Sentinel Lymph Node Biopsy (SLNB) for Early-Stage Head and Neck Squamous-Cell Carcinoma of the Tongue: Twenty Years of Experience at I.N.T. “G.Pascale”
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Histologically confirmed early-stage OTSCC (T1-T2).
- OTSCC with no clinically or radiographically detectable regional or distant metastasis at presentation (cN0-cM0), assessed through neck ultrasound, CT, and/or MRI.
- Treatment involved the excision of the primary tongue tumor and SLNB.
- Primary OTSCC that had not undergone prior treatment.
- Absence of radiotherapy or chemotherapy in the clinical history.
- No previous occurrence of cancers at other sites.
- A minimum follow-up period of 1 year.
- Clinical examination.
- Routine blood sample analysis, including liver and renal function tests.
- Preoperative flexible fibropharyngoscopy.
- Neck ultrasound with Doppler.
- Head and neck CT or MRI with contrast.
- Lymphoscintigraphy to identify the sentinel lymph node.
- Histopathological examination.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kågedal, Å.; Margolin, G.; Held, C.; da Silva, P.F.N.; Piersiala, K.; Munck-Wikland, E.; Jacobsson, H.; Häyry, V.; Cardell, L.O. A Novel Sentinel Lymph Node Approach in Oral Squamous Cell Carcinoma. Curr. Pharm. Des. 2020, 26, 3834–3839. [Google Scholar] [CrossRef]
- Esce, A.R.; Baca, A.L.; Redemann, J.P.; Rebbe, R.W.; Schultz, F.; Agarwal, S.; Hanson, J.A.; Olson, G.T.; Martin, D.R.; Boyd, N.H. Predicting nodal metastases in squamous cell carcinoma of the oral tongue using artificial intelligence. Am. J. Otolaryngol. 2024, 45, 104102. [Google Scholar] [CrossRef]
- Konishi, M.; Kakimoto, N. Radiomics analysis of intraoral ultrasound images for prediction of late cervical lymph node metastasis in patients with tongue cancer. Head Neck 2023, 45, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Committeri, U.; Fusco, R.; Di Bernardo, E.; Abbate, V.; Salzano, G.; Maglitto, F.; Orabona, G.D.; Piombino, P.; Bonavolontà, P.; Arena, A.; et al. Radiomics Metrics Combined with Clinical Data in the Surgical Management of Early-Stage (cT1-T2 N0) Tongue Squamous Cell Carcinomas: A Preliminary Study. Biology 2022, 11, 468. [Google Scholar] [CrossRef]
- Brandwein-Gensler, M.; Teixeira, M.S.; Lewis, C.M.; Lee, B.; Rolnitzky, L.; Hille, J.J.; Genden, E.; Urken, M.L.; Wang, B.Y. Oral squamous cell carcinoma: Histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am. J. Surg. Pathol. 2005, 29, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022, 16, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hingsammer, L.; Seier, T.; Zweifel, D.; Huber, G.; Rücker, M.; Bredell, M.; Lanzer, M. Sentinel lymph node biopsy for early stage tongue cancer-a 14-year single-centre experience. Int. J. Oral Maxillofac. Surg. 2019, 48, 437–442. [Google Scholar] [CrossRef]
- Woolgar, J.A.; Rogers, S.N.; Lowe, D.; Brown, J.S.; Vaughan, E.D. Cervical lymph node metastasis in oral cancer: The importance of even microscopic extracapsular spread. Oral Oncol. 2003, 39, 130–137. [Google Scholar] [CrossRef]
- Amit, M.; Yen, T.C.; Liao, C.T.; Binenbaum, Y.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Ebrahimi, A.; Clark, J.R.; Cernea, C.R.; et al. Clinical nodal stage is a significant predictor of outcome in patients with oral cavity squamous cell carcinoma and pathologically negative neck metastases: Results of the international consortium for outcome research. Ann. Surg. Oncol. 2013, 20, 3575–3581. [Google Scholar] [CrossRef]
- Junn, J.; Baugnon, K.; Lacayo, E.; Hudgins, P.; Patel, M.; Magliocca, K.; Corey, A.; El-Deiry, M.; Wadsworth, J.; Beitler, J.; et al. CT Accuracy of Extrinsic Tongue Muscle Invasion in Oral Cavity Cancer. AJNR Am. J. Neuroradiol. 2017, 38, 364–370. [Google Scholar] [CrossRef]
- Hoang, T.A.; Hasso, A.N. Magnetic resonance imaging of the oral cavity and tongue. Top. Magn. Reson. Imaging 1994, 6, 241–253. [Google Scholar] [CrossRef]
- Abu-Ghanem, S.; Yehuda, M.; Carmel, N.N.; Leshno, M.; Abergel, A.; Gutfeld, O.; Fliss, D.M. Elective Neck Dissection vs Observation in Early-Stage Squamous Cell Carcinoma of the Oral Tongue With No Clinically Apparent Lymph Node Metastasis in the Neck: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 857–865. [Google Scholar] [CrossRef]
- Missale, F.; Marchi, F.; Iandelli, A.; Subramaniam, N.; Dokhe, Y.; Sampieri, C.; Mattavelli, D.; Bresciani, L.; Carobbio, A.L.C.; Grammatica, A.; et al. Oncological outcomes of compartmental surgery and wide local excision in oral tongue and floor of the mouth cancer. Oral Oncol. 2022, 135, 106210. [Google Scholar] [CrossRef]
- D’cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Yii, N.W.; Patel, S.G.; Rhys-Evans, P.H.; Breach, N.M. Management of the N0 neck in early cancer of the oral tongue. Clin. Otolaryngol. Allied Sci. 1999, 24, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Hernando, J.; Villarreal, P.; Álvarez-Marcos, F.; Gallego, L.; García-Consuegra, L.; Junquera, L. Comparison of related complications: Sentinel node biopsy versus elective neck dissection. Int. J. Oral Maxillofac. Surg. 2014, 43, 1307–1312. [Google Scholar] [CrossRef]
- Schilling, C.; Stoeckli, S.J.; Vigili, M.G.; de Bree, R.; Lai, S.Y.; Alvarez, J.; Christensen, A.; Cognetti, D.M.; D’Cruz, A.K.; Frerich, B.; et al. Surgical consensus guidelines on sentinel node biopsy (SNB) in patients with oral cancer. Head Neck 2019, 41, 2655–2664. [Google Scholar] [CrossRef]
- Schilling, C.; Stoeckli, S.J.; Haerle, S.K.; Broglie, M.A.; Huber, G.F.; Sorensen, J.A.; Bakholdt, V.; Krogdahl, A.; von Buchwald, C.; Bilde, A.; et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur. J. Cancer 2015, 51, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt-Nordenvall, L.; Bark, R.; Elliot, A.; Von Beckerath, M.; Gahm, C. Distribution of sentinel nodes from parotid tumors-A feasibility study. Cancer Med. 2023, 12, 19667–19672. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Moore, J.; Soyer, H.P.; Mar, V.; Smithers, B.M. Trends and patterns of care of sentinel node biopsy in cutaneous melanoma: A population-based study in Queensland. ANZ J. Surg. 2023, 93, 2172–2179. [Google Scholar] [CrossRef]
- Christensen, A.; Wessel, I.; Charabi, B.W.; Juhl, K.; Kiss, K.; Lelkaitis, G.; Mortensen, J.; Kjaer, A.; von Buchwald, C.; Tvedskov, J.F. Diagnostic accuracy of combined optical- and radio-guided SNB for neck staging of oral squamous cell carcinoma lesions in the anterior oral cavity. Eur. Arch. Otorhinolaryngol. 2023, 280, 3393–3403. [Google Scholar] [CrossRef]
- Vaish, R.; Mittal, N.; Mahajan, A.; Rane, S.U.; Agrawal, A.; D’Cruz, A.K. Sentinel node biopsy in node negative early oral cancers: Solution to the conundrum! Oral Oncol. 2022, 134, 106070. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.J.; Yang, X.; Peng, H. Diagnostic Efficacy of Sentinel Lymph Node Biopsy in Early Oral Squamous Cell Carcinoma: A Meta-Analysis of 66 Studies. PLoS ONE 2017, 12, e0170322. [Google Scholar] [CrossRef]
- Civantos, F.J.; Zitsch, R.P.; Schuller, D.E.; Agrawal, A.; Smith, R.B.; Nason, R.; Petruzelli, G.; Gourin, C.G.; Wong, R.J.; Ferris, R.L.; et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: Results of a prospective multi-institutional trial. J. Clin. Oncol. 2010, 28, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, J.; Wu, H. Diagnostic value of sentinel lymph node biopsy for cT1/T2N0 tongue squamous cell carcinoma: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2017, 274, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Togo, G.; Maffia, F.; Vaira, L.A.; Maglitto, F.; Committeri, U.; Fusco, R.; Maglione, M.G.; Nocini, R.; De Luca, P.; et al. Early-Stage Oral Tongue Squamous Cell Carcinoma and a Positive Sentinel Lymph Node Biopsy: Description of a Prognostic Correlation between Pre-Treatment Inflammatory Biomarkers, the Depth of Invasion and the Worst Pattern of Invasion. J. Pers. Med. 2022, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.F.; Zeng, Z.Y.; Peng, H.W.; Guo, Z.M.; Wang, S.L.; Zhang, Q. Sentinel lymph node biopsy versus elective neck dissection in patients with cT1-2N0 oral tongue squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 186–190. [Google Scholar] [CrossRef]
- Thompson, C.F.; St John, M.A.; Lawson, G.; Grogan, T.; Elashoff, D.; Mendelsohn, A.H. Diagnostic value of sentinel lymph node biopsy in head and neck cancer: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2013, 270, 2115–2122. [Google Scholar] [CrossRef]
- van den Bosch, S.; Czerwinski, M.; Govers, T.; Takes, R.P.; de Bree, R.; Al-Mamgani, A.; Hannink, G.; Kaanders, J.H.A.M. Diagnostic test accuracy of sentinel lymph node biopsy in squamous cell carcinoma of the oropharynx, larynx, and hypopharynx: A systematic review and meta-analysis. Head Neck 2022, 44, 2621–2632. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kang, M.J.; Ahn, H.S.; Hwang, S.H. Comparison of sentinel lymph node biopsy and elective neck dissection for early oral cavity squamous cell carcinoma patients with clinically node-negative necks: Systematic review and meta-analysis. J. Laryngol. Otol. 2023, 137, 599–607. [Google Scholar] [CrossRef]
- Gupta, T.; Maheshwari, G.; Kannan, S.; Nair, S.; Chaturvedi, P.; Agarwal, J.P. Systematic review and meta-analysis of randomized controlled trials comparing elective neck dissection versus sentinel lymph node biopsy in early-stage clinically node-negative oral and/or oropharyngeal squamous cell carcinoma: Evidence-base for practice and implications for research. Oral Oncol. 2022, 124, 105642. [Google Scholar] [PubMed]
- Park, W.; Jin, H.; Heo, Y.; Jeong, H.S.; Son, Y.I.; Chung, M.K.; Baek, C.-H. Sentinel Lymph Node Biopsy Versus Elective Neck Dissection: Long-Term Oncologic Outcomes in Clinically Node-Negative Tongue Cancer. Clin. Exp. Otorhinolaryngol. 2022, 15, 107–114. [Google Scholar] [CrossRef]
- Cramer, J.D.; Sridharan, S.; Ferris, R.L.; Duvvuri, U.; Samant, S. Sentinel Lymph Node Biopsy Versus Elective Neck Dissection for Stage I to II Oral Cavity Cancer. Laryngoscope 2019, 129, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rigual, N.; Loree, T.; Frustino, J.; Jayaprakash, V.; Cohan, D.; Sullivan, M.; Kuriakose, M.A. Sentinel node biopsy in lieu of neck dissection for staging oral cancer. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Flach, G.B.; Bloemena, E.; Klop, W.M.C.; Van Es, R.J.J.; Schepman, K.P.; Hoekstra, O.S.; Castelijns, J.A.; Leemans, C.R.; de Bree, R. Sentinel lymph node biopsy in clinically N0 T1-T2 staged oral cancer: The Dutch multicenter trial. Oral Oncol. 2014, 50, 1020–1024. [Google Scholar] [CrossRef]
- Miura, K.; Hirakawa, H.; Uemura, H.; Yoshimoto, S.; Shiotani, A.; Sugasawa, M.; Homma, A.; Yokoyama, J.; Tsukahara, K.; Yoshizaki, T.; et al. Sentinel node biopsy for oral cancer: A prospective multicenter Phase II trial. Auris Nasus Larynx 2017, 44, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Doll, C.; Steffen, C.; Amthauer, H.; Thieme, N.; Elgeti, T.; Huang, K.; Kreutzer, K.; Koerdt, S.; Heiland, M.; Beck-Broichsitter, B. Sentinel Lymph Node Biopsy in Early Stages of Oral Squamous Cell Carcinoma Using the Receptor-Targeted Radiotracer 99mTc-Tilmanocept. Diagnostics 2021, 11, 1231. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.S.; Zheng, S.; Gao, H.; Wu, Z.Y.; Xu, J.W. Trans-lymphatic contrast-enhanced ultrasound with sentinel lymph node biopsy for detecting cervical skip metastasis to lymph nodes in early-stage oral tongue squamous cell carcinoma. Dentomaxillofacial Radiol. 2022, 51, 20210107. [Google Scholar] [CrossRef]
- Bredell, M.G. Sentinel lymph node mapping by indocyanin green fluorescence imaging in oropharyngeal cancer—Preliminary experience. Head Neck Oncol. 2010, 2, 31. [Google Scholar] [CrossRef]
- Al-Dam, A.; Precht, C.; Barbe, A.; Kohlmeier, C.; Hanken, H.; Wikner, J.; Schön, G.; Heiland, M.; Assaf, A.T. Sensitivity and specificity of sentinel lymph node biopsy in patients with oral squamous cell carcinomas using indocyanine green fluorescence imaging. J. Cranio-Maxillofac. Surg. 2018, 46, 1379–1384. [Google Scholar] [CrossRef]
- Zhou, B.; Long, Y.; Lü, C.; Yi, L.; Zhou, X.; Li, Z. Application value of indocyanine green fluorescence in sentinel lymph node biopsy for early-stage tongue cancer and oropharyngeal cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2022, 47, 1683–1688. [Google Scholar] [PubMed]
- De Ravin, E.; Venkatesh, S.; Harmsen, S.; Delikatny, E.J.; Husson, M.A.; Lee, J.Y.K.; Newman, J.G.; Rajasekaran, K. Indocyanine green fluorescence-guided surgery in head and neck cancer: A systematic review. Am. J. Otolaryngol. 2022, 43, 103570. [Google Scholar] [CrossRef] [PubMed]
- Costantino, A.; Canali, L.; Festa, B.M.; Spriano, G.; Mercante, G.; De Virgilio, A. Sentinel lymph node biopsy in high-risk cutaneous squamous cell carcinoma of the head and neck: Systematic review and meta-analysis. Head Neck 2022, 44, 2288–2300. [Google Scholar] [CrossRef] [PubMed]
- Van den Brekel, M.W.; Castelijns, J.A.; Stel, H.V.; Luth, W.J.; Valk, J.; van der Waal, I.; Snow, G.B. Occult metastatic neck disease: Detection with US and US-guided fine-needle aspiration cytology. Radiology 1991, 180, 457–461, Erratum in Radiology 1992, 182, 288. [Google Scholar] [CrossRef] [PubMed]
- Van den Brekel, M.W.; Castelijns, J.A.; Reitsma, L.C.; Leemans, C.R.; van der Waal, I.; Snow, G.B. Outcome of observing the N0 neck using ultrasonographic-guided cytology for follow-up. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 153–156. [Google Scholar] [CrossRef]
- Borgemeester, M.C.; van den Brekel, M.W.; van Tinteren, H.; Smeele, L.E.; Pameijer, F.A.; van Velthuysen, M.L.; Balm, A.J. Ultrasound-guided aspiration cytology for the assessment of the clinically N0 neck: Factors influencing its accuracy. Head Neck 2008, 30, 1505–1513. [Google Scholar] [CrossRef]
- Postema, R.J.; van Velthuysen, M.L.; van den Brekel, M.W.; Balm, A.J.; Peterse, J.L. Accuracy of fine-needle aspiration cytology of salivary gland lesions in The Netherlands cancer institute. Head Neck 2004, 26, 418–424. [Google Scholar] [CrossRef]
| Characteristic | Overall | SLN− Patients | SLN+ Patients | p Value at Yates’ Chi-Square Test or Mann–Whitney Test | |||
|---|---|---|---|---|---|---|---|
| N° | % | N° | % | N° | % | ||
| Patients | 122 | 100.0% | 92 | 75.4% | 30 | 24.6% | |
| Sex | |||||||
| Male | 61 | 50.0% | 51 | 55.4% | 10 | 33.4% | |
| Female | 61 | 50.0% | 41 | 44.6% | 20 | 66.6% | 0.06 |
| Age | 59 | 20–85 | 60 | 20–85 | 57 | 31–79 | 0.23 |
| T Classification | |||||||
| T1 | 73 | 59.8% | 64 | 69.6% | 9 | 30.0% | |
| T2 | 49 | 40.2% | 28 | 30.4% | 21 | 70.0% | 0.0003 |
| G Classification | |||||||
| G1 | 13 | 10.7% | 11 | 12.0% | 2 | 6.6% | |
| G2 | 63 | 51.6% | 55 | 59.8% | 8 | 26.7% | |
| G2–G3 | 9 | 7.4% | 6 | 6.5% | 3 | 10.0% | |
| G3 | 37 | 30.3% | 20 | 21.7% | 17 | 56.7% | 0.006 |
| Location of SLN | |||||||
| Level I | 12 | 7.1% | 12 | 9.1% | 0 | 0.0% | |
| Level II | 94 | 55.6% | 74 | 56.1% | 20 | 54.1% | |
| Level III | 52 | 30.8% | 42 | 31.8% | 10 | 27.0% | |
| Level IV | 5 | 3.0% | 4 | 3.0% | 1 | 2.7% | |
| Level V | 6 | 3.5% | 0 | 0.0% | 6 | 16.2% | |
| Total | 169 | 100.0% | 132 | 78.1% | 37 | 21.9% | 0.0006 |
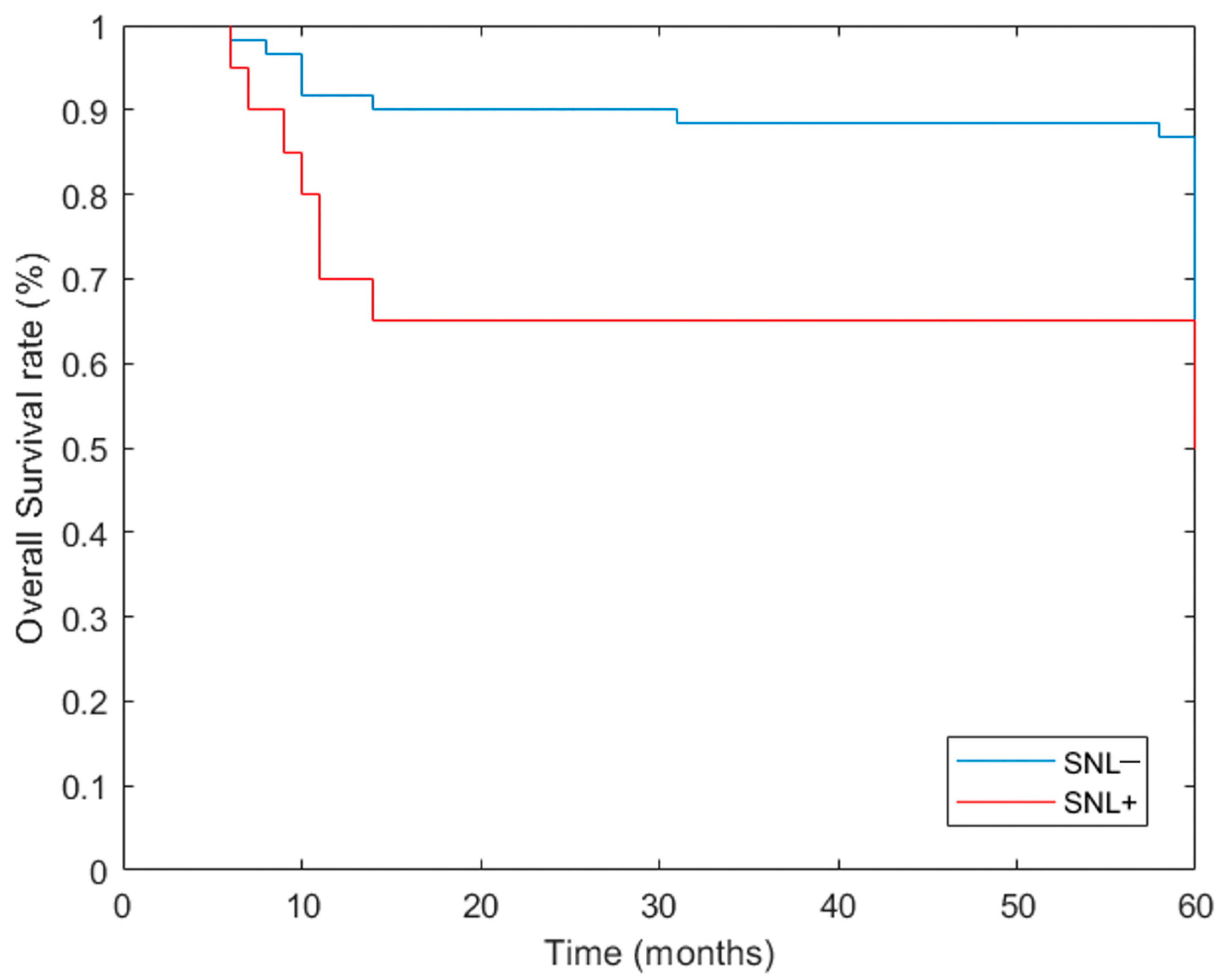
| 5 Years | OS | DFS | DSS | Relapse | ||||
|---|---|---|---|---|---|---|---|---|
| N° | % | N° | % | N° | % | N° | % | |
| SLN− | 53/61 | 86.89% | 54/61 | 88.52% | 58/61 | 95.08% | 7/61 | 11.66% |
| SLN+ | 13/20 | 65.00% | 12/20 | 60.00% | 16/20 | 80.00% | 8/20 | 40.00% |
| p value at Yates’ chi-square test | 0.06 | 0.11 | 0.1 | 0.01 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionna, F.; Pavone, E.; Aversa, C.; Maffia, F.; Spinelli, R.; Carraturo, E.; Salzano, G.; Maglitto, F.; Sarcinella, M.; Fusco, R.; et al. Sentinel Lymph Node Biopsy (SLNB) for Early-Stage Head and Neck Squamous-Cell Carcinoma of the Tongue: Twenty Years of Experience at I.N.T. “G.Pascale”. Cancers 2024, 16, 1153. https://doi.org/10.3390/cancers16061153
Ionna F, Pavone E, Aversa C, Maffia F, Spinelli R, Carraturo E, Salzano G, Maglitto F, Sarcinella M, Fusco R, et al. Sentinel Lymph Node Biopsy (SLNB) for Early-Stage Head and Neck Squamous-Cell Carcinoma of the Tongue: Twenty Years of Experience at I.N.T. “G.Pascale”. Cancers. 2024; 16(6):1153. https://doi.org/10.3390/cancers16061153
Chicago/Turabian StyleIonna, Franco, Ettore Pavone, Corrado Aversa, Francesco Maffia, Raffaele Spinelli, Emanuele Carraturo, Giovanni Salzano, Fabio Maglitto, Marco Sarcinella, Roberta Fusco, and et al. 2024. "Sentinel Lymph Node Biopsy (SLNB) for Early-Stage Head and Neck Squamous-Cell Carcinoma of the Tongue: Twenty Years of Experience at I.N.T. “G.Pascale”" Cancers 16, no. 6: 1153. https://doi.org/10.3390/cancers16061153
APA StyleIonna, F., Pavone, E., Aversa, C., Maffia, F., Spinelli, R., Carraturo, E., Salzano, G., Maglitto, F., Sarcinella, M., Fusco, R., Granata, V., Lastoria, S., Del Prato, F., & Maglione, M. G. (2024). Sentinel Lymph Node Biopsy (SLNB) for Early-Stage Head and Neck Squamous-Cell Carcinoma of the Tongue: Twenty Years of Experience at I.N.T. “G.Pascale”. Cancers, 16(6), 1153. https://doi.org/10.3390/cancers16061153









