Simple Summary
Lung cancer is one of the deadliest cancers worldwide, and the prognosis is poor. The disease is potentially curable if detected at an early stage, but currently, the only widely implemented screening tool is low-dose computed tomography. Biomarkers such as methylated tumor DNA may be used for the early detection of lung cancer, and sputum is an appealing, non-invasive sample type. With this systematic review and meta-analysis, we aimed to identify all studies evaluating the quantitative methylation of tumor DNA in sputum samples for lung cancer detection. A systematic overview of all the available evidence may highlight areas of improvement as well as certain high-performing genes to prioritize in future studies.
Abstract
Lung cancer is the leading cause of cancer-related mortality worldwide. Early diagnosis is pivotal for the prognosis. There is a notable overlap between lung cancer and chronic bronchitis, and the potential use of methylated tumor DNA in sputum as a biomarker for lung cancer detection is appealing. This systematic review and meta-analysis followed the PRISMA 2020 statement. A comprehensive search was conducted in Embase, Medline, Web of Science, and the Cochrane Library, using these search strings: Lung cancer, sputum, and methylated tumor DNA. A total of 15 studies met the eligibility criteria. Studies predominantly utilized a case–control design, with sensitivity ranging from 10 to 93% and specificity from 8 to 100%. A meta-analysis of all genes across studies resulted in a summary sensitivity of 54.3% (95% CI 49.4–59.2%) and specificity of 79.7% (95% CI 75.0–83.7%). Notably, two less explored genes (TAC1, SOX17) demonstrated sensitivity levels surpassing 85%. The study’s findings highlight substantial variations in the sensitivity and specificity of methylated tumor DNA in sputum for lung cancer detection. Challenges in reproducibility could stem from differences in tumor site, sample acquisition, extraction methods, and methylation measurement techniques. This meta-analysis provides a foundation for prioritizing high-performing genes, calling for a standardization and refinement of methodologies before potential application in clinical trials.
1. Introduction
Lung cancer is a global health challenge and the leading cause of cancer death world-wide [1]. Diagnosis at an early stage is pivotal for prognosis [2]. Unfortunately, a substantial portion of lung cancer cases receive a late-stage diagnosis, limiting curative treatment options [3]. This primarily stems from the fact that the majority of early-stage lung cancer is incidentally discovered [4], as symptoms typically manifest only in advanced stages [5].
Computed tomography (CT) screening has emerged as a valuable tool for the early detection of lung cancer and has been implemented in various countries, leading to a shift in diagnosing lung cancer at earlier stages [6,7,8,9]. However, CT screening is a costly and time-consuming process for radiologists [10], creating a demand for a biomarker capable of detecting lung cancer or identifying high-risk individuals [11].
In many countries, there is a notable overlap between lung cancer and chronic bronchitis [12], a condition marked by daily sputum production. Previous attempts at lung cancer screening using sputum cytology demonstrated low sensitivity [13], and it is only favored in specific countries [14]. Assessing the presence of methylated tumor DNA in sputum has demonstrated potential as a more efficient substitute for conventional cytology techniques [15]. Methylated tumor DNA can be quantified using various molecular techniques, often relying on polymerase chain reaction (PCR) methods like real-time PCR, quantitative methylation-specific PCR (QMSP), and digital PCR [16]. Additional approaches include pyrosequencing and next-generation sequencing (NGS) methods [17].
Over the years, multiple studies have evaluated the diagnostic accuracy of methylated tumor DNA in the sputum of a large number of genes for lung cancer detection [18,19,20]. These studies have provided considerable variation in sensitivity and specificity, possibly due to differences in sputum sample collection methods. Furthermore, the method used to analyze methylated tumor DNA can profoundly impact the results, emphasizing the importance of clearly reporting the methodology for result reproducibility and comparison. An up-to-date systematic review and meta-analysis of the current evidence are necessary for a more comprehensive understanding of the diagnostic performance of methylated DNA in sputum.
The primary objective of this systematic review and meta-analysis on methylated tumor DNA in sputum for lung cancer diagnosis was to systematically identify and analyze all relevant studies investigating the diagnostic accuracy of this approach.
2. Materials and Methods
2.1. Study Protocol and Registration
The present systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [21]. The study protocol was registered in the PROSPERO database (registration number CRD42023453066, University of York, York, UK) on 21 August 2023 [22].
2.2. Data Sources and Search Strategy
Systematic literature searches were performed in the online databases Embase and Embase Classic, Ovid Medline, Web of Science, and Cochrane Library. The search strings consisted of three main concepts divided into search blocks: (A) lung cancer, (B) sputum, and (C) methylated tumor DNA. Each block contained the relevant Subject Headings (Embase and Medline) or Medical Subject Headings (Cochrane Library) and free-text keywords. All keywords were included by incorporating the Boolean operator “OR”. The three blocks were then combined using the Boolean operator “AND”, and the resulting references were retrieved for each database on 21 August 2023. We conducted citation searches (forward and backward) on all studies included after full-text evaluation using the Web of Science database. These references were extracted on 3 October 2023.
The searches were not limited by language, article type, or year of publication. The search strings were constructed by the authors assisted by a research librarian, and the search strings for each database are available in the Supplementary Materials.
2.3. Reference Screening and Eligibility Criteria
All references were imported into the web-based systematic review platform Covidence (Covidence, Melbourne, Australia). Duplicates were automatically identified and removed by Covidence. All references were screened by title and abstract by two independent reviewers (MB and SW), and consensus was reached by discussion with the option to involve a third reviewer (RA) in case of persistent disagreement. All potentially relevant studies were reviewed in full by two independent reviewers (MB and SW) and scored according to the following inclusion criteria: (A) adult patients with a diagnosis of lung cancer or patients undergoing diagnostic work-up or screening for lung cancer, and adult healthy control subjects or non-cancer control patients; (B) spontaneous or induced sputum collected for analysis of methylated tumor DNA using a quantitative analysis method; (C) tumor cytology or histopathology used as the reference standard; (D) diagnostic performance of the biomarker(s) reported as contingency data with sufficient information to calculate sensitivity and specificity. The exclusion criteria comprised (A) case reports, meeting abstracts, editorials, comments, notes, letters, and literature reviews; (B) studies in languages other than English; (C) in silico analyses of public data repositories.
2.4. Data Extraction and Quality Assessment
The data extraction form was pilot-tested in Covidence with three studies. Basic study characteristics included the study ID (the first author’s last name and the year of publication), geographic region, study design, number and description of lung cancer patients including histology and stage, number and description of control subjects, and the choice of reference standard. Based on previous experience, we included an assessment of how the Methods section was reported inspired by the Minimum Information for publication of Quantitative real-time PCR Experiments (MIQE) guidelines [23]. These items included sample collection, which part of the sputum sample was used for analysis, DNA extraction, primer and probe sequences, reaction volume and amount of DNA, thermocycling parameters, assay type, calibration curves, and diagnostic cutoff. Extracted outcomes included gene name(s), diagnostic sensitivity and specificity, and the number of true positives, false negatives, true negatives, and false positives in a contingency table.
The overall study quality was evaluated according to the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool, which contains four domains covering patient selection, index test, reference standard, and flow and timing [24]. We also included study funding and author conflicts of interest.
All data extraction and quality assessments were carried out independently and blindly by two reviewers (SW and MB), and disputes were settled by discussion. In case of methodology specific issues, we consulted an expert (RA), who had the deciding vote.
2.5. Statistical Analysis
Study characteristics and selected MIQE items were collated in summary tables. Diagnostic sensitivity was calculated by the following formula: True positive/(true positive + false negative). Diagnostic specificity was calculated as follows: True negative/(true negative + false positive). A diagnostic test meta-analysis was carried out on all studies with sufficient data. The summary effect estimates were derived from the STATA command metandi, which was also used for the hierarchical summary receiver operating characteristics (HSROC) plot [25]. This procedure consists of a two-level mixed-effect logistic regression model based on an independent binomial distribution. Single-gene performances across the included studies were illustrated by forest plots of sensitivity and specificity using the STATA command midas [26]. Deek’s funnel plot and Deek’s funnel plot asymmetry test were used for evaluating the risk of publication bias with a level of significance set at 0.05. All analyses were performed in STATA BE version 18 (StataCorp LLC, College Station, TX, USA), and forest plot graphics were executed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
3. Results
3.1. Search Results and Study Selection
The results of the database searches and the subsequent selection process are illustrated in Figure 1. The initial searches, carried out in Embase, Medline, Web of Science, and Cochrane Library, identified 1299 potentially eligible records. Forward and backward citation searches of the 15 included studies resulted in 1154 additional references, many of which were duplicates. A total of 1234 references were eliminated based on title and abstract screening, which left 67 studies eligible for full-text assessment. Eventually, 15 studies were included in the review.
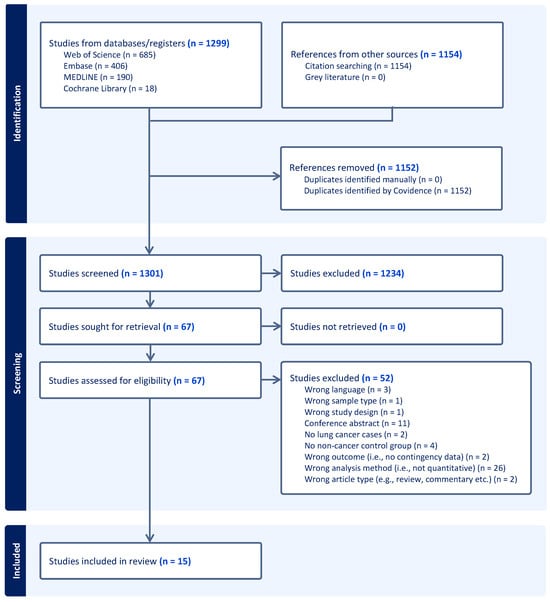
Figure 1.
PRISMA flowchart illustrating the study selection process.
3.2. Study Characteristics
The included studies were published between 2007 and 2021 (Table 1 and Supplementary Figure S1). The majority of the studies (9/15) originated from European countries, 3/15 were from North America, and 3/15 were from Asia (Table 1). Only two of the studies were cohort studies [27,28], while the remaining were of a case–control design. Four studies presented both a training and a validation cohort [18,19,29,30]. The number of cases and controls ranged from 13 to 159 and 24 to 159, respectively, with a median number of 56 cases and 68 controls. The reference standard was histology or cytology in 9/19 cohorts, histopathology of a surgery specimen alone or in combination with a tissue biopsy in 2/19 and 2/19 cohorts, respectively, while the reference standard was not described for 6/19 cohorts.

Table 1.
Study characteristics for all independent cohorts included. Stage I-IV as reported in the studies. LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; SCLC, small-cell lung cancer.
3.3. Biological Sample Types and Collection Method
Studies analyzing both spontaneously produced sputum and induced sputum were included in the present review. Spontaneous sputum constituted the main sample type in the studies (11/15), induced sputum was used in 3/15 studies, and the specific induction method Lung Flute was used in 1/15 studies. The Lung Flute used in the study by Li et al. is a handheld therapy device employing positive expiratory pressure in combination with sound waves to mobilize mucus in the airways [30,39]. Induced sputum is produced by letting the patient inhale a nebulized hypertonic saline solution to help sputum production [40].
Only 3/15 studies used a single-day sample, 11/15 used pooled sputum samples from multiple days, and one study did not describe the timing of sputum collection. The specific part of the sputum sample used for methylated DNA analysis was generally not described (11/15), but 3/15 studies reported using the cell pellet, and one study performed a comparison of pellet and supernatant. Detailed information for each study can be viewed in Supplementary Table S1.
3.4. The Reporting of Key Domains of the Analysis Methods
Various methods were applied for methylated tumor DNA analysis by the included studies, but they were mainly PCR-based, and only one study applied a sequencing approach [35]. We identified eight key domains from the MIQE guidelines to aid in assessing how well the methods were reported by the included studies [23]. These eight domains were assessed in a simplified manner by a score of either “Yes” (reported) or “No” (not reported) for each item, and the results are presented in Table 2. Overall, the reporting quality was good, yet only 5/15 studies reported adequate information for all eight domains [27,30,31,34,37]. The remaining 10/15 studies failed to report information on at least one key domain. One study did report most of the required information; however, the information was reported in multiple references and it was therefore difficult to obtain with certainty [29]. More details regarding DNA extraction kits and the amount of DNA used can be found in Supplementary Table S1.

Table 2.
Reporting of PCR-based methods. N/A, not applicable; PCR, polymerase chain reaction; QMSP, quantitative methylation-specific PCR; ref, reference.
3.5. Methylated DNA Analysis in Sputum for the Diagnosis of Lung Cancer
A total number of 31 genes were investigated in the 15 included studies with frequencies ranging from 1 (17 genes) to 13 (1 gene, RASSF1A). The comprehensive list of genes is available in the Supplementary Materials Table S2. Besides RASSF1A, APC and CYGB were the most frequent genes targeted in nine and eight cohorts, respectively. The diagnostic sensitivity reported by the studies varied and ranged from 10 to 93%, and the specificity had a similar range of 8–100%.
The sensitivity of aberrantly methylated RASSF1A ranged from 17 to 57% as reported in the 13 independent cohorts investigating this gene with a pooled sensitivity estimate of 39% (95% CI 14–68%). The individual results are presented in Figure 2a. The diagnostic specificity of RASSF1A was generally high with a range of 75–100% and a pooled estimate of 94% (95% CI 90–96%) (Figure 2b).
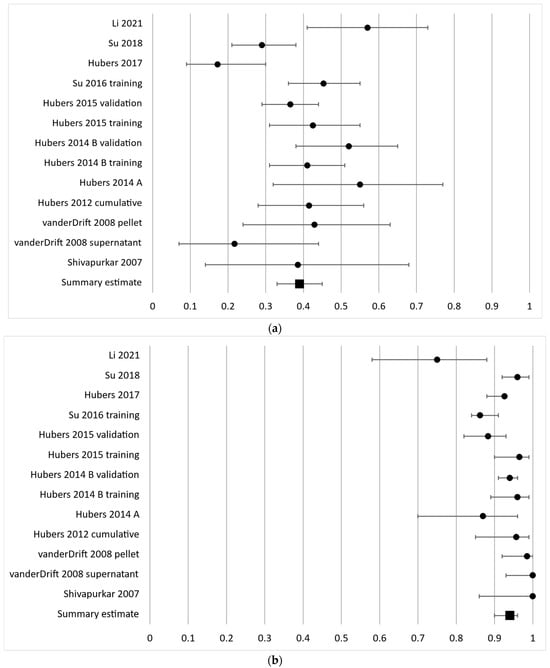
Figure 2.
Forest plot of sensitivity (a) and specificity (b) for the most frequently analyzed gene, RASSF1A. The black circles represent the diagnostic performance estimates, and the error bars represent the calculated 95% confidence intervals. The black square represents the summary estimate for all independent cohorts [18,20,27,29,30,31,33,36,37,38].
As the second most frequently investigated gene, APC had a diagnostic sensitivity of 17–63% in nine independent cohorts and a pooled sensitivity estimate of 44% (95% CI 32–56%) (Figure 3a). The specificity ranged from 55 to 100% with a pooled effect estimate of 82% (95% CI 66–92%) (Figure 3b).
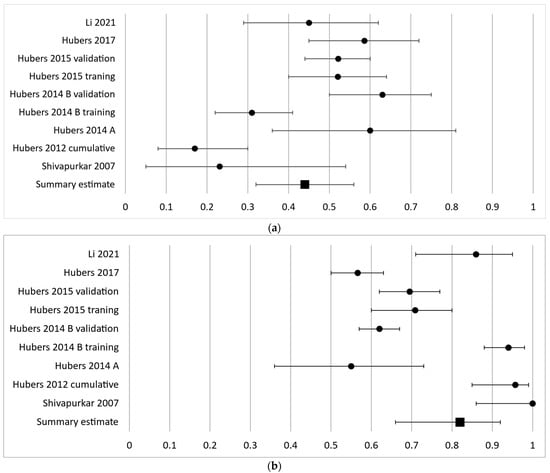
Figure 3.
Forest plot of sensitivity (a) and specificity (b) for the second most frequently analyzed gene, APC. The black circles represent the diagnostic performance estimates, and the error bars represent the calculated 95% confidence intervals. The black square represents the summary estimate for all independent cohorts [18,27,29,30,31,36,37].
The third most frequently analyzed gene in eight cohorts was CYGB with a diagnostic sensitivity ranging from 31 to 57% with a summary estimate of 47% (95% CI 40–54%) and a specificity ranging from 46 to 100% with a summary estimate of 82% (95% CI 64–92%) (Figure 4a,b).
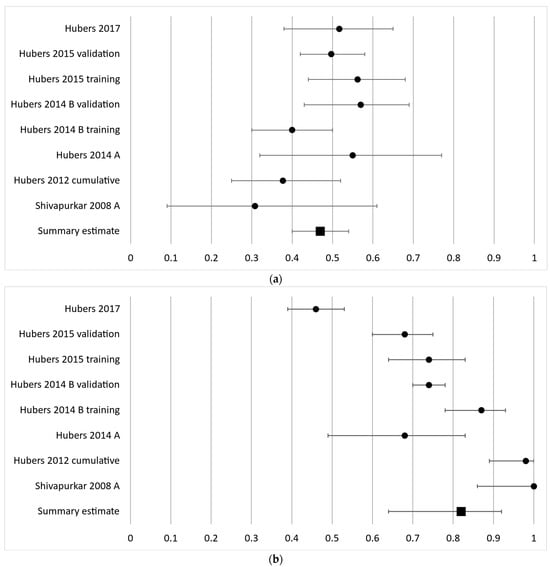
Figure 4.
Forest plot of sensitivity (a) and specificity (b) for the third most frequently analyzed gene, CYGB. The black circles represent the diagnostic performance estimates, and the error bars represent the calculated 95% confidence intervals. The black square represents the summary estimate for all independent cohorts [18,27,29,32,36,37].
A meta-analysis of the comprehensive range of genes analyzed in all independent cohorts resulted in 87 discrete data points and summary sensitivity and specificity estimates of 54.3% (95% CI 49.4–59.2%) and 79.7% (95% CI 75.0–83.7%), respectively. The corresponding diagnostic odds ratio was 4.7 (95% CI 3.8–5.7). The area under the HSROC curve was 0.71 (95% CI 0.67–0.75), and the 95% confidence region as well as the 95% prediction region are illustrated in Figure 5. Two of the less frequently investigated genes, SOX17 and TAC1, showed a high diagnostic sensitivity >85% with a corresponding specificity of >70% in three studies [28,30,38]. The comprehensive contingency data can be accessed in Supplementary Table S3, and the HSROC graph with an ID for each data point can be viewed in Supplementary Figure S2 and Table S4.
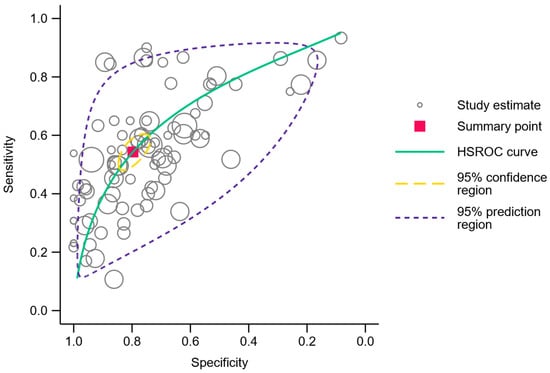
Figure 5.
Hierarchical summary receiver operating characteristics plot. Each open circle represents a gene analyzed in an independent cohort. The summary point is represented by the red square, and the 95% confidence region and 95% prediction region are outlined in dashed yellow and purple lines, respectively.
3.6. Quality Assessment and Risk of Bias
We used the QUADAS-2 checklist to evaluate the risk of bias in the included studies as recommended for test accuracy studies by the Cochrane Handbook for Systematic Reviews of Interventions [41]. The studies were of an acceptable quality overall, but issues in the “Patient selection” domain were frequent (Figure 6). Only two studies received “Low risk” in all seven judgments across the four domains [28,30]. The comprehensive quality assessments are reported in the Supplementary Materials (Tables S5–S9).
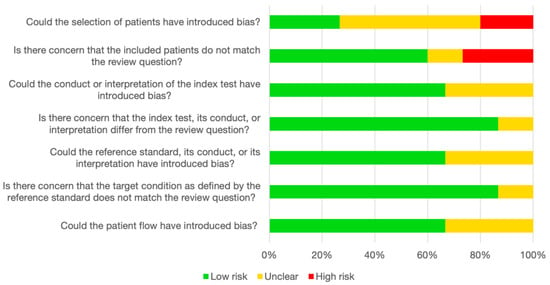
Figure 6.
Assessment of study quality according to QUADAS-2. The stacked bar chart represents the consensus judgments in each of the four domains covered by QUADAS-2. Green: low risk of bias/low level of concern. Yellow: unclear risk of bias/unclear level of concern. Red: high risk of bias/high level of concern. QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies 2.
We evaluated the potential risk of small study effects/publication bias using Deek’s funnel plot asymmetry test (p = 0.096) and the corresponding graph (Supplementary Figure S3) and found no statistically significant risk of publication bias.
4. Discussion
This systematic review and meta-analysis aimed to evaluate the quantitative detection of methylated tumor DNA in sputum samples as a diagnostic tool in lung cancer patients. A total of 15 studies met the eligibility criteria for inclusion in this review. The studies displayed varying sensitivity and specificity in the detection of methylated tumor DNA, with a summary sensitivity estimate of 54.3% (95% CI 49.4–59.2%) and a summary specificity estimate of 79.7% (95% CI 75.0–83.7%). The area under the HSROC curve was 0.71 (95% CI 0.67–0.75).
The use of tumor DNA methylation has been proposed for screening applications in both blood [42] and sputum [43]. Sputum analysis as a screening approach for detecting lung cancer is appealing. It is non-invasive and bears no significant risk or inconveniences for the participants. Nevertheless, past research has indicated that cytological evaluation of sputum, even when combined with concurrent chest X-ray assistance, may not be sufficient for effective screening [44]. The current meta-analysis examining the detection of lung cancer through the analysis of methylated tumor DNA in sputum reveals considerable variations in sensitivity, strongly influenced by the specific gene under investigation. Even when focusing on the three most extensively studied genes (RASSF1A, APC, and CYGB), variations persist, underscoring the overall challenges in reproducibility. It is imperative to scrutinize factors such as the site and stage of lung cancer, sputum production method, DNA extraction technique, and analysis methodology. Each of these steps holds equal importance in assessing the viability of a particular gene as a feasible screening target for clinical application.
Most frequently, the studies employed a case–control design (12 out of 15 studies). This design confers distinct advantages, allowing for the execution of retrospective studies using readily available sputum samples and the possibility of matching the controls and the cases based on various parameters. The retrospective study design, however, entails a higher risk of introducing bias. Although prospective cohort studies entail greater costs and time investment, they offer a superior level of evidence [45]. No randomized clinical trials were identified.
The objective of lung cancer screening is to identify early-stage cases amenable to curative treatment options [46]. However, a significant portion of stage I/II lung cancer tumors [47] may not be in close proximity to large bronchi, and sputum production may not originate from the tumor site. Unfortunately, the large majority of studies lacked the provision of sensitivity and specificity specific to stage, histological type, or central/peripheral location of lung cancer. Consequently, the creation of a meta-analysis categorized by stage, histology, or location was not possible. Additionally, the predominant use of spontaneous sputum samples may not adequately represent deep bronchial origins [48]. These factors potentially contribute to the relatively low sensitivity observed in the studies.
Another important factor is the pre-analytical handling of the sputum samples. Optimal pre-analytical conditions are pivotal for the results when analyzing tumor DNA in blood samples [49]. To our knowledge, no standardized guidelines exist for the optimal pre-analytical handling of sputum samples intended for tumor DNA analysis, but some of the recommendations regarding circulating tumor DNA may reasonably be applied to sputum analysis. Methylated tumor DNA is present in sputum in very limited quantities, and the final amount retrieved from the sample may vary depending on the chosen collection method and the part of the sample used for analysis. Only the study by van der Drift et al. from 2008 compared supernatant and pellet; they found that RASSF1A was methylated in 20% and 42.9% of the lung cancer patients for cell-free and cellular DNA, respectively [33]. This indicates that the cell pellet may achieve better sensitivity. However, as this was only reported in one study, further investigation is needed.
The sample storage time can also affect the DNA yield as shown in a study by Sozzi et al. who estimated a yearly DNA decay rate of around 30% for both plasma and purified DNA frozen at −80 °C [50]. The study by Leng et al. utilized a patient cohort from a study initiated in 1993 and the study was published in 2012, resulting in a potential storage time of up to 19 years [19]. Several other studies also reported potentially extensive storage times of 10 years or more [28,36,37]. The kits or methods used for DNA extraction should always be reported in studies investigating methylated tumor DNA, as stated in the MIQE guidelines, as the DNA yield can vary depending on the kit, and only two studies failed to report these data [23]. Most of the included studies used DNA extraction kits intended for blood, which are optimized for longer DNA fragments. This might affect the diagnostic sensitivity, since tumor DNA tends to be more fragmented than normal DNA [51].
Variations in results may also arise from differences in the analysis of sputum methylation. An inclusion criterion for this study was the adoption of quantitative measurements of methylated DNA. Among the selected studies, the majority (12 out of 15) employed QMSP, while 2 out of 15 utilized digital PCR, and 1 out of 15 employed pyrosequencing. The prevalence of QMSP may be attributed to the fact that it is an older and very well-established technology, and the studies in this review date from 2007 and onward. Digital PCR is a more current technique, which was used by two studies in the current review [30,38]. Acquiring a digital PCR platform is costly, and quantitative PCR can produce high-quality, quantitative results if the right guidelines for sample processing and data analysis are followed [52]. Previously, both QMSP and digital PCR using MethyLight have demonstrated a robust correlation between expected and observed methylation values [16]. However, digital PCR exhibits higher sensitivity, as illustrated previously, with a 20-fold lower detection limit with the droplet digital PCR technique compared to standard quantitative PCR [53]. The study by Su et al. from 2018 compared droplet digital PCR with QMSP and found a lower limit of quantification for the digital PCR approach, albeit not a 20-fold difference as discussed above [38]. An extensive interlaboratory study found that droplet digital PCR could achieve highly reproducible absolute quantification of a specific DNA target, with an inter-laboratory difference of less than 12% [54]. Taken together, digital PCR seems a favorable method for analyzing methylated tumor DNA.
Methylation analysis in sputum samples is generally not very widely investigated, and we only identified 15 studies for the present review as opposed to 33 studies evaluating the use of methylated tumor DNA in blood samples for lung cancer detection [42]. Sputum may be considered a material with limited potential for this purpose, since only one study was published in the period from 2020 to 2022, whereas 13 of the 33 studies of methylated tumor DNA in blood were published in the same time period. Blood plasma is a more standardized material compared to sputum, and there are several DNA extraction kits aimed specifically at cell-free DNA, which is not the case for sputum. Not all patients are able to produce a sputum sample, while blood can be sampled in almost any situation. As a result, plasma may be a better biological sample type than sputum for analyzing minimally invasive biomarkers. However, sputum, bronchial lavage, and other sample types collected in closer proximity to the tumor might be relevant in specific situations.
While most genes in the present review exhibited a relatively low sensitivity of around 50%, a subset of less explored genes demonstrated sensitivity levels surpassing 85%, with specificity consistently above 70%. Notably, these genes (TAC1, SOX17; Supplementary Table S3) were all investigated in the same three studies, which were three of the most recently published, and two of them used digital PCR [28,30,38]. It is also worth noting that two of these studies were the only ones to receive a score of “Low risk” across all four QUADAS-2 domains [28,30]. Future research on methylated sputum DNA could advantageously prioritize these genes and focus on optimizing and standardizing sample types, extraction methods, and methylation analysis techniques. The diagnostic potential may be further improved by incorporating other types of biomarkers such as miRNA, as performed by Li 2021 [30] and Su 2016 [20], or as a combination of biomarkers and other characteristics [55].
Our search strategy employed broad criteria, scanning major databases to identify a substantial number of potentially relevant studies. Nevertheless, we acknowledge the possibility of not capturing all pertinent research, as some studies may have used gene names without broader ctDNA terms in their titles or abstracts, resulting in their exclusion from our search results. Additionally, the review did not encompass gray literature, congress abstracts, unpublished results, or studies in a language other than English.
Pooling data from multiple studies in this review and meta-analysis enhances sample size and statistical power, thereby improving the precision and reliability of the findings. This approach supplies a comprehensive synthesis of the available evidence on the diagnostic accuracy of methylated DNA in sputum of lung cancer, facilitating the identification of possible patterns or tendencies across studies. However, it is imperative to acknowledge that the results may not be universally applicable to all populations, given differences in the race and smoking prevalence of lung cancer patients. This caveat is particularly noteworthy as methylation has been established to be heavily influenced by smoking [56]. Thus, caution is advised in interpreting the summary estimates, considering the extensive and diverse array of investigated genes and cohorts.
The included studies were of varying quality, with potential limitations or biases in design, conduct, or reporting affecting the validity and reliability of the results. The predominance of case–control studies with healthy subjects as the control group introduces spectrum bias, emphasizing the need for more accurate effect estimates from cohort studies or case–control studies involving patients with benign diseases. The risk of publication bias was considered; however, Deek’s funnel plot asymmetry test did not indicate a significant concern. Heterogeneity across studies, stemming from variations in case and control populations, analysis methods, cutoff values, and study designs, introduces a level of uncertainty in the generalizability of the findings. It is crucial to recognize these limitations and exercise caution when interpreting and applying the results of this meta-analysis.
5. Conclusions
In summary, the accuracy in identifying lung cancer through the assessment of methylated tumor DNA in sputum exhibited notable discrepancies among various studies. These variations are believed to stem from differences in tumor location, lung cancer stage, sample procurement, DNA extraction methodologies, and approaches to measuring methylation. With an overall sensitivity estimate of 55% (specificity at 80%), it is evident that improvements are required before contemplating inclusion in a randomized controlled trial. Uniform and consistent reporting of materials and methods are essential for the interpretation and reproducibility of results, and this research area would benefit from standardization. Nonetheless, this meta-analysis establishes a foundation for pinpointing genes with comparatively higher sensitivity and specificity, urging further exploration with a focus on refining the mentioned factors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16030506/s1, Figure S1: Number of studies published per year; Table S1: Sample type, collection method, and DNA extraction; Table S2: Comprehensive list of genes; Table S3: Sensitivity, specificity, and contingency data for the 15 studies included in the meta-analysis; Figure S2: Hierarchical summary receiver operating characteristics plot; Table S4: ID number, study ID, and gene name corresponding to Figure S2; Table S5: Quality Assessment of Diagnostic Accuracy Studies 2, domain 1—Patient selection; Table S6: Quality Assessment of Diagnostic Accuracy Studies 2, domain 2—Index test; Table S7: Quality Assessment of Diagnostic Accuracy Studies 2, domain 3—Reference standard; Table S8: Quality Assessment of Diagnostic Accuracy Studies 2, domain 4—Flow and timing; Table S9: Funding and conflicts of interest; Figure S3: Deek’s funnel plot. References [17,18,19,20,27,28,29,30,31,32,33,34,35,36,37,38] are cited in the supplementary materials.
Author Contributions
Conceptualization, M.B., S.W.C.W., T.F.H., O.H. and R.F.A.; methodology, M.B., S.W.C.W., R.F.A. and S.T.; formal analysis, M.B., S.W.C.W. and S.T.; investigation, M.B. and S.W.C.W.; data curation, M.B., S.W.C.W. and R.F.A.; writing—original draft preparation, M.B. and S.W.C.W.; writing—review and editing, all authors; supervision, O.H. and R.F.A.; project administration, M.B. and S.W.C.W.; funding acquisition, O.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Danish Cancer Society via the Danish Comprehensive Cancer Centers: “Danish Research Center for Lung Cancer” and “ctDNA Research Center—The Danish Research Center for Circulating Tumor DNA Guided Cancer Management, Danish Cancer Society (grant No. R257-A14700)”.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors would like to thank research librarian Mette Brandt Eriksen for assistance with the databases and search strategy.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217. [Google Scholar] [CrossRef]
- Woodard, G.A.; Jones, K.D.; Jablons, D.M. Lung Cancer Staging and Prognosis. In Lung Cancer; Reckamp, K.L., Ed.; Cancer Treatment and Research; Springer International Publishing: Cham, Switzerland, 2016; Volume 170, pp. 47–75. ISBN 978-3-319-40387-8. [Google Scholar]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Hilberg, O.; Andersen, M.B.; Weinreich, U.M.; Rasmussen, T.R. Increased Use of Computed Tomography in Denmark: Stage Shift toward Early Stage Lung Cancer through Incidental Findings. Acta Oncol. 2022, 61, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Bredtoft, E.N.; Madsen, H.H.; Rasmussen, T.R. Stage I Lung Cancer Patients with or without Symptoms—Are the Patients Different and Should We Treat Them Differently? Acta Oncol. 2021, 60, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.L.; Rosenstein, A.L.; Kiang, M.V.; Shah, S.A.; Gaissert, H.A.; Chang, D.C.; Fintelmann, F.J.; Yang, C.-F.J. Association of Computed Tomography Screening with Lung Cancer Stage Shift and Survival in the United States: Quasi-Experimental Study. BMJ 2022, 376, e069008. [Google Scholar] [CrossRef]
- Field, J.K.; Vulkan, D.; Davies, M.P.A.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung Cancer Mortality Reduction by LDCT Screening: UKLS Randomised Trial Results and International Meta-Analysis. Lancet Reg. Health Eur. 2021, 10, 100179. [Google Scholar] [CrossRef]
- The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Gill, R.R.; Jaklitsch, M.T.; Jacobson, F.L. Controversies in Lung Cancer Screening. J. Am. Coll. Radiol. 2016, 13, R2–R7. [Google Scholar] [CrossRef]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef]
- Henriksen, M.B.; Jensen, L.H.; Hilberg, O.; Hansen, T.F.; Brasen, C.L. 1284P Lung Cancer among Patients with Chronic Obstructive Pulmonary Disease: A Danish 10-Year Observational Study of the Overlapping Population. Ann. Oncol. 2023, 34, S741. [Google Scholar] [CrossRef]
- Thunnissen, F.B.J.M. Sputum Examination for Early Detection of Lung Cancer. J. Clin. Pathol. 2003, 56, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, H.; Kusumoto, M.; Yatabe, Y.; Aokage, K.; Watanabe, S.; Ishikura, S. Lung Cancer in Japan. J. Thorac. Oncol. 2022, 17, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Soukiasian, H.J.; Leung, A.; Imai, T.; Bose, S.; Kim, S.; Mosenifar, Z.; Gupta, N.K.; Tajbakhsh, J. Highly Sensitive Noninvasive Early Lung Cancer Detection Using DNA Methylation Topology in Sputum-Derived Epithelial Cells. JTCVS Open 2023, 13, 389–410. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, N.; Huggett, J.F.; Taylor, M.S.; Foy, C.A.; Devonshire, A.S. Quantification of Epigenetic Biomarkers: An Evaluation of Established and Emerging Methods for DNA Methylation Analysis. BMC Genom. 2014, 15, 1174. [Google Scholar] [CrossRef] [PubMed]
- McGinn, S.; Gut, I.G. DNA Sequencing—Spanning the Generations. N. Biotechnol. 2013, 30, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hubers, A.J.; Heideman, D.A.M.; Burgers, S.A.; Herder, G.J.M.; Sterk, P.J.; Rhodius, R.J.; Smit, H.J.; Krouwels, F.; Welling, A.; Witte, B.I.; et al. DNA Hypermethylation Analysis in Sputum for the Diagnosis of Lung Cancer: Training Validation Set Approach. Br. J. Cancer 2015, 112, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Do, K.; Yingling, C.M.; Picchi, M.A.; Wolf, H.J.; Kennedy, T.C.; Feser, W.J.; Baron, A.E.; Franklin, W.A.; Brock, M.V.; et al. Defining a Gene Promoter Methylation Signature in Sputum for Lung Cancer Risk Assessment. Clin. Cancer Res. 2012, 18, 3387–3395. [Google Scholar] [CrossRef]
- Su, Y.; Fang, H.; Jiang, F. Integrating DNA Methylation and microRNA Biomarkers in Sputum for Lung Cancer Detection. Clin. Epigenetics 2016, 8, 109. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Borg, M.; Wen, S.W.C. Methylated Cell Free Tumor DNA in Sputum as a Tool for Diagnosing Lung Cancer. PROSPERO 2023 CRD42023453066. 2023. Available online: https://www.Crd.York.Ac.Uk/Prospero/Display_record.Php?ID=CRD42023453066 (accessed on 1 October 2023).
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2 Group QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Whiting, P. Metandi: Meta-Analysis of Diagnostic Accuracy Using Hierarchical Logistic Regression. Stata J. Promot. Commun. Stat. Stata 2009, 9, 211–229. [Google Scholar] [CrossRef]
- Dwamena, B. MIDAS: Stata Module for Meta-Analytical Integration of Diagnostic Test Accuracy Studies; Boston College Department: Chestnut Hill, MA, USA, 2007. [Google Scholar]
- Hubers, A.J.; Heideman, D.A.M.; Herder, G.J.M.; Burgers, S.A.; Sterk, P.J.; Kunst, P.W.; Smit, H.J.; Postmus, P.E.; Witte, B.I.; Duin, S.; et al. Prolonged Sampling of Spontaneous Sputum Improves Sensitivity of Hypermethylation Analysis for Lung Cancer. J. Clin. Pathol. 2012, 65, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.; Jusue-Torres, I.; Stark, A.; Chen, C.; Rodgers, K.; Lee, B.; Griffin, C.; Yang, A.; Huang, P.; Wrangle, J.; et al. Early Detection of Lung Cancer Using DNA Promoter Hypermethylation in Plasma and Sputum. Clin. Cancer Res. 2017, 23, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Hubers, A.J.; Van Der Drift, M.A.; Prinsen, C.F.M.; Witte, B.I.; Wang, Y.; Shivapurkar, N.; Stastny, V.; Bolijn, A.S.; Hol, B.E.A.; Feng, Z.; et al. Methylation Analysis in Spontaneous Sputum for Lung Cancer Diagnosis. Lung Cancer 2014, 84, 127–133. [Google Scholar] [CrossRef]
- Li, N.; Dhilipkannah, P.; Jiang, F. High-Throughput Detection of Multiple miRNAs and Methylated DNA by Droplet Digital PCR. J. Pers. Med. 2021, 11, 359. [Google Scholar] [CrossRef]
- Shivapurkar, N.; Stastny, V.; Suzuki, M.; Wistuba, I.I.; Li, L.; Zheng, Y.; Feng, Z.; Hol, B.; Prinsen, C.; Thunnissen, F.B.; et al. Application of a Methylation Gene Panel by Quantitative PCR for Lung Cancers. Cancer Lett. 2007, 247, 56–71. [Google Scholar] [CrossRef]
- Shivapurkar, N.; Stastny, V.; Okumura, N.; Girard, L.; Xie, Y.; Prinsen, C.; Thunnissen, F.B.; Wistuba, I.I.; Czerniak, B.; Frenkel, E.; et al. Cytoglobin, the Newest Member of the Globin Family, Functions as a Tumor Suppressor Gene. Cancer Res. 2008, 68, 7448–7456. [Google Scholar] [CrossRef]
- van der Drift, M.A.; Prinsen, C.F.M.; Hol, B.E.A.; Bolijn, A.S.; Jeunink, M.a.F.; Dekhuijzen, P.N.R.; Thunnissen, F.B.J.M. Can Free DNA Be Detected in Sputum of Lung Cancer Patients? Lung Cancer Amst. Neth. 2008, 61, 385–390. [Google Scholar] [CrossRef]
- Shivapurkar, N.; Stastny, V.; Xie, Y.; Prinsen, C.; Frenkel, E.; Czerniak, B.; Thunnissen, F.B.; Minna, J.D.; Gazdar, A.F. Differential Methylation of a Short CpG-Rich Sequence within Exon 1 of TCF21 Gene: A Promising Cancer Biomarker Assay. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-H.; Kim, K.U.; Kim, J.-E.; Kim, H.-H.; Lee, M.K.; Lee, C.H.; Lee, S.-Y.; Oh, T.; An, S. Detection of HOXA9 Gene Methylation in Tumor Tissues and Induced Sputum Samples from Primary Lung Cancer Patients. Clin. Chem. Lab. Med. 2011, 49, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Hubers, A.J.; Brinkman, P.; Boksem, R.J.; Rhodius, R.J.; Witte, B.I.; Zwinderman, A.H.; Heideman, D.A.M.; Duin, S.; Koning, R.; Steenbergen, R.D.M.; et al. Combined Sputum Hypermethylation and eNose Analysis for Lung Cancer Diagnosis. J. Clin. Pathol. 2014, 67, 707–711. [Google Scholar] [CrossRef]
- Hubers, A.J.; Heideman, D.A.M.; Duin, S.; Witte, B.I.; De Koning, H.J.; Groen, H.J.M.; Prinsen, C.F.M.; Bolijn, A.S.; Wouters, M.; Van Der Meer, S.E.; et al. DNA Hypermethylation Analysis in Sputum of Asymptomatic Subjects at Risk for Lung Cancer Participating in the NELSON Trial: Argument for Maximum Screening Interval of 2 Years. J. Clin. Pathol. 2017, 70, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Fang, H.B.; Jiang, F. An Epigenetic Classifier for Early Stage Lung Cancer. Clin. Epigenetics 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Anjuman, N.; Li, N.; Guarnera, M.; Stass, S.A.; Jiang, F. Evaluation of Lung Flute in Sputum Samples for Molecular Analysis of Lung Cancer. Clin. Transl. Med. 2013, 2, e15. [Google Scholar] [CrossRef] [PubMed]
- Weiszhar, Z.; Horvath, I. Induced Sputum Analysis: Step by Step. Breathe 2013, 9, 300–306. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.4 (updated August 2023); Cochrane: Calgary, AB, Canada, 2023. [Google Scholar]
- Borg, M.; Wen, S.W.C.; Andersen, R.F.; Timm, S.; Hansen, T.F.; Hilberg, O. Methylated Circulating Tumor DNA in Blood as a Tool for Diagnosing Lung Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3959. [Google Scholar] [CrossRef]
- Park, J.A.; Suzuki, K. Novel Screening Tools for Lung Cancer. Thorac. Surg. Clin. 2023, 33, 215–226. [Google Scholar] [CrossRef]
- Usman Ali, M.; Miller, J.; Peirson, L.; Fitzpatrick-Lewis, D.; Kenny, M.; Sherifali, D.; Raina, P. Screening for Lung Cancer: A Systematic Review and Meta-Analysis. Prev. Med. 2016, 89, 301–314. [Google Scholar] [CrossRef]
- Mokhles, S.; Takkenberg, J.J.; Treasure, T. Evidence-Based and Personalized Medicine. It’s [AND] Not [OR]. Ann. Thorac. Surg. 2017, 103, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Salfity, H.V.N.; Tong, B.C.; Kocher, M.R.; Tailor, T.D. Historical Perspective on Lung Cancer Screening. Thorac. Surg. Clin. 2023, 33, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Ridge, C.A.; Nicholson, A.G.; Mirsadraee, S. The 8th Lung Cancer TNM Classification and Clinical Staging System: Review of the Changes and Clinical Implications. Quant. Imaging Med. Surg. 2018, 8, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Thurlbeck, W.M. Pathophysiology of Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 1990, 11, 389–403. [Google Scholar] [CrossRef]
- Meddeb, R.; Pisareva, E.; Thierry, A.R. Guidelines for the Preanalytical Conditions for Analyzing Circulating Cell-Free DNA. Clin. Chem. 2019, 65, 623–633. [Google Scholar] [CrossRef]
- Sozzi, G.; Roz, L.; Conte, D.; Mariani, L.; Andriani, F.; Verderio, P.; Pastorino, U. Effects of Prolonged Storage of Whole Plasma or Isolated Plasma DNA on the Results of Circulating DNA Quantification Assays. JNCI J. Natl. Cancer Inst. 2005, 97, 1848–1850. [Google Scholar] [CrossRef]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, Structures, and Functions of Circulating DNA in Oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]
- Yu, M.; Carter, K.T.; Makar, K.W.; Vickers, K.; Ulrich, C.M.; Schoen, R.E.; Brenner, D.; Markowitz, S.D.; Grady, W.M. MethyLight Droplet Digital PCR for Detection and Absolute Quantification of Infrequently Methylated Alleles. Epigenetics 2015, 10, 803–809. [Google Scholar] [CrossRef]
- Whale, A.S.; Devonshire, A.S.; Karlin-Neumann, G.; Regan, J.; Javier, L.; Cowen, S.; Fernandez-Gonzalez, A.; Jones, G.M.; Redshaw, N.; Beck, J.; et al. International Interlaboratory Digital PCR Study Demonstrating High Reproducibility for the Measurement of a Rare Sequence Variant. Anal. Chem. 2017, 89, 1724–1733. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Marsh, T.; Irajizad, E.; Patel, N.; Murage, E.; Vykoukal, J.; Dennison, J.B.; Do, K.-A.; Ostrin, E.; Spitz, M.R.; et al. Blood-Based Biomarker Panel for Personalized Lung Cancer Risk Assessment. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Leenen, F.A.D.; Muller, C.P.; Turner, J.D. DNA Methylation: Conducting the Orchestra from Exposure to Phenotype? Clin. Epigenetics 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).