MACC1 Regulates LGR5 to Promote Cancer Stem Cell Properties in Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Organoids
2.2. PDX Generation and the Tumor Dissociation Procedure
2.3. CD44-APC Tagging and Flow Cytometry Cell Sorting (FACS) Analysis
2.4. Aldefluor Assay
2.5. RNA Sequencing
2.6. In Vivo Tumorigenicity Assay
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Tumor Sphere Formation Assay
2.9. Clonogenic Formation Assay
2.10. Chromatin Immunoprecipitation (ChIP)
2.11. Patient Sample Analysis
2.12. Statistical Analysis
3. Results
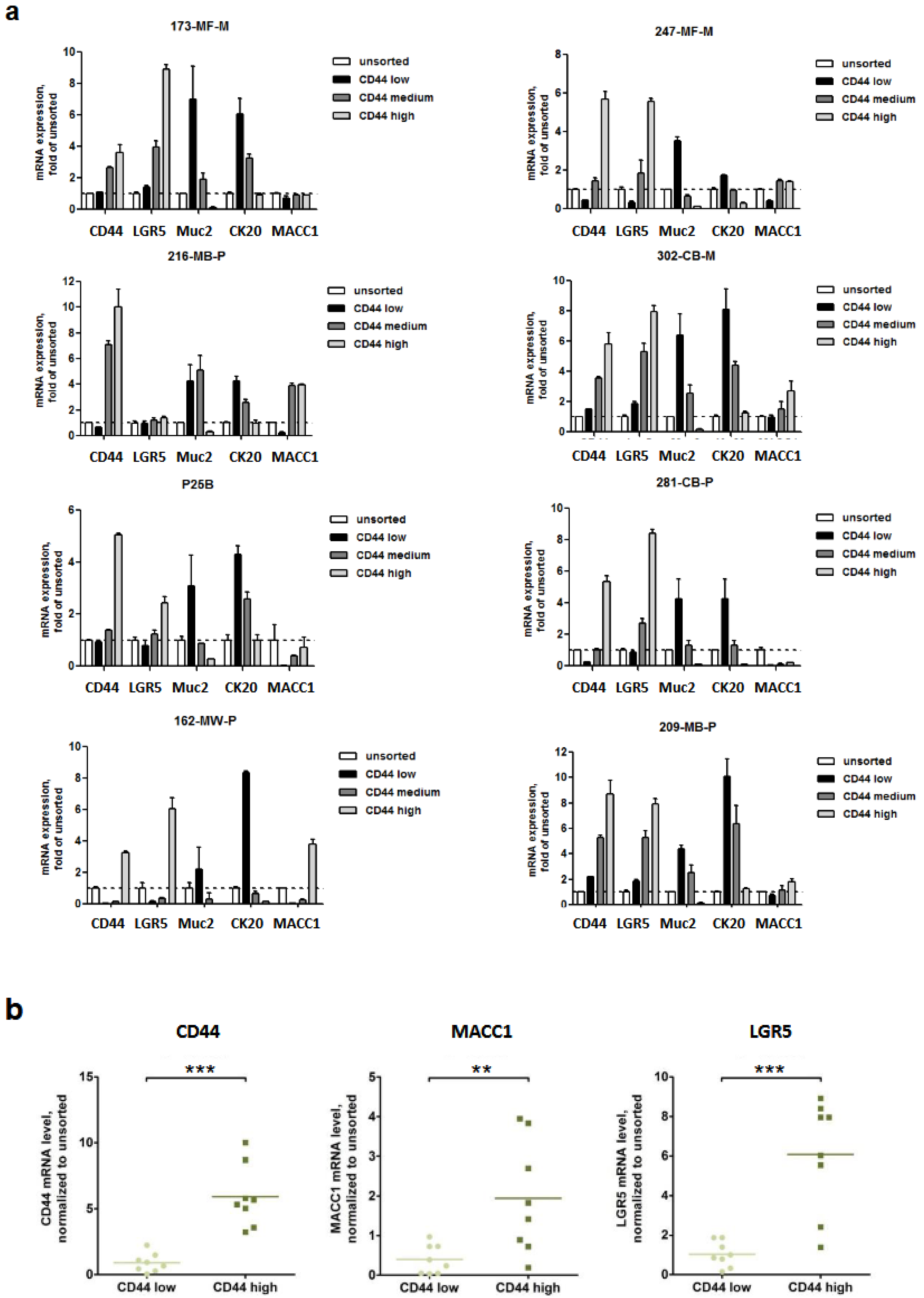
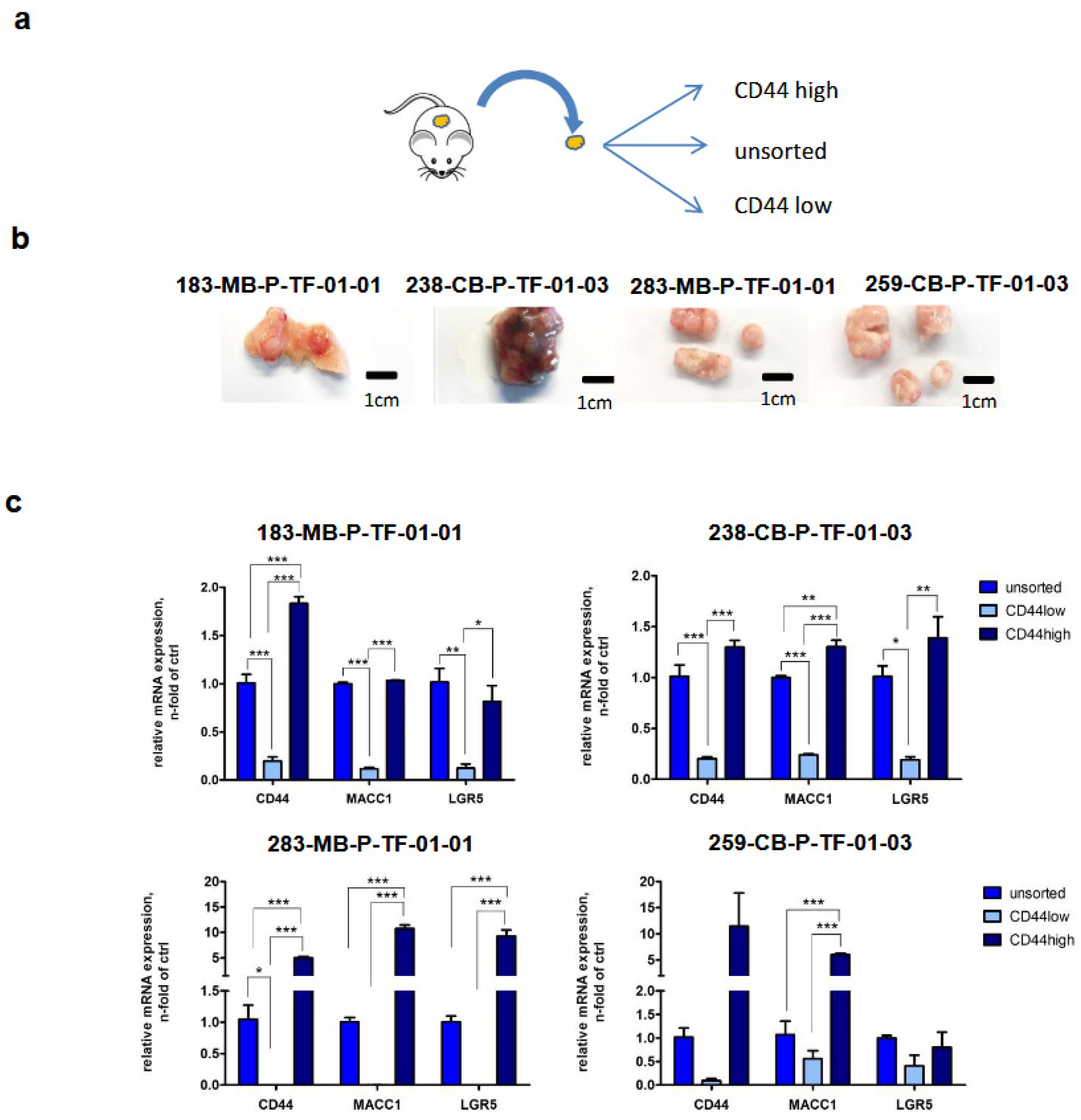
3.1. Expression of MACC1 and LGR5 Are Elevated in CD44-High Stem Cell-Enriched Populations of Patient-Derived 3D Organoids
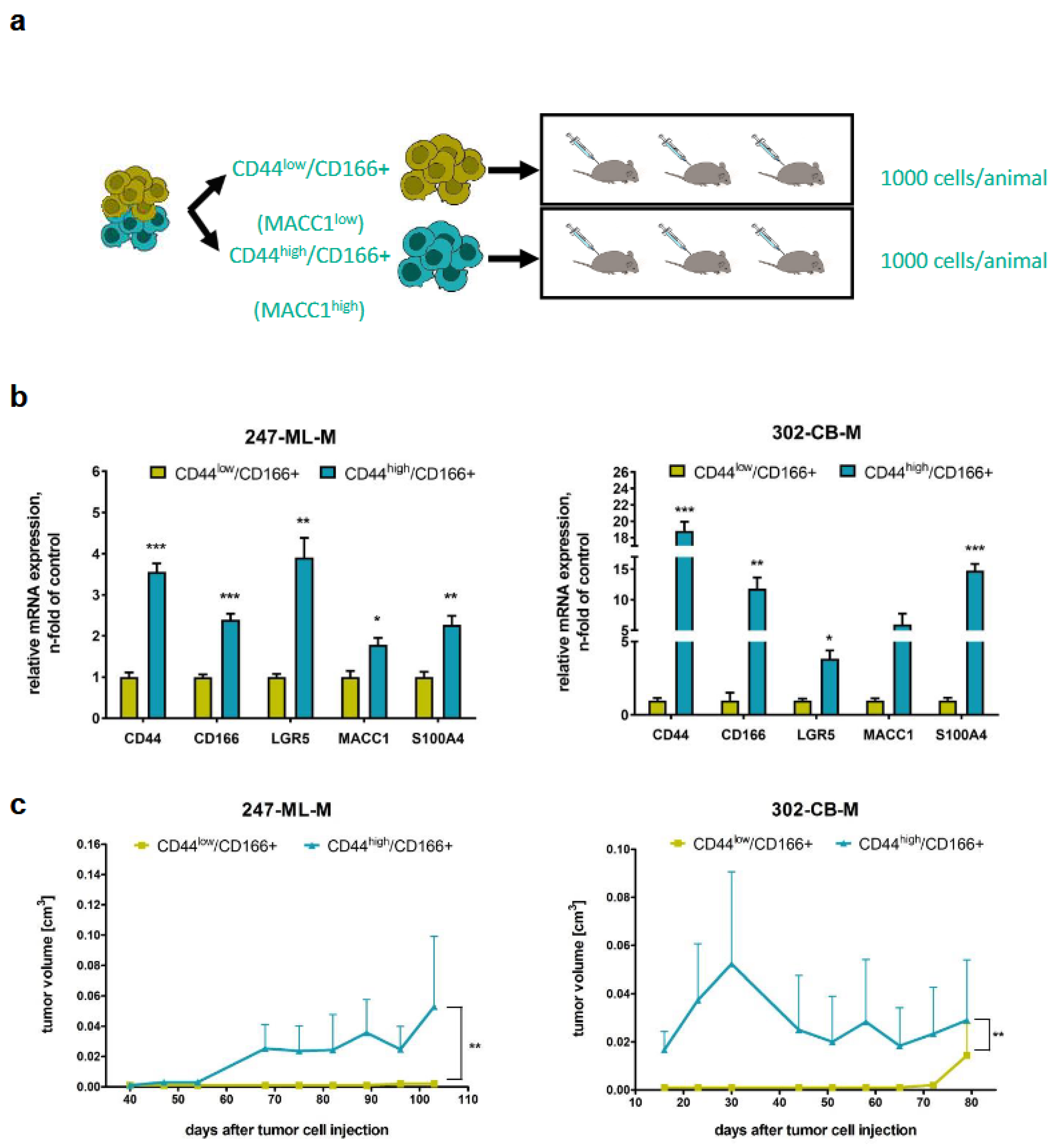
3.2. Tumorigenicity of CD44-High/CD166+ Subpopulations of PDO-Derived PDXs
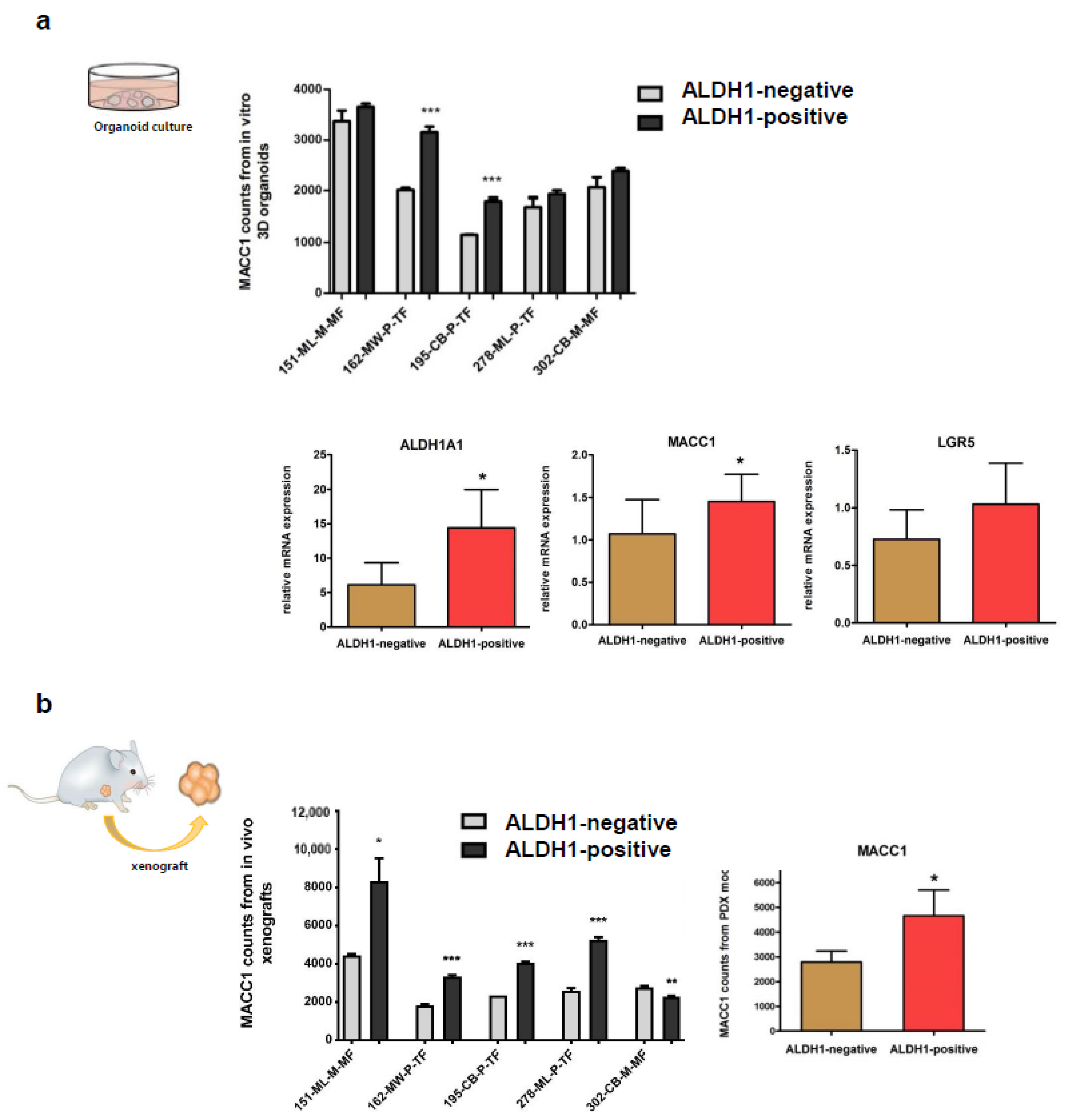
3.3. Expressions of MACC1 and LGR5 Are Elevated in ALDH1-Positive Stem Cell-Enriched Populations of Patient-Derived 3D Organoids
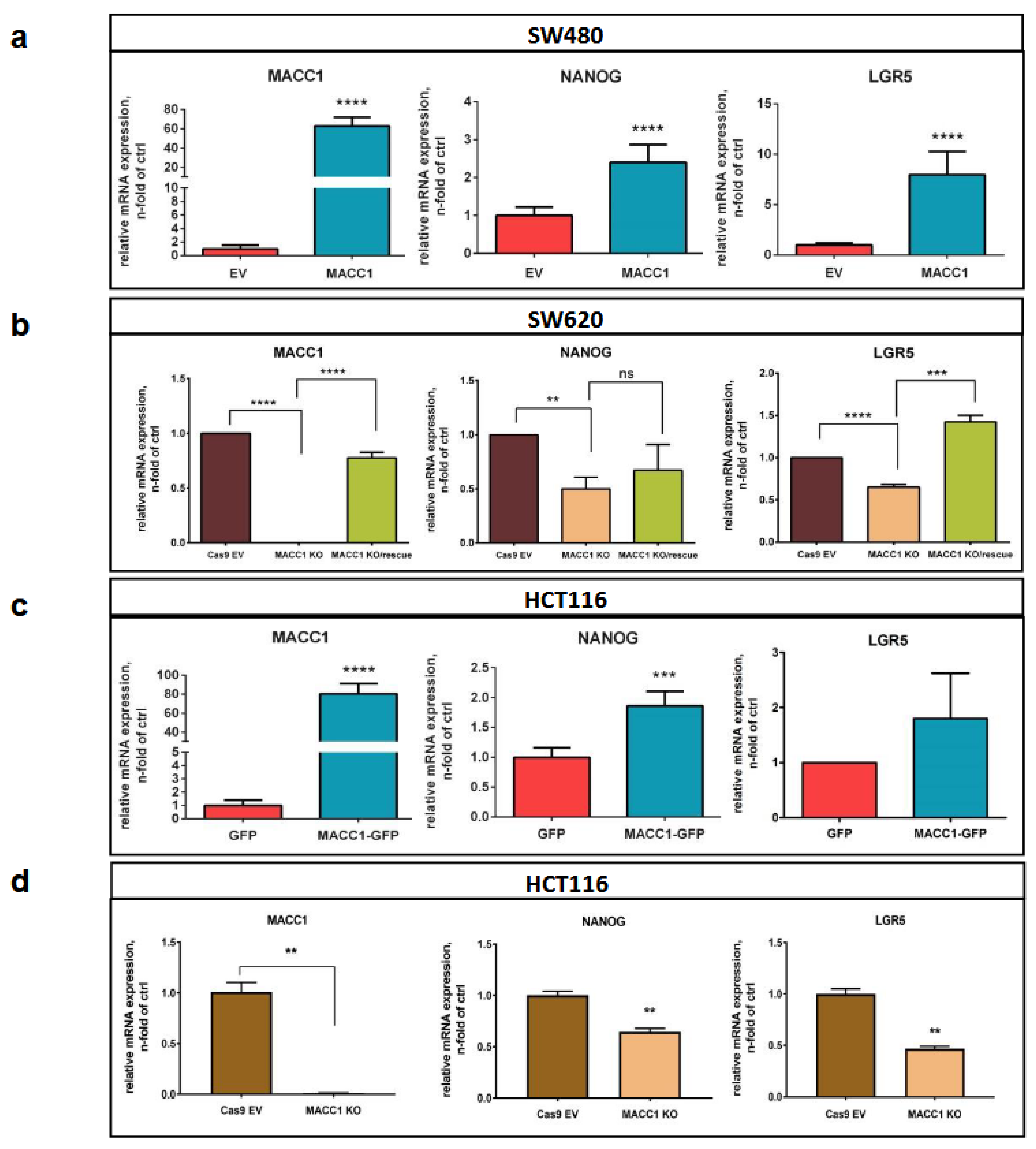
3.4. The MACC1—Stemness Marker Link in CRC 2D Cell Lines
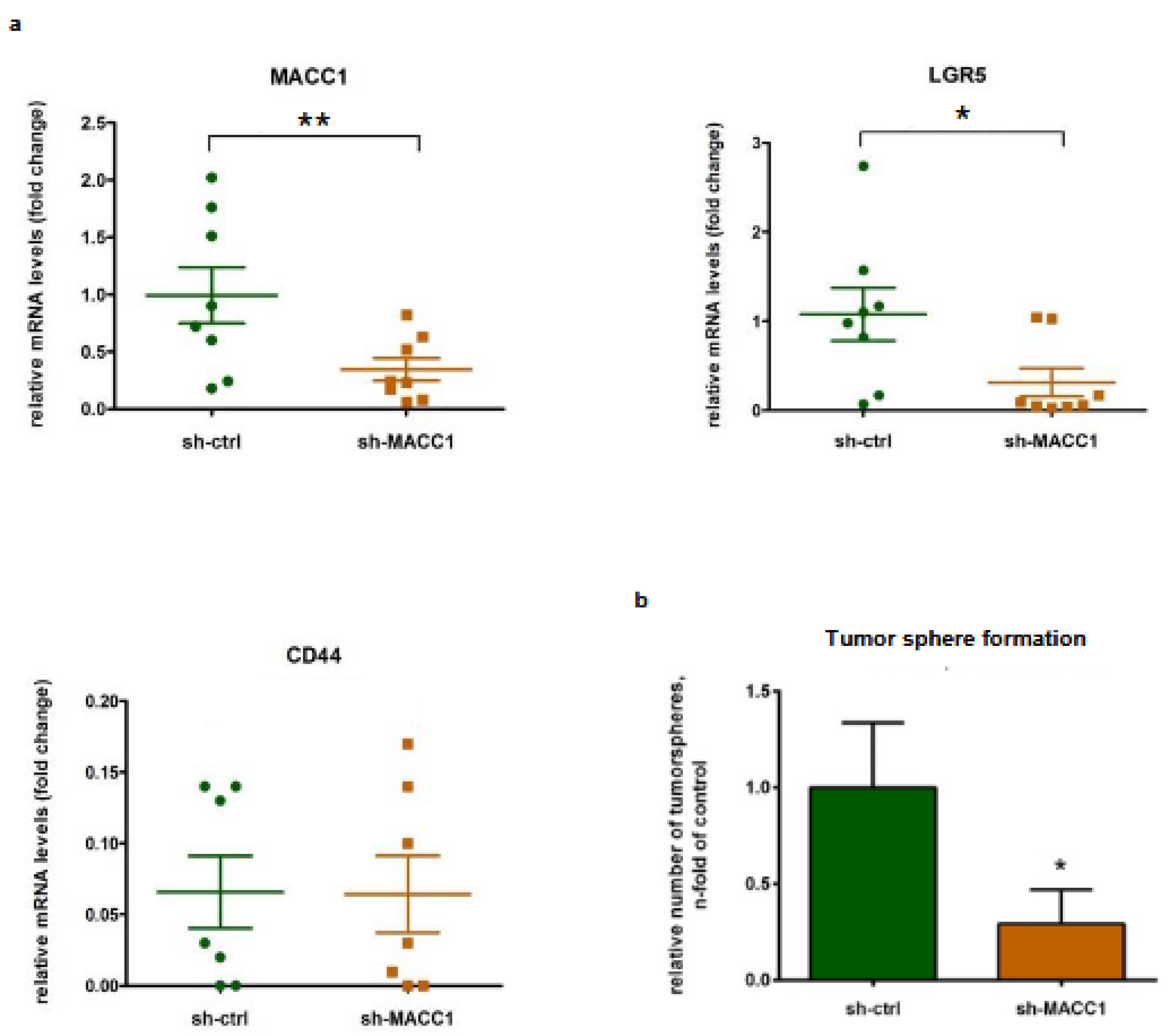
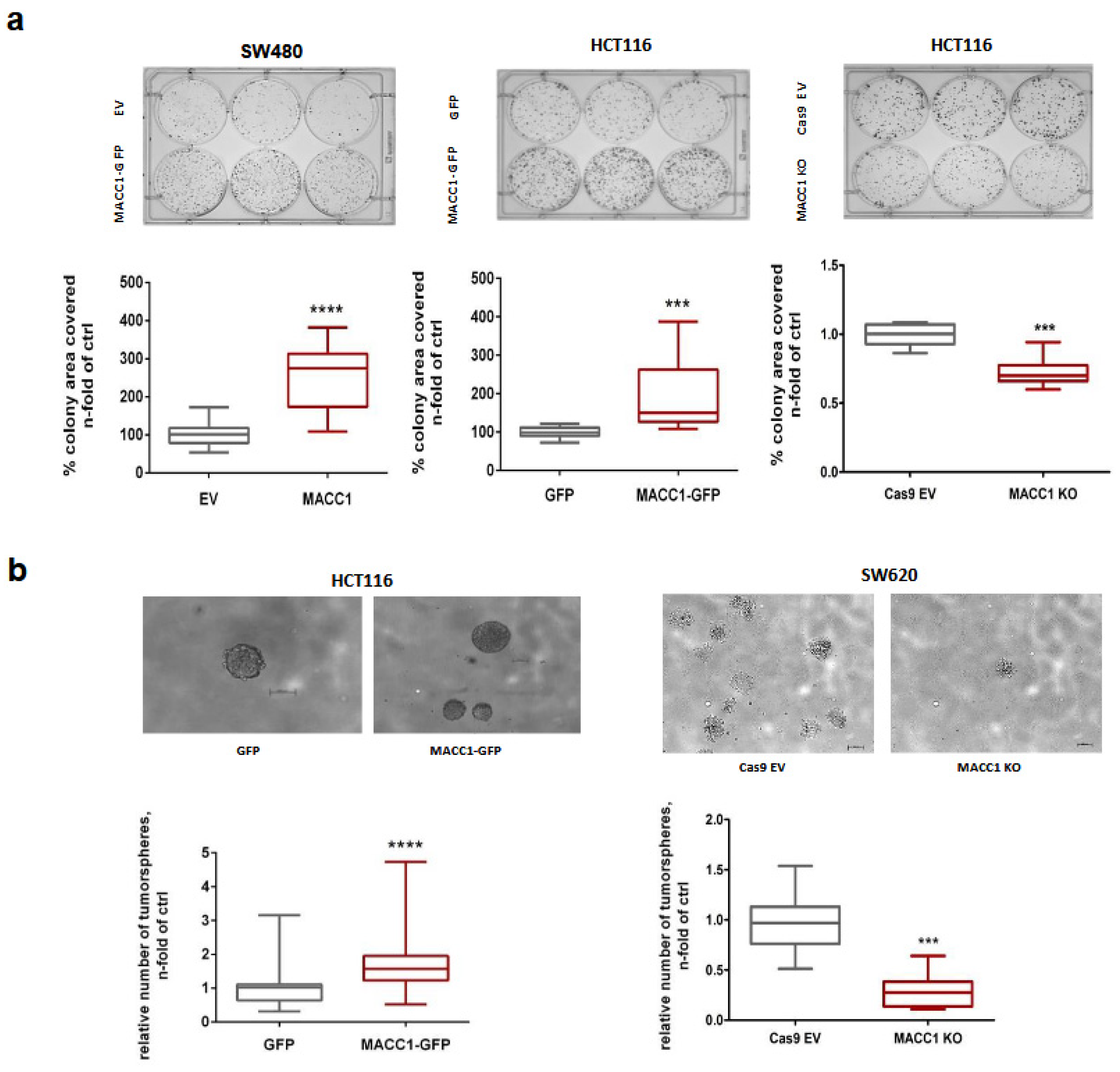
3.5. The MACC1—Stemness Link in CRC 2D Cell Lines: Phenotypic Assays
3.6. Back Translation of the MACC1—LGR5 Association in CRC-PDX Models
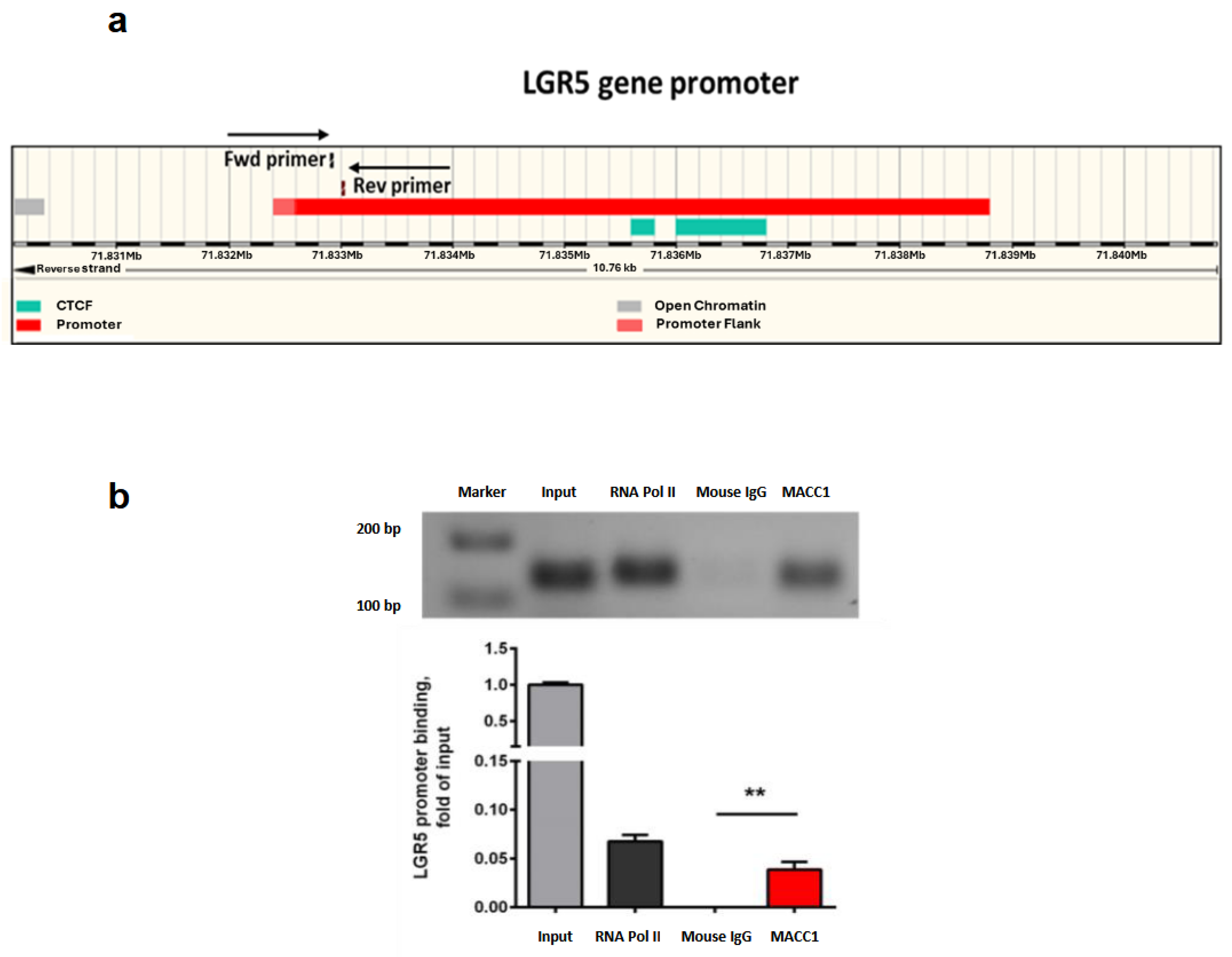
3.7. MACC1 Increases Cancer Stemness via Transcriptional Activation of the LGR5 Target Gene
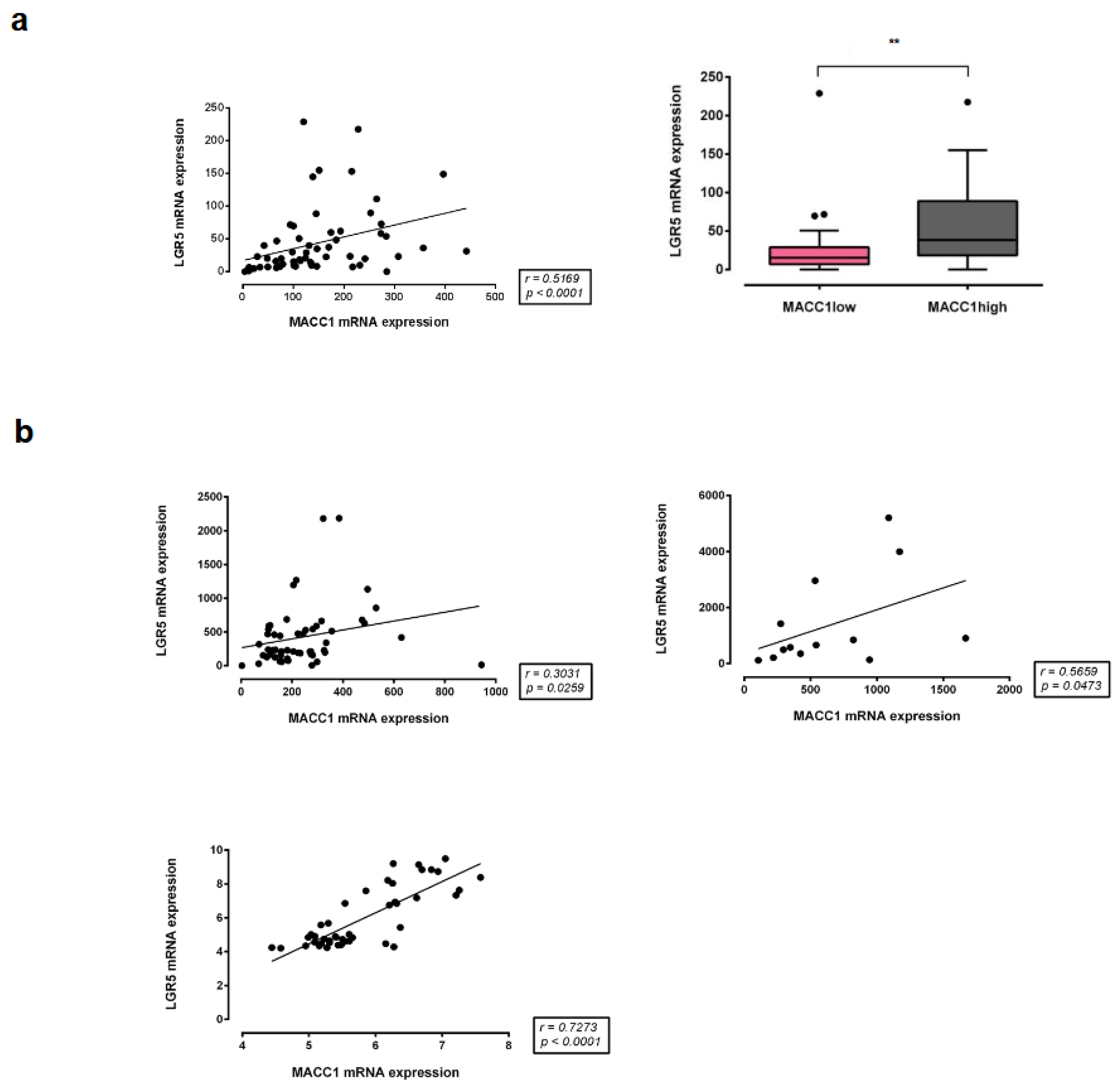
3.8. Correlation of MACC1 with LGR5 Expression in Different CRC Patient Cohorts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Stein, U.; Walther, W.; Arlt, F.; Schwabe, H.; Smith, J.; Fichtner, I.; Birchmeier, W.; Schlag, P.M. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat. Med. 2009, 15, 59–67. [Google Scholar] [CrossRef]
- Radhakrishnan, H.; Walther, W.; Zincke, F.; Kobelt, D.; Imbastari, F.; Erdem, M.; Kortüm, B.; Dahlmann, M.; Stein, U. MACC1—The first decade of a key metastasis molecule from gene discovery to clinical translation. Cancer Metastasis Rev. 2018, 37, 805–820. [Google Scholar] [CrossRef]
- Lemos, C.; Hardt, M.S.; Juneja, M.; Voss, C.; Förster, S.; Jerchow, B.; Haider, W.; Bläker, H.; Stein, U. MACC1 induces tumor progression in transgenic mice and colorectal cancer patients via increased pluripotency markers Nanog and Oct4. Clin. Cancer Res. 2016, 22, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef]
- Anderson, E.C.; Hessman, C.; Levin, T.G.; Monroe, M.M.; Wong, M.H. The role of colorectal cancer stem cells in metastatic disease and therapeutic response. Cancers 2011, 3, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Feras, J.; Khalek, A.; Gallicano, G.I.; Mishra, L. Colon Cancer Stem Cells. Gastrointest. Cancer Res. 2010, (Suppl. 1), S16–S23. [Google Scholar]
- Barker, N.; van de Wetering, M.; Clevers, H. The intestinal stem cell. Genes Dev. 2008, 22, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.Y.; Liang, S.G.; Hsueh, A.J. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol. Endocrinol. 1998, 12, 1830–1845. [Google Scholar] [CrossRef]
- Barker, N.; Tan, S.; Clevers, H. Lgr proteins in epithelial stem cell biology. Development 2013, 140, 2484–2494. [Google Scholar] [CrossRef]
- Barker, N.; Clevers, H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 2010, 138, 1681–1696. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Merlos-Suárez, A.; Barriga, F.M.; Jung, P.; Iglesias, M.; Céspedes, M.V.; Rossell, D.; Sevillano, M.; Hernando-Momblona, X.; da Silva-Diz, V.; Muñoz, P.; et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 2011, 8, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Kortüm, B.; Radhakrishnan, H.; Zincke, F.; Sachse, C.; Burock, S.; Keilholz, U.; Dahlmann, M.; Walther, W.; Dittmar, G.; Kobelt, D.; et al. Combinatorial treatment with statins and niclosamide prevents CRC dissemination by unhinging the MACC1-β-catenin-S100A4 axis of metastasis. Oncogene 2022, 41, 4446–4458. [Google Scholar] [CrossRef] [PubMed]
- Schütte, M.; Risch, T.; Abdavi-Azar, N.; Boehnke, K.; Schumacher, D.; Keil, M.; Yildiriman, R.; Jandrasits, C.; Borodina, T.; Amstislavskiy, V.; et al. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat. Commun. 2017, 8, 14262. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.L.; Schumacher, D.; Staudte, S.; Steffen, A.; Haybaeck, J.; Keilholz, U.; Schweiger, C.; Golob-Schwarzl, N.; Mumberg, D.; Henderson, D.; et al. Non-canonical hedgehog signaling is a positive regulator of the WNT pathway and is required for the survival of colon cancer stem cells. Cell Rep. 2017, 21, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.L.; Schumacher, D.; Staudte, S.; Steffen, A.; Lesche, R.; Toedling, J.; Jourdan, T.; Haybaeck, J.; Golob-Schwarzl, N.; Mumberg, D.; et al. Identification of a neural development gene expression signature in colon cancer stem cells reveals a role for EGR2 in tumorigenesis. iScience 2022, 25, 104498. [Google Scholar] [CrossRef]
- Li, C.; Zuo, D.; Yin, L.; Lin, Y.; Li, C.; Liu, T.; Wang, L. Prognostic value of MUC2 expression in colorectal cancer: A systematic review and meta-Analysis. Gastroenterol. Res. Pract. 2018, 2018, 6986870. [Google Scholar] [CrossRef]
- Li, W.X.; Xiao, H.W.; Hong, X.Q.; Niu, W.X. Predictive value of CK20 in evaluating the efficacy of treatment and prognosis after surgery for colorectal cancer. Genet. Mol. Res. 2015, 14, 5823–5829. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Q.; Jiang, B.; Chang, W.; Yuan, W.; Ma, Z.; Liu, Z.; Shu, X. Prognostic value of cancer stem cell marker ALDH1 expression in colorectal cancer: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0145164. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Chen, Y.; Liu, P.; Huang, S.; Zhang, Y.; Sun, Y.; Wu, Z.; Hu, M.; Wu, Q.; et al. ALDH1: A potential therapeutic target for cancer stem cells in solid tumors. Front. Oncol. 2022, 12, 1026278. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Ishikawa, T.; Iida, S.; Ishiguro, M.; Mogushi, K.; Mizushima, H.; Uetake, H.; Tanaka, H.; Sugihara, K. Clinical significance of osteoprotegerin expression in human colorectal cancer. Clin. Cancer Res. 2011, 17, 2444–2450. [Google Scholar] [CrossRef]
- Tsuji, S.; Midorikawa, Y.; Takahashi, T.; Yagi, K.; Takayama, T.; Yoshida, K.; Sugiyama, Y.; Aburatani, H. Potential responders to FOLFOX therapy for colorectal cancer by Random Forests analysis. Br. J. Cancer 2012, 106, 126–132. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Astolfi, A.; Nannini, M.; Paterini, P.; Piazzi, G.; Ercolani, G.; Brandi, G.; Martinelli, G.; Pession, A.; Pinna, A.D.; et al. Gene expression profiling of liver metastases from colorectal cancer as potential basis for treatment choice. Br. J. Cancer 2008, 99, 1729–1734. [Google Scholar] [CrossRef]
- Pereira, L.P.; Silva, P.; Duarte, M.; Rodrigues, L.; Duarte, C.M.; Albuquerque, C.; Serra, A.T. Targeting colorectal cancer proliferation, stemness and metastatic potential using brassicaceae extracts enriched in isothiocyanates: A 3D cell model-based study. Nutrients 2017, 9, 368. [Google Scholar] [CrossRef]
- Zeng, L.; Luo, X.; Zhang, Z.; Wang, Z.; Qian, J. Long non-coding FAM201A accelerates the tumorigenesis and progression of colorectal cancer through miR-3163/MACC1 axis. Transl. Oncol. 2022, 25, 101490. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.; Gao, H.; Li, J.; Ma, X.; Liu, X.; Gong, P.; Cui, X.; Li, Y. Forkhead-box A3 (FOXA3) represses cancer stemness and partially potentiates chemosensitivity by targeting metastasis-associated in colon cancer 1 (MACC1) signaling pathway in colorectal cancer cells. Curr. Cancer Drug Targets 2020, 21, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Blasiak, J. The role of microRNA in metastatic colorectal cancer and its significance in cancer prognosis and treatment. Acta Biochim. Pol. 2012, 59, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhu, C.; Pan, L.; Li, M. MACC1 regulates the AKT/STAT3 signaling pathway to induce migration, invasion, cancer stemness, and suppress apoptosis in cervical cancer cells. Bioengineered 2022, 13, 61–70. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Wei, W.; Liu, Y. Long noncoding RNA MACC1-AS1 promotes the stemness of nonsmall cell lung cancer cells through promoting UPF1-mediated destabilization of LATS1/2. Environ. Toxicol. 2020, 35, 998–1006. [Google Scholar] [CrossRef]
- Guo, Y.; Zhong, J.; Wu, F.; Zhan, Z. Long noncoding RNA MACC1-AS1 promotes the stemness of hepatocellular carcinoma cells by antagonizing miR-145. J. Int. Med. Res. 2020, 48, 300060520920411. [Google Scholar] [CrossRef]
- Chen, S.; Luo, X.; Wu, W.; Li, Y.; Yu, H.; Wang, Y.; Yan, J. The long non-coding RNA MACC1-AS1 promotes nasopharyngeal carcinoma cell stemness via suppressing miR-145-mediated inhibition on SMAD2/MACC1-AS1 axis. Biomed. Pharmacother. 2020, 125, 109986. [Google Scholar] [CrossRef]
- He, W.; Liang, B.; Wang, C.; Li, S.; Zhao, Y.; Huang, Q.; Liu, Z.; Yao, Z.; Wu, Q.; Liao, W.; et al. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene 2019, 38, 4637–4654. [Google Scholar] [CrossRef]
- de Sousa e Melo, F.; Kurtova, A.V.; Harnoss, J.M.; Kljavin, N.; Hoeck, J.D.; Hung, J.; Anderson, J.E.; Storm, E.E.; Modrusan, Z.; Koeppen, H.; et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 2017, 543, 676–680. [Google Scholar] [CrossRef]
- Imbastari, F.; Dahlmann, M.; Sporbert, A.; Mattioli, C.C.; Mari, T.; Scholz, F.; Timm, L.; Twamley, S.; Migotti, R.; Walther, W.; et al. MACC1 regulates clathrin-mediated endocytosis and receptor recycling of transferrin receptor and EGFR in colorectal cancer. Cell Mol. Life Sci. 2021, 78, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.C.; Rochelle, L.K.; Ray, C.; Pack, T.F.; Bock, C.B.; Lubkov, V.; Lyerly, H.K.; Waggoner, A.S.; Barak, L.S.; Caron, M.G. Inhibiting clathrin-mediated endocytosis of the leucine-rich G protein-coupled receptor-5 diminishes cell fitness. J. Biol. Chem. 2017, 292, 7208–7222. [Google Scholar] [CrossRef] [PubMed]
- Koelzer, V.H.; Herrmann, P.; Zlobec, I.; Karamitopoulou, E.; Lugli, A.; Stein, U. Heterogeneity analysis of Metastasis Associated in Colon Cancer 1 (MACC1) for survival prognosis of colorectal cancer patients: A retrospective cohort study. BMC Cancer 2015, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Sadek, S.A.; Rehim, D.M.; Fatima, S. The role of tumor budding in colorectal adenocarcinoma: Possible involvement of the intestinal cancer stem cell marker Lgr5. Indian J. Pathol. Microbiol. 2020, 63, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Basti, A.; Malhan, D.; Dumbani, M.; Dahlmann, M.; Stein, U.; Relógio, A. Core-Clock genes regulate proliferation and invasion via a reciprocal interplay with MACC1 in colorectal cancer cells. Cancers 2022, 14, 3458. [Google Scholar] [CrossRef]
- Cheng, L.T.; Tan, G.Y.T.; Chang, F.P.; Wang, C.K.; Chou, Y.C.; Hsu, P.H.; Hwang-Verslues, W.W. Core clock gene BMAL1 and RNA-binding protein MEX3A collaboratively regulate Lgr5 expression in intestinal crypt cells. Sci. Rep. 2023, 13, 17597. [Google Scholar] [CrossRef]
- Melvin, V.S.; Feng, W.; Hernandez-Lagunas, L.; Artinger, K.B.; Williams, T. A morpholino-based screen to identify novel genes involved in craniofacial morphogenesis. Dev. Dyn. 2013, 242, 817–831. [Google Scholar] [CrossRef]
- Olbertová, K.; Hrčkulák, D.; Kříž, V.; Jesionek, W.; Kubovčiak, J.; Ešner, M.; Kořínek, V.; Buchtová, M. Role of LGR5-positive mesenchymal cells in craniofacial development. Front. Cell Dev. Biol. 2022, 10, 810527. [Google Scholar] [CrossRef] [PubMed]
- Pachmayr, E.; Treese, C.; Stein, U. Underlying mechanisms for distant metastasis—Molecular biology. Visc. Med. 2017, 33, 11–20. [Google Scholar] [CrossRef] [PubMed]
- King, C.M.; Marx, O.M.; Ding, W.; Koltun, W.A.; Yochum, G.S. TCF7L1 Regulates LGR5 Expression in Colorectal Cancer Cells. Genes 2023, 14, 481. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.G.; Kim, H.S.; Chang, W.Y.; Bae, J.M.; Kim, W.H.; Kang, G.H. Expression profile of LGR5 and its prognostic significance in colorectal cancer progression. Am. J. Pathol. 2018, 188, 2236–2250. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, B.O.; Zincke, F.; Eckert, A.; Kobelt, D.; Preissner, S.; Liebeskind, J.M.; Gunkel, N.; Putzker, K.; Lewis, J.; Preissner, S.; et al. Real-world evidence for preventive effects of statins on cancer incidence: A trans-Atlantic analysis. Clin. Transl. Med. 2022, 12, e726. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Schöpe, P.C.; Lewis, J.; Putzker, K.; Uhrig, U.; Specker, E.; von Kries, J.P.; Lindemann, P.; Omran, A.; Sanchez-Ibarra, H.E.; et al. Discovery of tetrazolo-pyridazine-based small molecules as inhibitors of MACC1-driven cancer metastasis. Biomed. Pharmacother. 2023, 168, 115698. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Sato, T. Defining the role of Lgr5+ stem cells in colorectal cancer: From basic research to clinical applications. Genome Med. 2017, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, M.; Ohta, Y.; Nishikori, S.; Matano, M.; Takano, A.; Fujii, M.; Date, S.; Sugimoto, S.; Kanai, T.; Sato, T. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature 2017, 545, 187–192. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdem, M.; Lee, K.H.; Hardt, M.; Regan, J.L.; Kobelt, D.; Walther, W.; Mokrizkij, M.; Regenbrecht, C.; Stein, U. MACC1 Regulates LGR5 to Promote Cancer Stem Cell Properties in Colorectal Cancer. Cancers 2024, 16, 604. https://doi.org/10.3390/cancers16030604
Erdem M, Lee KH, Hardt M, Regan JL, Kobelt D, Walther W, Mokrizkij M, Regenbrecht C, Stein U. MACC1 Regulates LGR5 to Promote Cancer Stem Cell Properties in Colorectal Cancer. Cancers. 2024; 16(3):604. https://doi.org/10.3390/cancers16030604
Chicago/Turabian StyleErdem, Müge, Kyung Hwan Lee, Markus Hardt, Joseph L. Regan, Dennis Kobelt, Wolfgang Walther, Margarita Mokrizkij, Christian Regenbrecht, and Ulrike Stein. 2024. "MACC1 Regulates LGR5 to Promote Cancer Stem Cell Properties in Colorectal Cancer" Cancers 16, no. 3: 604. https://doi.org/10.3390/cancers16030604
APA StyleErdem, M., Lee, K. H., Hardt, M., Regan, J. L., Kobelt, D., Walther, W., Mokrizkij, M., Regenbrecht, C., & Stein, U. (2024). MACC1 Regulates LGR5 to Promote Cancer Stem Cell Properties in Colorectal Cancer. Cancers, 16(3), 604. https://doi.org/10.3390/cancers16030604







