Perioperative Chemotherapy Including Bevacizumab in Potentially Curable Metastatic Colorectal Cancer: Long-Term Follow-Up of the ASSO-LM1 Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
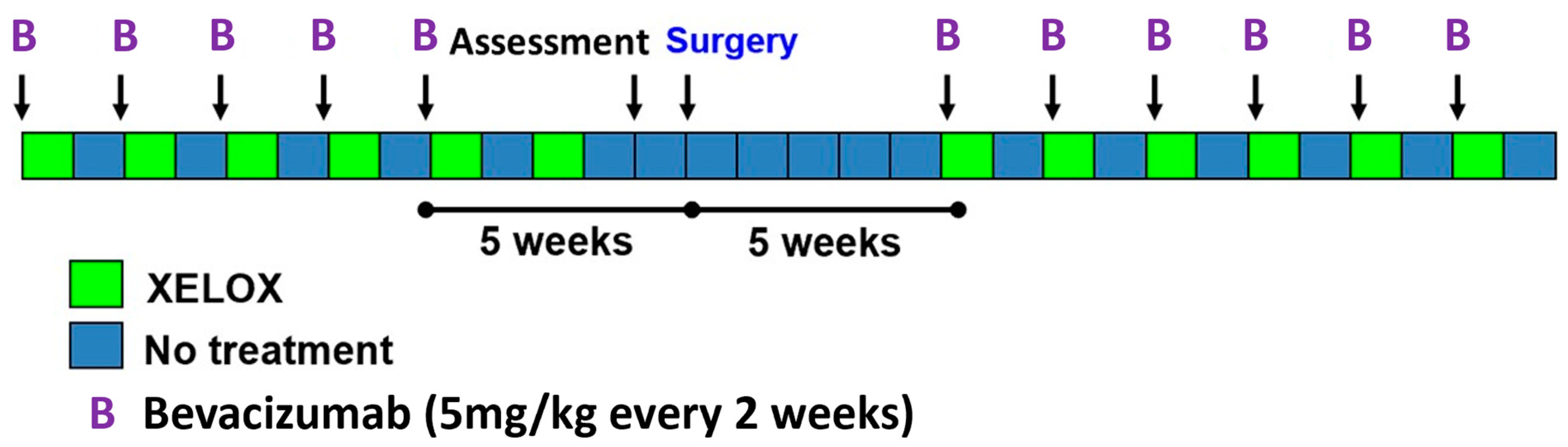
2.1. Study Design
2.2. Data Management and Statistical Analysis
3. Results
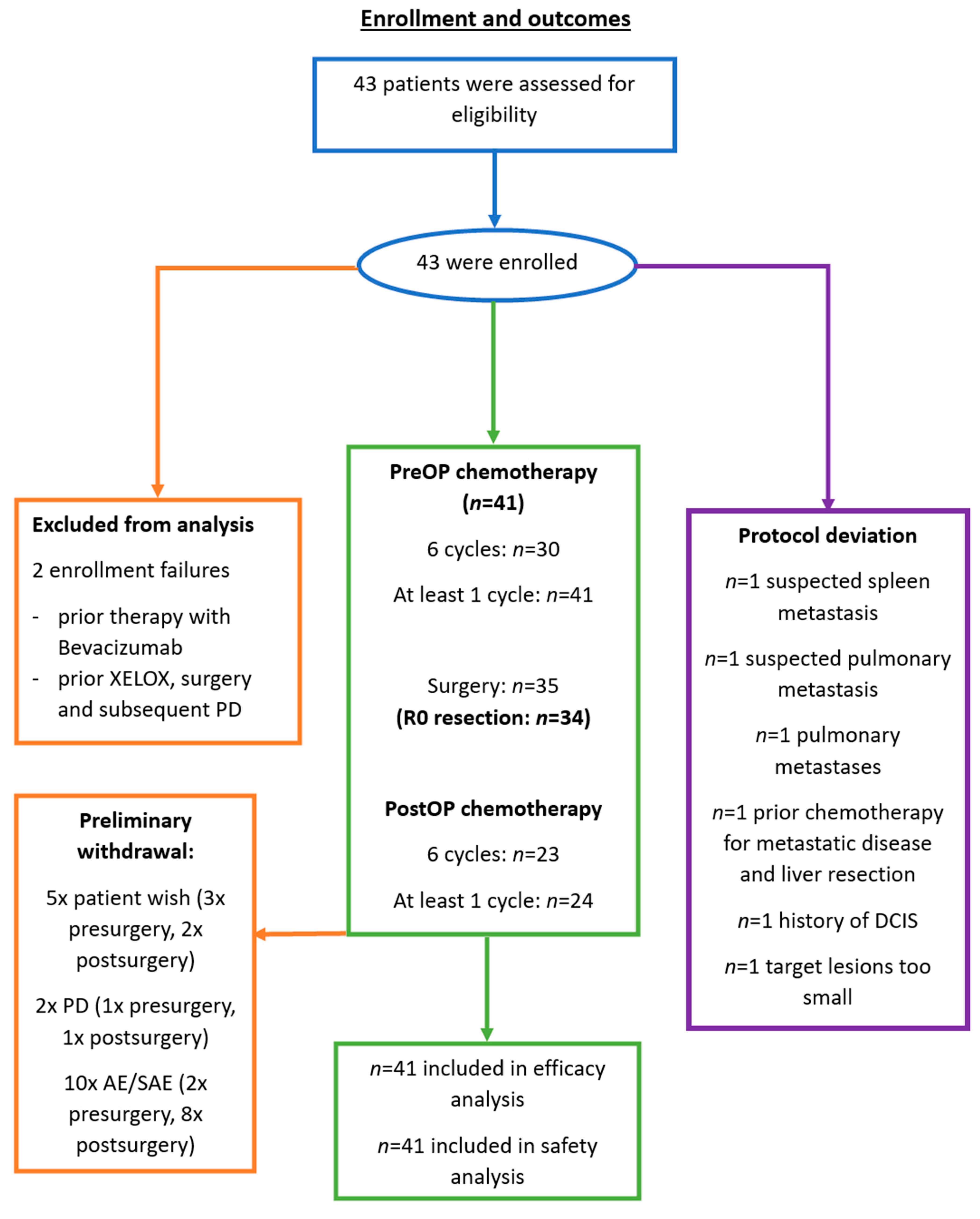
3.1. Patient Characteristics
3.2. Primary Outcome: Resectability Rate
3.3. Secondary Outcomes: Complications, Safety and Response Rate
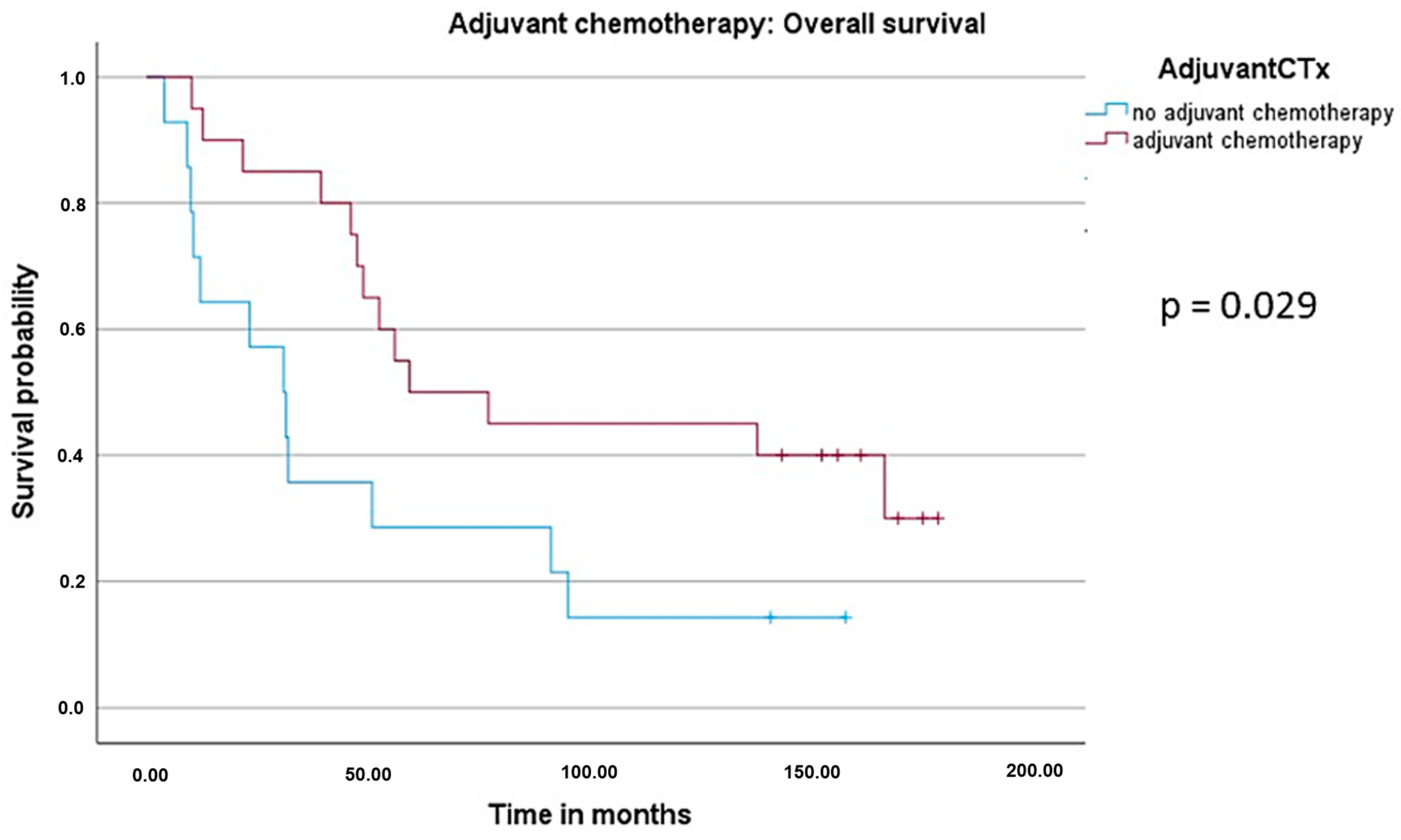
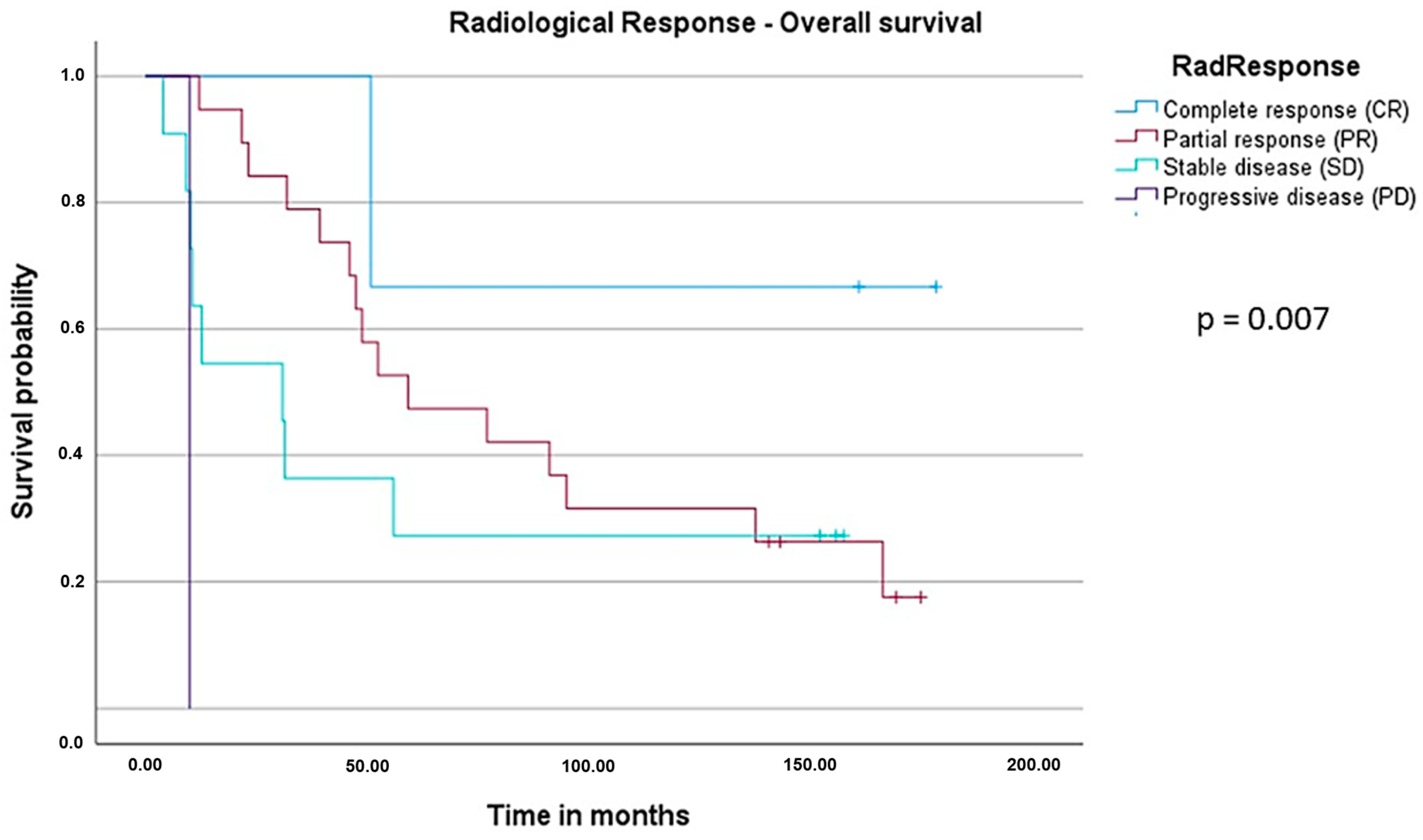
3.4. Survival Analysis: Overall Survival (OS) and Recurrence-Free Survival (RFS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- De Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–447. [Google Scholar] [CrossRef]
- Benoist, S.; Nordlinger, B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann. Surg. Oncol. 2009, 16, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Kemeny, N.; Jarnagin, W.; DeMatteo, R.; Blumgart, L.; Fong, Y.; Becker, J.M.; Bilchik, A.; Porter, G.; Hoffman, J.P.; et al. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J. Gastrointest. Surg. 2003, 7, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Shimizu, Y.; Mizusawa, J.; Inaba, Y.; Hamaguchi, T.; Shida, D.; Ohue, M.; Komori, K.; Shiomi, A.; Shiozawa, M.; et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone for liver metastasis from colorectal cancer: JCOG0603 study. J. Clin. Oncol. 2020, 38, 4005. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhong, Y.; Wei, Y.; Ye, L.; Lin, Q.; Ren, L.; Ye, Q.; Liu, T.; Xu, J.; Qin, X. Effect of neoadjuvant chemotherapy in patients with resectable colorectal liver metastases. PLoS ONE 2014, 9, e86543. [Google Scholar] [CrossRef]
- Nordlinger, B.; Van Cutsem, E.; Rougier, P.; Köhne, C.H.; Ychou, M.; Sobrero, A.; Adam, R.; Arvidsson, D.; Carrato, A.; Georgoulias, V.; et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur. J. Cancer 2007, 43, 2037–2045. [Google Scholar] [CrossRef]
- Gruenberger, T.; Bridgewater, J.; Chau, I.; García Alfonso, P.; Rivoire, M.; Mudan, S.; Lasserre, S.; Hermann, F.; Waterkamp, D.; Adam, R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: The OLIVIA multinational randomised phase II trial. Ann. Oncol. 2015, 26, 702–708. [Google Scholar] [CrossRef]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial Therapy with FOLFOXIRI and Bevacizumab for Metastatic Colorectal Cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef]
- Tomasello, G.; Petrelli, F.; Ghidini, M.; Russo, A.; Passalacqua, R.; Barni, S. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: A systematic review and pooled analysis. JAMA Oncol. 2017, 3, e170278. [Google Scholar] [CrossRef]
- Ivey, G.D.; Johnston, F.M.; Azad, N.S.; Christenson, E.S.; Lafaro, K.J.; Shubert, C.R. Current Surgical Management Strategies for Colorectal Cancer Liver Metastases. Cancers 2022, 14, 1063. [Google Scholar] [CrossRef]
- Feng, Q.Y.; Wei, Y.; Chen, J.W.; Chang, W.J.; Ye, L.C.; Zhu, D.X.; Xu, J.M. Anti-EGFR and anti-VEGF agents: Important targeted therapies of colorectal liver metastases. World J. Gastroenterol. 2014, 20, 4263–4275. [Google Scholar] [CrossRef]
- US Food and Drug Administration. New treatments for colorectal cancer. FDA Consum. 2004, 38, 17. [Google Scholar]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Muro, K.; Shitara, K.; Yamazaki, K.; Shiozawa, M.; Ohori, H.; Takashima, A.; Yokota, M.; Makiyama, A.; Akazawa, N.; et al. Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival among Patients with RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2023, 329, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Mitchell, E.; Chidiac, T.; Scroggin, C.; Hagenstad, C.; Spigel, D.; Marshall, J.; Cohn, A.; McCollum, D.; Stella, P.; et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J. Clin. Oncol. 2009, 27, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.; Koopman, M.; Cats, A.; Rodenburg, C.J.; Creemers, G.J.M.; Schrama, J.G.; Erdkamp, F.L.G.; Vos, A.H.; van Groeningen, C.J.; Sinnige, H.A.M.; et al. Chemotherapy, Bevacizumab, and Cetuximab in Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol. 2014, 15, 601–611. [Google Scholar] [CrossRef]
- Hubert, C.; Lucidi, V.; Weerts, J.; Dili, A.; Demetter, P.; Massart, B.; Komuta, M.; Navez, J.; Reding, R.; Gigot, J.F.; et al. Impact of biological agents on the prevalence of chemotherapy associated liver injury (CALI): Multicentric study of patients operated for colorectal liver metastases. Eur. J. Surg. Oncol. 2018, 44, 1532–1538. [Google Scholar] [CrossRef]
- Zhao, J.; van Mierlo, K.M.C.; Gómez-Ramírez, J.; Kim, H.; Pilgrim, C.H.C.; Pessaux, P.; Rensen, S.S.; van der Stok, E.P.; Schaap, F.G.; Soubrane, O.; et al. Systematic review of the influence of chemotherapy-associated liver injury on outcome after partial hepatectomy for colorectal liver metastases. Br. J. Surg. 2017, 104, 990–1002. [Google Scholar] [CrossRef]
- Massani, M.; Capovilla, G.; Ruffolo, C.; Bonariol, R.; Maccatrozzo, P.; Tuci, F.; Battistella, G.; Grazi, G.L.; Bassi, N. Blood transfusions and steatohepatitis are independent risk factors for complications following liver resection for colorectal cancer liver metastases. Mol. Clin. Oncol. 2017, 7, 529–538. [Google Scholar] [CrossRef]
- Viganò, L.; Rubbia-Brandt, L.; De Rosa, G.; Majno, P.; Langella, S.; Toso, C.; Mentha, G.; Capussotti, L. Nodular Regenerative Hyperplasia in Patients Undergoing Liver Resection for Colorectal Metastases after Chemotherapy: Risk Factors, Preoperative Assessment and Clinical Impact. Ann. Surg. Oncol. 2015, 22, 4149–4157. [Google Scholar] [CrossRef]
- Wicherts, D.A.; De Haas, R.J.; Sebagh, M.; Ciacio, O.; Lévi, F.; Paule, B.; Giacchetti, S.; Guettier, C.; Azoulay, D.; Castaing, D.; et al. Regenerative nodular hyperplasia of the liver related to chemotherapy: Impact on outcome of liver surgery for colorectal metastases. Ann. Surg. Oncol. 2011, 18, 659–669. [Google Scholar] [CrossRef]
- Søreide, J.A.; Deshpande, R. Post hepatectomy liver failure (PHLF)—Recent advances in prevention and clinical management. Eur. J. Surg. Oncol. 2021, 47, 216–224. [Google Scholar] [CrossRef]
- Klinger, M.; Eipeldauer, S.; Hacker, S.; Herberger, B.; Tamandl, D.; Dorfmeister, M.; Koelblinger, C.; Gruenberger, B.; Gruenberger, T. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur. J. Surg. Oncol. 2009, 35, 515–520. [Google Scholar] [CrossRef]
- Tamandl, D.; Klinger, M.; Eipeldauer, S.; Herberger, B.; Kaczirek, K.; Gruenberger, B.; Gruenberger, T. Sinusoidal obstruction syndrome impairs long-term outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann. Surg. Oncol. 2011, 18, 421–430. [Google Scholar] [CrossRef]
- Stremitzer, S.; Stift, J.; Singh, J.; Starlinger, P.; Gruenberger, B.; Tamandl, D.; Gruenberger, T. Histological response, pattern of tumor destruction and clinical outcome after neoadjuvant chemotherapy including bevacizumab or cetuximab in patients undergoing liver resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2015, 41, 868–874. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Z.; Zou, X.; Liu, T. Bevacizumab and wound-healing complications: A systematic review and meta-analysis of randomized controlled trials. Oncotarget 2016, 7, 82473–82481. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.J.G.; Bolhuis, K.; Loosveld, O.J.L.; de Groot, J.W.B.; Droogendijk, H.; Helgason, H.H.; Hendriks, M.P.; Klaase, J.M.; Kazemier, G.; Liem, M.S.L.; et al. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): An open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. 2023, 24, 757–771. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Descriptive Statistics | |
|---|---|---|
| Total number of patients | 41 | |
| Gender | Male | 28 (68.3%) |
| Female | 13 (31.7%) | |
| Age | Median 66 years (38–80 years) | |
| Weight | Median 75 kg (47–120 kg) | |
| Height | Median 170 cm (152–190 cm) | |
| ECOG | 0 | 26 (63.4%) |
| 1 | 15 (36.6%) | |
| Medical history | Smoking | 13 (31.7%) |
| Arterial hypertension | 20 (48.8%) | |
| Diabetes mellitus | 3 (7.3%) | |
| Thromboembolic disease | 2 (4.9%)—not active | |
| Cardiac events | 7 (17.1%) | |
| Gastrointestinal ulcers | 2 (4.9%) | |
| Hematological disease | 1 (2.4%) | |
| Primary tumor location | Left-sided | 28 (68.3%) |
| Right-sided | 10 (24.4%) | |
| Missing | 3 (7.3%) | |
| CRLM diagnosis | Synchronous | 32 (78.0%) |
| Metachronous | 9 (22.0%) | |
| Surgery of the primary (prior to study enrollment) | 23 (56.1%) | |
| Pathological T stage (n = 23) | 1 | 3 (13.04%) |
| 2 | 3(13.04%) | |
| 3 | 14 (60.87%) | |
| 4 | 3 (13.04%) | |
| Pathological N stage (n = 23) | 0 | 8 (34.8%) |
| 1 | 11 (47.8%) | |
| 2 | 3 (13.0%) | |
| X | 1 (4.4%) | |
| Pathological M stage (n = 23) | 0 | 7 (30.4%) |
| 1 | 15 (65.2%) | |
| X | 1 (4.4%) | |
| Grading of primary tumor (n = 23) | 1 | 1 (4.4%) |
| 2 | 16 (69.5%) | |
| 3 | 6 (26.1%) | |
| Characteristics | Descriptive Statistics | |
|---|---|---|
| Blood transfusion | 3 (8.6%) | |
| ICU admission | 25 (71.4%) | |
| Number of CRLM > 1 | 19 (54.3%) | |
| Largest CRLM > 5 cm | 5 (14.3%) | |
| Synchronous metastases | 27 (77.1%) | |
| Lymph node positive primary | 18 (51.4%) | |
| CEA above normal range | 18 (51.4%) | |
| FONG clinical risk score | 0 | 3 (8.6%) |
| 1 | 5 (14.3%) | |
| 2 | 6 (17.1%) | |
| 3 | 18 (51.4%) | |
| 4 | 3 (8.6%) | |
| 5 | 0 (0%) | |
| Mutational profile | Any KRAS mutation | 7 (20.0%) |
| KRAS wildtype | 17 (48.6%) | |
| KRAS missing | 11 (31.4%) | |
| BRAF mutation | 0 (0%) | |
| BRAF wildtype | 11 (31.4%) | |
| BRAF missing | 24 (68.6%) | |
| Postoperative complications | Pulmonary | 6 (17.1%) |
| Biliary fistula | 1 (2.9%) | |
| Hepatic failure | 1 (2.9%) | |
| Wound infection | 3 (8.6%) | |
| Wound healing | 3 (8.6%) | |
| Operative revisions (all due to anastomotic leak) | 3 (8.6%):
| |
| Resection margin | R0 | 34 (97.1%) |
| R1 | 1 (2.9%) | |
| AE Preoperative | Grade 3 and 4 n = 41 | AE Postoperative | Grade 3 and 4 n = 24 |
|---|---|---|---|
| Diarrhea | 7 (17.1%) | Neuropathy | 3 (12.5%) |
| Hand foot syndrome (HFS) | 2 (4.9%) | Hyperglycemia | 1 (4.2%) |
| Neuropathy | 1 (2.4%) | Leukocytosis | 1 (4.2%) |
| Thromboembolic events | 2 (4.9%) | Diarrhea | 1 (4.2%) |
| N | mOS (Months) | HR (CI) | p Value | ||
|---|---|---|---|---|---|
| Gender | Male | 24 | 52.3 | 1.026 (0.424–2.483) | 0.954 |
| Female | 10 | 48.8 | |||
| Age | ≤66 | 20 | 55.8 | 1.481 (0.672–3.263) | 0.330 |
| >66 | 14 | 48.8 | |||
| Primary location | Left-sided | 25 | 50.7 | 0.871 (0.345–2.196) | 0.770 |
| Right-sided | 9 | 55.8 | |||
| Syn vs. meta | Synchronous | 25 | 55.8 | 1.382 (0.575–3.321) | 0.469 |
| Metachronous | 9 | 31.9 | |||
| Performance status | ECOG 0 | 24 | 52.3 | 1.336 (0.573–3.118) | 0.503 |
| ECOG 1 | 10 | 31.9 | |||
| RAS status (missing n = 12) | Wildtype | 16 | 50.7 | 1.774 (0.620–5.082) | 0.285 |
| Mutant | 6 | 21.7 | |||
| Fong score (missing n = 2) | 0–3 (low) | 29 | 55.8 | 1.584 (0.464–5.402) | 0.463 |
| 4–5 (high) | 3 | 48.8 | |||
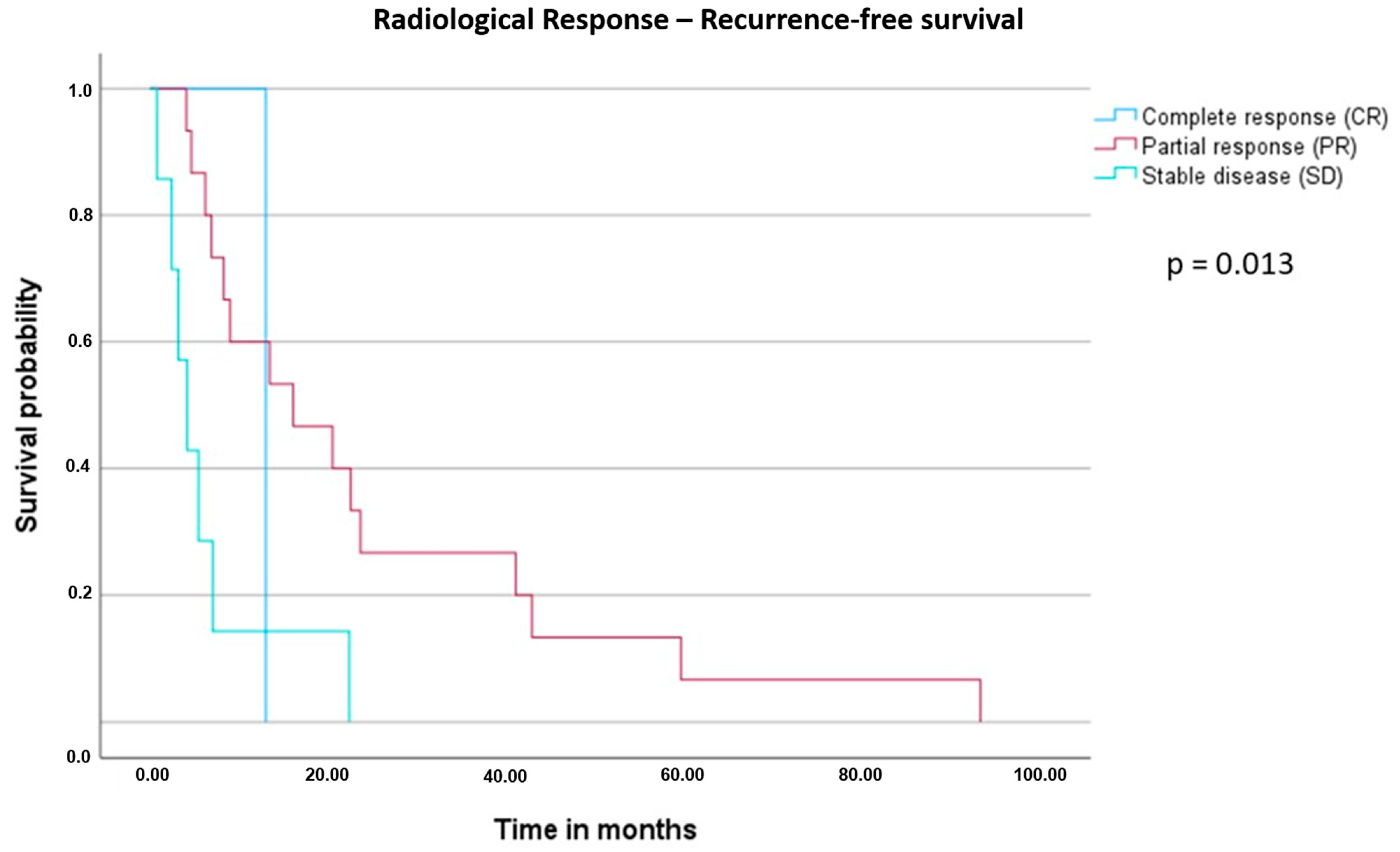
| Radiological response | Complete response (CR) | 3 | NR | Reference | 0.007 |
| Partial response (PR) | 19 | 59.1 | 3.178 (0.419–24.127) | ||
| Stable disease (SD) | 11 | 30.8 | 4.902 (0.606–39.665) | ||
| Progressive disease (PD) | 1 | 10.0 | 56.156 (2.538–1242.455) | ||
| Surgery | Yes | 32 | 52.3 | 0.179 (0.038–0.851) | 0.031 |
| No | 2 | 10.0 | |||
| Chemotherapy (CTx) | Complete periOP CTx | 20 | 59.1 | 0.418 (0.186–0.938) | 0.034 |
| No adjuvant CTx | 14 | 30.8 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Santol, J.; Gruenberger, B.; Lenauer, A.; Laengle, F.; Thaler, J.; Piringer, G.; Eisterer, W.; Djanani, A.; Stift, J.; et al. Perioperative Chemotherapy Including Bevacizumab in Potentially Curable Metastatic Colorectal Cancer: Long-Term Follow-Up of the ASSO-LM1 Trial. Cancers 2024, 16, 857. https://doi.org/10.3390/cancers16050857
Dong Y, Santol J, Gruenberger B, Lenauer A, Laengle F, Thaler J, Piringer G, Eisterer W, Djanani A, Stift J, et al. Perioperative Chemotherapy Including Bevacizumab in Potentially Curable Metastatic Colorectal Cancer: Long-Term Follow-Up of the ASSO-LM1 Trial. Cancers. 2024; 16(5):857. https://doi.org/10.3390/cancers16050857
Chicago/Turabian StyleDong, Yawen, Jonas Santol, Birgit Gruenberger, Alfred Lenauer, Friedrich Laengle, Josef Thaler, Gudrun Piringer, Wolfgang Eisterer, Angela Djanani, Judith Stift, and et al. 2024. "Perioperative Chemotherapy Including Bevacizumab in Potentially Curable Metastatic Colorectal Cancer: Long-Term Follow-Up of the ASSO-LM1 Trial" Cancers 16, no. 5: 857. https://doi.org/10.3390/cancers16050857
APA StyleDong, Y., Santol, J., Gruenberger, B., Lenauer, A., Laengle, F., Thaler, J., Piringer, G., Eisterer, W., Djanani, A., Stift, J., & Gruenberger, T. (2024). Perioperative Chemotherapy Including Bevacizumab in Potentially Curable Metastatic Colorectal Cancer: Long-Term Follow-Up of the ASSO-LM1 Trial. Cancers, 16(5), 857. https://doi.org/10.3390/cancers16050857





