The Clinical Value of Pre-Diagnostic Thrombocytosis for the Detection of Lung Cancer in Primary Care
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
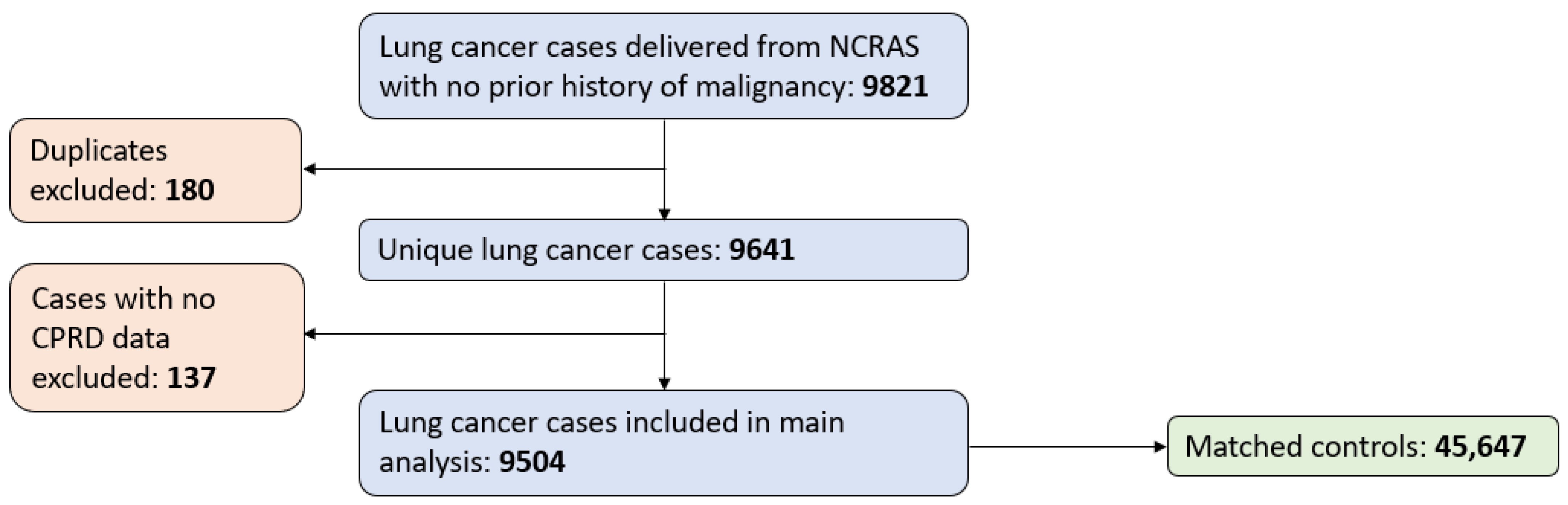
2.1. Data Sources and Patients
2.2. Study Definitions
2.3. Sample Size Calculations
2.4. Statistical Analysis of Data
3. Results
3.1. Patient Characteristics
3.2. Pre-Diagnostic Thrombocytosis
3.3. Stage at Diagnosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Research UK. Lung Cancer Incidence Statistics [Internet]. 2022. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence (accessed on 20 November 2023).
- Cancer Research UK. Early Diagnosis Data Hub [Internet]. 2023. Available online: https://crukcancerintelligence.shinyapps.io/EarlyDiagnosis/ (accessed on 20 November 2023).
- National Health Service. Online Version of the NHS Long Term Plan [Internet]. Available online: https://www.longtermplan.nhs.uk/ (accessed on 20 November 2023).
- Sunak RBS. Press Release: New Lung Cancer Screening Roll Out to Detect Cancer Sooner [Internet]. June 2023. Available online: https://www.gov.uk/government/news/new-lung-cancer-screening-roll-out-to-detect-cancer-sooner (accessed on 5 March 2024).
- NHS England. Routes to Diagnosis, 2018 [Internet]. 2022. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/routes-to-diagnosis/2018 (accessed on 9 March 2024).
- Bailey, S.E.; Ukoumunne, O.C.; Shephard, E.A.; Hamilton, W. Clinical relevance of thrombocytosis in primary care: A prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br. J. Gen. Pract. 2017, 67, e405–e413. [Google Scholar] [CrossRef] [PubMed]
- Giannakeas, V.; Kotsopoulos, J.; Cheung, M.C.; Rosella, L.; Brooks, J.D.; Lipscombe, L.; Akbari, M.R.; Austin, P.C.; Narod, S.A. Analysis of Platelet Count and New Cancer Diagnosis Over a 10-Year Period. JAMA Netw. Open 2022, 5, e2141633. [Google Scholar] [CrossRef] [PubMed]
- Giannakeas, V.; Narod, S.A. Incidence of Cancer Among Adults with Thrombocytosis in Ontario, Canada. JAMA Netw. Open 2021, 4, e2120633. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health and Care Excellence. Lung and Pleural Cancers—Recognition and Referral [Internet]. 2021. Available online: https://cks.nice.org.uk/topics/lung-pleural-cancers-recognition-referral/ (accessed on 20 November 2023).
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Wistuba, I. The 2015 World Health Organization Classification of Lung Tumors. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Raso, M.G.; Bota-Rabassedas, N.; Wistuba, I.I. Pathology and Classification of SCLC. Cancers 2021, 13, 820. [Google Scholar] [CrossRef] [PubMed]
- Blandin Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef] [PubMed]
- Herrett, E.; Gallagher, A.M.; Bhaskaran, K.; Forbes, H.; Mathur, R.; van Staa, T.; Smeeth, L. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int. J. Epidemiol. 2015, 44, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Dedman, D.; Campbell, J.; Booth, H.; Lunn, D.; Chapman, J.; Myles, P. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int. J. Epidemiol. 2019, 48, 1740–1740g. [Google Scholar] [CrossRef] [PubMed]
- Henson, K.E.; Elliss-Brookes, L.; Coupland, V.H.; Payne, E.; Vernon, S.; Rous, B.; Rashbass, J. Data Resource Profile: National Cancer Registration Dataset in England. Int. J. Epidemiol. 2020, 49, 16–16h. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Hyde, C.; Hamilton, W. Risk of uterine cancer in symptomatic women in primary care: Case–control study using electronic records. Br. J. Gen. Pract. 2013, 63, e643–e648. [Google Scholar] [CrossRef] [PubMed]
- Shephard, E.; Neal, R.; Rose, P.; Walter, F.; Hamilton, W.T. Clinical features of kidney cancer in primary care: A case-control study using primary care records. Br. J. Gen. Pract. 2013, 63, e250–e255. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.; Peters, T.; Round, A.; Sharp, D. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax 2005, 60, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Lung Cancer Foundation of America. Types of Lung Cancer [Internet]. 2022. Available online: https://lcfamerica.org/about-lung-cancer/diagnosis/types/ (accessed on 25 November 2023).
- Stata Corp. Ci—Confidence Intervals for Means, Proportions, and Variances [Internet]. 2023. Available online: https://www.stata.com/manuals/rci.pdf (accessed on 9 March 2024).
- StataCorp. Stata Statistical Software; Release 16; StataCorp LLP: College Station, TX, USA, 2019. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. Available online: https://pubmed.ncbi.nlm.nih.gov/18064739/ (accessed on 8 September 2022). [CrossRef] [PubMed]
- Barlow, M.; Hamilton, W.; Ukoumunne, O.C.; Bailey, S.E.R. The association between thrombocytosis and subtype of lung cancer: A systematic review and meta-analysis. Transl. Cancer Res. 2021, 10, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhong, H.; Ye, L.; Li, Q.; Fang, S.; Gu, W.; Qian, Y. Prognostic value of pretreatment platelet counts in lung cancer: A systematic review and meta-analysis. BMC Pulm. Med. 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N.; Winther-Larsen, A. Pretreatment Platelet Count is a Prognostic Marker in Lung Cancer: A Danish Registry-based Cohort Study. Clin. Lung Cancer 2023, 24, 175–183. [Google Scholar] [CrossRef]
- Zhou, Y.; Walter, F.M.; Mounce, L.; Abel, G.A.; Singh, H.; Hamilton, W.; Stewart, G.D.; Lyratzopoulos, G. Identifying opportunities for timely diagnosis of bladder and renal cancer via abnormal blood tests: A longitudinal linked data study. Br. J. Gen. Pract. 2022, 72, e19–e25. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.J.; Louie, A.V.; Sutradhar, R.; Paszat, L.; Brooks, D.; Gershon, A.S. Association between COPD and Stage of Lung Cancer Diagnosis: A Population-Based Study. Curr. Oncol. 2023, 30, 6397–6410. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Cancer Incidence for All Cancers Combined [Internet]. 2018. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/all-cancers-combined#heading-Zero (accessed on 9 March 2024).
- Chen, Y.; Zhong, H.; Zhao, Y.; Luo, X.; Gao, W. Role of platelet biomarkers in inflammatory response. Biomark. Res. 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Henson:, L.A.; Chukwusa, E.; Ng Yin Ling, C.; Khan, S.A.; Gao, W. Lung cancer deaths (England 2001–2017)—Comorbidities: A national population-based analysis. BMJ Support. Palliat. Care 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
| Lung Cancer Cases n = 9504 | Matched Controls n = 45,647 | ||||||
|---|---|---|---|---|---|---|---|
| Median age (IQR) | 72 (65 to 79) | 72 (65 to 79) | |||||
| Male sex, % | 52.6% | 52.3% | |||||
| Histological subtype distribution, n (%) | ADC | 3260 (34.3) | |||||
| SCC | 2020 (21.3) | ||||||
| LCC | 70 (0.7) | ||||||
| SCLC | 1098 (11.6) | ||||||
| Other or missing | 3056 (32.2) | ||||||
| n | % | 95% CI | n | % | 95% CI | ||
| Diagnosed at an early stage | 2149 | 23.7 | 22.9 to 24.7 | ||||
| Ever smokers | 9132 | 96.1 | 95.7 to 96.5 | 35,344 | 77.4 | 77.0 to 77.8 | |
| Anti-platelet prescriptions | 3030 | 31.9 | 30.9 to 32.8 | 10,420 | 22.8 | 22.4 to 23.2 | |
| COPD diagnosis | 2211 | 23.3 | 22.4 to 24.1 | 2041 | 4.5 | 4.3 to 4.7 | |
| Patients with a pre-diagnostic platelet record | 6921 | 72.8 | 71.9 to 73.7 | 21,302 | 46.7 | 46.2 to 47.1 | |
| All Patients, % (95% CI) | Males, % (95% CI) | Females, % (95% CI) | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | |
| All patients | 13.3 (12.6 to 14.0) | 1.4 (1.3 to 1.5) | 11.1 (10.2 to 12.0) | 0.8 (0.6 to 0.9) | 15.8 (14.7 to 16.9) | 2.0 (1.9 to 2.2) |
| ADC | 10.7 (9.7 to 11.8) | 1.3 (1.1 to 1.5) | 8.6 (7.3 to 10.1) | 0.7 (0.5 to 0.9) | 12.3 (11.2 to 14.5) | 1.9 (1.6 to 2.2) |
| SCC | 16.9 (15.3 to 18.6) | 1.2 (1.0 to 1.5) | 15.1 (13.2 to 17.2) | 0.8 (0.6 to 1.1) | 19.9 (17.1 to 22.9) | 1.9 (1.5 to 2.4) |
| SCLC | 11.8 (10.0 to 13.9) | 1.1 (0.8 to 1.5) | 10.2 (7.8 to 13.1) | 0.7 (0.4 to 1.1) | 13.4 (10.7 to 16.6) | 1.5 (1.1 to 2.1) |
| Adj. OR | 95% CI | p-Value | Adj. Incidence (%) | 95% CI | |
|---|---|---|---|---|---|
| Subtype | |||||
| ADC | 1 * | 10.8 | 9.7 to 11.8 | ||
| SCC | 1.7 | 1.5 to 2.0 | <0.001 | 17.2 | 15.5 to 18.8 |
| SCLC | 1.1 | 0.9 to 1.3 | 0.6 | 11.4 | 9.5 to 13.2 |
| Sex | |||||
| Male | 1 * | 11.0 | 10.0 to 12.0 | ||
| Female | 1.5 | 1.3 to 1.7 | <0.001 | 15.1 | 13.8 to 16.4 |
| Previous COPD diagnosis | |||||
| No | 1 * | 11.5 | 10.6 to 12.4 | ||
| Yes | 1.7 | 1.4 to 2.0 | <0.001 | 17.9 | 15.8 to 19.9 |
| Age group | |||||
| 40 to 49 | 1 * | 15.0 | 9.3 to 20.7 | ||
| 50 to 59 | 1.0 | 0.63 to 1.69 | 0.9 | 15.4 | 12.8 to 18.0 |
| 60 to 69 | 0.88 | 0.55 to 1.41 | 0.6 | 13.5 | 12.0 to 14.9 |
| 70 to 79 | 0.82 | 0.51 to 1.31 | 0.4 | 12.6 | 11.3 to 14.0 |
| 80+ | 0.63 | 0.38 to 1.03 | 0.06 | 10.0 | 8.2 to 11.9 |
| Adj. OR | 95% CI | p-Value | Adj. Incidence (%) | 95% CI | |
|---|---|---|---|---|---|
| Subtype | |||||
| ADC | 1 * | 26.4 | 24.9 to 28.0 | ||
| SCC | 1.3 | 1.1 to 1.4 | 0.001 | 31.6 | 29.5 to 33.7 |
| SCLC | 0.17 | 0.14 to 0.23 | <0.001 | 6.5 | 5.1 to 8.0 |
| Pre-diagnostic thrombocytosis status | |||||
| Normal | 1 * | 26.2 | 25.0 to 27.3 | ||
| Thrombocytosis | 0.40 | 0.29 to 0.55 | <0.001 | 14.5 | 12.1 to 16.9 |
| Interaction between thrombocytosis status and subtype | |||||
| Normal ADC | 28.4 | 26.7 to 30.0 | |||
| Thrombocytosis ADC | 13.8 | 10.2 to 17.4 | |||
| Normal SCC | 33.4 | 31.1 to 35.7 | |||
| Thrombocytosis SCC | 20.0 | 15.9 to 24.3 | |||
| Normal SCLC | 6.6 | 5.0 to 8.1 | |||
| Thrombocytosis SCLC | 6.4 | 2.4 to 10.5 | |||
| Sex | |||||
| Male | 1 * | 20.7 | 20.7 to 23.4 | ||
| Female | 1.4 | 1.2 to 1.6 | <0.001 | 26.2 | 26.2 to 29.5 |
| Age group | |||||
| 40 to 49 | 1 * | 18.9 | 12.7 to 25.0 | ||
| 50 to 59 | 1.3 | 0.82 to 2.0 | 0.26 | 22.9 | 19.9 to 25.9 |
| 60 to 69 | 1.5 | 0.95 to 2.2 | 0.09 | 24.9 | 23.1 to 26.7 |
| 70 to 79 | 1.5 | 0.98 to 2.3 | 0.07 | 25.4 | 23.7 to 27.2 |
| 80+ | 1.4 | 0.91 to 2.2 | 0.13 | 24.4 | 21.9 to 27.0 |
| Previous COPD diagnosis | |||||
| No | 1 * | 23.2 | 22.1 to 24.4 | ||
| Yes | 1.4 | 1.2 to 1.6 | <0.001 | 29.7 | 27.3 to 32.2 |
| Anti-platelet drug prescription | |||||
| No | 1 * | 37.4 | 22.5 to 25.0 | ||
| Yes | 1.2 | 1.0 to 1.3 | 0.015 | 26.1 | 24.7 to 28.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barlow, M.; Hamilton, W.; Bailey, S.E.R. The Clinical Value of Pre-Diagnostic Thrombocytosis for the Detection of Lung Cancer in Primary Care. Cancers 2024, 16, 1154. https://doi.org/10.3390/cancers16061154
Barlow M, Hamilton W, Bailey SER. The Clinical Value of Pre-Diagnostic Thrombocytosis for the Detection of Lung Cancer in Primary Care. Cancers. 2024; 16(6):1154. https://doi.org/10.3390/cancers16061154
Chicago/Turabian StyleBarlow, Melissa, Willie Hamilton, and Sarah E. R. Bailey. 2024. "The Clinical Value of Pre-Diagnostic Thrombocytosis for the Detection of Lung Cancer in Primary Care" Cancers 16, no. 6: 1154. https://doi.org/10.3390/cancers16061154
APA StyleBarlow, M., Hamilton, W., & Bailey, S. E. R. (2024). The Clinical Value of Pre-Diagnostic Thrombocytosis for the Detection of Lung Cancer in Primary Care. Cancers, 16(6), 1154. https://doi.org/10.3390/cancers16061154






