Determination of the Prevalence of Microsatellite Instability, BRAF and KRAS/NRAS Mutation Status in Patients with Colorectal Cancer in Slovakia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Cohort Specification
2.3. Sample Preparation
2.4. Detection of Microsatellite Instability
2.5. Identification of BRAF and KRAS/NRAS Mutations
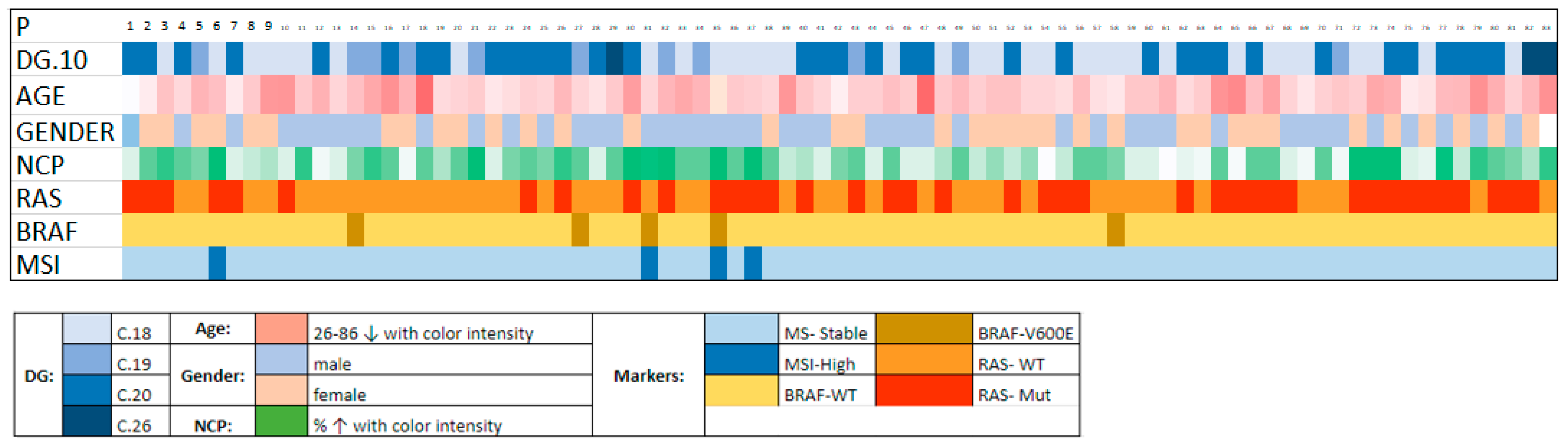
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 20 June 2023).
- Available online: https://gco.iarc.fr/today/data/factsheets/populations/703-slovakia-fact-sheets.pdf (accessed on 28 June 2023).
- Babela, R.; Orsagh, A.; Ricova, J.; Lansdorp-Vogelaar, I.; Csanadi, M.; De Koning, H.; Reckova, M. Cost-effectiveness of colorectal cancer screening in Slovakia. Eur. J. Cancer Prev. 2022, 31, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Forgacova, N.; Gazdarica, J.; Budis, J.; Radvanszky, J.; Szemes, T. Repurposing non-invasive prenatal testing data: Population study of single nucleotide variants associated with colorectal cancer and Lynch syndrome. Oncol. Lett. 2021, 22, 779. [Google Scholar] [CrossRef]
- Pham, P.T.; Pekarcikova, J.; Edelstein, R.; Majdan, M. Joinpoint analysis of colorectal cancer trend in the Slovakia. Bratisl. Lek. Listy. 2023, 124, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Ghias, K. Colorectal cancer carcinogenesis: A review of mechanisms. Cancer Biol. Med. 2016, 13, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Nazemalhosseini Mojarad, E.; Kuppen, P.J.; Aghdaei, H.A.; Zali, M.R. The CpG island methylator phenotype (CIMP) in colorectal cancer. Gastroenterol. Hepatol. Bed Bench. 2013, 6, 120–128. [Google Scholar] [PubMed]
- Kunkel, T.A.; Erie, D.A. DNA mismatch repair. Annu. Rev. Biochem. 2005, 74, 681–710. [Google Scholar] [CrossRef]
- Al-Sukhni, W.; Aronson, M.; Gallinger, S. Hereditary colorectal cancer syndromes: Familial adenomatous polyposis and lynch syndrome. Surg. Clin. N. Am. 2008, 88, 819–844, vii. [Google Scholar] [CrossRef]
- Garrido-Ramos, M.A. Satellite DNA: An Evolving Topic. Genes 2017, 8, 230. [Google Scholar] [CrossRef]
- Lower, S.S.; McGurk, M.P.; Clark, A.G.; Barbash, D.A. Satellite DNA evolution: Old ideas, new approaches. Curr. Opin. Genet. Dev. 2018, 49, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Thurin, M.; Cesano, A.; Marincola, F.M. Biomarkers for Immunotherapy of Cancer: Methods and Protocols; Humana: New York, NY, USA, 2019. [Google Scholar]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Pećina-Šlaus, N.; Kafka, A.; Salamon, I.; Bukovac, A. Mismatch Repair Pathway, Genome Stability and Cancer. Front. Mol. Biosci. 2020, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Peltomäki, P. Lynch syndrome genes. Fam. Cancer 2005, 4, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.N.; Kopetz, E.S. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: Clinical characteristics, clinical behavior, and response to targeted therapies. J. Gastrointest. Oncol. 2015, 6, 660–667. [Google Scholar] [PubMed]
- Rajagopalan, H.; Bardelli, A.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B.; Velculescu, V.E. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 2002, 418, 934. [Google Scholar] [CrossRef] [PubMed]
- Poynter, J.N.; Siegmund, K.D.; Weisenberger, D.J.; Long, T.I.; Thibodeau, S.N.; Lindor, N.; Young, J.; Jenkins, M.A.; Hopper, J.L.; Baron, J.A.; et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.E.; Johnson, B.; Kugathasan, L.; Morris, V.K.; Raghav, K.; Swanson, L.; Lim, H.J.; Renouf, D.J.; Gill, S.; Wolber, R.; et al. Population-based Screening for in Metastatic Colorectal Cancer Reveals Increased Prevalence and Poor Prognosis. Clin. Cancer Res. 2020, 26, 4599–4605. [Google Scholar] [CrossRef]
- Parikh, C.; Subrahmanyam, R.; Ren, R. Oncogenic NRAS, KRAS, and HRAS exhibit different leukemogenic potentials in mice. Cancer Res. 2007, 67, 7139–7146. [Google Scholar] [CrossRef]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef]
- Saeed, O.; Lopez-Beltran, A.; Fisher, K.W.; Scarpelli, M.; Montironi, R.; Cimadamore, A.; Massari, F.; Santoni, M.; Cheng, L. RAS genes in colorectal carcinoma: Pathogenesis, testing guidelines and treatment implications. J. Clin. Pathol. 2019, 72, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Tamosaitis, L.; Kvederaviciute, K.; Tarvydas, R.; Staniute, G.; Kalyan, K.; Meskinyte-Kausiliene, E.; Stankevicius, V.; Valius, M. KRAS, NRAS and BRAF mutations in colorectal cancer and melanoma. Med. Oncol. 2017, 34, 26. [Google Scholar] [CrossRef] [PubMed]
- Bożyk, A.; Krawczyk, P.; Reszka, K.; Krukowska, K.; Kolak, A.; Mańdziuk, S.; Wojas-Krawczyk, K.; Ramlau, R.; Milanowski, J. Correlation between, and mutations and tumor localizations in patients with primary and metastatic colorectal cancer. Arch. Med. Sci. 2022, 18, 1221–1230. [Google Scholar] [PubMed]
- Larki, P.; Gharib, E.; Yaghoob Taleghani, M.; Khorshidi, F.; Nazemalhosseini-Mojarad, E.; Asadzadeh Aghdaei, H. Coexistence of and Mutations in Colorectal Cancer: A Case Report Supporting The Concept of Tumoral Heterogeneity. Cell J. 2017, 19, 113–117. [Google Scholar] [PubMed]
- Roth, A.D.; Tejpar, S.; Delorenzi, M.; Yan, P.; Fiocca, R.; Klingbiel, D.; Dietrich, D.; Biesmans, B.; Bodoky, G.; Barone, C.; et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 2010, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, N.; Martinez-Useros, J.; Li, W.; Orta, A.; Perez, N.; Carames, C.; Hernandez, T.; Moreno, I.; Serrano, G.; Garcia-Foncillas, J. KRAS and BRAF Mutations as Prognostic and Predictive Biomarkers for Standard Chemotherapy Response in Metastatic Colorectal Cancer: A Single Institutional Study. Cells 2020, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 28 June 2023).
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Peltomäki, P.; Nyström, M.; Mecklin, J.-P.; Seppälä, T.T. Lynch Syndrome Genetics and Clinical Implications. Gastroenterology 2023, 164, 783–799. [Google Scholar] [CrossRef]
- Helderman, N.C.; Bajwa-ten Broeke, S.W.; Morreau, H.; Suerink, M.; Terlouw, D.; van der Werf, A.S.; van Wezel, T.; Nielsen, M. The diverse molecular profiles of lynch syndrome-associated colorectal cancers are (highly) dependent on underlying germline mismatch repair mutations. Crit. Rev. Oncol. Hematol. 2021, 163, 103338. [Google Scholar] [CrossRef]
- Sorich, M.J.; Wiese, M.D.; Rowland, A.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta-analysis of randomized, controlled trials. Ann. Oncol. 2015, 26, 13–21. [Google Scholar] [CrossRef]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020, 80, 2969–2974. [Google Scholar] [CrossRef]
- De la Chapelle, A.; Hampel, H. Clinical relevance of microsatellite instability in colorectal cancer. J. Clin. Oncol. 2010, 28, 3380–3387. [Google Scholar] [CrossRef] [PubMed]
- Lhermitte, B.; Egele, C.; Weingertner, N.; Ambrosetti, D.; Dadone, B.; Kubiniek, V.; Burel-Vandenbos, F.; Coyne, J.; Michiels, J.-F.; Chenard, M.-P.; et al. Adequately defining tumor cell proportion in tissue samples for molecular testing improves interobserver reproducibility of its assessment. Virchows Arch. 2017, 470, 21–27. [Google Scholar] [CrossRef]
- Guyot D’Asnières De Salins, A.; Tachon, G.; Cohen, R.; Karayan-Tapon, L.; Junca, A.; Frouin, E.; Godet, J.; Evrard, C.; Randrian, V.; Duval, A.; et al. Discordance between immunochemistry of mismatch repair proteins and molecular testing of microsatellite instability in colorectal cancer. ESMO Open 2021, 6, 100120. [Google Scholar] [CrossRef]
- Forbes, S.; Clements, J.; Dawson, E.; Bamford, S.; Webb, T.; Dogan, A.; Flanagan, A.; Teague, J.; Wooster, R.; Futreal, P.A.; et al. COSMIC 2005. Br. J. Cancer 2006, 94, 318–322. [Google Scholar] [CrossRef]
- Gularte-Mérida, R.; Smith, S.; Bowman, A.S.; da Cruz Paula, A.; Chatila, W.; Bielski, C.M.; Vyas, M.; Borsu, L.; Zehir, A.; Martelotto, L.G.; et al. Same-Cell Co-Occurrence of RAS Hotspot and BRAF V600E Mutations in Treatment-Naive Colorectal Cancer. JCO Precis. Oncol. 2022, 6, e2100365. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Chatila, W.K.; Arora, A.; Sanchez-Vega, F.; Kantheti, H.S.; Rojas Zamalloa, J.A.; Krieger, P.; Callahan, M.K.; Warner, A.B.; Postow, M.A.; et al. Therapeutic Implications of Detecting MAPK-Activating Alterations in Cutaneous and Unknown Primary Melanomas. Clin. Cancer Res. 2021, 27, 2226–2235. [Google Scholar] [CrossRef]
- Ros, J.; Baraibar, I.; Sardo, E.; Mulet, N.; Salvà, F.; Argilés, G.; Martini, G.; Ciardiello, D.; Cuadra, J.L.; Tabernero, J.; et al. and inhibition as treatment strategies in V600E metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835921992974. [Google Scholar] [CrossRef]
- Vittal, A.; Middinti, A.; Kasi Loknath Kumar, A. Are All Mutations the Same? A Rare Case Report of Coexisting Mutually Exclusive KRAS and BRAF Mutations in a Patient with Metastatic Colon Adenocarcinoma. Case Rep. Oncol. Med. 2017, 2017, 2321052. [Google Scholar] [CrossRef]
- Cree, I.A.; Deans, Z.; Ligtenberg, M.J.L.; Normanno, N.; Edsjö, A.; Rouleau, E.; Solé, F.; Thunnissen, E.; Timens, W.; Schuuring, E.; et al. Guidance for laboratories performing molecular pathology for cancer patients. J. Clin. Pathol. 2014, 67, 923–931. [Google Scholar] [CrossRef]
- Cafiero, C.; Re, A.; D’Amato, G.; Surico, P.L.; Surico, G.; Pirrelli, M.; Pisconti, S. KRAS and BRAF Concomitant Mutations in a Patient with Metastatic Colon Adenocarcinoma: An Interesting Case Report. Case Rep. Oncol. 2020, 13, 595–600. [Google Scholar] [CrossRef]
- Sargent, D.J.; Shi, Q.; Yothers, G.; Tejpar, S.; Bertagnolli, M.M.; Thibodeau, S.N.; Andre, T.; Labianca, R.; Gallinger, S.; Hamilton, S.R.; et al. Prognostic impact of deficient mismatch repair (dMMR) in 7,803 stage II/III colon cancer (CC) patients (pts): A pooled individual pt data analysis of 17 adjuvant trials in the ACCENT database. J. Clin. Oncol. 2014, 32, 3507. [Google Scholar] [CrossRef]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Zhou, X.; Ma, Y.; Fu, W. Is microsatellite instability-high really a favorable prognostic factor for advanced colorectal cancer? A meta-analysis. World J. Surg. Oncol. 2019, 17, 169. [Google Scholar] [CrossRef]

| Diagnosis | ICD-10 Code | No. of Patients |
|---|---|---|
| Malignant neoplasm of colon | C18 | 3 |
| Malignant neoplasm of cecum | C18.0 | 6 |
| Malignant neoplasm of ascending colon | C18.2 | 4 |
| Malignant neoplasm of splenic flexure | C18.5 | 3 |
| Malignant neoplasm of sigmoid colon | C18.7 | 13 |
| Malignant neoplasm of overlapping sites of colon | C18.8 | 1 |
| Malignant neoplasm of colon, unspecified | C18.9 | 4 |
| Malignant neoplasm of rectosigmoid junction | C19 | 11 |
| Malignant neoplasm of rectum | C20 | 29 |
| Malignant neoplasm of anus, unspecified | C21.0 | 6 |
| Malignant neoplasm of intestinal tract, part unspecified | C26.0 | 2 |
| Malignant neoplasm of ill-defined sites within the digestive system | C26.9 | 1 |
| MSI-H | BRAF | KRAS/NRAS | |
|---|---|---|---|
| Positive | 4 (4.8%) | 5 (6.0%) | KRAS: 33 (39.8%) |
| NRAS: 6 (7.2%) | |||
| Negative | 79 (95.2%) | 78 (94.0%) | 44 (53.0%) |
| P | ICD-10 | Age | Gender | NCP | RAS | BRAF | MSI | MMR |
|---|---|---|---|---|---|---|---|---|
| 6 | C18.2 | 62 | F | 100 | KRAS: c.38G > A, p.G13D | N | MSI-H | MLH1-, PMS2- |
| 31 | C18 | 74 | M | 80 | N | p.V600E | MSI-H | MLH1-, PMS2- |
| 35 | C18.2 | 79 | F | 70 | KRAS: c.35G > A, p.G12D | p.V600E | MSI-H | MLH1-, MSH2-, PMS2- |
| 37 | C18.2 | 67 | M | 90 | KRAS: c.35G > A, p.Gly12Asp | N | MSI-H | MLH1- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendek, T.; Saade, R.; Pos, O.; Kolnikova, G.; Urbanova, M.; Budis, J.; Mihok, L.; Tomas, M.; Szemes, T.; Repiska, V. Determination of the Prevalence of Microsatellite Instability, BRAF and KRAS/NRAS Mutation Status in Patients with Colorectal Cancer in Slovakia. Cancers 2024, 16, 1128. https://doi.org/10.3390/cancers16061128
Rendek T, Saade R, Pos O, Kolnikova G, Urbanova M, Budis J, Mihok L, Tomas M, Szemes T, Repiska V. Determination of the Prevalence of Microsatellite Instability, BRAF and KRAS/NRAS Mutation Status in Patients with Colorectal Cancer in Slovakia. Cancers. 2024; 16(6):1128. https://doi.org/10.3390/cancers16061128
Chicago/Turabian StyleRendek, Tomas, Rami Saade, Ondrej Pos, Georgina Kolnikova, Monika Urbanova, Jaroslav Budis, Luboslav Mihok, Miroslav Tomas, Tomas Szemes, and Vanda Repiska. 2024. "Determination of the Prevalence of Microsatellite Instability, BRAF and KRAS/NRAS Mutation Status in Patients with Colorectal Cancer in Slovakia" Cancers 16, no. 6: 1128. https://doi.org/10.3390/cancers16061128
APA StyleRendek, T., Saade, R., Pos, O., Kolnikova, G., Urbanova, M., Budis, J., Mihok, L., Tomas, M., Szemes, T., & Repiska, V. (2024). Determination of the Prevalence of Microsatellite Instability, BRAF and KRAS/NRAS Mutation Status in Patients with Colorectal Cancer in Slovakia. Cancers, 16(6), 1128. https://doi.org/10.3390/cancers16061128






