Simple Summary
This article reviews the value of Positron Emission Tomography/Magnetic Resonance Imaging (PET/MRI) in evaluating female pelvic cancers. It also provides a comparative analysis of PET/MRI with other imaging modalities in the context of female pelvic malignancies and outlines their respective strengths and limitations. The aim of this narrative review is to introduce to clinicians up and coming technology and how it may be valuable to their assessment of female pelvic cancers.
Abstract
The diagnosis, treatment, and management of gynecologic malignancies benefit from both positron emission tomography/computed tomography (PET/CT) and MRI. PET/CT provides important information on the local extent of disease as well as diffuse metastatic involvement. MRI offers soft tissue delineation and loco-regional disease involvement. The combination of these two technologies is key in diagnosis, treatment planning, and evaluating treatment response in gynecological malignancies. This review aims to assess the performance of PET/MRI in gynecologic cancer patients and outlines the technical challenges and clinical advantages of PET/MR systems when specifically applied to gynecologic malignancies.
1. Introduction
Gynecological pelvic cancers, including cervical, endometrial, ovarian, vaginal, and vulvar cancers, account for approximately 15% of cancers diagnosed in women worldwide [1,2]; in the United States, 114,810 new cases and 34,020 deaths are estimated for 2023 [3]. When staging gynecological pelvic cancers, the local tumor extent, metastases to nearby lymph nodes, and distant metastases each need to be characterized. This usually requires multiple imaging modalities, including both PET/CT and MRI. PET/CT characterizes both local tumor extent and distant metastasis [4,5,6]. MRI provides important soft-tissue detail and information on the local–regional extent of disease. PET/MRI has been increasingly utilized in clinical practice and offers advantages over PET/CT or MRI alone [7]. These advantages include combining each modalities’ strength into one exam, with direct anatomic correlation such as improved soft-tissue contrast, co-registration of metabolic and MRI anatomic images, and reduced radiation exposure.
PET/MRI demonstrates higher sensitivity and specificity than either PET/CT or MRI alone in staging, detecting gynecologic cancer recurrence, and the assessment of post-treatment response [8,9,10,11,12,13,14].
This review outlines the current and potential utilization of PET/MR for gynecological cancer imaging and how the technology can be applied in diagnosing, staging, restaging, and monitoring treatment responses for female gynecological cancer. A relevant overview of the PET/MR technique, including systems, biomarkers, advantages, challenges, and prospects, is provided.
2. PET/MRI
Cross-sectional imaging provides helpful information for more accurate diagnosis, staging, treatment planning, and monitoring of gynecologic malignancies (Table 1), each technique with its benefits and pitfalls [15]. The first clinical CT scanner was installed in 1971 [16]. By the early 1980s, 1.5 T MRI scanners became clinically available [17]. PET/CT and PET/MRI became clinically available in the mid-1990s and 2010s, respectively [18].

Table 1.
Benefits and pitfalls of imaging modalities in gynecologic malignancies.
The combination of whole-body PET and MRI, with targeted MRI of the specific organ of interest, offers a unified solution for local staging and detection of distant metastases. Systems are available in three main designs: tri-modality, sequential, and integrated (Table 2). Of the three designs, integrated PET/MR offers a comprehensive acquisition where the MRI and PET can be acquired simultaneously. However, not all facilities offer the integrative design, and similar diagnostic information can be provided from the tri-modality and sequential designs. The main benefit of the integrative approach regards image registration and increased convenience for the patient for a single diagnostic exam.

Table 2.
PET/MR Systems.
2.1. Oncologic PET Tracers and Patient Preparation
The primary radiotracer in gynecologic oncology PET/MRI (and PET/CT) is 18F-fluorodeoxyglucose (FDG), which targets increased glucose metabolism in malignancy. Some research studies have highlighted the benefits of prostate-specific membrane antigen (PSMA)-targeted 18F-DCFPyL and 68Ga-FAPI-04 (fibroblast activating protein inhibitor) in diagnosing and assessing the treatment of ovarian cancer [48,49]. Additionally, 68Ga-FAPI-04, 68Ga-RGD (arginine–glycine–aspartic acid peptide) and 18F-EF5 have shown promise in diagnosing and planning radiation treatment for cervical cancer [50,51,52].
Proper patient preparation enhances the interpretative accuracy of PET/MRI. Minimizing the influence of metabolic changes and peristalsis are key objectives. The primary radiotracer in the oncologic PET/MRI of the female pelvis is FDG. Metabolic activity can be influenced by several factors, including diet, extreme physical activity, trauma, medications, infection/inflammation, and environmental temperature [45]. Therefore, a consideration of these factors when performing a PET/MRI may be helpful to ensuring the quality and reliability of results. FDG-PET imaging is optimized by patients fasting for at least four hours before injecting the radiotracer to achieve a target blood glucose level below 160 mg/dL. To improve image quality, an antiperistaltic agent such as butylscopolamine bromide (Buscopan) or glucagon can be administered [53,54]. Patients may undergo PET scanning one hour after the radiotracer injection.
2.2. Quantitative Imaging Biomarkers
Both PET scanning and MRI offer quantitative biomarkers (Table 3) that individually provide valuable information about the region of interest, including cellular density and metabolic activity. The main quantitative biomarker for MRI is derived from diffusion-weighted imaging (DWI).
Several pathological prognostic variables including tumor stage and overall survival (OS) are correlated either individually or in combination with PET/MRI biomarkers. According to a prospective study by Shih et al., SUVmax and ADCmin are both independent predictors of progression-free survival (PFS) and OS [55]. They also found MTV/ADCmin is the strongest predictive biomarker for tumor stage, and ADCmin is significantly lower in advanced cancer stages (≥IB3), while MTV, TLG, MTV/ADCmin, and TLG/ADCmin are higher. Furthermore, Steiner et al. indicated the potential predictive power of SUVmax/ADC for determining nodal status by reporting higher tumor SUVmax/ADC in patients with metastatic pelvic lymph nodes [56].
2.3. Advantages of PET/MRI
PET/MRI has several advantages compared with alternative stand-alone imaging modalities and PET/CT. First, it provides complementary information from both PET and MRI, resulting in improved diagnostic accuracy for detecting pelvic malignancies. Second, PET/MRI reduces ionizing radiation exposure compared with PET/CT, making it a preferable option for patients requiring multiple follow-up examinations or those who are more sensitive to radiation exposure, such as children or women of childbearing age [57]. Third, PET/MRI shows higher reader diagnostic confidence in discriminating between benign and malignant lesions compared with PET/CT in cases of recurrent female pelvic malignancies [8]. PET/MRI enhances TNM staging, allowing a more accurate evaluation of the primary tumor locoregional extent, lymph node involvement, and metastasis compared with PET/CT [58]. Another advantage of PET/MRI is its ability to detect liver metastasis with higher accuracy compared with PET/CT [59,60]. Based on the study conducted by Gardner et al., the liver metastasis rates for stage IV ovarian, uterine, and cervical cancers were 57%, 22%, and 16%, respectively [61]. MRI is more sensitive to liver lesions than CT, and this allows for more accurate detection and differentiation between benign and malignant lesions [62]. Consequently, PET/MRI offers higher lesion conspicuity and diagnostic confidence compared with PET/CT for the characterization of liver lesions [60].

Table 3.
Quantitative biomarkers.
Table 3.
Quantitative biomarkers.
| Biomarker | Description | Clinical Interpretation |
|---|---|---|
| PET Scan | ||
| SUV (Standardized Uptake Value) | Measure the uptake of the radioactive tracer in a specific region of interest (ROI) to assess the activity and metabolism of tissues SUV = Tracer concentration in ROI (kBq/mL)/Injected dose per body weight (kBq/g) | Inversely correlated with ADC [63,64,65,66,67,68,69,70,71,72,73] A higher SUV indicates higher metabolic activity in the ROI |
| SUVmean (Mean Standardized Uptake Value) | Calculating the average tracer uptake in the selected ROI A comprehensive assessment of the overall tracer uptake within the ROI, useful for areas with varying tracer uptake (e.g., tumors) | Monitoring treatment response: a decrease in SUV from baseline indicates metabolic response to treatment [37] Prognosis: Overall survival is better in metabolic responders compared with metabolic non-responders [37] |
| SUVmax (Maximum Standardized Uptake Value) | Indicating the highest level of tracer uptake within a defined ROI Notable inverse correlation with ADCmin [55,74] | Diagnosis and staging: distinguish malignant (higher SUVmax) and benign adnexal lesions [75] Treatment planning: Higher SUVmax values may indicate a more aggressive tumor [68] Monitoring treatment response: changes in SUVmax and especially the percent change value may have the potential to predict response to chemotherapy or chemoradiotherapy [36,38,76] Prognosis: changes in SUVmax predict the patient outcomes, disease recurrence, PFS [36,76,77] |
| MTV (metabolic tumor volume) | The metabolically active volume of the tumor (i.e., the portion of the tumor with a high SUV) | Staging: baseline MTV is a predictor of tumor characteristics such as MI and cervical stromal invasion, and lymph node metastasis; it is higher in cases with lymph node metastasis compared with those without such a metastasis Treatment planning: helps in determining the appropriate dosage and target volume for radiation treatment, ensuring that the radiation is delivered precisely to the areas containing tumor cells [78] Monitoring treatment response: the percentage of post-treatment changes in MTV is associated with the overall tumor response [35] Prognosis: the baseline MTV and the percentage of changes in MTV are predictive factors for OS, and PFS, recurrence [35,77,79] |
| TLG (Total Lesion Glycolysis) | provides a more comprehensive measure of tumor activity than SUVmax or SUVmean alone TLG = SUVmean × MTV | Staging: baseline TLG is a predictor of tumor characteristics, such as MI and cervical stromal invasion, and lymph node metastasis [77,80] Treatment planning: useful for radiation therapy planning by comprehensive assessment of the tumor burden [78] Monitoring treatment: change in TLG after treatment may have the potential to predict response to treatment [39,79] Prognosis: baseline TLG is prognostic factor of OS and PSF [39,77,78,79,81] |
| DWI | ||
| ADC (Apparent Diffusion Coefficient) | Provides valuable information about tissue microstructure and cellular integrity [63,64,65,66,67,68,69,70,71,72,73] Inversely correlated with SUV | Helpful in differentiating between benign and malignant lesions, assessing tumor aggressiveness, and monitoring treatment response |
| ADCmin (Minimum Apparent Diffusion Coefficient) | Represents the region with the most restricted diffusion or the highest tumor cellularity Notable inverse correlation with SUVmax [67,74] | Diagnosis and staging: malignant tumors and regions with high cellular density tend to have lower ADC values, while benign or necrotic regions have higher ADC values Monitoring treatment: a decrease in ADCmin values after therapy can indicate a positive treatment response [55] Prognosis: independent predictor of OS [55] |
3. Applications to Gynecologic Cancers
3.1. Cervical Cancer
MRI and FDG-PET/CT are commonly used in the staging of invasive cervical cancer, characterizing local and distant disease, and predicting the likelihood of survival [82,83] (Figure 1, Figure 2, Figure 3 and Figure 4). Several studies attest to the overall benefits of PET/MRI compared with other modalities [56,84,85]. One retrospective study demonstrated that both tri-modality PET/MRI (PET/CT- and MR-fused images) and contrasted enhanced pelvic MRI (ceMRI) had significantly higher T-staging accuracy (both 83.3%) than PET/CT (with contrast-enhanced CT, ceCT) 53.3% [84].
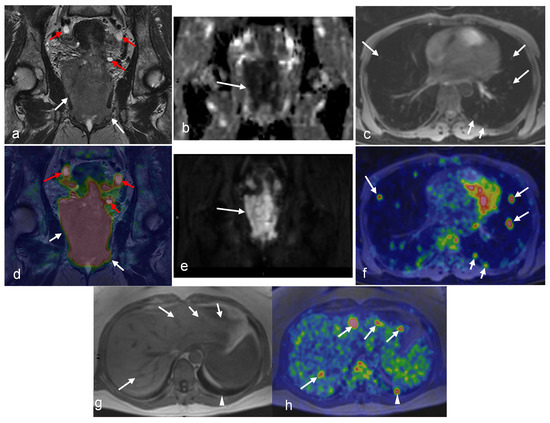
Figure 1.
A 49-year-old woman with stage IVB cervical cancer. Both the coronal T2-weighted image (a) and fused PET/MRI (d) demonstrate the tumor with parametrial involvement and almost the entire vaginal vault (white arrows in (a,d)). There is also involvement of the bladder, ovaries, and proximal ureters (not imaged). Bilateral hydronephrosis is partially visualized (red arrows in (a) and (d)). The mass demonstrates diffusion restriction on the ADC map (arrow in (b)) and bright signal on the DWI (arrow in (e)). Axial Dixon water MRI (c) and axial-fused PET/MRI (f) show lung metastasis (arrows). Liver metastasis (arrows) and additional lung metastasis (arrowhead) are demonstrated in axial Dixon in-phase MRI (g) with high FDG uptakes on axial-fused PET/MRI (h). (Courtesy of Elisabeth Hedlund, MD, Håkan Ahlström, MD, and Björg Jónsdóttir, MD, PhD, Uppsala University, Uppsala, Sweden).
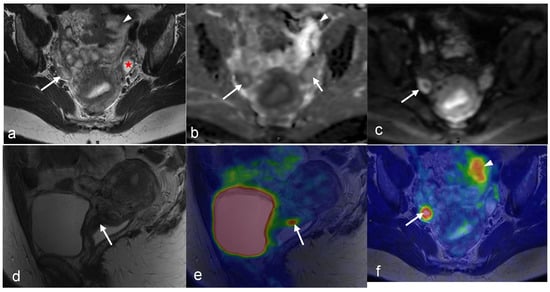
Figure 2.
A 43-year-old woman with stage IB1 cervical cancer found to have a corpus luteum cyst in her right ovary. The cyst is visualized as a peripherally low-intensity structure with central high intensity (arrow in (b)) on ADC map and a peripherally hyperintense structure with low central intensity (arrow in (c)) on the axial diffusion-weighted image. The corresponding FDG uptake (arrow in (f)) on the fused PET/MRI is determined to be benign. In addition, the benign ovarian cyst in the left ovary is seen (star in (a)) with no pathological FDG uptake. The arrowhead in (a,b,f) shows part of the left superior corner of the bladder with corresponding FDG uptake in the urine. Sagittal T2-weighted MRI (d) and sagittal-fused PET/MRI (e) show the cervical mass (arrow in (d,e)). (Courtesy of Elisabeth Hedlund, MD, Håkan Ahlström, MD, and Björg Jónsdóttir, MD, PhD, Uppsala University, Uppsala, Sweden).
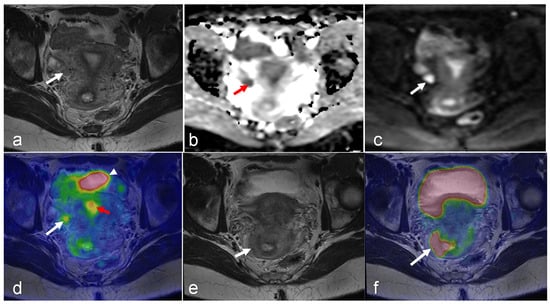
Figure 3.
A 22-year-old woman with stage IB2 cervical cancer clinically or stage IIIC1 cancer as determined via PET/MRI due to a metastatic lymph node. The metastatic lymph node (arrow) is hypointense on the axial T2-weighted MRI (a); low signal on the ADC map (b); high signal on the DWI (c), with FDG uptake on fused PET/MRI (d) images. Physiologic FDG uptake in bladder (arrowheads in (d)) and endometrium (red arrow in (d)) can be seen. Both axial T2-wighted MRI (e) and axial-fused PET/MRI (f) show the cervical tumor with a suspicious irregular right margin (arrow in (e)) with pathologic FDG uptake interpreted as parametrial invasion (arrow in (f)). (Courtesy of Elisabeth Hedlund, MD, Håkan Ahlström, MD, and Björg Jónsdóttir, MD, PhD, Uppsala University, Uppsala, Sweden).
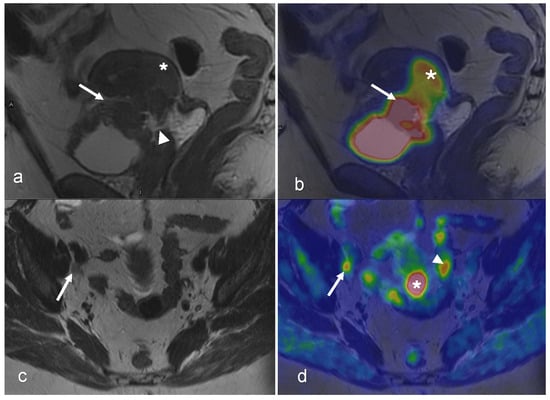
Figure 4.
A 54-year-old woman with squamous cell carcinoma of the cervix, FIGO stage IVA. Sagittal T2-weighted MRI (a) shows a 43 mm tumor (star) with indications of invasion into the upper vagina and bladder (arrow) with suspected vesicovaginal fistula (arrowhead). Sagittal-fused PET/MRI (b) shows the FDG uptake of the tumor (star) and bladder wall (arrow). Both axial T2-weighted image (c) and axial-fused PET/MRI (d) demonstrate an 8 mm lymph node with irregular margins and pathologic FDG uptake (arrow in (c,d)). On axial-fused PET/MRI, (d) there is pathologic FDG uptake corresponding to the cervical tumor (star in (d)), which is difficult to distinguish on the axial T2-weighted image. Physiological FDG uptake in the bowel is seen on axial-fused PET/MRI (arrowhead in (d)). (Courtesy of Elisabeth Hedlund, MD, Håkan Ahlström, MD, and Björg Jónsdóttir, MD, PhD, Uppsala University, Uppsala, Sweden).
In cervical cancer, undiagnosed lymph node metastases present unique challenges during clinical staging. PET/MRI has shown promise as a non-surgical alternative for staging these metastases, offering advantages such as avoiding surgical risks, while reducing the time and costs associated with the procedure. Kim et al. evaluated 79 cervical cancer patients who underwent both MRI and PET/CT prior to lymphadenectomy. They found that tri-modality PET/MRI outperformed PET/CT in detecting regional lymph node metastases due to superior lymph node characterization via MRI [85]. Another study by Steiner et al. found that pre-treatment PET/MRI had a higher correlation between tumor size in imaging and pathology in patients with primary cervical cancer than did MRI alone (rs = 0.87 vs. 0.58) [56]. In terms of N staging, PET/MRI and MRI were equally effective (areas under curve, AUC of 0.73). However, for M staging, PET/MRI performed better than MRI (AUC 0.80 vs. 0.67). This can be related to the higher specificity of PET/MRI (100% vs. 74%) [56].
For radiation treatment planning, PET accurately characterizes tumor volume and assesses lymph node status [86]. Combined with MRI, PET/MRI estimates tumor volume more accurately than PET/CT does due to the more precise identification of tumor margins via MRI [56]. PET/MRI improves treatment planning by providing a precise assessment of parametrial invasion compared with MRI alone (AUC 0.89 vs. 0.73) [56]. Acute therapy-induced edema and inflammation can lead to false-positive findings on post-treatment PET due to the increased uptake of FDG [87]. Advanced MRI techniques such as restriction spectrum imaging (RSI) may addresses this problem of false positives as edema may be distinguished from the residual tumor [88].
PET/MRI may be useful in distinguishing tumor recurrence from radiation-induced anatomical and tissue changes, such as fibrosis and scarring, during post-treatment assessment [89,90]. Schwarz et al. found that the 3-month post-treatment FDG-PET metabolic response is more prognostic of survival outcomes than the pretreatment lymph node status, with 3-year progression-free survival (PFS) rates of 78%, 33%, and 0% for complete- and partial-metabolic response, and progressive disease, respectively (p < 0.001) [91]. Moreover, Kidd et al. demonstrated pelvic lymph node SUVmax, independent of the primary cervical tumor SUVmax, was a prognostic biomarker for treatment response, recurrence, and survival [76].
3.2. Endometrial Cancer
Studies have demonstrated that PET/MRI offers quantitative assessment data, aiding in the evaluation of disease extent and the selection of appropriate treatment plans for endometrial cancer [67,68,92]. Tsuyoshi et al. compared imaging biomarkers of pretreatment-integrated PET/MRI, using a reduced FOV (rFOV) DWI, between low- and high-risk endometrial cancers [92]. The SUV/ADC, characterizing tumor aggressiveness, demonstrated the greatest diagnostic accuracy compared with ADC and SUV alone (AUCs were 0.83, 0.72, and 0.66, p < 0.05, respectively). This finding can be valuable for selecting an appropriate treatment plan. Another study found a notable inverse correlation between SUVmax and ADCmin in 47 endometrial cancer patients who underwent integrated PET/MRI (r = −0.53; p = 0.001) [67]. Additionally, tumors of an advanced stage, and with deep myometrial invasion, cervical invasion, lymphovascular space invasion (LVSI), and lymph node metastasis exhibited significantly higher SUVmax/ADCmin.
Both MRI and PET/CT are useful in evaluating endometrial cancer (in addition to standard female pelvic ultrasound) [93]. The NCCN 2020 guidelines recommend MRI for initial locoregional assessment [94]. Whole-body PET/CT is used to assess lymph nodes and distant metastases in clinically suspected low-grade and all high-grade tumors [95,96,97]. For the post-therapy evaluation of clinically suspected recurrence, either a ceCT of the chest, abdomen, and pelvis or a whole-body FDG-PET/CT, along with an MRI of the pelvis or an MRI of the abdomen, are considered suitable options [31]. However, the combined modality of PET/MRI adds value when analyzing menstrual changes and during endometrial cancer staging.
PET/MRI plays a crucial role in endometrial cancer staging by facilitating precise evaluation of myometrial invasion and lymph node involvement, both of which are vital for the accurate staging of the disease (Figure 5). Tsuyoshi et al. demonstrated that non-contrast-integrated PET/MRI had comparable performance to that of ceMRI for T staging and to that of ceCT for N and M staging [98]. The sensitivities of PET/MRI and ceCT for detecting regional nodal metastasis were 100% and 14.3%, respectively. Kitajima et al. compared tri-modality PET/MRI with PET/CT (ceCT) and reported that PET/MRI exhibited significantly higher accuracy for T staging (80% vs. 60%) and comparable accuracy for N staging in endometrial cancer patients [99]. Ironi et al. found that integrated PET/MRI had 77% accuracy in detecting myometrial invasion (MI) with a positive predictive value (PPV) of 89%, and 91% accuracy with a high negative predictive value (NPV) 96% in detecting lymph nodes [100]. The study also revealed that volume-derived MRI variables, such as volume index (VI), total tumor volume (TTV), and tumor volume ratio (TVR), as well as PET parameters (e.g., MTV and TLG), were significant predictors of LVSI. Furthermore, these volume-derived MRI variables were found to be accurate predictors of the risk group (high-risk vs. low-risk). In another study conducted by Bian et al., integrated PET/MRI was observed to be more accurate than PET/CT in detecting myometrial invasion (81.8% vs. 45.9%) [58]. Additionally, PET/MRI showed higher sensitivity and specificity in detecting regional lymph node metastases compared with PET/CT (sensitivity: 50% vs. 33.3% and specificity: 100% vs. 91.2%, respectively). All of these technologies are improved compared with standard female pelvic ultrasound, where the sensitivity, specificity, and accuracy of finding myometrial invasion ≥50% were 65.6%, 80.3%, and 75.8% [101]. Therefore, PET/MRI can be considered an alternative diagnostic strategy to conventional imaging modalities for preoperative staging, particularly for patients who are unable to receive contrast agents. PET/MRI can also be helpful in identifying recurrent disease during post-treatment assessment (Figure 6).
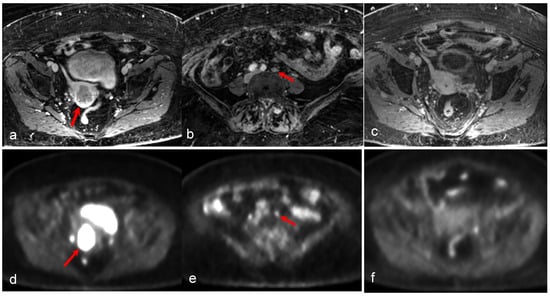
Figure 5.
An 81-year-old woman with high-grade serous carcinoma of an endometrial origin. FDG PET/MRI was obtained for staging. Axial-focused PET image (d) demonstrating focal intense FDG uptake within the lower uterus and upper cervix, corresponding to a hypoenhancing mass seen on the axial T1-weighted post-contrast image (a). Additionally, an 8 mm short axis left common iliac chain node with mild FDG uptake was noted (b,e). There was no evidence of more distant metastatic disease. The patient was treated with pelvic radiation including boost to the left iliac chain lymph node, and subsequent chemotherapy. Follow-up axial T1-weighted image (c) and PET/MR (f) revealing complete metabolic response with absent FDG uptake within the mass, and complete resolution of abnormal enhancement with only a small amount of non-enhancing fluid in the endometrial canal. (Courtesy of Eric C. Ehman, MD, Department of Radiology, Mayo Clinic, Rochester, MN, USA).
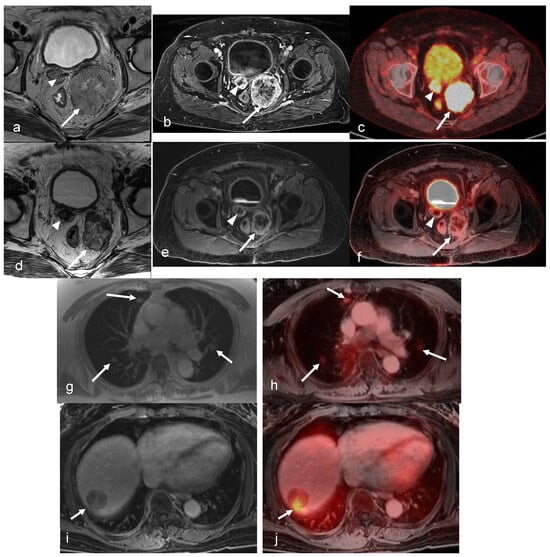
Figure 6.
A 68-year-old female’s history of stage IVb endometrioid endometrial cancer who underwent systemic therapy, hysterectomy, and bilateral salpingo-oophorectomy with recurrent disease and pelvic implants in the vaginal cuff. Axial oblique small FOV T2-weighted (a) and axial post-contrast T1-weighted fat-saturated images (b) show two T2 intermediate signals, heterogeneously enhancing pelvic implants in the vaginal cuff (arrow and arrowhead). Fused PET/CT (c) images show intense FDG uptake in the pelvic implants (arrow and arrowhead). After external beam radiation therapy and brachytherapy, axial oblique small FOV T2-weighted (d) and axial post contrast T1-weighted fat-saturated images (e) show a decrease in size and the enhancement of the two pelvic implants in the vaginal cuff (arrow and arrowhead). Fused PET/MRI images (f) show a decrease in FDG uptake in the pelvic implants (arrow and arrowhead) with the small residual rim of a viable tumor, compatible with the partial local treatment response. (g) The axial in-phase image of the chest shows three new pulmonary nodules, most likely metastasis (arrowheads). A fused PET/MRI image (h) shows FDG uptake in the pulmonary nodules (arrowheads). (i) An axial post-contrast T1-weighted fat-saturated image shows a new heterogeneously enhancing metastatic liver mass with focal intense FDG uptake on fused PET/MRI images (arrowhead in (j)). (Courtesy of Vipul Sheth, MD, PhD and Negaur Iranpour, MD, Department of Radiology, Stanford University, Stanford, CA, USA).
The combined modality of PET/MRI circumvents challenges associated with FDG uptake due to menstrual changes. The premenopausal endometrium may have physiologically low FDG uptake, with two peaks of high FDG uptake during each menstrual cycle: one during the first three days of menstruation and another mid-cycle [25,26,27,102,103,104,105]. Two potential explanations for these peaks are peristaltic motions of the sub-endometrial myometrium and endometrial degeneration/narcotization [106,107,108]. Thus, elevated FDG uptake in the endometrium adjacent to a cervical cancer region does not always indicate endometrial tumor invasion [27]. Physiologic FDG uptake can be distinguished from abnormal uptake via PET/MRI through a comparison of PET and MR images (Figure 3).
3.3. Ovarian Cancer
While ultrasound and CT are often the primary imaging techniques for detecting malignant ovarian tumors, pelvic MRI and PET/CT are utilized during staging. In the case of advanced epithelial ovarian cancer, complementary whole-body staging is recommended. PET/MRI is helpful in the evaluation of ovarian lesions, TNM staging, the identification of patients who are not candidates for optimal surgery, and the differentiation of malignant FDG-avid lesions from benign ones.
PET/MRI has demonstrated higher sensitivity and specificity in the assessment of ovarian lesions (Figure 7), compared with PET/CT [109]. In a retrospective study, the findings indicated that PET/MRI had higher sensitivity (94%) and specificity (100%) compared with PET/CT (sensitivity: 74%, specificity: 80%) and MRI (sensitivity: 84%, specificity: 60%) [109]. Furthermore, the NPVs for PET/MRI, PET/CT, and MRI were 83%, 44%, and 50%, respectively. The PPVs were 100%, 93%, and 89%, respectively. Additionally, the diagnostic accuracy of PET/MRI using TNM staging is comparable with that of PET/CT, while showing no differences in detecting regional lymph node involvement and abdominal metastases [12]. Another challenge in ovarian cancer patients with carcinomatosis is estimating the total tumor burden since this is crucial for the decision of whether primary surgery should be suggested or not. PET/MRI has demonstrated superior accuracy to that of MRI in determining the peritoneal cancer index (PCI) in patients with a high tumor load and in defining patients not suitable for optimal surgery [101].
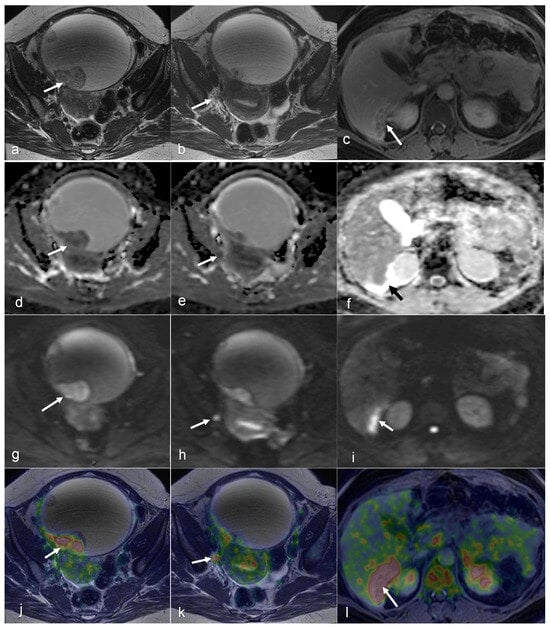
Figure 7.
A 50-year-old female patient with peritoneal carcinomatosis secondary to ovarian clear cell carcinoma has a surgical peritoneal cancer index (PCI) score of 14 and a minimal amount of free fluid. On an axial T2-weighted image (a), a large cystic mass (hyperintense lesion) with a solid component appears slightly hypointense (arrow on (a)), shows restricted diffusion on the DWI and ADC map (arrows on (g,d)), and has high FDG uptake on fused PET/MRI (arrow in (j)). A 5 mm lymph node near the right internal iliac vessels is visibly hypointense on T2WI (arrow on (b)), shows restricted diffusion on DWI and ADC map (arrows in (h,e)), and has high FDG uptake (arrow on (k)). Notably, there is a peritoneal implant in the dorsal right liver lobe (arrows in (c,f,i,l)). (Courtesy of Elisabeth Hedlund, MD, Håkan Ahlström, MD, and Björg Jónsdóttir, MD, PhD, Uppsala University, Uppsala, Sweden).
PET/MRI may also be useful to overcome limitations of PET in ovarian lesions (Figure 8 and Figure 9), particularly with FDG uptake in non-malignant pathologies. Focal ovarian FDG uptake in women of reproductive age may be physiological rather than pathological [25,26,27]. Researchers have observed oval-shaped FDG uptake during the late follicular to early luteal phase, with an SUV greater than 3.0 [25,26]. During the luteinizing hormone (LH) peak, an increase in energy is required to grow a dominant follicle. In addition, there is a surge of macrophages and the production of numerous cytokines. Thus, corpus luteum formation following ovulation (Figure 2) is an inflammatory reaction that leads to a significant accumulation of FDG in macrophages [15,110]. However, in postmenopausal women, normal ovaries are non-FDG-avid, and any uptake in the ovaries or adnexa warrants further evaluation [27]. With the addition of MR, these non-malignant pathologies can be stratified while still receiving the benefit of PET in ovarian lesions.
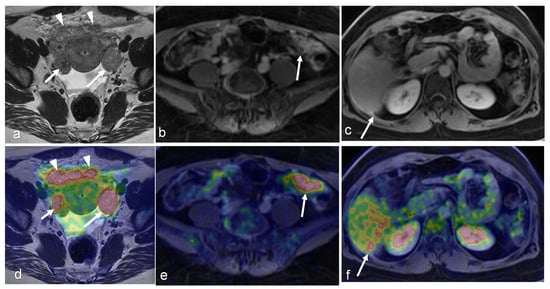
Figure 8.
A 43-year-old woman with peritoneal carcinomatosis secondary to high-grade serous carcinoma has a surgical PCI score of 22. On an axial T2-weighted image, the bilateral ovarian tumors appear moderately hypointense (arrows in (a)) and show uptake of FDG on fused PET/MRI (arrows in (d)). Large omental caking ventral to the uterus and ovaries is also visible (arrowheads on (a,d)). Spread of the peritoneal implant to the left the paracolic gutter (in the lower abdomen (arrows in (b,e)) and the medial border of the right liver lobe can be observed (arrows in (c,f)) (Courtesy of Elisabeth Hedlund, MD, Håkan Ahlström, MD, and Björg Jónsdóttir, MD, PhD, Uppsala University, Uppsala, Sweden).
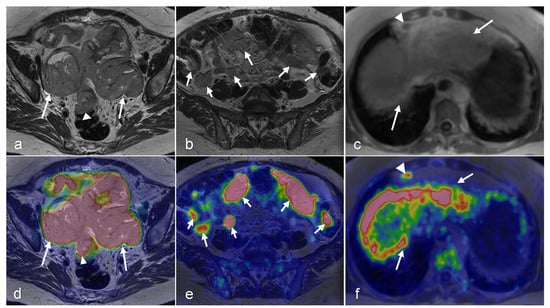
Figure 9.
A 63-year-old woman with peritoneal carcinomatosis secondary to bilateral high-grade serous carcinoma with a PCI score of 39 with invasion of the rectal wall (arrowhead in (a,d)). On axial T2-weighted images, massive infiltration of the greater omentum is visible (arrows in (a,b)) with high FDG uptake (arrows in (d,e)) on axial-fused PET/MRI images. Both axial T1-weighted in-phase (c) and axial-fused PET/MRI (f) demonstrate multiple peritoneal implants spread across the surface of the liver, carcinomatosis implants on the diaphragm (arrows in (c,f)), and one supradiaphragmatic lymph node metastasis (arrowhead in (c,f)). (Courtesy of Elisabeth Hedlund, MD, Håkan Ahlström, MD, and Björg Jónsdóttir, MD, PhD, Uppsala University, Uppsala, Sweden).
3.4. Vaginal and Vulvar Cancers
The ACR recommends MRI for locoregional assessment and PET/CT for the evaluation of nodal and distant metastatic involvement in the pretreatment assessment of recurrent vaginal cancer [111]. In addition, imaging is used for radiation planning to protect the surrounding healthy tissue from being irradiated.
PET/MRI can provide significant value in evaluating vaginal and vulvar tumors. For instance, PET/MRI can aid in distinguishing between recurrent disease and post-treatment or postsurgical changes [90] (Figure 10). Lymph node metastasis is the most important prognostic factor in vulvar cancer, despite the limited progress in the detection of lymph node involvement at an earlier stage over the past four decades [112]. According to Cohn et al., PET has high specificity but relatively low sensitivity and NPV in the detection of groin lymph node metastases arising from vulvar cancer [43]. In contrast, a retrospective study of 160 vulvar cancer patients assessed preoperative PET/CT for predicting groin and pelvic lymph node involvement [113]. PET/CT exhibited strong sensitivity and NPV, with a groin-level sensitivity of 78.9%, specificity of 78.2%, accuracy of 78.4%, PPV of 61.2%, and NPV of 89.4% [113].
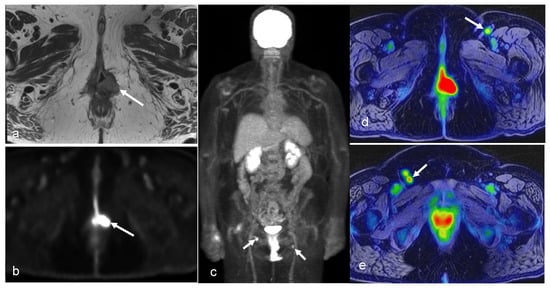
Figure 10.
A 64-year-old woman with history of vulvar cancer diagnosed 4 years prior and initially treated with left vulvectomy. The patient was re-evaluated due to new symptoms of pain and itching, and a biopsy was performed, revealing a recurrent high-grade squamous neoplasm. PET/MRI was ordered for restaging. Axial PET (b) and T2-weighted (a) images from dedicated pelvis MRI with dedicated pelvic PET show an FDG-avid, nodular, intermediate T2 signal in the left perineum compatible with local recurrence. The whole-body survey MIP image (c) and axial-fused PET/MRI images (d,e) demonstrate bilateral FDG-avid inguinal lymph nodes without more distant metastatic disease. The patient went on to undergoing wide local excision with adjuvant radiation and chemotherapy. (Courtesy of Eric C. Ehman, MD, Department of Radiology, Mayo Clinic, Rochester, MN, USA).
4. PET/MR Considerations
4.1. Challenges
While PET/MRI offers significant advantages, it also poses unique challenges that require careful consideration. The physiologic activity of FDG by the bladder and intestines can potentially obscure the detection of pathological findings or lead to a false-positive diagnosis on PET/MRI of gynecologic malignancies. This can be reduced by correlating PET images with MR images. Another related challenge is high FDG uptake in non-malignant pathologies (e.g., infection and inflammation), potentially leading to false-positive PET findings [114]. Fortunately, dynamic contrast-enhanced (DCE) MRI and novel techniques such as DWI may aid in differentiation to overcome these challenges.
Additionally, both sequential and integrated systems require significant modifications to PET and MR hardware and software to maintain similar performance to that of the standalone techniques (with most compromises on the MRI side). An example is the development of magnetic field-insensitive avalanche photodiodes and silicon photomultipliers (SiPMs) for the replacement of the conventional photomultiplier tubes of the PET detectors in integrated systems [115]. In general, the design and materials of PET and MR components are carefully considered to prevent negative impacts on PET from MRI and vice versa, including appropriate shielding designs to avoid eddy currents and using materials of low magnetic susceptibility to reduce susceptibility artifacts.
The ability of using MRI for the attenuation correction of PET in sequential and integrated systems has been a topic of discussion. For adequate interpretation and quantification, PET data need correction for photon attenuation. This is because the photon count depends not only on the number of photons emitted but also on the linear attenuation coefficient (μ) and tissue thickness. Integrated and sequential PET/MR systems lack the ability for CT-based attenuation correction, so their attenuation maps (μ-maps) are estimated from MRI. T1-weighted or water–fat (i.e., Dixon) MR sequences are utilized for the generation of μ-maps in whole-body applications, including pelvic imaging. The images are typically segmented into up to four classes: air, soft tissue, fat, and lung tissue [116,117,118]. However, MR voxel intensities reflect proton density and MR relaxation properties, which are not directly convertible into μ-values. Instead, MR-derived μ-maps are created by assigning predefined μ-values (fixed or continuous) to the voxels of each class, rather than patient-specific values [117,118].
A challenge with the described MR-based attenuation correction (MR-AC) is its inability to visualize bone, metal implants, and MR hardware within the PET FOV, despite their significant photon attenuation. Bone is typically substituted with soft tissue, leading to an underestimation of bone attenuation and the SUV in regions within or near the skeleton [119,120,121]. In gynecologic imaging, the SUV quantification of lesions in the vicinity of the pelvic bone could be affected. To reduce such errors, there are two MR-AC methods that include bone for whole-body applications. The first method utilizes a deep neural network to segment bone, along with air, lung, fat, and lean tissue in the Dixon MR images [117]. The second method adds bone from a model-based bone segmentation algorithm to the Dixon-derived μ-map [121]. Additionally, there are specific alternatives available for the bone attenuation of the skull, such as ultrashort or zero-echo-time sequences [116,122]. In PET/MRI, metal implants give rise to artifacts on MRI, which degrade both the MR and PET images’ quality. The implants cause MR signal loss and artifacts due to their low proton density and strong magnetic susceptibility compared with adjacent tissue [117,123].
Due to MR hardware limitations (decreasing B0 homogeneity and gradient linearity with distance from the isocenter), the maximum achievable MR FOV is smaller than the PET FOV (~50 cm vs. ~60–70 cm), which might cause truncation artifacts in the MR-AC. Patients with arms positioned alongside the body and obese patients can give rise to such artifacts, affecting PET reconstruction in various body regions, including the pelvis [124]. This issue has been reduced to clinically acceptable levels via two main approaches: (1) B0 homogenization using gradient enhancement (HUGE), in which an optimal readout gradient field for the minimization of B0 inhomogeneities and gradient nonlinearities outside the MR FOV is determined [125]; (2) estimation of the contour of tissue outside the MR FOV from non-attenuation corrected PET images [117]. B0 homogeneity is also affected by metal implants, which cause MR signal loss and artifacts [123]. The affected image region often exceeds the implant size, leading to incorrect attenuation correction in its vicinity. Currently, no reliable and robust MR-based attenuation correction method, accounting for metal implants, is commercially available. The manufacturers have solved the issue of the attenuation of hardware (e.g., receiver coils) within the PET FOV by using predefined μ-maps from CT-based templates for fixed coils, and made efforts to displace highly attenuating material to outside the PET FOV [126,127]. It is recommended to always use the most current software version and attenuation correction protocols on the designated PET/MRI system due to the complexity and increasing variety of attenuation correction methods [128].
As reported from the International Workshop on PET/MRI in 2017, MR-AC is generally considered acceptable for most routine clinical situations, with uncertainties comparable to those of PET/CT [122]. However, the PET/MRI oncology community still requested efforts to reduce residual bias from MR-AC and truncation artifacts [122]. According to Eiber et al., both PET/CT and PET/MRI demonstrated a strong correlation in SUV estimation across various tumors (r = 0.9975, p < 0.0001) [129]. Variations can occur in measured tracer uptake between PET/MRI and PET/CT due to the utilization of distinct PET quantification methods in each modality. Thus, caution is advised when comparing results or conducting repeated examinations, such as those undertaken pre- and post-therapy. Consistently using the same modality is preferable for more comparable results.
A further challenge with PET/MRI is the high cost. The purchase price of the PET/MR scanner is more than that of standalone PET or MRI systems, and the former is not as widely available in healthcare facilities [130]. However, the ability of integrated and sequential PET/MR systems to produce multiparametric PET and MR images simultaneously, as well as to function as standalone MRI or PET methods when the other imaging outcomes are not required, can potentially aid in the establishment of this hybrid modality as a cost-effective imaging alternative in oncology [130]. Examples of such multiparametric images in gynecologic malignancies of the female pelvis are provided in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10.
4.2. Future Directions
A future prospect specific to integrated PET/MR systems, compared with tri-modality and sequential systems, is that the static magnetic field (B0) shortens the range of the positron, leading to an increased PET spatial resolution in the plane perpendicular to the direction of B0, compared with that of PET/CT [131]. The effect increases with increased magnetic field strength and positron energy. For the most common radiotracer FDG, the effect is minimal because of the relatively low energy of the emitted positron, but would be substantial for medium- and high-energy positrons (e.g., 68Ga and 120I, respectively) [132]. The development of novel tracers for gynecologic malignancies of the female pelvis, based on molecules with higher disease specificity compared to FDG and radionuclides with medium/high-energy positrons, has potential to increase the possibility to differentiate between malignant and surrounding tissue.
Due to considerable scan times, PET images can be influenced by motion (e.g., cardiorespiratory motion; movement of other internal organs) [133]. With gating techniques, artifacts from periodic motion are usually reduced by limiting the PET acquisition or reconstruction to a predefined phase, which results in a loss of valuable data and a decreased signal-to-noise ratio. This issue can be addressed via motion tracking followed by retrospective correction through image registration, or via prospective motion correction incorporated already at image reconstruction [133]. For such methods, simultaneously acquired MR data from integrated PET/MR systems provide anatomical details that can be used alone or in combination with PET for improved motion correction [134,135,136,137], as compared with sequential PET/CT [138]. MR-based retrospective correction for respiratory and/or cardiac motion has been implemented by PET/MR manufacturers [139,140,141]. Prospective motion correction has been used within research for oncological applications [134,142]. While motion correction has shown notable impacts in cancer imaging, particularly for lung and liver lesions, its implementation in clinical practice is limited and remains a prospect for integrated PET/MRI [133]. Methods for the correction of non-periodic motion, such as those associated with bladder filling, are also topics for future development [133]. In the PET/MRI of female pelvic malignancies, a whole-body PET is commonly acquired simultaneously with a whole-body MRI. The latter is used for both diagnostic purposes and as an anatomical reference to the PET images. A dedicated MRI of the pelvic region, consisting of multiple pulse sequences, is usually performed separately within the same examination. To improve the anatomical alignment between the whole-body PET and the dedicated pelvic MRI, any motion-related disparities (e.g., those arising from bladder filling) can be effectively addressed and corrected by using the MR data.
Artificial intelligence (AI) has emerged as a powerful tool in medical imaging. PET images often suffer from noise and limited spatial resolution. AI models, including convolutional neural networks, U-Nets, and generative adversarial networks, have demonstrated improvements in denoising and image enhancement [143,144,145]. These advancements can potentially reduce radiotracer doses and scan times, as well as improve workflow efficiency [144,145]. Deep learning methods can be utilized to transform MR images into pseudo-CT images, which are necessary for attenuation correction in PET/MRI [145]. However, challenges remain, including poor model generalizability, which may result in variable performance across different scanners or protocols [146]. Multidisciplinary collaborations between clinicians and AI experts are essential for the practical application of AI in routine clinical practice.
5. Conclusions
PET/MRI evaluation is becoming increasingly popular as it provides complementary physiological and molecular information from PET with anatomical and physiological information from MRI. In the case of female pelvic malignancies, FDG-PET/MRI has shown to be more accurate than FDG-PET/CT, in the assessment of staging (local tumor extent, lymphadenopathy, and extra pelvic metastases at diagnosis) and therapy evaluation. This multi-modality approach can help minimize false positives and/or false negatives, and consequently improve diagnosis and reduce the use of unnecessary treatments. Integrated PET/MR systems allow for simultaneous PET and MRI with benefits such as improved anatomical alignment between the modalities, reduced total scan time, and reduced doses of ionizing radiation. Future technological improvements, with respect to the development of novel tracers for female gynecological malignancies, MR-based attenuation correction, and motion correction, could further enhance the use of these systems. To justify the higher expenses associated with PET/MRI, it is necessary to conduct research demonstrating the impact of this combined modality on patient management and the improvement in outcomes of pelvic malignancies. Overall, PET/MR imaging has the potential to become a valuable tool in the clinical management of pelvic malignancies and may offer several advantages over other imaging modalities.
Author Contributions
Conceptualization, S.E., E.L. and R.R.-P.; Writing—original draft preparation, S.E. and E.L.; writing—review and editing, S.E., E.L., S.J.B., A.S., S.L. and R.R.-P.; visualization, E.H., K.S., E.C.E., V.R.S. and N.I.; supervision, R.R.-P.; project administration, S.E.; funding acquisition, R.R.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research and its article processing charges (APC) were funded by NIH R37CA249659, GE Healthcare, and Krueger v. Wyeth Research Award, Swedish Research Council (Vetenskapsrådet): dnr 2021-00427, Lions Cancer Fund Mellansverige Uppsala-Örebro, and Makarna Eriksson Foundation (G och E Erikssons stiftelse för cancerforskning), Urogenital Radiology Fellowship of the European School of Radiology (ESOR)/European Society of Urogenital Radiology (ESUR).
Institutional Review Board Statement
Permission was granted by both an ethical committee and radiation protection committee in Uppsala (DNR 2015-003). The cases provided by Mayo Clinic and Stanford were retrospectively reviewed in a HIPAA-compliant manner for educational purposes.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study, ensuring all patient data were anonymized and confidentiality was maintained.
Data Availability Statement
All data relevant to this study are contained within the manuscript. No additional data beyond those presented in the manuscript can be provided.
Conflicts of Interest
S.E., S.J.B., K.S., V.R.S., N.I., S.L., E.C.E., and A.S. declare no conflicts of interest. R.R.-P. received research funding from GE Healthcare and has received a speaker honorarium from Efficiency Learning Systems (2022) and Educational Symposia (2022). She has stock options in Cortech Labs and Curemetrix and has been involved as a consultant in Human Longevity Inc and Curemetrix. She serves on the scientific advisory board for Imagine Scientific (as well as an SBIR grant). She also has received honoraria from Bayer. E.L. is a former employee of Antaros Medical AB. The funders had no role in the design, collection, analyses, writing, or the decision to publish the results of this study.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Antoch, G.; Vogt, F.M.; Freudenberg, L.S.; Nazaradeh, F.; Goehde, S.C.; Barkhausen, J.; Dahmen, G.; Bockisch, A.; Debatin, J.F.; Ruehm, S.G. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA 2003, 290, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; Townsend, D.W.; Brun, T.; Kinahan, P.E.; Charron, M.; Roddy, R.; Jerin, J.; Young, J.; Byars, L.; Nutt, R. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 2000, 41, 1369–1379. [Google Scholar] [PubMed]
- Bar-Shalom, R.; Yefremov, N.; Guralnik, L.; Gaitini, D.; Frenkel, A.; Kuten, A.; Altman, H.; Keidar, Z.; Israel, O. Clinical performance of PET/CT in evaluation of cancer: Additional value for diagnostic imaging and patient management. J. Nucl. Med. 2003, 44, 1200–1209. [Google Scholar] [PubMed]
- Delso, G.; Furst, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Beiderwellen, K.; Grueneisen, J.; Ruhlmann, V.; Buderath, P.; Aktas, B.; Heusch, P.; Kraff, O.; Forsting, M.; Lauenstein, T.C.; Umutlu, L. [18F]FDG PET/MRI vs. PET/CT for whole-body staging in patients with recurrent malignancies of the female pelvis: Initial results. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Kirchner, J.; Sawicki, L.M.; Suntharalingam, S.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Deuschl, C.; Herrmann, K.; Antoch, G.; Forsting, M.; et al. Whole-body staging of female patients with recurrent pelvic malignancies: Ultra-fast 18F-FDG PET/MRI compared to 18F-FDG PET/CT and CT. PLoS ONE 2017, 12, e0172553. [Google Scholar] [CrossRef]
- Sawicki, L.M.; Kirchner, J.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Schaarschmidt, B.M.; Forsting, M.; Herrmann, K.; Antoch, G.; Umutlu, L. Comparison of 18F-FDG PET/MRI and MRI alone for whole-body staging and potential impact on therapeutic management of women with suspected recurrent pelvic cancer: A follow-up study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Makihara, N.; Ebina, Y.; Yamada, H.; Takahashi, S.; Sugimura, K. Value of fusion of PET and MRI in the detection of intra-pelvic recurrence of gynecological tumor: Comparison with 18F-FDG contrast-enhanced PET/CT and pelvic MRI. Ann. Nucl. Med. 2014, 28, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Schaarschmidt, B.M.; Heubner, M.; Suntharalingam, S.; Milk, I.; Kinner, S.; Heubner, A.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; et al. Implementation of FAST-PET/MRI for whole-body staging of female patients with recurrent pelvic malignancies: A comparison to PET/CT. Eur. J. Radiol. 2015, 84, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Beiderwellen, K.; Heusch, P.; Gratz, M.; Schulze-Hagen, A.; Heubner, M.; Kinner, S.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; et al. Simultaneous positron emission tomography/magnetic resonance imaging for whole-body staging in patients with recurrent gynecological malignancies of the pelvis: A comparison to whole-body magnetic resonance imaging alone. Investig. Radiol. 2014, 49, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, D.; Dufour, P.; Tordjeman-Rizzi, N.; Prolongeau, J.F.; Depret-Moser, S.; Monnier, J.C. Immunological aspects of ovarian function: Role of the cytokines. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 63, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.A.; Stein, J.A.; Pelc, N.J. How CT happened: The early development of medical computed tomography. J. Med. Imaging 2021, 8, 052110. [Google Scholar] [CrossRef] [PubMed]
- Kabasawa, H. MR Imaging in the 21st Century: Technical Innovation over the First Two Decades. Magn. Reson. Med. Sci. 2022, 21, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Townsend, D. History and future technical innovation in positron emission tomography. J. Med. Imaging 2017, 4, 011013. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, C.; Faria, S.; Devine, C.; Patnana, M.; Sagebiel, T.; Iyer, R.B.; Bhosale, P.R. [18F]-2-Fluoro-2-Deoxy-D-glucose-PET Assessment of Cervical Cancer. PET Clin. 2018, 13, 165–177. [Google Scholar] [CrossRef]
- Mahajan, A.; Sable, N.P.; Popat, P.B.; Bhargava, P.; Gangadhar, K.; Thakur, M.H.; Arya, S. Magnetic Resonance Imaging of Gynecological Malignancies: Role in Personalized Management. Semin. Ultrasound CT MR 2017, 38, 231–268. [Google Scholar] [CrossRef]
- Sala, E.; Rockall, A.G.; Freeman, S.J.; Mitchell, D.G.; Reinhold, C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: What the radiologist needs to know. Radiology 2013, 266, 717–740. [Google Scholar] [CrossRef] [PubMed]
- Kusmirek, J.; Robbins, J.; Allen, H.; Barroilhet, L.; Anderson, B.; Sadowski, E.A. PET/CT and MRI in the imaging assessment of cervical cancer. Abdom. Imaging 2015, 40, 2486–2511. [Google Scholar] [CrossRef] [PubMed]
- Monteil, J.; Maubon, A.; Leobon, S.; Roux, S.; Marin, B.; Renaudie, J.; Genet, D.; Fermeaux, V.; Aubard, Y.; Tubiana-Mathieu, N. Lymph node assessment with 18F-FDG-PET and MRI in uterine cervical cancer. Anticancer Res. 2011, 31, 3865–3871. [Google Scholar] [PubMed]
- Otero-García, M.M.; Mesa-Álvarez, A.; Nikolic, O.; Blanco-Lobato, P.; Basta-Nikolic, M.; de Llano-Ortega, R.M.; Paredes-Velázquez, L.; Nikolic, N.; Szewczyk-Bieda, M. Role of MRI in staging and follow-up of endometrial and cervical cancer: Pitfalls and mimickers. Insights Imaging 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, S.; Inubushi, M.; Okada, H. Physiological 18F-FDG uptake in the ovaries and uterus of healthy female volunteers. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H.; Cheng, W.Y.; Cheng, X.; Dang, Y.H. Characteristics of physiological uptake of uterus and ovaries on 18F-fluorodeoxyglucose positron emission tomography. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2007, 29, 124–129. [Google Scholar] [PubMed]
- Lerman, H.; Metser, U.; Grisaru, D.; Fishman, A.; Lievshitz, G.; Even-Sapir, E. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: Assessment by PET/CT. J. Nucl. Med. 2004, 45, 266–271. [Google Scholar] [PubMed]
- Reinhardt, M.J.; Ehritt-Braun, C.; Vogelgesang, D.; Ihling, C.; Högerle, S.; Mix, M.; Moser, E.; Krause, T.M. Metastatic lymph nodes in patients with cervical cancer: Detection with MR imaging and FDG PET. Radiology 2001, 218, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.C.; Sagebiel, T.; Balachandran, A.; Devine, C.; Lal, C.; Bhosale, P.R. Imaging in endometrial carcinoma. Indian J. Radiol. Imaging 2015, 25, 137–147. [Google Scholar] [CrossRef]
- Soper, J.T. Radiographic imaging in gynecologic oncology. Clin. Obstet. Gynecol. 2001, 44, 485–494. [Google Scholar] [CrossRef]
- Expert Panel on GYN and OB Imaging; Reinhold, C.; Ueno, Y.; Akin, E.A.; Bhosale, P.R.; Dudiak, K.M.; Jhingran, A.; Kang, S.K.; Kilcoyne, A.; Lakhman, Y.; et al. ACR Appropriateness Criteria(R) Pretreatment Evaluation and Follow-Up of Endometrial Cancer. J. Am. Coll. Radiol. 2020, 17, S472–S486. [Google Scholar] [CrossRef] [PubMed]
- Engbersen, M.P.; Van Driel, W.; Lambregts, D.; Lahaye, M. The role of CT, PET-CT, and MRI in ovarian cancer. Br. J. Radiol. 2021, 94, 20210117. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Liang, K.; Xiao, Z.; Yang, Q.; Yang, S. A meta-analysis on the diagnostic value of diffusion-weighted imaging on ovarian cancer. J. Buon 2019, 24, 2333–2340. [Google Scholar] [PubMed]
- Timmerman, D.; Planchamp, F.; Bourne, T.; Landolfo, C.; du Bois, A.; Chiva, L.; Cibula, D.; Concin, N.; Fischerova, D.; Froyman, W.; et al. ESGO/ISUOG/IOTA/ESGE Consensus Statement on pre-operative diagnosis of ovarian tumors. Int. J. Gynecol. Cancer 2021, 31, 961–982. [Google Scholar] [CrossRef] [PubMed]
- Vallius, T.; Hynninen, J.; Kemppainen, J.; Alves, V.; Auranen, K.; Matomaki, J.; Oksa, S.; Virtanen, J.; Grenman, S.; Auranen, A.; et al. 18F-FDG-PET/CT based total metabolic tumor volume change during neoadjuvant chemotherapy predicts outcome in advanced epithelial ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Kim, H.S.; Lee, J.Y.; Kang, W.J.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Early Assessment of Response to Neoadjuvant Chemotherapy with 18F-FDG-PET/CT in Patients with Advanced-Stage Ovarian Cancer. Cancer Res. Treat. 2020, 52, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Avril, N.; Sassen, S.; Schmalfeldt, B.; Naehrig, J.; Rutke, S.; Weber, W.A.; Werner, M.; Graeff, H.; Schwaiger, M.; Kuhn, W. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J. Clin. Oncol. 2005, 23, 7445–7453. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Yamamoto, Y.; Kanenishi, K.; Ohno, M.; Hata, T.; Kushida, Y.; Haba, R.; Ohkawa, M. Monitoring the neoadjuvant therapy response in gynecological cancer patients using FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Cho, A.; Lee, J.H.; Yun, M.; Lee, J.D.; Kim, Y.T.; Kang, W.J. The role of metabolic tumor volume and total lesion glycolysis on (1)(8)F-FDG PET/CT in the prognosis of epithelial ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1898–1906. [Google Scholar] [CrossRef]
- Martoni, A.A.; Fanti, S.; Zamagni, C.; Rosati, M.; De Iaco, P.; D’Errico Grigioni, A.; Castellucci, P.; Quercia, S.; Musto, A.; Ricci Maccarini, L.; et al. [18F]FDG-PET/CT monitoring early identifies advanced ovarian cancer patients who will benefit from prolonged neo-adjuvant chemotherapy. Q. J. Nucl. Med. Mol. Imaging 2011, 55, 81–90. [Google Scholar]
- Sohaib, S.A.; Reznek, R.H. MR imaging in ovarian cancer. Cancer Imaging 2007, 7, S119–S129. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Tsui, B.Q.; Bahrami, S.; Masamed, R.; Memarzadeh, S.; Raman, S.S.; Patel, M.K. Gynecologic tumor board: A radiologist’s guide to vulvar and vaginal malignancies. Abdom. Radiol. 2021, 46, 5669–5686. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.E.; Dehdashti, F.; Gibb, R.K.; Mutch, D.G.; Rader, J.S.; Siegel, B.A.; Herzog, T.J. Prospective evaluation of positron emission tomography for the detection of groin node metastases from vulvar cancer. Gynecol. Oncol. 2002, 85, 179–184. [Google Scholar] [CrossRef]
- Veit-Haibach, P.; Kuhn, F.P.; Wiesinger, F.; Delso, G.; von Schulthess, G. PET-MR imaging using a tri-modality PET/CT-MR system with a dedicated shuttle in clinical routine. Magma 2013, 26, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.; Friedman, K.P.; Shah, S.N.; Chandarana, H. Practical guide for implementing hybrid PET/MR clinical service: Lessons learned from our experience. Abdom. Imaging 2015, 40, 1366–1373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vandenberghe, S.; Moskal, P.; Karp, J.S. State of the art in total body PET. EJNMMI Phys. 2020, 7, 35. [Google Scholar] [CrossRef]
- Musafargani, S.; Ghosh, K.K.; Mishra, S.; Mahalakshmi, P.; Padmanabhan, P.; Gulyas, B. PET/MRI: A frontier in era of complementary hybrid imaging. Eur. J. Hybrid. Imaging 2018, 2, 12. [Google Scholar] [CrossRef]
- Sadowski, E.A.; Lees, B.; McMillian, A.B.; Kusmirek, J.E.; Cho, S.Y.; Barroilhet, L.M. Distribution of prostate specific membrane antigen (PSMA) on PET-MRI in patients with and without ovarian cancer. Abdom. Radiol. 2023, 48, 3643–3652. [Google Scholar] [CrossRef]
- Xi, Y.; Sun, L.; Che, X.; Huang, X.; Liu, H.; Wang, Q.; Meng, H.; Miao, Y.; Qu, Q.; Hai, W.; et al. A comparative study of [(68)Ga]Ga-FAPI-04 PET/MR and [18F]FDG PET/CT in the diagnostic accuracy and resectability prediction of ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2885–2898. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W.; Qin, C.; Song, Y.; Liu, F.; Hu, F.; Lan, X. Uterine Uptake of 68Ga-FAPI-04 in Uterine Pathology and Physiology. Clin. Nucl. Med. 2022, 47, 7–13. [Google Scholar] [CrossRef]
- Ahangari, S.; Littrup Andersen, F.; Liv Hansen, N.; Jakobi Nøttrup, T.; Berthelsen, A.K.; Folsted Kallehauge, J.; Richter Vogelius, I.; Kjaer, A.; Espe Hansen, A.; Fischer, B.M. Multi-parametric PET/MRI for enhanced tumor characterization of patients with cervical cancer. Eur. J. Hybrid. Imaging 2022, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Narva, S.I.; Seppänen, M.P.; Raiko, J.R.H.; Forsback, S.J.; Orte, K.J.; Virtanen, J.M.; Hynninen, J.; Hietanen, S. Imaging of Tumor Hypoxia With 18F-EF5 PET/MRI in Cervical Cancer. Clin. Nucl. Med. 2021, 46, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Maucherat, B.; Movassaghi, R.; Fleury, V.; Le Thiec, M.; Geffroy, D.; Labbe-Devilliers, C.; Rousseau, C. Is Glucagon Administration Compatible With FDG PET/MRI? Clin. Nucl. Med. 2022, 47, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Taylor, M.B.; Carrington, B.M.; Bonington, S.C.; Swindell, R. The value of hyoscine butylbromide in pelvic MRI. Clin. Radiol. 2007, 62, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.L.; Yen, R.F.; Chen, C.A.; Cheng, W.F.; Chen, B.B.; Chang, Y.H.; Cheng, M.F.; Shih, T.T. PET/MRI in Cervical Cancer: Associations Between Imaging Biomarkers and Tumor Stage, Disease Progression, and Overall Survival. J. Magn. Reson. Imaging 2021, 53, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.; Narva, S.; Rinta-Kiikka, I.; Hietanen, S.; Hynninen, J.; Virtanen, J. Diagnostic efficiency of whole-body 18F-FDG PET/MRI, MRI alone, and SUV and ADC values in staging of primary uterine cervical cancer. Cancer Imaging 2021, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.C.; Seghers, V.; Paldino, M.J.; Dodge, C.; Krishnamurthy, R.; Krishnamurthy, R.; Rohren, E.M. Assessment of Sequential PET/MRI in Comparison With PET/CT of Pediatric Lymphoma: A Prospective Study. AJR Am. J. Roentgenol. 2016, 206, 623–631. [Google Scholar] [CrossRef]
- Bian, L.H.; Wang, M.; Gong, J.; Liu, H.H.; Wang, N.; Wen, N.; Fan, W.S.; Xu, B.X.; Wang, M.Y.; Ye, M.X.; et al. Comparison of integrated PET/MRI with PET/CT in evaluation of endometrial cancer: A retrospective analysis of 81 cases. PeerJ 2019, 7, e7081. [Google Scholar] [CrossRef] [PubMed]
- Donati, O.F.; Hany, T.F.; Reiner, C.S.; von Schulthess, G.K.; Marincek, B.; Seifert, B.; Weishaupt, D. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: Comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J. Nucl. Med. 2010, 51, 692–699. [Google Scholar] [CrossRef]
- Beiderwellen, K.; Gomez, B.; Buchbender, C.; Hartung, V.; Poeppel, T.D.; Nensa, F.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Depiction and characterization of liver lesions in whole body [(1)(8)F]-FDG PET/MRI. Eur. J. Radiol. 2013, 82, e669–e675. [Google Scholar] [CrossRef]
- Gardner, A.B.; Charo, L.M.; Mann, A.K.; Kapp, D.S.; Eskander, R.N.; Chan, J.K. Ovarian, uterine, and cervical cancer patients with distant metastases at diagnosis: Most common locations and outcomes. Clin. Exp. Metastasis 2020, 37, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Niekel, M.C.; Bipat, S.; Stoker, J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: A meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 2010, 257, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Floberg, J.M.; Fowler, K.J.; Fuser, D.; DeWees, T.A.; Dehdashti, F.; Siegel, B.A.; Wahl, R.L.; Schwarz, J.K.; Grigsby, P.W. Spatial relationship of 2-deoxy-2-[18F]-fluoro-D-glucose positron emission tomography and magnetic resonance diffusion imaging metrics in cervical cancer. EJNMMI Res. 2018, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Schaarschmidt, B.M.; Heubner, M.; Aktas, B.; Kinner, S.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; Umutlu, L. Integrated PET/MRI for whole-body staging of patients with primary cervical cancer: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Brandmaier, P.; Purz, S.; Bremicker, K.; Hockel, M.; Barthel, H.; Kluge, R.; Kahn, T.; Sabri, O.; Stumpp, P. Simultaneous [18F]FDG-PET/MRI: Correlation of Apparent Diffusion Coefficient (ADC) and Standardized Uptake Value (SUV) in Primary and Recurrent Cervical Cancer. PLoS ONE 2015, 10, e0141684. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Beiderwellen, K.; Heusch, P.; Buderath, P.; Aktas, B.; Gratz, M.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; Umutlu, L. Correlation of standardized uptake value and apparent diffusion coefficient in integrated whole-body PET/MRI of primary and recurrent cervical cancer. PLoS ONE 2014, 9, e96751. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.L.; Yen, R.F.; Chen, C.A.; Chen, B.B.; Wei, S.Y.; Chang, W.C.; Sheu, B.C.; Cheng, W.F.; Tseng, Y.H.; Chen, X.J.; et al. Standardized uptake value and apparent diffusion coefficient of endometrial cancer evaluated with integrated whole-body PET/MR: Correlation with pathological prognostic factors. J. Magn. Reson. Imaging 2015, 42, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Joja, I.; Fukushima, C.; Haruma, T.; Hayashi, C.; Kusumoto, T.; Seki, N.; Hongo, A.; Hiramatsu, Y. The preoperative SUVmax is superior to ADCmin of the primary tumour as a predictor of disease recurrence and survival in patients with endometrial cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lee, E.Y.; Lai, V.; Chan, Q. Correlation between tissue metabolism and cellularity assessed by standardized uptake value and apparent diffusion coefficient in peritoneal metastasis. J. Magn. Reson. Imaging 2014, 40, 99–105. [Google Scholar] [CrossRef]
- Er, H.C.; Erden, A.; Kucuk, N.O.; Gecim, E. Correlation of minimum apparent diffusion coefficient with maximum standardized uptake on fluorodeoxyglucose PET-CT in patients with rectal adenocarcinoma. Diagn. Interv. Radiol. 2014, 20, 105–109. [Google Scholar] [CrossRef]
- Rakheja, R.; Chandarana, H.; DeMello, L.; Jackson, K.; Geppert, C.; Faul, D.; Glielmi, C.; Friedman, K.P. Correlation between standardized uptake value and apparent diffusion coefficient of neoplastic lesions evaluated with whole-body simultaneous hybrid PET/MRI. AJR Am. J. Roentgenol. 2013, 201, 1115–1119. [Google Scholar] [CrossRef]
- Olsen, J.R.; Esthappan, J.; DeWees, T.; Narra, V.R.; Dehdashti, F.; Siegel, B.A.; Schwarz, J.K.; Grigsby, P.W. Tumor volume and subvolume concordance between FDG-PET/CT and diffusion-weighted MRI for squamous cell carcinoma of the cervix. J. Magn. Reson. Imaging 2013, 37, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Schob, S.; Höhn, A.K.; Bremicker, K.; Exner, M.; Stumpp, P.; Purz, S. Parameters of simultaneous 18F-FDG-PET/MRI predict tumor stage and several histopathological features in uterine cervical cancer. Oncotarget 2017, 8, 28285–28296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, J.; Wang, N.; Bian, L.; Wang, M.; Ye, M.; Wen, N.; Fu, M.; Fan, W.; Meng, Y. Cervical cancer evaluated with integrated 18F-FDG PET/MR. Oncol. Lett. 2019, 18, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.A.; Kubik-Huch, R.A.; Hauser, N.; Freiwald-Chilla, B.; von Schulthess, G.; Froehlich, J.M.; Veit-Haibach, P. PET/MRI and PET/CT in advanced gynaecological tumours: Initial experience and comparison. Eur. Radiol. 2015, 25, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer 2010, 116, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Yanarateş, A.; Budak, E.; Budak, A.; Inan, A.H.; Kanmaz, A.G.; Oral, A.; Yazici, B. Clinical value of metabolic PET parameters of primary vulvar carcinoma. Rev. Esp. Med. Nucl. Imagen Mol. (Engl. Ed.) 2021, 40, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Bradshaw, T.; Solaiyappan, M.; Cho, S.Y. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One is Better? Nucl. Med. Mol. Imaging 2018, 52, 5–15. [Google Scholar] [CrossRef]
- Yoo, J.; Choi, J.Y.; Moon, S.H.; Bae, D.S.; Park, S.B.; Choe, Y.S.; Lee, K.H.; Kim, B.T. Prognostic significance of volume-based metabolic parameters in uterine cervical cancer determined using 18F-fluorodeoxyglucose positron emission tomography. Int. J. Gynecol. Cancer 2012, 22, 1226–1233. [Google Scholar] [CrossRef]
- Vural Topuz, Ö.; Aksu, A.; Erinç, S.R.; Tokgözoğlu, N.; Tamam, M. The Evaluation of Preoperative 18F-FDG PET/CT in Patients with Endometrial Cancer and the Correlation Between PET Parameters and Postoperative Pathology Results. Mol. Imaging Radionucl. Ther. 2022, 31, 16–22. [Google Scholar] [CrossRef]
- De Cuypere, M.; Lovinfosse, P.; Gennigens, C.; Hermesse, J.; Rovira, R.; Duch, J.; Goffin, F.; Hustinx, R.; Kridelka, F. Tumor total lesion glycolysis and number of positive pelvic lymph nodes on pretreatment positron emission tomography/computed tomography (PET/CT) predict survival in patients with locally advanced cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Mirpour, S.; Mhlanga, J.C.; Logeswaran, P.; Russo, G.; Mercier, G.; Subramaniam, R.M. The role of PET/CT in the management of cervical cancer. AJR Am. J. Roentgenol. 2013, 201, W192–W205. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Atri, M. 2018 FIGO Staging System for Uterine Cervical Cancer: Enter Cross-sectional Imaging. Radiology 2019, 292, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Deguchi, M.; Ebina, Y.; Yamada, H.; Takahashi, S.; Sugimura, K. Fusion of PET and MRI for staging of uterine cervical cancer: Comparison with contrast-enhanced 18F-FDG PET/CT and pelvic MRI. Clin. Imaging 2014, 38, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Choi, H.J.; Park, S.Y.; Lee, H.Y.; Seo, S.S.; Yoo, C.W.; Jung, D.C.; Kang, S.; Cho, K.S. Additional value of MR/PET fusion compared with PET/CT in the detection of lymph node metastases in cervical cancer patients. Eur. J. Cancer 2009, 45, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Grigsby, P.W. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.T.; Wale, D.J.; Viglianti, B.L.; Townsend, D.M.; Manganaro, M.S.; Gross, M.D.; Wong, K.K.; Rubello, D. The impact of infection and inflammation in oncologic 18F-FDG PET/CT imaging. Biomed. Pharmacother. 2019, 117, 109168. [Google Scholar] [CrossRef] [PubMed]
- White, N.S.; McDonald, C.R.; Farid, N.; Kuperman, J.M.; Kesari, S.; Dale, A.M. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using “restriction spectrum imaging”: Quantitative comparison with high B-value DWI and ADC. AJNR Am. J. Neuroradiol. 2013, 34, 958–964, S951. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, J.; Gao, J.; Guo, L.; Zhou, H.; Hu, Y.; Zhu, C.; Li, Q.; Ma, X. Diagnostic role of 18F-FDG PET/MRI in patients with gynecological malignancies of the pelvis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175401. [Google Scholar] [CrossRef]
- McGettigan, M.; Zulfiqar, M.; Shetty, A.S. Imaging of Vaginal and Vulvar Malignancy. Radiol. Clin. N. Am. 2023, 61, 651–670. [Google Scholar] [CrossRef]
- Schwarz, J.K.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA 2007, 298, 2289–2295. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Chino, Y.; Shinagawa, A.; Kurokawa, T.; Okazawa, H.; Yoshida, Y. FDG-PET/MRI with high-resolution DWI characterises the distinct phenotypes of endometrial cancer. Clin. Radiol. 2020, 75, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Creutzberg, C.L.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.A.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Arch. 2021, 478, 153–190. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. Uterine Neoplasms, Version 2.2023, NCCN Clincal Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 8 January 2024).
- Gadducci, A.; Cosio, S.; Fanucchi, A.; Cristofani, R.; Genazzani, A.R. An intensive follow-up does not change survival of patients with clinical stage I endometrial cancer. Anticancer Res. 2000, 20, 1977–1984. [Google Scholar]
- Bollineni, V.R.; Ytre-Hauge, S.; Bollineni-Balabay, O.; Salvesen, H.B.; Haldorsen, I.S. High Diagnostic Value of 18F-FDG PET/CT in Endometrial Cancer: Systematic Review and Meta-Analysis of the Literature. J. Nucl. Med. 2016, 57, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Terai, Y.; Yamamoto, K.; Yamada, T.; Ohmichi, M. The diagnostic accuracy of fluorodeoxyglucose-positron emission tomography/computed tomography and sentinel node biopsy in the prediction of pelvic lymph node metastasis in patients with endometrial cancer: A retrospective observational study. Medicine 2018, 97, e12522. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Okazawa, H.; Yoshida, Y. Diagnostic value of 18F-FDG PET/MRI for staging in patients with endometrial cancer. Cancer Imaging 2020, 20, 75. [Google Scholar] [CrossRef]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Takahashi, S.; Ebina, Y.; Miyahara, Y.; Yamada, H.; Sugimura, K. Value of fusion of PET and MRI for staging of endometrial cancer: Comparison with ¹⁸F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur. J. Radiol. 2013, 82, 1672–1676. [Google Scholar] [CrossRef]
- Ironi, G.; Mapelli, P.; Bergamini, A.; Fallanca, F.; Candotti, G.; Gnasso, C.; Taccagni, G.L.; Sant’Angelo, M.; Scifo, P.; Bezzi, C.; et al. Hybrid PET/MRI in Staging Endometrial Cancer: Diagnostic and Predictive Value in a Prospective Cohort. Clin. Nucl. Med. 2022, 47, e221–e229. [Google Scholar] [CrossRef]
- Jónsdóttir, B.; Marcickiewicz, J.; Borgfeldt, C.; Bjurberg, M.; Dahm-Kähler, P.; Flöter-Rådestad, A.; Hellman, K.; Holmberg, E.; Kjølhede, P.; Rosenberg, P.; et al. Preoperative and intraoperative assessment of myometrial invasion in endometrial cancer-A Swedish Gynecologic Cancer Group (SweGCG) study. Acta Obstet. Gynecol. Scand. 2021, 100, 1526–1533. [Google Scholar] [CrossRef]
- Gordon, B.A.; Flanagan, F.L.; Dehdashti, F. Whole-body positron emission tomography: Normal variations, pitfalls, and technical considerations. AJR Am. J. Roentgenol. 1997, 169, 1675–1680. [Google Scholar] [CrossRef]
- Shreve, P.D.; Anzai, Y.; Wahl, R.L. Pitfalls in oncologic diagnosis with FDG PET imaging: Physiologic and benign variants. Radiographics 1999, 19, 61–77; quiz 150–151. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Ide, M.; Takagi, S.; Shohtsu, A. Intrauterine accumulation of F-18 FDG during menstruation. Clin. Nucl. Med. 1997, 22, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Chander, S.; Meltzer, C.C.; McCook, B.M. Physiologic uterine uptake of FDG during menstruation demonstrated with serial combined positron emission tomography and computed tomography. Clin. Nucl. Med. 2002, 27, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Kunz, G.; Leyendecker, G. Uterine peristaltic activity during the menstrual cycle: Characterization, regulation, function and dysfunction. Reprod. Biomed. Online 2002, 4 (Suppl. S3), 5–9. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Togashi, K.; Yamaoka, T.; Fujiwara, T.; Ueda, H.; Koyama, T.; Kobayashi, H.; Kagimura, T.; Fujii, S.; Konishi, J. Uterine peristalsis shown on cine MR imaging using ultrafast sequence. J. Magn. Reson. Imaging 2003, 18, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Togashi, K.; Yamaoka, T.; Nakai, A.; Kido, A.; Nishio, S.; Yamamoto, T.; Kitagaki, H.; Fujii, S. Kinematics of the uterus: Cine mode MR imaging. Radiographics 2004, 24, e19. [Google Scholar] [CrossRef]
- Fiaschetti, V.; Calabria, F.; Crusco, S.; Meschini, A.; Nucera, F.; Schillaci, O.; Simonetti, G. MR-PET fusion imaging in evaluating adnexal lesions: A preliminary study. Radiol. Med. 2011, 116, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Yamada, S.; Kubota, K.; Ishiwata, K.; Tamahashi, N.; Ido, T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: High accumulation in macrophages and granulation tissues studied by microautoradiography. J. Nucl. Med. 1992, 33, 1972–1980. [Google Scholar]
- Expert Panel on GYN and OB Imaging; Kilcoyne, A.; Gottumukkala, R.V.; Kang, S.K.; Akin, E.A.; Hauck, C.; Hindman, N.M.; Huang, C.; Khanna, N.; Paspulati, R.; et al. ACR Appropriateness Criteria(R) Staging and Follow-up of Primary Vaginal Cancer. J. Am. Coll. Radiol. 2021, 18, S442–S455. [Google Scholar] [CrossRef]
- Preti, M.; Bucchi, L.; Micheletti, L.; Privitera, S.; Corazza, M.; Cosma, S.; Gallio, N.; Borghi, A.; Bevilacqua, F.; Benedetto, C. Four-decade trends in lymph node status of patients with vulvar squamous cell carcinoma in northern Italy. Sci. Rep. 2021, 11, 5661. [Google Scholar] [CrossRef] [PubMed]
- Rufini, V.; Garganese, G.; Ieria, F.P.; Pasciuto, T.; Fragomeni, S.M.; Gui, B.; Florit, A.; Inzani, F.; Zannoni, G.F.; Scambia, G.; et al. Diagnostic performance of preoperative [18F]FDG-PET/CT for lymph node staging in vulvar cancer: A large single-centre study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Berchiolli, R.; Kirienko, M.; Rossi, A.; Glaudemans, A.; Slart, R.; Erba, P.A. PET/MRI in Infection and Inflammation. Semin. Nucl. Med. 2018, 48, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Disselhorst, J.A.; Bezrukov, I.; Kolb, A.; Parl, C.; Pichler, B.J. Principles of PET/MR Imaging. J. Nucl. Med. 2014, 55, 2S–10S. [Google Scholar] [CrossRef] [PubMed]
- Rausch, I.; Rischka, L.; Ladefoged, C.N.; Furtner, J.; Fenchel, M.; Hahn, A.; Lanzenberger, R.; Mayerhoefer, M.E.; Traub-Weidinger, T.; Beyer, T. PET/MRI for Oncologic Brain Imaging: A Comparison of Standard MR-Based Attenuation Corrections with a Model-Based Approach for the Siemens mMR PET/MR System. J. Nucl. Med. 2017, 58, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Catana, C. Attenuation correction for human PET/MRI studies. Phys. Med. Biol. 2020, 65, 23TR02. [Google Scholar] [CrossRef] [PubMed]
- Wallstén, E.; Axelsson, J.; Jonsson, J.; Karlsson, C.T.; Nyholm, T.; Larsson, A. Improved PET/MRI attenuation correction in the pelvic region using a statistical decomposition method on T2-weighted images. EJNMMI Phys. 2020, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moller, A.; Souvatzoglou, M.; Delso, G.; Bundschuh, R.A.; Chefd’hotel, C.; Ziegler, S.I.; Navab, N.; Schwaiger, M.; Nekolla, S.G. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: Evaluation with PET/CT data. J. Nucl. Med. 2009, 50, 520–526. [Google Scholar] [CrossRef]
- Hofmann, M.; Bezrukov, I.; Mantlik, F.; Aschoff, P.; Steinke, F.; Beyer, T.; Pichler, B.J.; Scholkopf, B. MRI-based attenuation correction for whole-body PET/MRI: Quantitative evaluation of segmentation- and atlas-based methods. J. Nucl. Med. 2011, 52, 1392–1399. [Google Scholar] [CrossRef]
- Paulus, D.H.; Quick, H.H.; Geppert, C.; Fenchel, M.; Zhan, Y.; Hermosillo, G.; Faul, D.; Boada, F.; Friedman, K.P.; Koesters, T. Whole-Body PET/MR Imaging: Quantitative Evaluation of a Novel Model-Based MR Attenuation Correction Method Including Bone. J. Nucl. Med. 2015, 56, 1061–1066. [Google Scholar] [CrossRef]
- Bailey, D.L.; Pichler, B.J.; Guckel, B.; Antoch, G.; Barthel, H.; Bhujwalla, Z.M.; Biskup, S.; Biswal, S.; Bitzer, M.; Boellaard, R.; et al. Combined PET/MRI: Global Warming-Summary Report of the 6th International Workshop on PET/MRI, March 27–29, 2017, Tubingen, Germany. Mol. Imaging Biol. 2018, 20, 4–20. [Google Scholar] [CrossRef]
- Schramm, G.; Ladefoged, C.N. Metal artifact correction strategies in MRI-based attenuation correction in PET/MRI. BJR Open 2019, 1, 20190033. [Google Scholar] [CrossRef]
- Lindemann, M.E.; Gratz, M.; Blumhagen, J.O.; Jakoby, B.; Quick, H.H. MR-based truncation correction using an advanced HUGE method to improve attenuation correction in PET/MR imaging of obese patients. Med. Phys. 2022, 49, 865–877. [Google Scholar] [CrossRef]
- Blumhagen, J.O.; Braun, H.; Ladebeck, R.; Fenchel, M.; Faul, D.; Scheffler, K.; Quick, H.H. Field of view extension and truncation correction for MR-based human attenuation correction in simultaneous MR/PET imaging. Med. Phys. 2014, 41, 022303. [Google Scholar] [CrossRef]
- Paulus, D.H.; Quick, H.H. Hybrid Positron Emission Tomography/Magnetic Resonance Imaging: Challenges, Methods, and State of the Art of Hardware Component Attenuation Correction. Investig. Radiol. 2016, 51, 624–634. [Google Scholar] [CrossRef]
- Quick, H.H. Integrated PET/MR. J. Magn. Reson. Imaging 2014, 39, 243–258. [Google Scholar] [CrossRef]
- Veit-Haibach, P.; Ahlström, H.; Boellaard, R.; Delgado Bolton, R.C.; Hesse, S.; Hope, T.; Huellner, M.W.; Iagaru, A.; Johnson, G.B.; Kjaer, A.; et al. International EANM-SNMMI-ISMRM consensus recommendation for PET/MRI in oncology. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3513–3537. [Google Scholar] [CrossRef]
- Eiber, M.; Martinez-Moller, A.; Souvatzoglou, M.; Holzapfel, K.; Pickhard, A.; Loffelbein, D.; Santi, I.; Rummeny, E.J.; Ziegler, S.; Schwaiger, M.; et al. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1691–1701. [Google Scholar] [CrossRef]
- Miles, K.A.; Voo, S.A.; Groves, A.M. Additional Clinical Value for PET/MRI in Oncology: Moving beyond Simple Diagnosis. J. Nucl. Med. 2018, 59, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.E.; Christensen, N.L.; Heil, B.G. Use of a magnetic field to increase the spatial resolution of positron emission tomography. Med. Phys. 1994, 21, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Herzog, H.; Weirich, C.; Tellmann, L.; Kaffanke, J.; Caldeira, L.; Kops, E.R.; Qaim, S.M.; Coenen, H.H.; Iida, H. Effects of magnetic fields of up to 9.4 T on resolution and contrast of PET images as measured with an MR-BrainPET. PLoS ONE 2014, 9, e95250. [Google Scholar] [CrossRef]
- Polycarpou, I.; Soultanidis, G.; Tsoumpas, C. Synergistic motion compensation strategies for positron emission tomography when acquired simultaneously with magnetic resonance imaging. Philos. Trans. A Math. Phys. Eng. Sci. 2021, 379, 20200207. [Google Scholar] [CrossRef]
- Manber, R.; Thielemans, K.; Hutton, B.F.; Barnes, A.; Ourselin, S.; Arridge, S.; O’Meara, C.; Wan, S.; Atkinson, D. Practical PET Respiratory Motion Correction in Clinical PET/MR. J. Nucl. Med. 2015, 56, 890–896. [Google Scholar] [CrossRef]
- Kolbitsch, C.; Neji, R.; Fenchel, M.; Schuh, A.; Mallia, A.; Marsden, P.; Schaeffter, T. Joint cardiac and respiratory motion estimation for motion-corrected cardiac PET-MR. Phys. Med. Biol. 2018, 64, 015007. [Google Scholar] [CrossRef]
- Küstner, T.; Schwartz, M.; Martirosian, P.; Gatidis, S.; Seith, F.; Gilliam, C.; Blu, T.; Fayad, H.; Visvikis, D.; Schick, F.; et al. MR-based respiratory and cardiac motion correction for PET imaging. Med. Image Anal. 2017, 42, 129–144. [Google Scholar] [CrossRef]
- Munoz, C.; Ellis, S.; Nekolla, S.G.; Kunze, K.P.; Vitadello, T.; Neji, R.; Botnar, R.M.; Schnabel, J.A.; Reader, A.J.; Prieto, C. MR-guided motion-corrected PET image reconstruction for cardiac PET-MR. J. Nucl. Med. 2021, 62, 1768–1774. [Google Scholar] [CrossRef]
- Fayad, H.; Schmidt, H.; Wuerslin, C.; Visvikis, D. Reconstruction-Incorporated Respiratory Motion Correction in Clinical Simultaneous PET/MR Imaging for Oncology Applications. J. Nucl. Med. 2015, 56, 884–889. [Google Scholar] [CrossRef]
- Munoz, C.; Neji, R.; Cruz, G.; Mallia, A.; Jeljeli, S.; Reader, A.J.; Botnar, R.M.; Prieto, C. Motion-corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency. Magn. Reson. Med. 2018, 79, 339–350. [Google Scholar] [CrossRef]
- Wurslin, C.; Schmidt, H.; Martirosian, P.; Brendle, C.; Boss, A.; Schwenzer, N.F.; Stegger, L. Respiratory motion correction in oncologic PET using T1-weighted MR imaging on a simultaneous whole-body PET/MR system. J. Nucl. Med. 2013, 54, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.M.; Trivieri, M.; Karakatsanis, N.A.; Padilla, M.; Abgral, R.; Dweck, M.R.; Kovacic, J.C.; Fayad, Z.A. Correction of respiratory and cardiac motion in cardiac PET/MR using MR-based motion modeling. Phys. Med. Biol. 2018, 63, 225011. [Google Scholar] [CrossRef] [PubMed]
- Petibon, Y.; Huang, C.; Ouyang, J.; Reese, T.G.; Li, Q.; Syrkina, A.; Chen, Y.-L.; El Fakhri, G. Relative role of motion and PSF compensation in whole-body oncologic PET-MR imaging. Med. Phys. 2014, 41, 042503. [Google Scholar] [CrossRef]
- Yang, J. AI applications for quantitative and qualitative PET in PET/MR-where do we stand? Eur. Radiol. 2023, 33, 7530–7531. [Google Scholar] [CrossRef]
- Balaji, V.; Song, T.A.; Malekzadeh, M.; Heidari, P.; Dutta, J. Artificial Intelligence for PET and SPECT Image Enhancement. J. Nucl. Med. 2024, 65, 4–12. [Google Scholar] [CrossRef]
- Zaharchuk, G.; Davidzon, G. Artificial Intelligence for Optimization and Interpretation of PET/CT and PET/MR Images. Semin. Nucl. Med. 2021, 51, 134–142. [Google Scholar] [CrossRef]
- Liu, J.; Malekzadeh, M.; Mirian, N.; Song, T.A.; Liu, C.; Dutta, J. Artificial Intelligence-Based Image Enhancement in PET Imaging: Noise Reduction and Resolution Enhancement. PET Clin. 2021, 16, 553–576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).