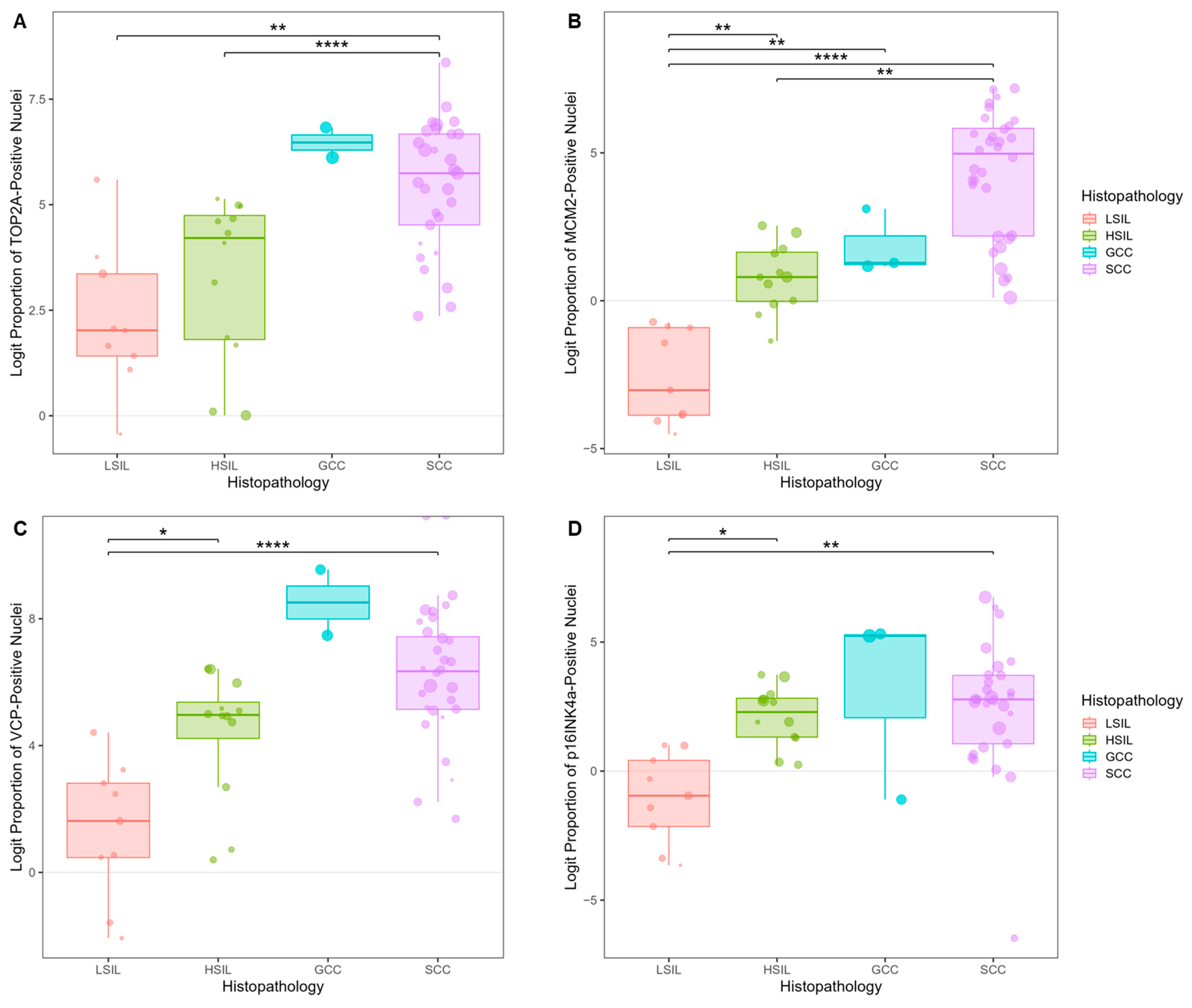
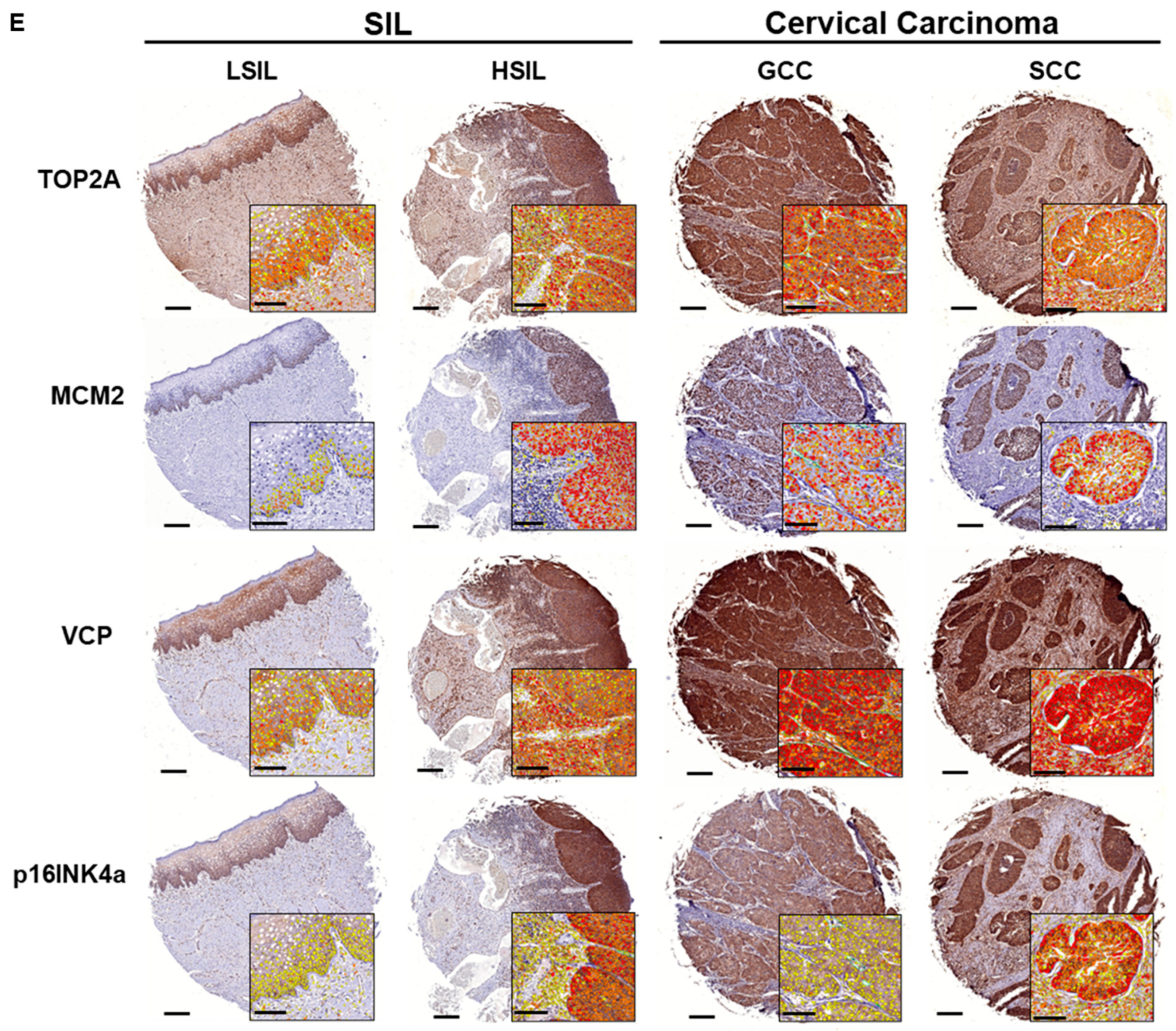
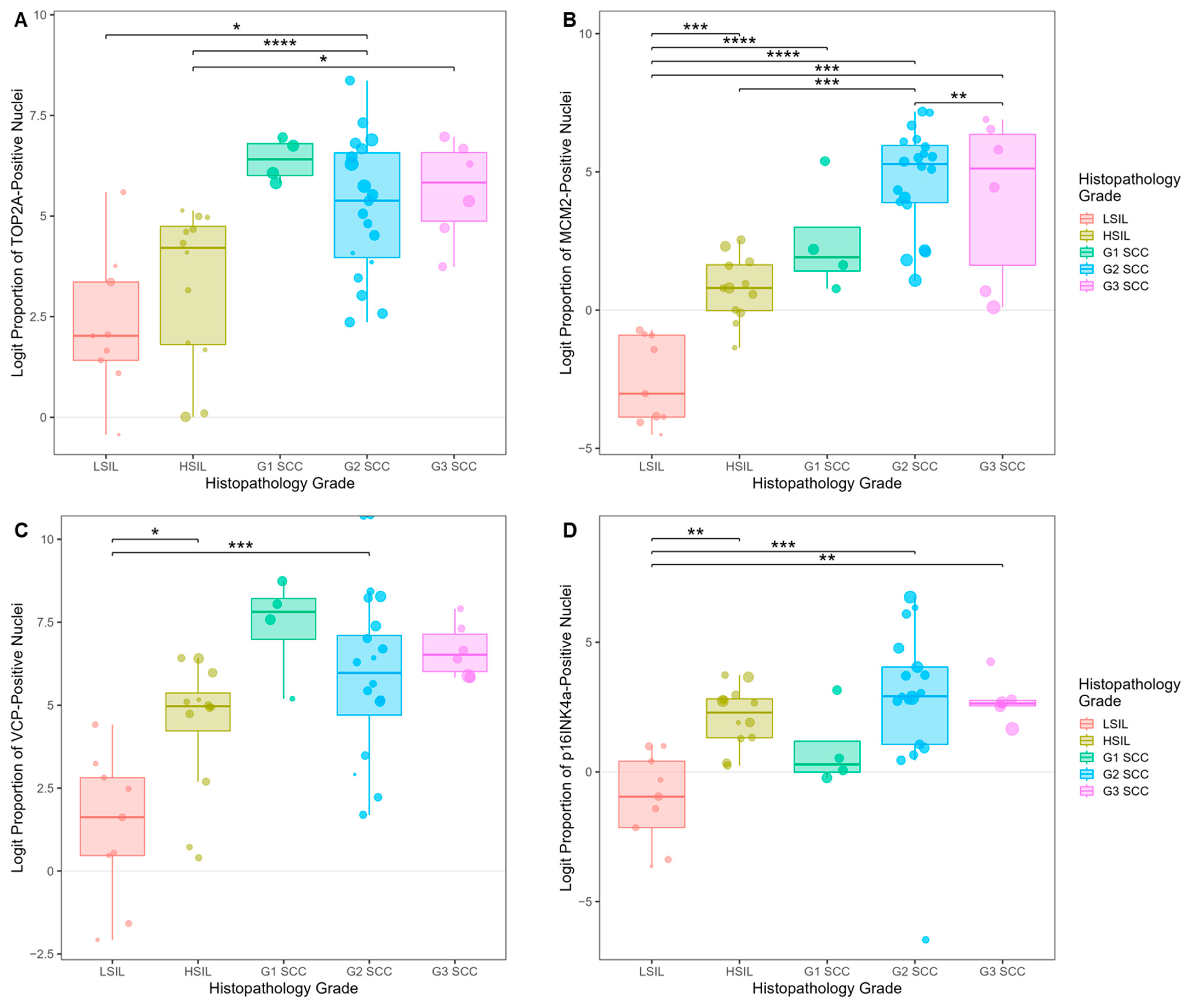
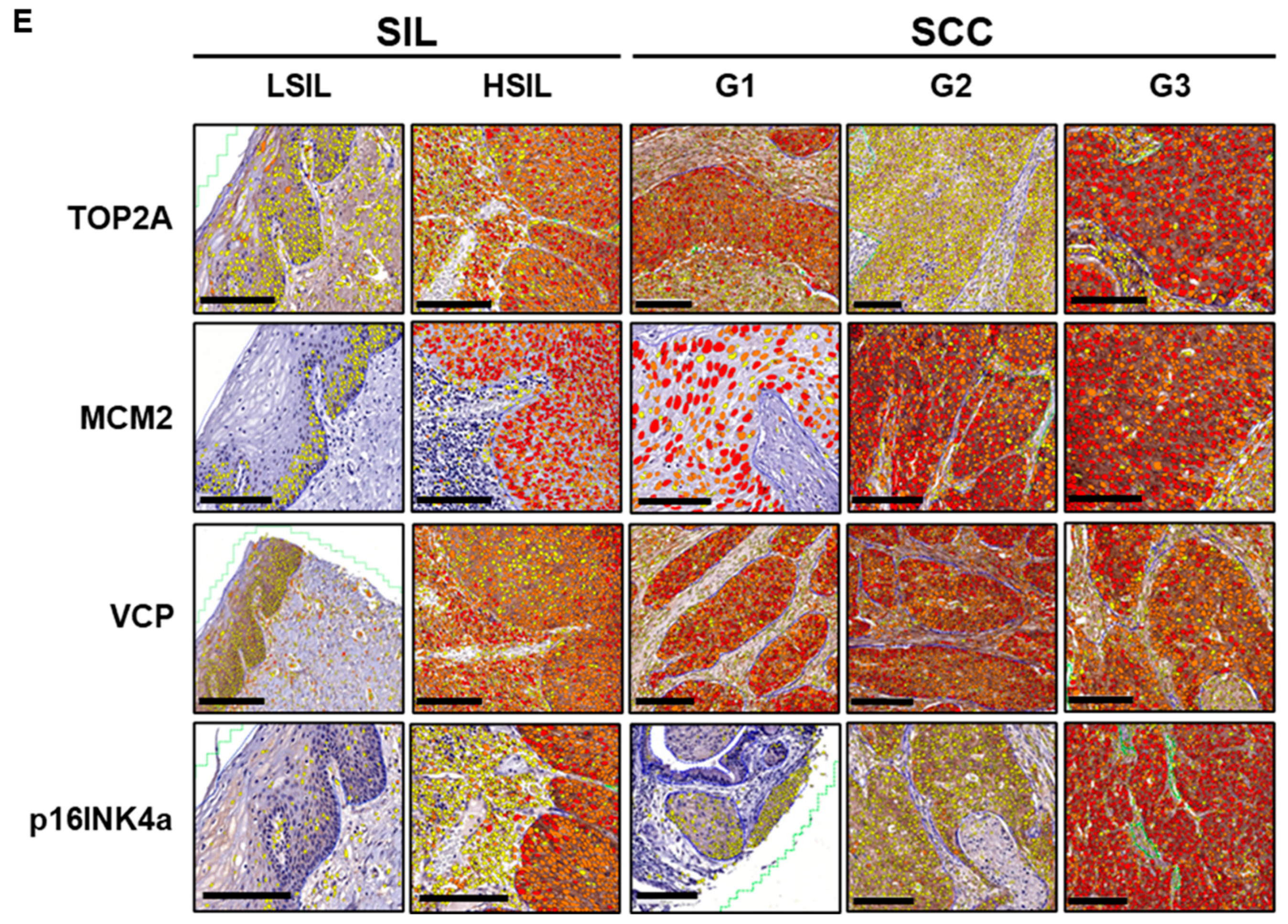
3.5. Biomarker Expression in Cervical Precancer and Cancer Tissues
3.5.1. Biomarker Expression Trends in Tissues of Cervical SILs and CC Subtypes
3.5.2. Biomarker Expression Trends in Tissues of Cervical SILs and Different Histopathology Grades of SCC
3.5.3. Evaluating the Classification Potential of the Four Biomarkers in Cervical Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Adenocarcinoma |
| ASC-US | Atypical squamous cells of undetermined significance |
| ATCC | American Type Culture Collection |
| BCA | Bicinchoninic acid |
| BER | Balanced error rate |
| BSA | Bovine serum albumin |
| CC | Cervical cancer/carcinoma |
| CIN | Cervical intraepithelial neoplasia |
| CK19 | Cytokeratin 19 |
| DAB | 3,3′-diaminobenzidine |
| ELISA | Enzyme-linked immunosorbent assay |
| GAM | Generalized additive model |
| GCC | Glassy cell carcinoma |
| GLMM | Generalized linear mixed model |
| HPV | Human papillomavirus |
| HRP | Horseradish peroxidase |
| HSIL | High-grade squamous intraepithelial lesion |
| ICC | Immunocytochemistry |
| IHC | Immunohistochemistry |
| IRB | Institutional Review Board |
| LBC | Liquid-based cytology |
| LMICs | Low- and middle-income countries |
| LSIL | Low-grade squamous intraepithelial lesion |
| MCM2 | Minichromosome maintenance complex component 2 |
| NILM | Negative for intraepithelial lesion or malignancy |
| p16INK4a/CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| Pap | Papanicolaou |
| PCA | Principal component analysis |
| PCS cells | Primary cervical epithelial cells |
| POC | Point-of-care |
| RCC | Reactive cellular changes |
| RIPA | Radioimmunoprecipitation assay |
| ROC | Receiver operating characteristic |
| SCC | Squamous cell carcinoma |
| SIL | Squamous intraepithelial lesion |
| TMA | Tissue microarray |
| TOP2A | Topoisomerase II alpha |
| VCP | Valosin-containing protein |
References
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries (Review). Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.I.; Ren, W.; Flowers, L.; Rajwa, B.; Chibwesha, C.J.; Parham, G.P.; Irudayaraj, J.M. Point-of-care test for cervical cancer in LMICs. Oncotarget 2016, 7, 18787–18797. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Ibrahim Khalil, A.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2021, 9, e161–e169. [Google Scholar] [CrossRef] [PubMed]
- Moitry, M.; Jégu, J.; Averous, G.; Velten, M.; Fender, M.; Akladios, C.; Baldauf, J.-J. Reporting reactive cellular changes on smears among women who undergo cervical cancer screening: Results of a cohort study after seven years of follow-up. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 216, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Pangarkar, M.A. The Bethesda System for reporting cervical cytology. Cytojournal 2022, 19, 28. [Google Scholar] [CrossRef]
- Ntamushigo, J.C.; Sebitloane, H.M. Review of triage strategies for atypical squamous cells of undetermined significance among young women. Int. J. Gynecol. Obstet. 2024, 168, 428–435. [Google Scholar] [CrossRef]
- Alrajjal, A.; Pansare, V.; Choudhury, M.S.R.; Khan, M.Y.A.; Shidham, V.B. Squamous intraepithelial lesions (SIL: LSIL, HSIL, ASCUS, ASC-H, LSIL-H) of Uterine Cervix and Bethesda System. Cytojournal 2021, 18, 16. [Google Scholar] [CrossRef]
- Kattoor, J.; Kamal, M.M. The gray zone squamous lesions: ASC-US/ASC-H. Cytojournal 2022, 19, 30. [Google Scholar] [CrossRef]
- de Abreu, F.P.; Casa, P.L.; Rossetto, M.V.; de Oliveira, N.S.; Benvenuti, J.L.; Cassol, M.P.; Brollo, J.; Sartor, I.T.S.; Silva, S.d.A.e. Gene expression analysis in cervical cancer progression: Towards unveiling alterations from normal to tumoral tissue. Hum. Gene 2022, 34, 201131. [Google Scholar] [CrossRef]
- Bitsouni, V.; Gialelis, N.; Stratis, I.G.; Tsilidis, V. From primary HPV infection to carcinoma in situ: A mathematical approach to cervical intraepithelial neoplasia. Stud. Appl. Math. 2024, 153, e12697. [Google Scholar] [CrossRef]
- Sahasrabuddhe, V.V. Cervical Cancer: Precursors and Prevention. Hematol. Oncol. Clin. N. Am. 2024, 38, 771–781. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240030824 (accessed on 18 January 2025).
- Campos-Parra, A.D.; Pérez-Quintanilla, M.; Martínez-Gutierrez, A.D.; Pérez-Montiel, D.; Coronel-Martínez, J.; Millan-Catalan, O.; De León, D.C.; Pérez-Plasencia, C. Molecular Differences between Squamous Cell Carcinoma and Adenocarcinoma Cervical Cancer Subtypes: Potential Prognostic Biomarkers. Curr. Oncol. 2022, 29, 4689–4702. [Google Scholar] [CrossRef] [PubMed]
- Zolciak-Siwinska, A.; Jonska-Gmyrek, J. Glassy cell carcinoma of the cervix: A literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.A.; Choi, J.; Capasso, A.; DiClemente, R.J. Improving HPV Vaccination Uptake Among Adolescents in Low Resource Settings: Sociocultural and Socioeconomic Barriers and Facilitators. Adolesc. Health Med. Ther. 2024, 15, 73–82. [Google Scholar] [CrossRef]
- Allanson, E.R.M.; Schmeler, K.M. Cervical cancer prevention in low- and middle-income countries. Clin. Obstet. Gynecol. 2021, 64, 501–518. [Google Scholar] [CrossRef]
- Dominik, P.; Sonja, M.-K.; Robert, J.; Marcin, P. Effect of vaccination against HPV in the HPV-positive patients not covered by primary prevention on the disappearance of infection. Sci. Rep. 2025, 15, 12642. [Google Scholar] [CrossRef]
- Zhou, X. Application of different methods for cervical cancer detection. Trans. Mater. Biotechnol. Life Sci. 2024, 3, 137–142. [Google Scholar] [CrossRef]
- Li, J.; Adobo, S.D.; Shi, H.; Judicael, K.A.W.; Lin, N.; Gao, L. Screening Methods for Cervical Cancer. ChemMedChem 2024, 19, e202400021. [Google Scholar] [CrossRef]
- Yusuf, M. Perspectives on Cervical Cancer: Insights into Screening Methodology and Challenges. Cancer Screen. Prev. 2024, 3, 47–55. [Google Scholar] [CrossRef]
- Regauer, S.; Reich, O.; Kashofer, K. HPV-negative Squamous Cell Carcinomas of the Cervix with Special Focus on Intraepithelial Precursor Lesions. Am. J. Surg. Pathol. 2022, 46, 147–158. [Google Scholar] [CrossRef]
- Del Moral-Hernández, O.; Hernández-Sotelo, D.; Alarcón-Romero, L.d.C.; Mendoza-Catalán, M.A.; Flores-Alfaro, E.; Castro-Coronel, Y.; Ortiz-Ortiz, J.; Leyva-Vázquez, M.A.; Ortuño-Pineda, C.; Castro-Mora, W.; et al. TOP2A/MCM2, p16INK4a, and cyclin E1 expression in liquid-based cytology: A biomarkers panel for progression risk of cervical premalignant lesions. BMC Cancer 2021, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Habbani, S.F.I.; Dowlatshahi, S.J.; Elumalai, M.; Bolton, S.C.; Tecle, L.T.; Thakkar, P.R.; Linnes, J.C.; Mohammed, S.I. Abstract 6531: Point of care test for cervical cancer screening. Cancer Res. 2023, 83, 6531. [Google Scholar] [CrossRef]
- Valand, J.H.; Twine, D.; Kyomukamaa, M.; Atino, R.; Buteme, G.M.; Muhahiria, S.; Nalwoga, R.; Omary, I.; Nabwami, A.G.; Otim, E.; et al. Role of Bioinformatics in Cancer Diagnosis. Res. Sq. 2022, 1–15. [Google Scholar] [CrossRef]
- Molina, M.A.; Diatricch, L.C.; Quintana, M.C.; Melchers, W.J.; Andralojc, K.M. Cervical cancer risk profiling: Molecular biomarkers predicting the outcome of hrHPV infection. Expert Rev. Mol. Diagn. 2020, 20, 1099–1120. [Google Scholar] [CrossRef]
- Raju, K.; Raghuveer, C.; Sheela, S.; Sharath, B. Association of IHC p16INK4a expression and ELISA Plasma p16INK4a Protein in Squamous Cell Carcinoma of Uterine Cervix: A Concept of Liquid Biopsy. J. Clin. Diagn. Res. 2022, 16, XC06–XC10. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means, The Comprehensive R Archive Network (CRAN): Contributed Packages, October 2017. Available online: https://doi.org/10.32614/CRAN.PACKAGE.EMMEANS (accessed on 10 January 2025). [CrossRef]
- Wood, S.N.; Pya, N.; Säfken, B. Smoothing Parameter and Model Selection for General Smooth Models. J. Am. Stat. Assoc. 2016, 111, 1548–1563. [Google Scholar] [CrossRef]
- Oždian, T.; Vodička, J.; Dostál, J.; Holub, D.; Václavková, J.; Ješeta, M.; Hamerníková, B.; Kouřilová, P.; Malchar, O.; Dvořák, V.; et al. Proteome Mapping of Cervical Mucus and Its Potential as a Source of Biomarkers in Female Tract Disorders. Int. J. Mol. Sci. 2023, 24, 1038. [Google Scholar] [CrossRef] [PubMed]
- Volgareva, G.; Zavalishina, L.; Andreeva, Y.; Frank, G.; Krutikova, E.; Golovina, D.; Bliev, A.; Spitkovsky, D.; Ermilova, V.; Kisseljov, F. Protein p16 as a marker of dysplastic and neoplastic alterations in cervical epithelial cells. BMC Cancer 2004, 4, 58. [Google Scholar] [CrossRef]
- Nickerson, J.L.; Doucette, A.A. Rapid and Quantitative Protein Precipitation for Proteome Analysis by Mass Spectrometry. J. Proteome Res. 2020, 19, 2035–2042. [Google Scholar] [CrossRef]
- Rinaldi, A.J. Simple TCA/Acetone Protein Extraction Protocol for Proteomics Studies. protocols.io, 17 May 2023. [Google Scholar] [CrossRef]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Human Protein Atlas. [Online]. Available online: https://www.proteinatlas.org (accessed on 17 April 2024).
- Ngoka, L.C.M. Sample prep for proteomics of breast cancer: Proteomics and gene ontology reveal dramatic differences in protein solubilization preferences of radioimmunoprecipitation assay and urea lysis buffers. Proteome Sci. 2008, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, Y.; Zou, Y.; Qi, Z.; Huang, G.; Xia, S.; Gao, R.; Li, F.; Huang, Z. TOP2A Promotes Cell Migration, Invasion and Epithelial–Mesenchymal Transition in Cervical Cancer via Activating the PI3K/AKT Signaling. Cancer Manag. Res. 2020, 12, 3807–3814. [Google Scholar] [CrossRef]
- Lu, X.; Song, X.; Hao, X.; Liu, X.; Zhang, X.; Yuan, N.; Ma, H.; Zhang, Z. MicroRNA-186-3p attenuates tumorigenesis of cervical cancer by targeting MCM2. Oncol. Lett. 2021, 22, 539. [Google Scholar] [CrossRef] [PubMed]
- Bourgo, R.J.; Braden, W.A.; Wells, S.I.; Knudsen, E.S. Activation of the Retinoblastoma Tumor Suppressor Mediates Cell Cycle Inhibition and Cell Death in Specific Cervical Cancer Cell Lines. Mol. Carcinog. 2009, 48, 45–55. [Google Scholar] [CrossRef]
- Bairoch, A. The Cellosaurus, a Cell-Line Knowledge Resource. J. Biomol. Tech. 2018, 29, 25–38. [Google Scholar] [CrossRef]
- ATCC (American Type Culture Collection), Primary Cervical Epithelial Cells (PCS-480-011TM) Product Sheet. 2021. [Online]. Available online: https://www.atcc.org/products/pcs-480-011 (accessed on 10 October 2022).
- Valle, C.W.; Min, T.; Bodas, M.; Mazur, S.; Begum, S.; Tang, D.; Vij, N. Critical Role of VCP/p97 in the Pathogenesis and Progression of Non-Small Cell Lung Carcinoma. PLoS ONE 2011, 6, e29073. [Google Scholar] [CrossRef]
- Li, J.; Poi, M.J.; Tsai, M.-D. Regulatory mechanisms of tumor suppressor P16INK4A and their relevance to cancer. Biochemistry 2011, 50, 5566–5582. [Google Scholar] [CrossRef]
- National Human Genome Research Institute, “Cell Cycle”. [Online]. Available online: https://www.genome.gov/genetics-glossary/Cell-Cycle (accessed on 18 December 2023).
- Diéguez, N.C.; Martí, C.; Rodríguiez-Trujillo, A.; Barnadas, E.; Torné, A.; Ordi, J.; del Pino, M. Detection of mRNA of CDKN2A, MKi67 and TOP2A in liquid-based cytology as biomarker of high grade lesions of uterine cervix. Int. J. Gynecol. Cancer 2019, 29, A37. [Google Scholar] [CrossRef]
- TriPath Imaging®, ProExTM C Immunocytochemical Test. 2006. [Online]. Available online: https://www.bd.com/documents/bd-legacy/directions-for-use/womens-health/DS_WH_BD-ProEx-C-ICC_DF_EN.pdf (accessed on 10 January 2025).
- Roche Diagnostics, CINtec PLUS Cytology Package Insert. 2020. [Online]. Available online: https://diagnostics.roche.com/content/dam/diagnostics/us/en/products/c/cintec-plus/CINtec-PLUS-Cytology-Package-Insert-US.pdf (accessed on 10 January 2025).
- Huy, N.V.Q.; Tam, L.M.; Tram, N.V.Q.; Thuan, D.C.; Vinh, T.Q.; Thanh, C.N.; Chuang, L. The value of visual inspection with acetic acid and Pap smear in cervical cancer screening program in low resource settings—A population-based study. Gynecol. Oncol. Rep. 2018, 24, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Vahedpoor, Z.; Behrashi, M.; Khamehchian, T.; Abedzadeh-Kalahroudi, M.; Moravveji, A.; Mohmadi-Kartalayi, M. Comparison of the diagnostic value of the visual inspection with acetic acid (VIA) and Pap smear in cervical cancer screening. Taiwan. J. Obstet. Gynecol. 2019, 58, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Izadi-Mood, N.; Asadi, K.; Shojaei, H.; Sarmadi, S.; Ahmadi, S.A.; Sani, S.; Chelavi, L.H. Potential diagnostic value of P16 expression in premalignant and malignant cervical lesions. J. Res. Med. Sci. 2012, 17, 428–433. [Google Scholar] [PubMed]
- Bergeron, C.; Ordi, J.; Schmidt, D.; Trunk, M.J.; Keller, T.; Ridder, R. Conjunctive p16INK4a Testing Significantly Increases Accuracy in Diagnosing High-Grade Cervical Intraepithelial Neoplasia. Am. J. Clin. Pathol. 2010, 133, 395–406. [Google Scholar] [CrossRef]
- Lin, C.-Q.; Zeng, X.; Cui, J.-F.; Liao, G.-D.; Wu, Z.-N.; Gao, Q.-Q.; Zhang, X.; Yu, X.-Z.; Chen, W.; Xi, M.-R.; et al. Stability Study of Cervical Specimens Collected by Swab and Stored Dry Followed by Human Papillomavirus DNA Detection Using the cobas 4800 Test. J. Clin. Microbiol. 2017, 55, 568–573. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habbani, S.F.; Dowlatshahi, S.; Lichti, N.; Broman, M.; Tecle, L.; Bolton, S.; Flowers, L.; Guerrero-Preston, R.; Linnes, J.C.; Mohammed, S.I. Protein Biomarkers Enable Sensitive and Specific Cervical Intraepithelial Neoplasia (CIN) II/III+ Detection: One Step Closer to Universal Cervical Cancer Screening. Cancers 2025, 17, 1763. https://doi.org/10.3390/cancers17111763
Habbani SF, Dowlatshahi S, Lichti N, Broman M, Tecle L, Bolton S, Flowers L, Guerrero-Preston R, Linnes JC, Mohammed SI. Protein Biomarkers Enable Sensitive and Specific Cervical Intraepithelial Neoplasia (CIN) II/III+ Detection: One Step Closer to Universal Cervical Cancer Screening. Cancers. 2025; 17(11):1763. https://doi.org/10.3390/cancers17111763
Chicago/Turabian StyleHabbani, Samrin F., Sayeh Dowlatshahi, Nathanael Lichti, Meaghan Broman, Lucy Tecle, Scott Bolton, Lisa Flowers, Rafael Guerrero-Preston, Jacqueline C. Linnes, and Sulma I. Mohammed. 2025. "Protein Biomarkers Enable Sensitive and Specific Cervical Intraepithelial Neoplasia (CIN) II/III+ Detection: One Step Closer to Universal Cervical Cancer Screening" Cancers 17, no. 11: 1763. https://doi.org/10.3390/cancers17111763
APA StyleHabbani, S. F., Dowlatshahi, S., Lichti, N., Broman, M., Tecle, L., Bolton, S., Flowers, L., Guerrero-Preston, R., Linnes, J. C., & Mohammed, S. I. (2025). Protein Biomarkers Enable Sensitive and Specific Cervical Intraepithelial Neoplasia (CIN) II/III+ Detection: One Step Closer to Universal Cervical Cancer Screening. Cancers, 17(11), 1763. https://doi.org/10.3390/cancers17111763








