Value of 11C-Methionine PET Imaging in High-Grade Gliomas: A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Gliomas
3. Imaging Techniques
3.1. Initial Diagnosis and Surgical Management
3.2. Post-Operative Imaging and Radiation Planning
3.3. Difficulties with Post-Treatment Imaging
4. Treatment
4.1. Surgery
4.2. Radiation Therapy
4.3. Chemotherapy
4.4. Targeted Therapy
4.5. Alternating Electric Field Therapy
5. Follow-Up
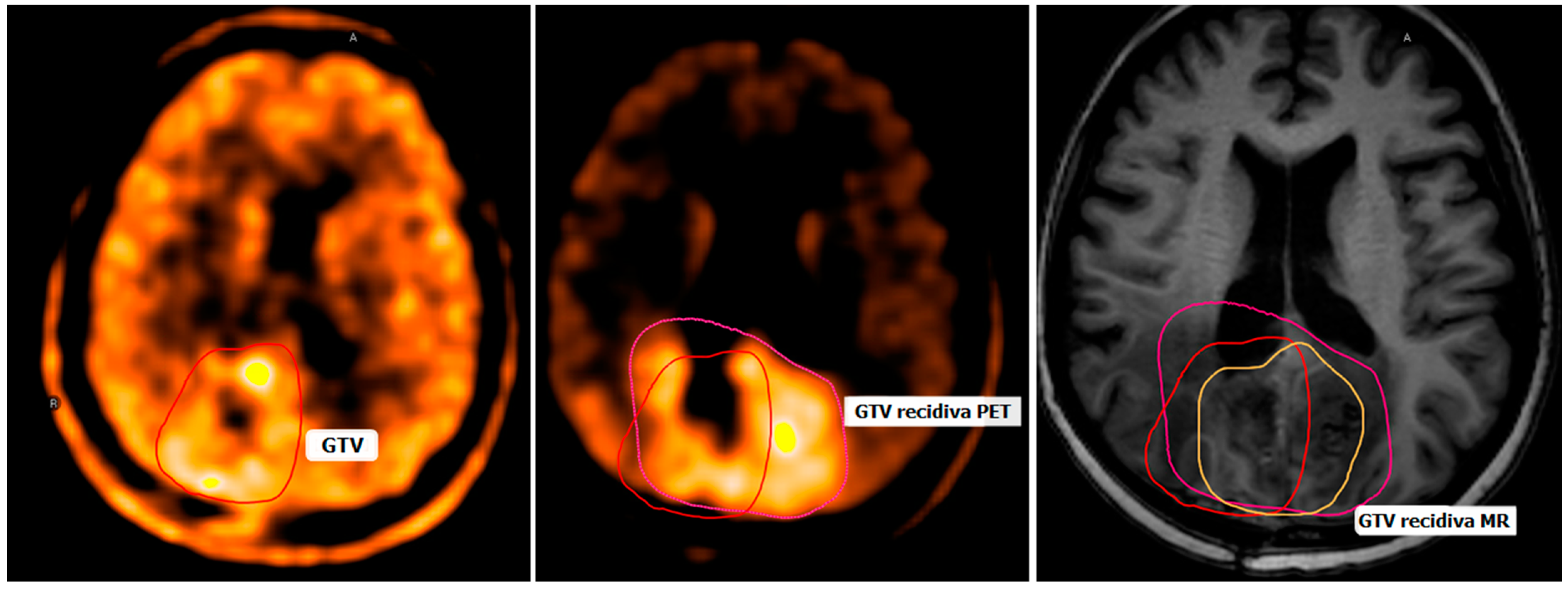
6. Recurrence and Progression
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gulyás, B.; Halldin, C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q. J. Nucl. Med. Mol. Imaging 2012, 56, 173–190. [Google Scholar] [PubMed]
- Galldiks, N.; Lohmann, P.; Fink, G.R.; Langen, K.J. Amino Acid PET in Neurooncology. J. Nucl. Med. 2023, 64, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, A.W.; Enting, R.H.; Heesters, M.A.; Dierckx, R.A.; van Rheenen, R.W.; Walenkamp, A.M.; Slart, R.H. Value of 11C-methionine PET in imaging brain tumors and metastases. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, C.; Kirchner, J.; Poeppel, T.D.; Schaarschmidt, B.; Kebir, S.; El Hindy, N.; Hense, J.; Quick, H.H.; Glas, M.; Herrmann, K.; et al. 11C-MET PET/MRI for detection of recurrent glioma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. L-[Methyl-11C] Methionine-Positron-Emission Tomography (MET-PET). Methods Mol. Biol. 2019, 1866, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Graber, J.J. Overview of prognostic factors in adult gliomas. Ann. Palliat. Med. 2021, 10, 863–874. [Google Scholar] [CrossRef]
- Lukács, G.; Tóth, Z.; Sipos, D.; Csima, M.; Hadjiev, J.; Bajzik, G.; Cselik, Z.; Semjén, D.; Repa, I.; Kovács, Á. Long-term follow-up results of concomitant chemoradiotherapy followed by adjuvant temozolomide therapy for glioblastoma multiforme patients. The importance of MRI information in survival: Single-center experience. Ideggyogy. Sz. 2018, 71, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoda, R.A.; Cimino, P.J. Classification and Grading of Central Nervous System Tumors According to the World Health Organization 5th Edition. Semin. Neurol. 2023, 43, 833–844. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Persico, M.; Matteoni, S.; Paggi, M.G. Molecular Biology in Glioblastoma Multiforme Treatment. Cells 2022, 11, 1850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Debreczeni-Máté, Z.; Törő, I.; Simon, M.; Gál, K.; Barabás, M.; Sipos, D.; Kovács, A. Recurrence Patterns after Radiotherapy for Glioblastoma with [(11)C]methionine Positron Emission Tomography-Guided Irradiation for Target Volume Optimization. Diagnostics 2024, 14, 964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pope, W.B.; Brandal, G. Conventional and advanced magnetic resonance imaging in patients with high-grade glioma. Q J Nucl Med. Mol. Imaging 2018, 62, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Alexander, G.S.; Bakas, S.; Nikam, R.; Talekar, K.; Palmer, J.D.; Shi, W. Advanced magnetic resonance imaging in glioblastoma: A review. Chin. Clin. Oncol. 2017, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Pirotte, B.; Goldman, S.; Massager, N.; David, P.; Wikler, D.; Vandesteene, A.; Salmon, I.; Brotchi, J.; Levivier, M. Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J. Nucl. Med. 2004, 45, 1293–1298. [Google Scholar] [PubMed]
- Singhal, T.; Narayanan, T.K.; Jacobs, M.P.; Bal, C.; Mantil, J.C. 11C-methionine PET for grading and prognostication in gliomas: A comparison study with 18F-FDG PET and contrast enhancement on MRI. J. Nucl. Med. 2012, 53, 1709–1715. [Google Scholar] [CrossRef]
- Niyazi, M.; Andratschke, N.; Bendszus, M.; Chalmers, A.J.; Erridge, S.C.; Galldiks, N.; Lagerwaard, F.J.; Navarria, P.; Munck af Rosenschöld, P.; Ricardi, U.; et al. ESTRO-EANO guideline on target delineation and radiotherapy details for glioblastoma. Radiother. Oncol. 2023, 184, 109663. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Central Nervous System Cancers. Version 1.2024. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425 (accessed on 15 June 2024).
- Galldiks, N. Diagnosing pseudoprogression in glioblastoma: A challenging clinical issue. Neurooncol. Pract. 2023, 11, 1–2. [Google Scholar] [CrossRef]
- Goldberg, M.; Mondragon-Soto, M.G.; Altawalbeh, G.; Baumgart, L.; Gempt, J.; Bernhardt, D.; Combs, S.E.; Meyer, B.; Aftahy, A.K. Enhancing outcomes: Neurosurgical resection in brain metastasis patients with poor Karnofsky performance score—A comprehensive survival analysis. Front Oncol. 2024, 13, 1343500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Datta, S.; Luthra, R.; Bharadvaja, N. Medicinal Plants for Glioblastoma Treatment. Anticancer Agents Med. Chem. 2022, 22, 2367–2384. [Google Scholar] [CrossRef] [PubMed]
- Sidibe, I.; Tensaouti, F.; Roques, M.; Cohen-Jonathan-Moyal, E.; Laprie, A. Pseudoprogression in Glioblastoma: Role of Metabolic and Functional MRI-Systematic Review. Biomedicines 2022, 10, 285. [Google Scholar] [CrossRef]
- Kim, Y.Z.; Kim, C.Y.; Lim, D.H. The Overview of Practical Guidelines for Gliomas by KSNO, NCCN, and EANO. Brain Tumor Res. Treat. 2022, 10, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ma, Y.; Chen, G.; Zeng, S.; Guo, T.; Yang, Z. Comparative analysis of the prognosis of external beam radiation therapy (EBRT) and EBRT plus brachytherapy for glioblastoma multiforme: A SEER population-based study. Radiat. Oncol. 2022, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, Y.; Koc, M. 3-D conformal radiotherapy with concomitant and adjuvant temozolomide for patients with glioblastoma multiforme and evaluation of prognostic factors. Radiol. Oncol. 2011, 45, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Yahara, K.; Ohguri, T.; Udono, H.; Yamamoto, J.; Tomura, K.; Onoda, T.; Imada, H.; Nishizawa, S.; Korogi, Y. Radiotherapy using IMRT boosts after hyperbaric oxygen therapy with chemotherapy for glioblastoma. J. Radiat. Res. 2017, 58, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Redmond, K.J.; Mehta, M. Stereotactic Radiosurgery for Glioblastoma. Cureus 2015, 7, e413. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Ruiz-Garcia, H.; Nehlsen, A.D.; Sindhu, K.K.; Estrada, R.S.; Borst, G.R.; Sheehan, J.P.; Quinones-Hinojosa, A.; Trifiletti, D.M. Preoperative Stereotactic Radiosurgery for Glioblastoma. Biology 2022, 11, 194. [Google Scholar] [CrossRef]
- Jiapaer, S.; Furuta, T.; Tanaka, S.; Kitabayashi, T.; Nakada, M. Potential Strategies Overcoming the Temozolomide Resistance for Glioblastoma. Neurol. Med.-Chir. 2018, 58, 405–421. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, S.; Kim, H.; Kang, H.; Youn, H.; Jo, S.; Youn, B.; Kim, H.Y. Revisiting Platinum-Based Anticancer Drugs to Overcome Gliomas. Int. J. Mol. Sci. 2021, 22, 5111. [Google Scholar] [CrossRef]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma targeted therapy: Insight into future of molecular approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef]
- Ghiaseddin, A.P.; Shin, D.; Melnick, K.; Tran, D.D. Tumor Treating Fields in the Management of Patients with Malignant Gliomas. Curr. Treat. Options Oncol. 2020, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Chang, S.M.; Van den Bent, M.J.; Vogelbaum, M.A.; Macdonald, D.R.; Lee, E.Q. Response Assessment in Neuro-Oncology Clinical Trials. J. Clin. Oncol. 2017, 35, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Andratschke, N.; Willmann, J.; Appelt, A.L.; Alyamani, N.; Balermpas, P.; Baumert, B.G.; Hurkmans, C.; Høyer, M.; Langendijk, J.A.; Kaidar-Person, O.; et al. European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus on re-irradiation: Definition, reporting, and clinical decision making. Lancet Oncol. 2022, 23, e469–e478, Erratum in Lancet Oncol. 2022, 23, e492. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Alexiou, G.A.; Fotopoulos, A.D.; Jabbour, P.; Kyritsis, A.P.; Sioka, C. Performance of 18F-FDG, 11C-Methionine, and 18F-FET PET for Glioma Grading: A Meta-analysis. Clin. Nucl. Med. 2019, 44, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zheng, J.; Xu, W.; Weng, J.; Gao, L.; Tao, L.; Liang, F.; Zhang, J. Accuracy of 18F-FDOPA Positron Emission Tomography and 18F-FET Positron Emission Tomography for Differentiating Radiation Necrosis from Brain Tumor Recurrence. World Neurosurg. 2018, 114, e1211–e1224. [Google Scholar] [CrossRef] [PubMed]
- Nikaki, A.; Angelidis, G.; Efthimiadou, R.; Tsougos, I.; Valotassiou, V.; Fountas, K.; Prasopoulos, V.; Georgoulias, P. 18F-fluorothymidine PET imaging in gliomas: An update. Ann. Nucl. Med. 2017, 31, 495–505. [Google Scholar] [CrossRef]
- Sipos, D.; László, Z.; Tóth, Z.; Kovács, P.; Tollár, J.; Gulybán, A.; Lakosi, F.; Repa, I.; Kovács, A. Additional Value of 18F-FDOPA Amino Acid Analog Radiotracer to Irradiation Planning Process of Patients with Glioblastoma Multiforme. Front. Oncol. 2021, 11, 699360. [Google Scholar] [CrossRef]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20 (Suppl. S5), S2–S8. [Google Scholar] [CrossRef]
- Kubota, K.; Matsuzawa, T.; Fujiwara, T.; Sato, T.; Tada, M.; Ido, T.; Ishiwata, K. Differential diagnosis of AH109A tumor and inflammation by radioscintigraphy with L-[methyl-11C]methionine. Jpn. J. Cancer Res. 1989, 80, 778–782. [Google Scholar] [CrossRef]
- Ng, D.; Jacobs, M.; Mantil, J. Combined C-11 methionine and F-18 FDG PET imaging in a case of neurosarcoidosis. Clin. Nucl. Med. 2006, 31, 373–375. [Google Scholar] [CrossRef]
- Hashimoto, S.; Inaji, M.; Nariai, T.; Kobayashi, D.; Sanjo, N.; Yokota, T.; Ishii, K.; Taketoshi, M. Usefulness of [11C] Methionine PET in the Differentiation of Tumefactive Multiple Sclerosis from High Grade Astrocytoma. Neurol. Med.-Chir. 2019, 59, 176–183. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debreczeni-Máté, Z.; Freihat, O.; Törő, I.; Simon, M.; Kovács, Á.; Sipos, D. Value of 11C-Methionine PET Imaging in High-Grade Gliomas: A Narrative Review. Cancers 2024, 16, 3200. https://doi.org/10.3390/cancers16183200
Debreczeni-Máté Z, Freihat O, Törő I, Simon M, Kovács Á, Sipos D. Value of 11C-Methionine PET Imaging in High-Grade Gliomas: A Narrative Review. Cancers. 2024; 16(18):3200. https://doi.org/10.3390/cancers16183200
Chicago/Turabian StyleDebreczeni-Máté, Zsanett, Omar Freihat, Imre Törő, Mihály Simon, Árpád Kovács, and David Sipos. 2024. "Value of 11C-Methionine PET Imaging in High-Grade Gliomas: A Narrative Review" Cancers 16, no. 18: 3200. https://doi.org/10.3390/cancers16183200
APA StyleDebreczeni-Máté, Z., Freihat, O., Törő, I., Simon, M., Kovács, Á., & Sipos, D. (2024). Value of 11C-Methionine PET Imaging in High-Grade Gliomas: A Narrative Review. Cancers, 16(18), 3200. https://doi.org/10.3390/cancers16183200






