Exploring the Language Used to Describe Older Patients at Multidisciplinary Cancer Conferences
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
2.2. Study Design and Setting
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Presentations in Overall Cohort
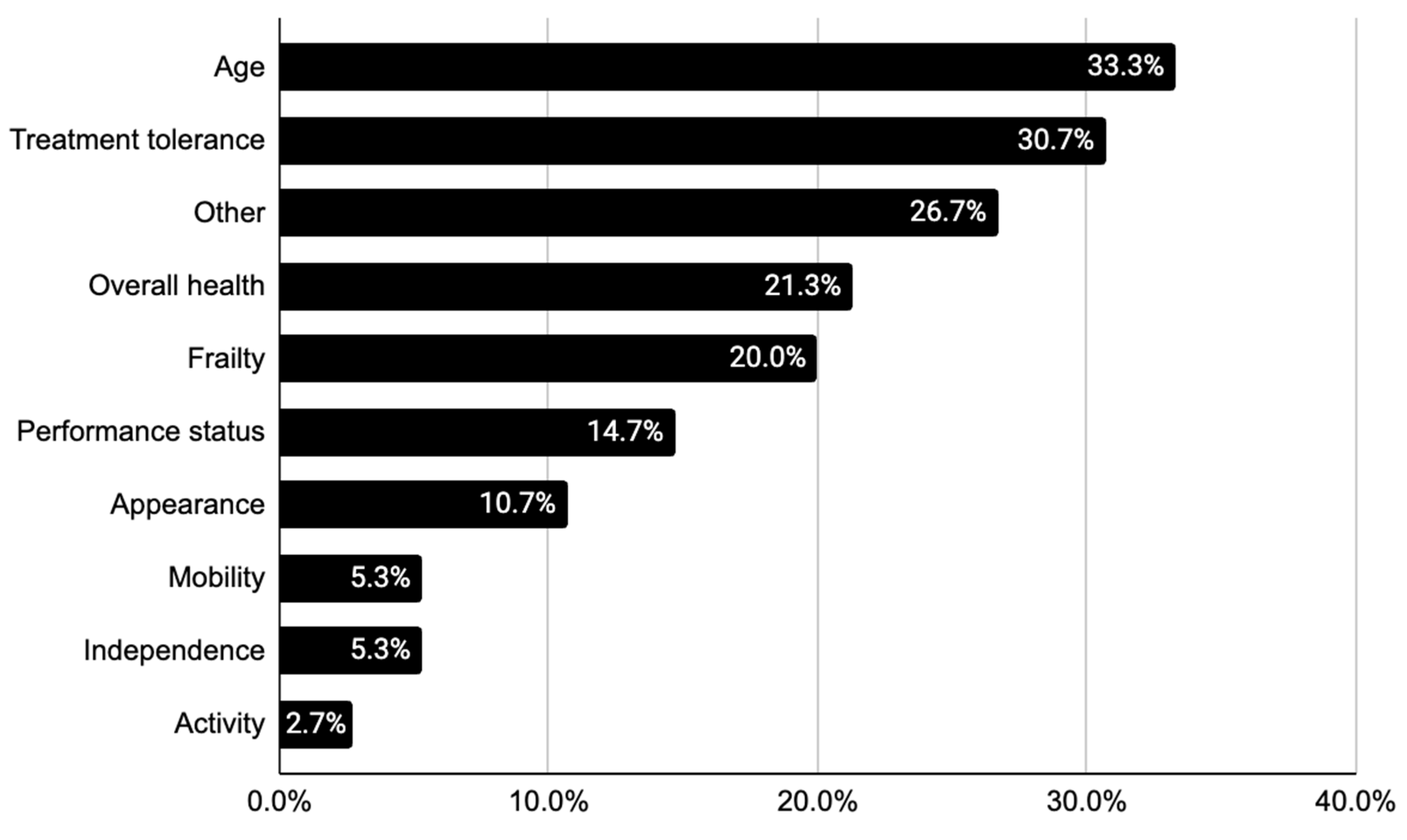
3.2. Descriptions of Frailty in Overall Cohort
3.3. Mentions of GA Domains in Overall Cohort
3.4. Case Presentations by MCC Site
3.5. Case Presentations by Presenter Specialty
3.6. Case Presentations by Presenter Training Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Date: | MCC site: | Presenter’s specialty:  MO MO  RO RO  SO SO | ||
| Patient study ID: | Patient age, sex: | Presenter:  Trainee Trainee  Staff Staff | ||
| For each case, check off the item(s) mentioned by the presenter (second column) during the MCC presentation and by the audience (third column) during the subsequent discussion. | By presenter | By audience | Notes from presentation | Notes from audience |
| Cancer-related items | ||||
| HPI |  |  | ||
| Stage and/or grade (suspected or confirmed) |  |  | ||
| Past treatment and modalities |  |  | ||
| Proposed treatment intent (curative or palliative) |  |  | ||
Proposed treatment modality—record all  Surgery Surgery  Chemotherapy Chemotherapy Radiotherapy Radiotherapy  Immunotherapy Immunotherapy Other—please specify Other—please specify |  |  | ||
| Non-cancer-related items | ||||
| Patient preference and/or goals of care |  |  | ||
| Family involvement in decision at hand |  |  | ||
| Smoking status and/or alcohol use |  |  | ||
| Socioeconomic status (e.g., education, income) |  |  | ||
| Living situation |  |  | ||
| Employment status |  |  | ||
| Race/Ethnicity |  |  | ||
| English-/Non-English speaking |  |  | ||
| Fitness/Frailty descriptions | ||||
| Fit/Vulnerable/Frail |  |  | ||
| Active/Inactive |  |  | ||
| Independent/Dependent |  |  | ||
| Performance status |  |  | ||
| Mobility |  |  | ||
| Appearance (e.g., “younger than stated age”) |  |  | ||
| Overall health |  |  | ||
| Other synonyms—record verbatim in space to the right |  |  | ||
| GA domains | ||||
| Functional status (with explicit mention of ADLs/IADLs) |  |  | ||
| Performance-based measures (e.g., grip strength, gait speed) |  |  | ||
| Falls |  |  | ||
| Comorbidities |  |  | ||
| Medications |  |  | ||
| Cognition |  |  | ||
| Mood |  |  | ||
| Social activity/support |  |  | ||
| Nutritional status |  |  | ||
References
- Marosi, C.; Köller, M. Challenge of cancer in the elderly. ESMO Open 2016, 1, e000020. [Google Scholar] [CrossRef] [PubMed]
- Sedrak, M.S.; Freedman, R.A.; Cohen, H.J.; Muss, H.B.; Jatoi, A.; Klepin, H.D.; Wildes, T.M.; Le-Rademacher, J.G.; Kimmick, G.G.; Tew, W.P.; et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J. Clin. 2021, 71, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Mohamed, M.R.; Xu, H.; Culakova, E.; Loh, K.P.; Magnuson, A.; A Flannery, M.; Obrecht, S.; Gilmore, N.; Ramsdale, E.; et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): A cluster-randomised study. Lancet 2021, 398, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Given, B.; Given, C.W. Older adults and cancer treatment. Cancer 2008, 113 (Suppl. 12), 3505–3511. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Torres, C.; Korc-Grodzicki, B.; Hsu, T. Models of Clinical Care Delivery for Geriatric Oncology in Canada and the United States: A Survey of Geriatric Oncology Care Providers. J. Geriatr. Oncol. 2022, 13, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Wright, F.; De Vito, C.; Langer, B.; Hunter, A.; Expert Panel on the Multidisciplinary Cancer Conference Standards. Multidisciplinary cancer conferences: A systematic review and development of practice standards. Eur. J. Cancer 2007, 43, 1002–1010. [Google Scholar] [CrossRef]
- Specchia, M.L.; Frisicale, E.M.; Carini, E.; Di Pilla, A.; Cappa, D.; Barbara, A.; Ricciardi, W.; Damiani, G. The impact of tumor board on cancer care: Evidence from an umbrella review. BMC Health Serv. Res. 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.P.; McLachlan, S.; Philip, J.A.M. “Pretty fit and healthy”: The discussion of older people in cancer multidisciplinary meetings. J. Geriatr. Oncol. 2019, 10, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, P.; Heitz, D.; Mathelin, C.; Polesi, H.; Asmane, I.; Litique, V.; Rob, L.; Bergerat, J.-P.; Kurtz, J.-E. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit. Rev. Oncol. Hematol. 2011, 79, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Festen, S.; Kok, M.; Hopstaken, J.S.; van der Wal-Huisman, H.; van der Leest, A.; Reyners, A.K.; de Bock, G.H.; de Graeff, P.; van Leeuwen, B.L. How to incorporate geriatric assessment in clinical decision-making for older patients with cancer. An implementation study. J. Geriatr. Oncol. 2019, 10, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Blanc, M.; Dialla, O.; Manckoundia, P.; Arveux, P.; Dabakuyo, S.; Quipourt, V. Influence of the geriatric oncology consultation on the final therapeutic decision in elderly subjects with cancer: Analysis of 191 patients. J. Nutr. Health Aging 2014, 18, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kyi, K.; Gilmore, N.; Kadambi, S.; Loh, K.P.; Magnuson, A. Stigmatizing language in caring for older adults with cancer: Common patterns of use and mechanisms to change the culture. J. Geriatr. Oncol. 2023, 14, 101593. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, R.; Yoong, J.; Joshi, S.; Yip, D.; Mileshkin, L.; Marx, G.M.; Dunlop, T.; Hovey, E.J.; Della Fiorentina, S.A.; Venkateswaran, L.; et al. Geriatric assessment of older patients with cancer in Australia—A multicentre audit. J. Geriatr. Oncol. 2015, 6, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Bolle, S.; Smets, E.M.A.; Hamaker, M.E.; Loos, E.F.; van Weert, J.C.M. Medical decision making for older patients during multidisciplinary oncology team meetings. J. Geriatr. Oncol. 2019, 10, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Kidger, J.; Murdoch, J.; Donovan, J.L.; Blazeby, J.M. Clinical decision-making in a multidisciplinary gynaecological cancer team: A qualitative study. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Billon, E.; Tassy, L.; Sarabi, M.; Braticevic, C.; Cecile, M.; Albrand, G.; Terret, C.; Rousseau, F. Use’s assessment of geriatric variables in the older patient with cancer’s multidisciplinary team meeting. J. Geriatr. Oncol. 2020, 11, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Festen, S.; Nijmeijer, H.; van Leeuwen, B.L.; van Etten, B.; van Munster, B.C.; de Graeff, P. Multidisciplinary decision-making in older patients with cancer, does it differ from younger patients? Eur. J. Surg. Oncol. 2021, 47, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Restivo, L.; Apostolidis, T.; Bouhnik, A.D.; Garciaz, S.; Aurran, T.; Julian-Reynier, C. Patients’ non-medical characteristics contribute to collective medical decision-making at multidisciplinary oncological team meetings. PLoS ONE 2016, 11, e0154969. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Velarde, C.; Hurria, A.; Magnuson, A.; Lowenstein, L.; Pandya, C.; O’Donovan, A.; Gorawara-Bhat, R.; Dale, W. Geriatric assessment-guided care processes for older adults: A delphi consensus of geriatric oncology experts. J. Natl. Compr. Cancer Netw. 2015, 13, 1120–1130. [Google Scholar] [CrossRef]
- Decoster, L.; Van Puyvelde, K.; Mohile, S.; Wedding, U.; Basso, U.; Colloca, G.; Rostoft, S.; Overcash, J.; Wildiers, H.; Steer, C.; et al. Screening tools for multidimentional health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations. Ann. Oncol. 2015, 26, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J.; The MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Coate, L.E.; Massey, C.; Hope, A.; Sacher, A.; Barrett, K.; Pierre, A.; Leighl, N.; Brade, A.; de Perrot, M.; Waddell, T.; et al. Treatment of the elderly when cure is the goal: The influence of age on treatment selection and efficacy for stage III non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Peake, M.D.; Thompson, S.; Lowe, D.; Pearson, M.G. Ageism in the management of lung cancer. Age Ageing 2003, 32, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Freyer, G.; Braud, A.-C.; Chaibi, P.; Spielmann, M.; Martin, J.-P.; Vilela, G.; Guerin, D.; Zelek, L. Dealing with metastatic breast cancer in elderly women: Results from a French study on a large cohort carried out by the “Observatory on elderly patients”. Ann. Oncol. 2006, 17, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Boyle, H.; Alibhai, S.; Decoster, L.; Efstathiou, E.; Fizazi, K.; Mottet, N.; Oudard, S.; Payne, H.; Prentice, M.; Puts, M.; et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur. J. Cancer 2019, 116, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, T.; Babic-Illman, G.; Ross, P.; Maisey, N.; Hughes, S.; Fields, P.E.; Martin, F.C.; Wang, Y.; Harari, D. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older patients. Br. J. Cancer 2015, 112, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Soo, W.K.; King, M.; Pope, A.; Parente, P.; Darzins, P.; Davis, I.D. Integrated geriatric assessment and treatment (INTEGRATE) in older people with cancer planned for systemic anticancer therapy. J. Clin. Oncol. 2020, 38 (Suppl. S15), 12011. [Google Scholar] [CrossRef]
- Mohile, S.G.; Epstein, R.M.; Hurria, A.; Heckler, C.E.; Canin, B.; Culakova, E.; Duberstein, P.; Gilmore, N.; Xu, H.; Plumb, S.; et al. Communication with older patients with cancer using geriatric assessment: A cluster-randomized clinical trial from the national cancer institute community oncology research program. JAMA Oncol. 2020, 6, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Marwell, J.G.; Khaki, A.R. Geriatric assessment in the older adult with genitourinary cancer: A narrative review. Front Oncol. 2023, 13, 1124309. [Google Scholar] [CrossRef] [PubMed]
- Wildes, T.M.; O’Donovan, A.; Colloca, G.F.; Cheung, K.L. Tumour boards in geriatric oncology. Age Ageing 2018, 47, 168–170. [Google Scholar] [CrossRef]

| Variables | Definition | Examples | |
|---|---|---|---|
| Frailty-Related | Frailty status | Description of patients as “frail”, “vulnerable”, or “fit” | “Quite frail”. “Pretty fit”. |
| Age | Reiteration of age to suggest or emphasize frailty/fitness status | “Fine for age”. “He’s 86, but an okay 86”. | |
| Activity level | Description of patients as “active” or “inactive” | “Pretty healthy, very active”. | |
| Appearance | Description of patients’ appearance | “Looks younger than 80”. “Does not look like a candidate for anything”. | |
| Independence | Description of patients as “independent” or “dependent” on others, without elaboration on specific ADLs or IADLs | “Fully independent”. | |
| Mobility | Description of patients’ ability to ambulate or move | “Very vigorous, moves well”. | |
| Overall health | Description of patients’ general health or well-being | “Otherwise quite healthy”. | |
| Performance status | Mention of patients’ performance status per ECOG | “ECOG 1”. “Performance status poor, very symptomatic”. | |
| Treatment tolerance | Prediction of a patient’s ability to tolerate treatment | “No way fit for [chemotherapy]”. | |
| GA-Related | Comorbidities | Mention of patients’ comorbidities (complete or incomplete) or comorbidity burden | “Many comorbidities, not a surgical candidate”. |
| Medications | Mention of patients’ medication list (complete or incomplete) | “On apixaban”. | |
| Cognition | Mention of the presence/absence of any cognitive impairment | “Baseline dementia”. | |
| Falls | Mention of patients’ falls or fall risk | “Had a fall and [subsequent] hip fracture with surgical repair”. | |
| Functional status | Description of patients’ ability to perform activities necessary for or desirable in daily life | “Fully independent, drives here for clinic appointments”. | |
| Mood | Mention of patients’ mood | “[Patient] has generalized low mood”. | |
| Physical performance | Mention of objective measures of patients’ physical function (for example, gait speed, grip strength) | N/A | |
| Nutrition | Mention of patients’ appetite or nutritional status | “Struggling with malnutrition”. | |
| Social support | Mention of a social support network, including mention of caregivers or loved ones involved in the patient’s life | “Difficult social situation, [patient] lives alone, [with] family in [another country]”. |
| Mean patient age, years (range) | 80.9 (75–97) | |
| Patient sex, n (%) | Male | 46 (61.3) |
| Female | 29 (38.7) | |
| Presenter specialty, n | Medical oncology | 22 |
| Radiation oncology | 11 | |
| Surgical oncology | 45 | |
| Presenter level, n | Trainee | 31 |
| Staff | 38 | |
| Both | 2 | |
| Case presentations by site, n (%) | General gastrointestinal | 4 (5.3) |
| Genitourinary | 19(25.3) | |
| Head and neck | 19 (25.3) | |
| Thoracic | 15 (20.0) | |
| Upper gastrointestinal | 18 (24.0) | |
| Presentation question, n (%) | Diagnostic | 4 (5.3) |
| Therapeutic | 65 (86.7) | |
| Both | 6 (8.0) | |
| Cancer diagnosis, n (%) | Symptomatic | 40 (53.3) |
| Incidental | 3 (4.0) | |
| Detection via surveillance | 17 (22.7) | |
| Unclear or unspecified | 15 (20.0) | |
| Cancer treatment history, n (%) | New diagnosis | 36 (48.0) |
| Prior treatment | 37 (49.3) | |
| Unclear or unspecified | 2 (2.7) | |
| Proposed treatment intent, n (%) | Curative | 1 (1.3) |
| Palliative | 12 (16.0) | |
| Both | 6 (8.0) | |
| Unclear or unspecified | 56 (74.7) | |
| Patient preferences, n (%) | Mentioned | 15 (20.0) |
| Proposed treatment modalities, n (%) | Chemotherapy | 35 (46.7) |
| Radiotherapy | 40 (53.3) | |
| Surgery | 37 (49.3) | |
| Immunotherapy | 4 (5.3) | |
| Best supportive care | 6 (8.0) | |
| Frailty-related descriptors | Frailty status, n (%) | 15 (20.0) |
| Age, n (%) | 24 (32.0) | |
| Activity level, n (%) | 2 (2.7) | |
| Appearance, n (%) | 8 (10.6) | |
| Appearance, n (%) | 4 (5.3) | |
| Independence, n (%) | 4 (5.3) | |
| Mobility, n (%) | 16 (21.3) | |
| Overall health, n (%) | 12 (16.0) | |
| Performance status, n (%) | 15 (20.0) | |
| Treatment tolerance, n (%) | 23 (30.7) | |
| GA domains | Comorbidities, n (%) | 39 (52.0) |
| Medications, n (%) | 16 (21.3) | |
| Cognition, n (%) | 4 (5.3) | |
| Falls, n (%) | 1 (1.3) | |
| Functional status, n (%) | 5 (6.7) | |
| Mood, n (%) | 1 (1.3) | |
| Physical performance, n (%) | 0 (0) | |
| Nutrition, n (%) | 3 (4.0) | |
| Social support, n (%) | 7 (9.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, V.S.; Carrozzi, A.; Papadopoulos, E.; Tejero, I.; Thiruparanathan, T.; Perlis, N.; Hope, A.J.; Jang, R.W.; Alibhai, S.M.H. Exploring the Language Used to Describe Older Patients at Multidisciplinary Cancer Conferences. Cancers 2024, 16, 1477. https://doi.org/10.3390/cancers16081477
Kim VS, Carrozzi A, Papadopoulos E, Tejero I, Thiruparanathan T, Perlis N, Hope AJ, Jang RW, Alibhai SMH. Exploring the Language Used to Describe Older Patients at Multidisciplinary Cancer Conferences. Cancers. 2024; 16(8):1477. https://doi.org/10.3390/cancers16081477
Chicago/Turabian StyleKim, Valerie S., Anthony Carrozzi, Efthymios Papadopoulos, Isabel Tejero, Thirisangi Thiruparanathan, Nathan Perlis, Andrew J. Hope, Raymond W. Jang, and Shabbir M. H. Alibhai. 2024. "Exploring the Language Used to Describe Older Patients at Multidisciplinary Cancer Conferences" Cancers 16, no. 8: 1477. https://doi.org/10.3390/cancers16081477
APA StyleKim, V. S., Carrozzi, A., Papadopoulos, E., Tejero, I., Thiruparanathan, T., Perlis, N., Hope, A. J., Jang, R. W., & Alibhai, S. M. H. (2024). Exploring the Language Used to Describe Older Patients at Multidisciplinary Cancer Conferences. Cancers, 16(8), 1477. https://doi.org/10.3390/cancers16081477





