Simple Summary
Hypersensitivity reactions (HSRs) to platinum and taxane are common, and desensitization can be used to complete the standard chemotherapy regimen with a good safety profile and high success rate. Our study showed that the use of desensitization for HSRs to taxane and platinum is low in clinical practice. Treatment of HSRs has been shown to be heterogeneous and dependent on the grade of the HSR. Guidelines for the treatment of HSRs to taxane and platinum in gynecologic cancers have been of great interest to clinicians. Our study highlights that the management of platinum and taxane HSRs in gynecological cancers could be standardized and that international guidelines need to be developed.
Abstract
Platinum and taxane chemotherapy is associated with the risk of hypersensitivity reactions (HSRs), which may require switching to less effective treatments. Desensitization to platinum and taxane HSRs can be used to complete chemotherapy according to the standard regimen. Therefore, we aimed to investigate the current management of HSRs to platinum and/or taxane chemotherapy in patients with gynecologic cancers. We conducted an online cross-sectional survey among gynecological and medical oncologists consisting of 33 questions. A total of 144 respondents completed the survey, and 133 respondents were included in the final analysis. Most participants were gynecologic oncologists (43.6%) and medical oncologists (33.8%), and 77.4% (n = 103) were involved in chemotherapy treatment. More than 73% of participants experienced >5 HSRs to platinum and taxane per year. Premedication and a new attempt with platinum or taxane chemotherapy were used in 84.8% and 92.5% of Grade 1–2 HSRs to platinum and taxane, respectively. In contrast, desensitization was used in 49.4% and 41.8% of Grade 3–4 HSRs to platinum and taxane, respectively. Most participants strongly emphasized the need to standardize the management of platinum and taxane HSRs in gynecologic cancer. Our study showed that HSRs in gynecologic cancer are common, but management is variable and the use of desensitization is low. In addition, the need for guidance on the management of platinum- and taxane-induced HSRs in gynecologic cancer was highlighted.
1. Introduction
Platinum- and taxane-based chemotherapy is the standard of care for patients with advanced gynecologic cancers [1,2,3,4]. Platinum-based anticancer drugs, including carboplatin, cisplatin, and oxaliplatin, induce apoptosis in cancer cells through DNA damage by disrupting DNA repair mechanisms, whereas taxanes (paclitaxel, docetaxel, and cabazitaxel) induce apoptosis by suppressing microtubule dynamics, preventing proper spindle formation and blocking mitosis [5,6,7,8]. However, multiple treatments with the same drug, such as platinum and taxanes, may result in oncologic resistance and hypersensitivity reactions (HSRs). This has an impact on further treatment and outcomes by necessitating a switch to a chemotherapy regimen that is less effective and more toxic [9,10,11,12]. Premedication with antihistamines and corticosteroids is usually recommended for mild HSRs and is routinely administered for taxane- and platinum-based chemotherapy [10,11,12,13]. However, premedication is not effective in preventing more severe allergic reactions, particularly those to platinum salts [14,15].
Desensitization is the establishment of a temporary tolerance to a substance that previously triggered an HSR [16]. Desensitization protocols for HSRs to chemotherapeutic agents are based on a stepwise increase in infusion rates of highly diluted drug solutions, starting as slowly as a few micrograms per milliliter of a drug in the first hour, with increasing doses over several hours to a few days until the total cumulative therapeutic dose is achieved and tolerated [12,16,17,18,19,20]. Importantly, patients remain allergic to the drug and must be desensitized for each course of treatment, as desensitization induces tolerance to a drug only temporarily, depending on continued exposure [20]. It should be considered in patients with HSRs as a safe alternative to platinum salts and taxanes in the use of standard chemotherapy, aiming at the best therapeutic results according to international standards [17,20,21,22].
Platinum hypersensitivity affects approximately 5% of the general oncologic population and 8 to 16% of women with gynecologic cancers, and taxane hypersensitivity affects 10 to 13% of the general oncologic population [17,19,21,23,24]. This is of clinical importance and justifies an optimal strategy for the maintenance of treatment. At present, desensitization techniques are well established and there are international guidelines for their administration [18]. However, clinical effects of desensitization are rarely studied and few specialized centers offer desensitization as part of standard practice.
Therefore, the aims of our cross-sectional study were to (1) reveal the current management of HSRs to platinum and/or taxane chemotherapy in patients with gynecologic cancer and (2) determine if there is a need for standardizing the management of HSRs for best patient care.
2. Materials and Methods
2.1. Study Design
We conducted a cross-sectional survey among gynecological and medical oncologists. SurveyMonkey software (https://www.surveymonkey.com/home/) was used to create and distribute the questionnaire. The survey was posted online on the European Network of Young Gynaecological Oncologists (ENYGO), European Society of Gynaecological Oncology (ESGO), and Oncoalert social media channels, Furthermore, we sent emails to the ENYGO members and subscribers of the Oncoalert Newsletter. We collected data between April 2023 and September 2023.
2.2. Variables
The survey consisted of 33 questions in English, prepared and based on the expertise of gynecological and medical oncologists treating patients with gynecological cancers and validated by the ESGO Scientific Committee (Supplementary File S1). The questionnaire contained four main sections including (1) general demographic information (nine questions), (2) HSRs and platinum-based chemotherapy (11 questions), (3) HSRs and chemotherapy with taxane (11 questions), and (4) future direction of HSRs and desensitization in gynecological cancer (two questions). HSR Grade was defined as Common Terminology for Adverse Events (CTCAEs) Grades 1–4. Briefly, CTCAE 1, 2, 3, and 4 refer to mild, moderate, severe, and life-threatening adverse events, respectively.
2.3. Data Sources/Measurement
The software SurveyMonkey was used for questionnaire creation and distribution. Data were collated from members and followers of European Network of ENYGO and ESGO (newsletter including HSR Survey received = 1938 and opened = 106). Additionally, the survey was placed online on ENYGO and Oncoalert social media channels (opened = 53).
2.4. Statistical Analyses
Descriptive statistics are presented as counts and frequencies for categorical data and medians (range) for metric or ordinal variables. Cases with median p-values correspond to the Kruskall–Wallis tests, and cases with categorical data p-values correspond to Fisher’s exact tests. p-values of group comparisons correspond to log-rank tests. A p value < 0.05 was considered significant. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Macintosh, Version 28.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Demographic Data
A total of 144 respondents from 33 countries completed the survey. The final analysis included 133 respondents, of whom 79 administered platinum-based and 67 taxane-based chemotherapy. We excluded respondents who were only involved in the treatment of breast cancer (n = 6), respondents who did not treat gynecological cancers (n = 5), and one respondent who was a nurse. The countries with the highest participation were Switzerland (10.5%), Italy (9%), and Germany (8.3%) (Table 1, Supplementary File S1). The genders of the participants were balanced, with 54.9% female and 45.1% male and a mean age of 38 years. The majority of participants were gynecological oncologists (43.6%) and medical oncologists (33.8%). Seventy-six participants (57.1%) worked mainly in university hospitals and 103 participants (77.4%) were involved in chemotherapy treatment. The clinical experience of the participants was well balanced, with 32.3% having less than 5 years, 33.8% 5–10 years, and 33.8% more than 10 years. Detailed demographic characteristics of these participants are shown in Table 1.

Table 1.
Demographic representation of the participants.
3.2. HSR and Platinum-Based Chemotherapy
Out of the 79 participants who administered platinum-based chemotherapy, more than half of them treated more than 100 gynecological cancer patients per year with platinum-based chemotherapy (Table 2). The majority (73.4%) of participants experienced more than five platinum HSRs per year. In 84.8% of Grade 1 and 2 HSRs (according to CTCAE), participants used premedication with antihistamines/steroids and made new attempts with standard infusions of platinum-based chemotherapy. However, 41.8% used desensitization in these patients, only 15.2% stopped chemotherapy, and 8.9% changed the treatment regimen. In contrast, in cases with Grade 3–4 HSRs, 35.4% of the participants suspended chemotherapy, while 34.1% changed the regimen. There was a minimal increase in the use of desensitization in patients with Grades 3–4 HSRs compared to patients with Grade 1–2 HSRs (49.4% vs. 41.8%). Desensitization was mainly performed by medical oncologists (40.5%) and allergologists (27.8%) in their own clinics. Sixty-seven percent of the participants were able to continue platinum-based chemotherapy after tolerance was achieved in more than 50% of the cases. However, 45.6% of participants experienced one or more critical events during tolerance induction, mainly due to recurrent HSR CTCAE Grade 1–2 (47.2%) and Grade 3–4 (66.7%).

Table 2.
Results of the questions about hypersensitivity reactions and platinum- and taxane-based chemotherapy.
3.3. HSR and Taxane-Based Chemotherapy
A total of 67 participants administered taxane-based chemotherapy, with the majority treating more than 100 gynecological cancer patients per year and regularly experiencing HSRs in their patients (Table 2). Premedication with antihistamines and steroids together with a retry of the standard infusion was the main mode of action for Grade 1–2 HSRs (92.5%). In contrast, in Grade 3–4 HSRs, the mode of action was balanced (Table 2). When desensitization was used, the majority were able to continue with standard taxane treatment. Desensitization was performed by medical oncologists (34.3%), allergologists (23.9%), and gynecological oncologists (10.4%). Of the participants, 52% never experienced a critical incident during tolerance induction, but 25.4% experienced more than one. The main reasons for critical incidents are HSRs to taxane CTCAE Grades 1 and 2 (34.5%) and Grade 3–4 (62.1%).
3.4. Desensitization of HSRs in Gynecological Cancers
The majority (53.3%) of participants without experience in chemotherapy treatment were not aware of desensitization of HSRs to platinum- and taxane-based chemotherapy, whereas the majority of participants involved in chemotherapy treatment were aware of desensitization. However, participants strongly emphasized the need to standardize the management of platinum and taxane HSRs in gynecological cancer and to develop international guidelines, regardless of their involvement in chemotherapy treatment (Table 3).

Table 3.
Results of the question on awareness and need for standardization of desensitization for hypersensitivity reactions in gynecological cancer.
3.5. Management of Hypersensitivity Reactions Based on Length of Clinical Practice Experience
No significant difference was evident in the results of the questions comparing participants with less than five years, five to ten years, or more than ten years of clinical practice experience (Figure 1). However, participants with more than ten years of experience were more likely to report experiencing <5 or 5–10 HSRs to taxane per year than the participants with five to ten years of experience or less than 5 years of experience (p = 0.38, 8 vs. 6 vs. 4 and 5 vs. 4 vs. 1, respectively) (Figure 1). In addition, there was a trend that the longer the experience in clinical practice, the more often the participants did not think there was a need for standardization and guidelines for managing HSRs to taxane and platinum (p = 0.26) (Figure 2).
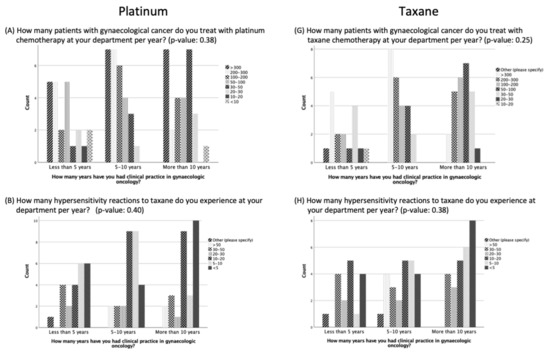
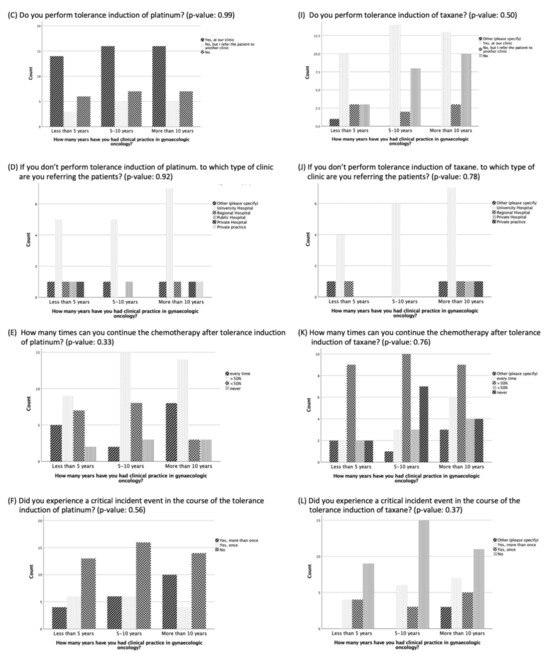
Figure 1.
Management of hypersensitivity reactions to platinum and taxane based on duration of clinical practice experience.
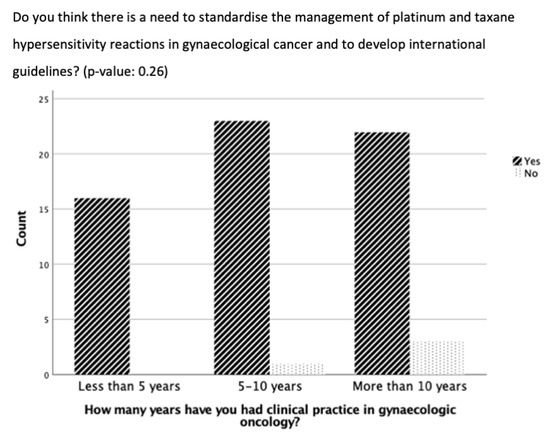
Figure 2.
Need for standardization of desensitization for hypersensitivity reactions in gynecological cancer based on duration of clinical practice experience.
4. Discussion
The majority of the participants experienced a high frequency of HSRs to taxane and platinum in their clinical practice, with more than five HSRs per year. Management of HSRs is heterogeneous and depends on the grade of the HSR. Overall, we found that the use of desensitization for HSRs to taxane and platinum in clinical practice is low at less than 50% and guidelines for the treatment of HSRs to taxane and platinum in gynecological cancers were of great interest to clinicians, regardless of their experience with chemotherapy.
Clinicians treating gynecological cancers regularly experience HSRs to platinum- and taxane-based chemotherapy (8–16% and 10–13%, respectively) [11,12,17,19,21,23,24], which is also due to the fact that more lines of treatment are being used in gynecological cancer than 1–2 decades ago [25]. Additionally, real HSR rates are likely to be underestimated, as oncologists often report only severe reactions [10,26]. Our study showed a high frequency of HSRs, with more than 73% of the participants treating more than five patients with HSRs to platinum- and taxane-based chemotherapy, which emphasizes the need to find a strategy to maintain the optimal treatment regimen in this large group of patients.
The treatment strategy for HSRs to taxanes and platinum often depends on its Grade. For mild HSRs, premedication with antihistamines and corticosteroids is typically recommended and routinely used [10,11,12,13]. This is well represented in our study, with more than 84.8% and 92% of patients with Grade 1–2 HSRs receiving platinum and taxane, respectively, regularly premedicated with antihistamines and corticosteroids. However, premedication is ineffective in preventing more severe HSRs (Grade 3–4) to platinum and taxane, and therefore, desensitization should be considered to continue standard chemotherapy for the best therapeutic outcome in these patients [14,15,17,20,21,22].
The results of our survey showed a higher use of desensitization in Grade 3–4 HSRs compared to Grade 1–2 HSRs with platinum and taxane (49.4% and 48.8% vs. 41.8% and 20.9%, respectively). Moreover, 31.6% and 44.8% of the participants regularly premedicate their patients with antihistamines and corticosteroids for Grade 3–4 HSRs to platinum and taxane, respectively. In addition, there is a high rate of switching to another treatment regimen, with 34.1% for platinum-based and 44.8% for taxane-based chemotherapy. This may be explained by the finding that only 67.1% of patients after platinum desensitization and 58.2% of patients after taxane desensitization had a high likelihood (>50%) of continuing platinum- or taxane-based chemotherapy. In addition, there was a high incidence of critical events (40%) during the desensitization process. This is in contrast to what is known about the safety of desensitization procedures and their management [17,18,19,20,21,22]. This is important to address, as an improved outcome for overall survival has been demonstrated in hypersensitive patients receiving carboplatin desensitization compared to non-hypersensitive patients in relapsed ovarian cancer, independent of the germline BRCA status [27]. However, standard desensitization protocols are not widely accepted or used, in part because they can be time consuming and in part because there are several different protocols available [17,20,21,22]. One way to address this important issue is to standardize the management of platinum and taxane HSRs in gynecological cancer by developing international guidelines. This was particularly emphasized by participants with (59.2%) and without (83.3%) experience in the medical treatment of gynecological cancer patients.
This survey provides a global representation of participants and their current management of platinum and taxane HSRs in gynecological cancer. An advantage is the direct feedback from clinicians regularly confronted with HSRs in their daily clinical practice on their awareness and views on this topic. However, the small cohort size is a weakness of this study and limits the statistical power of the results. This is an anticipated problem with survey studies, especially when the target group are clinicians with a heavy workload and limited time to complete a survey. However, a variety of methods were used to distribute the survey, including the official social media channels of ENYGO, ESGO, and Oncoalert, and distribution of the survey via an email system to the ENYGO, ESGO, and Oncoalert databases. However, despite the variety of methods used, there was still a low response rate of 5%. Possible solutions to this problem could be to distribute the survey directly at congresses and workshops or to distribute it on a personal level, which could improve the response rate. Additionally, the fact that 23% of the respondents were not involved in chemotherapy treatment is a major limitation, as this could bias the results. To account for this, we analyzed the results of the questionnaire on HSRs to platinum- and taxane-based chemotherapy only for those respondents involved in chemotherapy treatment.
Currently, only a limited number of cancer centers have established desensitization as part of their standard practice. However, desensitization protocols for patients with taxane and platinum HSRs are available and recommended [10,11,12,18,19]. Knowledge of desensitization procedures in gynecological oncology could be optimized by regular analysis and management of successful tolerance induction to platinum and taxanes in patients with HSRs. This is important to achieve optimal treatment in accordance with international standards [14,15]. However, since the goal is to provide the best treatment within the recommended timeframe, it is also important not to delay planned chemotherapy for desensitization. For this reason, patients who develop HSRs should be seen and tested within one to two weeks of the reaction. This underscores the importance of a multidisciplinary approach to gynecologic cancer care in specialized centers and adherence to clinical guidelines, which ensures a better prognosis and quality of life for patients [28,29,30,31,32,33,34,35]. In particular, it has been shown that the collaboration of different experts leads to an increased awareness of potential treatments and a better evaluation of diagnostic–therapeutic areas beyond their own competence, which ultimately improves the effectiveness of treatments [28,29,30,31,36]. Our study shows the willingness of the participants to use the guidelines for the treatment of HSRs when they become available.
5. Conclusions
Our cross-sectional survey showed that HSRs in gynecological cancer are common, but management is variable with low use of desensitization. In addition, clinical practitioners emphasized the need for standardization and guidelines for the management of HSRs to platinum and taxane in gynecological cancer.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers16061155/s1, Supplementary File S1: Survey with thirty-three questions, prepared and based on the expertise of gynecological and medical oncologists treating patients with gynecological cancers.
Author Contributions
Conceptualization, T.A.Z., E.B., N.B., C.M. and V.H.-S.; data curation, T.A.Z., E.B., K.G., A.C., J.K.-B., T.N., I.K., A.S. (Alexander Shushkevich) and T.C.; formal analysis, T.A.Z., E.B., K.G. and J.K.-B.; funding acquisition, T.A.Z.; investigation, T.A.Z., E.B., K.G., Z.R., T.N., A.P., I.K., A.S. (Alexander Shushkevich), A.S. (Aleksandra Strojna), C.T. and T.C.; methodology, T.A.Z., K.G., A.C., Z.R., T.N., C.M. and G.M.; project administration, T.A.Z., N.B., Z.R., I.K., A.S. (Alexander Shushkevich), T.C. and V.H.-S.; resources, T.A.Z., N.B., Z.R., A.S. (Aleksandra Strojna), C.T., T.C., M.V., G.M. and V.H.-S.; software, E.B. and T.C.; supervision, T.A.Z., N.B., A.P., A.S. (Aleksandra Strojna), M.V., C.M., G.M. and V.H.-S.; visualization, E.B.; writing—original draft, T.A.Z., E.B., K.G., A.C., C.T. and V.H.-S.; writing—review and editing, T.A.Z., E.B., K.G., A.C., N.B., Z.R., J.K.-B., T.N., A.P., I.K., A.S. (Alexander Shushkevich), A.S. (Aleksandra Strojna), C.T., M.V., C.M. and V.H.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Swiss National Foundation [P500PM_20726/1, 2021], Bangerter-Rhyner Stiftung [0297, 2021], and Freie Gesellschaft Basel [2022].
Institutional Review Board Statement
All study activities were conducted in accordance with Institutional Review Board (IRB) guidelines for exempt studies. All methods were carried out in accordance with relevant guidelines and regulations. A formal IRB certification of exemption (Req-2021-00692) was provided by the ethics committee of Northwest and Central Switzerland (EKNZ), on the 24th of June 2021. The EKNZ can confirm that the research project (Req-2021-00692) fulfilled the general ethical and scientific standards for research with human subjects. The anonymization of personal data was guaranteed.
Informed Consent Statement
An informed consent form was not needed because it was assumed that only participants who wanted to participate would complete the survey. Therefore, the informed consent was the first question to proceed with the survey.
Data Availability Statement
The datasets that have been used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to acknowledge the ESGO office’s support in all project stages.
Conflicts of Interest
The authors declare that they have no conflicts of interest or financial ties to disclose.
References
- Oaknin, A.; Bosse, T.; Creutzberg, C.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.; et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef]
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.; Pothuri, B.; Ray-Coquard, I.; Tan, D.; Bellet, E.; Oaknin, A.; et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 833–848. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N.; Committee, E.G. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv262. [Google Scholar] [CrossRef]
- Falzone, L.; Bordonaro, R.; Libra, M. SnapShot: Cancer chemotherapy. Cell 2023, 186, 1816.e1. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Maloney, S.M.; Hoover, C.A.; Morejon-Lasso, L.V.; Prosperi, J.R. Mechanisms of Taxane Resistance. Cancers 2020, 12, 3323. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Castells, M.; Sancho-Serra Mdel, C.; Simarro, M. Hypersensitivity to antineoplastic agents: Mechanisms and treatment with rapid desensitization. Cancer Immunol. Immunother. 2012, 61, 1575–1584. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Vetter, M.H.; Cohn, D.E.; Khan, A.; Hays, J.L. Outpatient desensitization in selected patients with platinum hypersensitivity reactions. Gynecol. Oncol. 2017, 145, 603–610. [Google Scholar] [CrossRef]
- Moon, D.H.; Lee, J.M.; Noonan, A.E.; Annunziata, C.M.; Minasian, L.; Houston, N.; Hays, J.L.; Kohn, E.C. Deleterious BRCA1/2 mutation is an independent risk factor for carboplatin hypersensitivity reactions. Br. J. Cancer 2013, 109, 1072–1078. [Google Scholar] [CrossRef]
- Pagani, M.; Bavbek, S.; Alvarez-Cuesta, E.; Dursun, A.B.; Bonadonna, P.; Castells, M.; Cernadas, J.; Chiriac, A.; Sahar, H.; Madrigal-Burgaleta, R.; et al. Hypersensitivity reactions to chemotherapy: An EAACI Position Paper. Allergy 2022, 77, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, J.; Boursiquot, J.N.; Cournoyer, G.; Lemieux, J.; Masse, M.S.; Almanric, K.; Guay, M.P. Management of hypersensitivity to platinum- and taxane-based chemotherapy: Cepo review and clinical recommendations. Curr. Oncol. 2014, 21, e630–e641. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M. The complex clinical picture of presumably allergic side effects to cytostatic drugs: Symptoms, pathomechanism, reexposure, and desensitization. Med. Clin. N. Am. 2010, 94, 835–852, xiii. [Google Scholar] [CrossRef] [PubMed]
- Dizon, D.S.; Sabbatini, P.J.; Aghajanian, C.; Hensley, M.L.; Spriggs, D.R. Analysis of patients with epithelial ovarian cancer or fallopian tube carcinoma retreated with cisplatin after the development of a carboplatin allergy. Gynecol. Oncol. 2002, 84, 378–382. [Google Scholar] [CrossRef]
- Cernadas, J.R.; Brockow, K.; Romano, A.; Aberer, W.; Torres, M.J.; Bircher, A.; Campi, P.; Sanz, M.L.; Castells, M.; Demoly, P.; et al. General considerations on rapid desensitization for drug hypersensitivity—A consensus statement. Allergy 2010, 65, 1357–1366. [Google Scholar] [CrossRef]
- Vetter, M.H.; Khan, A.; Backes, F.J.; Bixel, K.; Cohn, D.E.; Copeland, L.J.; Fowler, J.M.; Salani, R.; Li, Q.; O’Malley, D.M. Outpatient desensitization of patients with moderate (high-risk) to severe platinum hypersensitivity reactions. Gynecol. Oncol. 2019, 152, 316–321. [Google Scholar] [CrossRef]
- Scherer, K.; Brockow, K.; Aberer, W.; Gooi, J.H.C.; Demoly, P.; Romano, A.; Schnyder, B.; Whitaker, P.; Cernadas, J.S.R.; Bircher, A.J.; et al. Desensitization in delayed drug hypersensitivity reactions—An EAACI position paper of the Drug Allergy Interest Group. Allergy 2013, 68, 844–852. [Google Scholar] [CrossRef]
- Zwimpfer, T.A.; Scherer, K.; Schotzau, A.; Heinzelmann-Schwarz, V.; Hartmann, K.; Vetter, M.; Montavon, C. Desensitization in patients with hypersensitivity to platinum and taxane in gynecological cancers. Cancer Med. 2023, 13, e6840. [Google Scholar] [CrossRef]
- Rosello, S.; Blasco, I.; Garcia Fabregat, L.; Cervantes, A.; Jordan, K.; Committee, E.G. Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2017, 28 (Suppl. S4), iv100–iv118. [Google Scholar] [CrossRef]
- Tsao, L.R.; Young, F.D.; Otani, I.M.; Castells, M.C. Hypersensitivity Reactions to Platinum Agents and Taxanes. Clin. Rev. Allergy Immunol. 2022, 62, 432–448. [Google Scholar] [CrossRef]
- Sloane, D.; Govindarajulu, U.; Harrow-Mortelliti, J.; Barry, W.; Hsu, F.I.; Hong, D.; Laidlaw, T.; Palis, R.; Legere, H.; Bunyavanich, S.; et al. Safety, Costs, and Efficacy of Rapid Drug Desensitizations to Chemotherapy and Monoclonal Antibodies. J. Allergy Clin. Immunol. Pract. 2016, 4, 497–504. [Google Scholar] [CrossRef]
- Koshiba, H.; Hosokawa, K.; Kubo, A.; Miyagi, Y.; Oda, T.; Watanabe, A.; Honjo, H. Incidence of Carboplatin-related hypersensitivity reactions in Japanese patients with gynecologic malignancies. Int. J. Gynecol. Cancer 2009, 19, 460–465. [Google Scholar] [CrossRef]
- Sendo, T.; Sakai, N.; Itoh, Y.; Ikesue, H.; Kobayashi, H.; Hirakawa, T.; Nakano, H.; Oishi, R. Incidence and risk factors for paclitaxel hypersensitivity during ovarian cancer chemotherapy. Cancer Chemother. Pharmacol. 2005, 56, 91–96. [Google Scholar] [CrossRef]
- Kessous, R.; Wissing, M.D.; Laskov, I.; Abitbol, J.; Bitharas, J.; Agnihotram, V.R.; Yasmeen, A.; Salvador, S.; Lau, S.; Gotlieb, W.H. Multiple lines of chemotherapy for patients with high-grade ovarian cancer: Predictors for response and effect on survival. Int. J. Cancer 2021, 148, 2304–2312. [Google Scholar] [CrossRef]
- Shepherd, G.M. Hypersensitivity reactions to chemotherapeutic drugs. Clin. Rev. Allergy Immunol. 2003, 24, 253–262. [Google Scholar] [CrossRef]
- Altwerger, G.; Florsheim, E.B.; Menderes, G.; Black, J.; Schwab, C.; Gressel, G.M.; Nelson, W.K.; Carusillo, N.; Passante, T.; Huang, G.; et al. Impact of carboplatin hypersensitivity and desensitization on patients with recurrent ovarian cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Scandurra, G.; Lombardo, V.; Gattuso, G.; Lavoro, A.; Distefano, A.B.; Scibilia, G.; Scollo, P. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (Review). Int. J. Oncol. 2021, 59, 53. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Morgese, F.; Rinaldi, S.; Torniai, M.; Mentrasti, G.; Scortichini, L.; Giampieri, R. Benefits and Limitations of a Multidisciplinary Approach in Cancer Patient Management. Cancer Manag. Res. 2020, 12, 9363–9374. [Google Scholar] [CrossRef] [PubMed]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Heudel, P.E.; Devouassoux-Shisheboran, M.; Taieb, S.; Genestie, C.; Selle, F.; Morice, P.; Rouzier, R.; Ray-Coquard, I. Multidisciplinary management of advanced ovarian cancer for an optimal therapeutic strategy. Eur. J. Gynaecol. Oncol. 2017, 38, 175–180. [Google Scholar] [PubMed]
- Bjorn, S.F.; Schnack, T.H.; Lajer, H.; Christensen, I.J.; Lundvall, L.; Thomsen, L.N.; Hogdall, C. Classification of Ovarian Cancer Surgery Facilitates Treatment Decisions in a Gynecological Multidisciplinary Team. Int. J. Gynecol. Cancer 2017, 27, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Moterani, V.C.; Tiezzi, D.G.; de Andrade, J.M.; Candido Dos Reis, F.J. Analysis of the relationship between hospital characteristics and survival in ovarian cancer: A historical cohort. J. Surg. Oncol. 2020, 122, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Chen, L.; Hou, J.Y.; Burke, W.M.; Tergas, A.I.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Association of Hospital Volume and Quality of Care With Survival for Ovarian Cancer. Obstet. Gynecol. 2017, 130, 545–553. [Google Scholar] [CrossRef]
- Vernooij, F.; Heintz, A.P.; Coebergh, J.W.; Massuger, L.F.; Witteveen, P.O.; van der Graaf, Y. Specialized and high-volume care leads to better outcomes of ovarian cancer treatment in the Netherlands. Gynecol. Oncol. 2009, 112, 455–461. [Google Scholar] [CrossRef]
- Scott, R.; Hawarden, A.; Russell, B.; Edmondson, R.J. Decision-Making in Gynaecological Oncology Multidisciplinary Team Meetings: A Cross-Sectional, Observational Study of Ovarian Cancer Cases. Oncol. Res. Treat. 2020, 43, 70–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).