Development and Validation of a Concise Objectifiable Risk Evaluation Score for Non-Relapse Mortality after Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Statistical Analysis
2.3. Score Development
3. Results
3.1. Patient-Specific Risk Score
3.2. Survival and Relapse
3.3. Second Validation
3.4. CORE Score Subgroup Analysis
3.5. Multivariate Analysis for NRM including Donor- and Transplant-Related Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granot, N.; Storb, R. History of hematopoietic cell transplantation: Challenges and progress. Haematologica 2020, 105, 2716–2729. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef]
- Chang, Y.J.; Zhao, X.Y.; Huang, X.J. Strategies for Enhancing and Preserving Anti-leukemia Effects Without Aggravating Graft-Versus-Host Disease. Front. Immunol. 2018, 9, 3041. [Google Scholar] [CrossRef]
- Cooper, J.P.; Storer, B.E.; Granot, N.; Gyurkocza, B.; Sorror, M.L.; Chauncey, T.R.; Shizuru, J.; Franke, G.N.; Maris, M.B.; Boyer, M.; et al. Allogeneic hematopoietic cell transplantation with non-myeloablative conditioning for patients with hematologic malignancies: Improved outcomes over two decades. Haematologica 2021, 106, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- McClune, B.L.; Weisdorf, D.J.; Pedersen, T.L.; da Silva, G.T.; Tallman, M.S.; Sierra, J.; DiPersio, J.; Keating, A.; Gale, R.P.; George, B.; et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J. Clin. Oncol. 2010, 28, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- Aoudjhane, M.; Labopin, M.; Gorin, N.C.; Shimoni, A.; Ruutu, T.; Kolb, H.J.; Frassoni, F.; Boiron, J.M.; Yin, J.L.; Finke, J.; et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: A retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005, 19, 2304–2312. [Google Scholar] [PubMed]
- Oudin, C.; Chevallier, P.; Furst, S.; Guillaume, T.; El Cheikh, J.; Delaunay, J.; Castagna, L.; Faucher, C.; Granata, A.; Devillier, R.; et al. Reduced-toxicity conditioning prior to allogeneic stem cell transplantation improves outcome in patients with myeloid malignancies. Haematologica 2014, 99, 1762–1768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aoki, J.; Kanamori, H.; Tanaka, M.; Yamasaki, S.; Fukuda, T.; Ogawa, H.; Iwato, K.; Ohashi, K.; Okumura, H.; Onizuka, M.; et al. Impact of age on outcomes of allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in elderly patients with acute myeloid leukemia. Am. J. Hematol. 2016, 91, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, A.; Shem-Tov, N.; Volchek, Y.; Danylesko, I.; Yerushalmi, R.; Nagler, A. Allo-SCT for AML and MDS with treosulfan compared with BU-based regimens: Reduced toxicity vs reduced intensity. Bone Marrow Transpl. 2012, 47, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- McLornan, D.; Szydlo, R.; Koster, L.; Chalandon, Y.; Robin, M.; Wolschke, C.; Beelen, D.; Socié, G.; Bornhäuser, M.; Angelucci, E.; et al. Myeloablative and Reduced-Intensity Conditioned Allogeneic Hematopoietic Stem Cell Transplantation in Myelofibrosis: A Retrospective Study by the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transpl. 2019, 25, 2167–2171. [Google Scholar] [CrossRef]
- Nikolousis, E.; Nagra, S.; Pearce, R.; Perry, J.; Kirkland, K.; Byrne, J.; Dignan, F.; Tholouli, E.; Gilleece, M.; Russell, N.; et al. Impact of pre-transplant co-morbidities on outcome after alemtuzumab-based reduced intensity conditioning allo-SCT in elderly patients: A British Society of Blood and Marrow Transplantation study. Bone Marrow Transpl. 2015, 50, 82–86. [Google Scholar] [CrossRef]
- Gratwohl, A.; Hermans, J.; Goldman, J.M.; Arcese, W.; Carreras, E.; Devergie, A.; Frassoni, F.; Gahrton, G.; Kolb, H.J.; Niederwieser, D.; et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet 1998, 352, 1087–1092. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Gratwohl, A.; Stern, M.; Brand, R.; Apperley, J.; Baldomero, H.; de Witte, T.; Dini, G.; Rocha, V.; Passweg, J.; Sureda, A.; et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: A retrospective analysis. Cancer 2009, 115, 4715–4726. [Google Scholar] [CrossRef]
- Armand, P.; Gibson, C.J.; Cutler, C.; Ho, V.T.; Koreth, J.; Alyea, E.P.; Ritz, J.; Sorror, M.L.; Lee, S.J.; Deeg, H.J.; et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 2012, 120, 905–913. [Google Scholar] [CrossRef]
- Muffly, L.S.; Boulukos, M.; Swanson, K.; Kocherginsky, M.; Cerro, P.D.; Schroeder, L.; Pape, L.; Extermann, M.; Van Besien, K.; Artz, A.S. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol. Blood Marrow Transpl. 2013, 19, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Luft, T.; Benner, A.; Terzer, T.; Jodele, S.; Dandoy, C.E.; Storb, R.; Kordelas, L.; Beelen, D.; Gooley, T.; Sandmaier, B.M.; et al. EASIX and mortality after allogeneic stem cell transplantation. Bone Marrow Transpl. 2020, 55, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Eikema, D.J.; Stelljes, M.; Beelen, D.; de Wreede, L.; Mufti, G.; Knelange, N.S.; Niederwieser, D.; Friis, L.S.; Ehninger, G.; et al. Optimized EBMT transplant-specific risk score in myelodysplastic syndromes after allogeneic stem-cell transplantation. Haematologica 2019, 104, 929–936. [Google Scholar] [CrossRef]
- Sorror, M.L.; Storb, R.F.; Sandmaier, B.M.; Maziarz, R.T.; Pulsipher, M.A.; Maris, M.B.; Bhatia, S.; Ostronoff, F.; Deeg, H.J.; Syrjala, K.L.; et al. Comorbidity-age index: A clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2014, 32, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, J.E.; Storer, B.E.; Armand, P.; Raimondi, R.; Gibson, C.; Rambaldi, A.; Ciceri, F.; Oneto, R.; Bruno, B.; Martin, P.J.; et al. Design and Validation of an Augmented Hematopoietic Cell Transplantation-Comorbidity Index Comprising Pretransplant Ferritin, Albumin, and Platelet Count for Prediction of Outcomes after Allogeneic Transplantation. Biol. Blood Marrow Transpl. 2015, 21, 1418–1424. [Google Scholar] [CrossRef]
- Sorror, M.L.; Storer, B.E.; Fathi, A.T.; Gerds, A.T.; Medeiros, B.C.; Shami, P.; Brunner, A.M.; Sekeres, M.A.; Mukherjee, S.; Peña, E.; et al. Development and Validation of a Novel Acute Myeloid Leukemia-Composite Model to Estimate Risks of Mortality. JAMA Oncol. 2017, 3, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Bolon, Y.T.A.R.; Allbee-Johnson, M.; Estrada-Merly, N.; Lee, S.J. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. The US Summary Slides; CIBMTR: Milwaukee, WI, USA, 2022. [Google Scholar]
- Maakaron, J.E.; Zhang, M.J.; Chen, K.; Abhyankar, S.; Bhatt, V.R.; Chhabra, S.; El Jurdi, N.; Farag, S.S.; He, F.; Juckett, M.; et al. Age is no barrier for adults undergoing HCT for AML in CR1: Contemporary CIBMTR analysis. Bone Marrow Transpl. 2022, 57, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and acute myeloid leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef] [PubMed]
- Muffly, L.; Pasquini, M.C.; Martens, M.; Brazauskas, R.; Zhu, X.; Adekola, K.; Aljurf, M.; Ballen, K.K.; Bajel, A.; Baron, F.; et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood 2017, 130, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, S.; Ziagkos, D.; de Wreede, L.C.; van Biezen, A.; Finke, J.; Platzbecker, U.; Niederwieser, D.; Einsele, H.; Bethge, W.; Schleuning, M.; et al. Allogeneic Stem Cell Transplantation for Patients Age ≥ 70 Years with Myelodysplastic Syndrome: A Retrospective Study of the MDS Subcommittee of the Chronic Malignancies Working Party of the EBMT. Biol. Blood Marrow Transpl. 2017, 23, 44–52. [Google Scholar] [CrossRef]
- Carré, M.; Porcher, R.; Finke, J.; Ehninger, G.; Koster, L.; Beelen, D.; Ganser, A.; Volin, L.; Lozano, S.; Friis, L.; et al. Role of Age and Hematopoietic Cell Transplantation-Specific Comorbidity Index in Myelodysplastic Patients Undergoing an Allotransplant: A Retrospective Study from the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Biol. Blood Marrow Transpl. 2020, 26, 451–457. [Google Scholar]
- Majhail, N.S.; Brunstein, C.G.; McAvoy, S.; DeFor, T.E.; Al-Hazzouri, A.; Setubal, D.; Arora, M.; Le, C.T.; Wagner, J.E.; Weisdorf, D.J. Does the hematopoietic cell transplantation specific comorbidity index predict transplant outcomes? A validation study in a large cohort of umbilical cord blood and matched related donor transplants. Biol. Blood Marrow Transpl. 2008, 14, 985–992. [Google Scholar] [CrossRef]
- Farina, L.; Bruno, B.; Patriarca, F.; Spina, F.; Sorasio, R.; Morelli, M.; Fanin, R.; Boccadoro, M.; Corradini, P. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia 2009, 23, 1131–1138. [Google Scholar] [CrossRef]
- Barba, P.; Piñana, J.L.; Martino, R.; Valcárcel, D.; Amorós, A.; Sureda, A.; Briones, J.; Delgado, J.; Brunet, S.; Sierra, J. Comparison of two pretransplant predictive models and a flexible HCT-CI using different cut off points to determine low-, intermediate-, and high-risk groups: The flexible HCT-CI Is the best predictor of NRM and OS in a population of patients undergoing allo-RIC. Biol. Blood Marrow Transpl. 2010, 16, 413–420. [Google Scholar]
| Factor | Category | n (%) 1 Training | n (%) 1 Validation | p Chi2 (<0.05) 2 | p NRM (<0.1) 3 Training |
|---|---|---|---|---|---|
| Patients | NS | - | |||
| Number | 617 (67) | 298 (33) | |||
| Patient age (median, range) | NS | 0.017 | |||
| <58 | 310 (50) | 156 (52) | |||
| ≥58 | 307 (50) | 142 (48) | |||
| Range | 18–79 years | 18–77 years | |||
| Disease | NS | 0.03 | |||
| AML | 245 (40) | 97 (33) | |||
| MDS | 90 (15) | 52 (17) | |||
| NHL + HD | 64 (10) | 32 (11) | |||
| ALL + other AL | 49 (8) | 25 (8) | |||
| MM + PCL | 59 (10) | 35 (12) | |||
| OMF + MDS/MPN + CML | 110 (18) | 57 (19) | |||
| Disease risk | NS | NS | |||
| Low risk | 267 (43) | 109 (37) | |||
| High risk | 350 (57) | 189 (63) | |||
| Albumin (CTC) | NS | <0.001 | |||
| ≥LLN | 369 (60) | 186 (62) | |||
| <LLN–30 g/L | 153 (25) | 69 (23) | |||
| <30–20 g/L | 89 (14) | 40 (13) | |||
| <20 g/L | 5 (1) | 3 (1) | |||
| Creatinine (CTC) | NS | 0.003 | |||
| ≤ULN | 532 (86) | 253 (85) | |||
| >ULN–1.5xULN | 68 (11) | 35 (12) | |||
| >1.5–3xULN | 12 (2) | 7 (2) | |||
| >3–6xULN | 4 (1) | 1 (0) | |||
| >6xULN | 0 (0) | 1 (0) | |||
| Left ventricular ejection fraction (CTC) | NS | 0.037 | |||
| >50% | 541 (91) | 272 (94) | |||
| 50–40% | 43 (7) | 16 (6) | |||
| 39–20% | 11 (2) | 1 (0) | |||
| <20% | 1 (0) | 0 (0) | |||
| Vital capacity (CTC) | NS | 0.005 | |||
| >90% | 248 (44) | 126 (46) | |||
| 90–75% | 209 (37) | 101 (37) | |||
| 75–50% | 96 (17) | 43 (16) | |||
| <50% | 13 (2) | 2 (1) | |||
| FEV1 (CTC) | NS | 0.002 | |||
| >99% | 128 (22) | 72 (26) | |||
| 99–70% | 343 (60) | 157 (56) | |||
| 69–60% | 51 (9) | 29 (10) | |||
| 59–50% | 25 (4) | 14 (5) | |||
| <49% | 25 (4) | 6 (2) | |||
| CRP (median) | NS | 0.005 | |||
| <6 mg/L | 319 (52) | 164 (55) | |||
| ≥6 mg/L | 298 (48) | 134 (45) |
| Factor | HR (95% CI) | Weighting |
|---|---|---|
| Serum albumin | ||
| Serum albumin ≥ LLN | reference | 0 |
| Serum albumin < LLN–30 g/L | 1.29 (0.77–2.15) | 1 |
| Serum albumin < 30–20 g/L | 1.60 (0.91–2.81) | 1 |
| Serum albumin < 20 g/L | 3.47 (0.42–28.53) | 3 |
| Serum creatinine | ||
| Serum creatinine ≤ ULN | reference | 0 |
| Serum creatinine > ULN–1.5xULN | 1.86 (1.09–3.17) | 1 |
| Serum creatinine > 1.5xULN | 2.21 (0.66–7.35) | 2 |
| Left ventricular ejection fraction (LVEF) | ||
| LVEF > 50% | reference | 0 |
| LVEF 50–40% | 1.62 (0.77–3.4) | 1 |
| LVEF < 40% | 1.63 (0.52–5.13) | 1 |
| Patient age | ||
| Patient age < 60 years | reference | 0 |
| Patient age 60–69 years | 1.56 (0.95–2.55) | 1 |
| Patient age ≥ 70 years | 2.14 (1.23–3.75) | 2 |
| Forced expiratory volume (FEV1) | ||
| FEV1 ≥ 60% | reference | 0 |
| FEV1 < 60% | 1.45 (0.72–2.91) | 1 |
| Vital capacity (VC) | ||
| VC ≥ 75% | reference | 0 |
| VC < 75% | 1.43 (0.79–2.59) | 1 |
| C-reactive protein (CRP) | ||
| CRP < 6 mg/L | reference | 0 |
| CRP ≥ 6 mg/L | 1.25 (0.8–1.96) | 1 |
| Score | Training Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pat. (%) | NRM | OS | Relapse | |||||||
| HR (95% CI) | p (%) | 2-y (%) | HR (95% CI) | p (%) | 2-y (%) | HR (95% CI) | p (%) | 2-y (%) | ||
| Total | <0.001 | <0.001 | 0.243 | |||||||
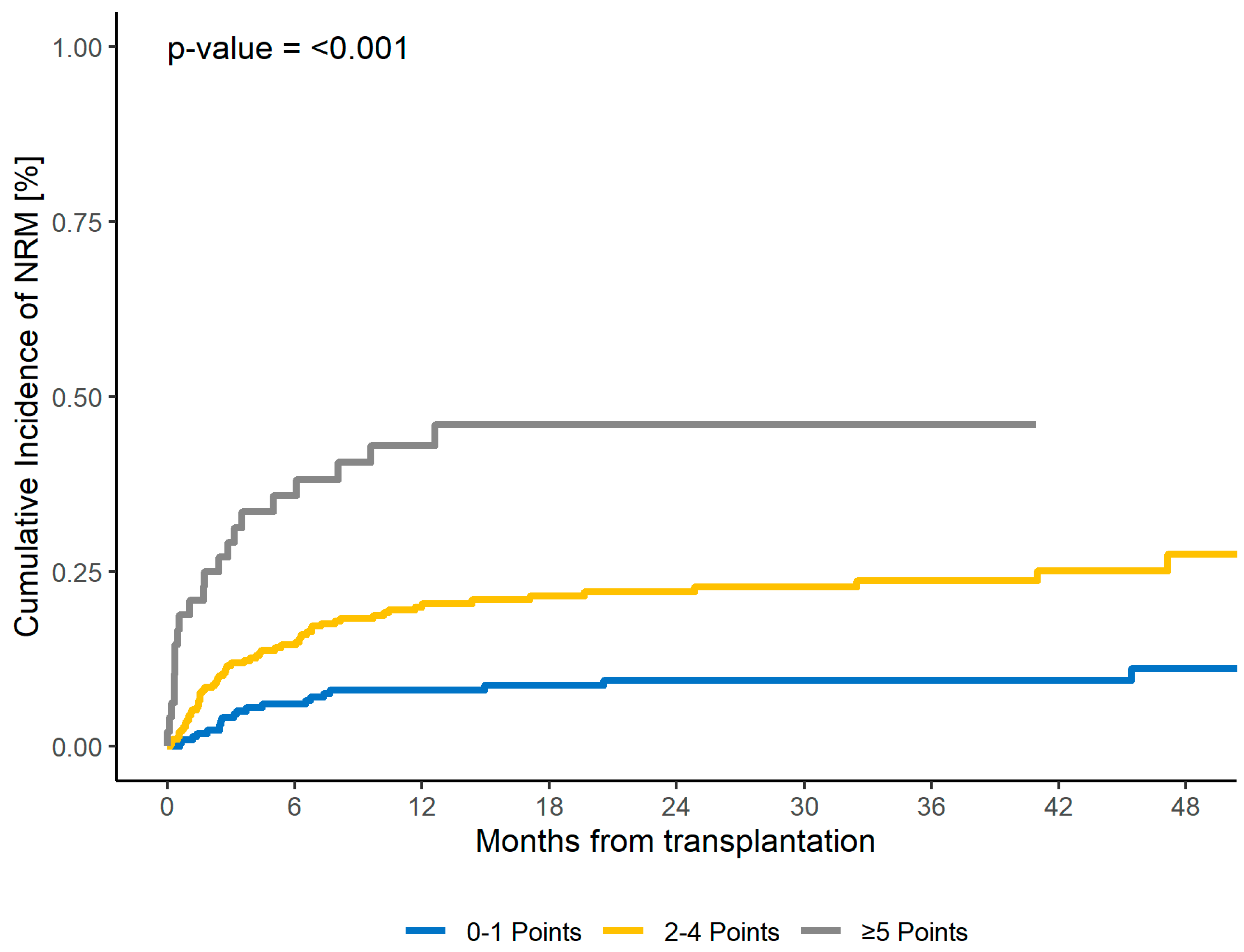
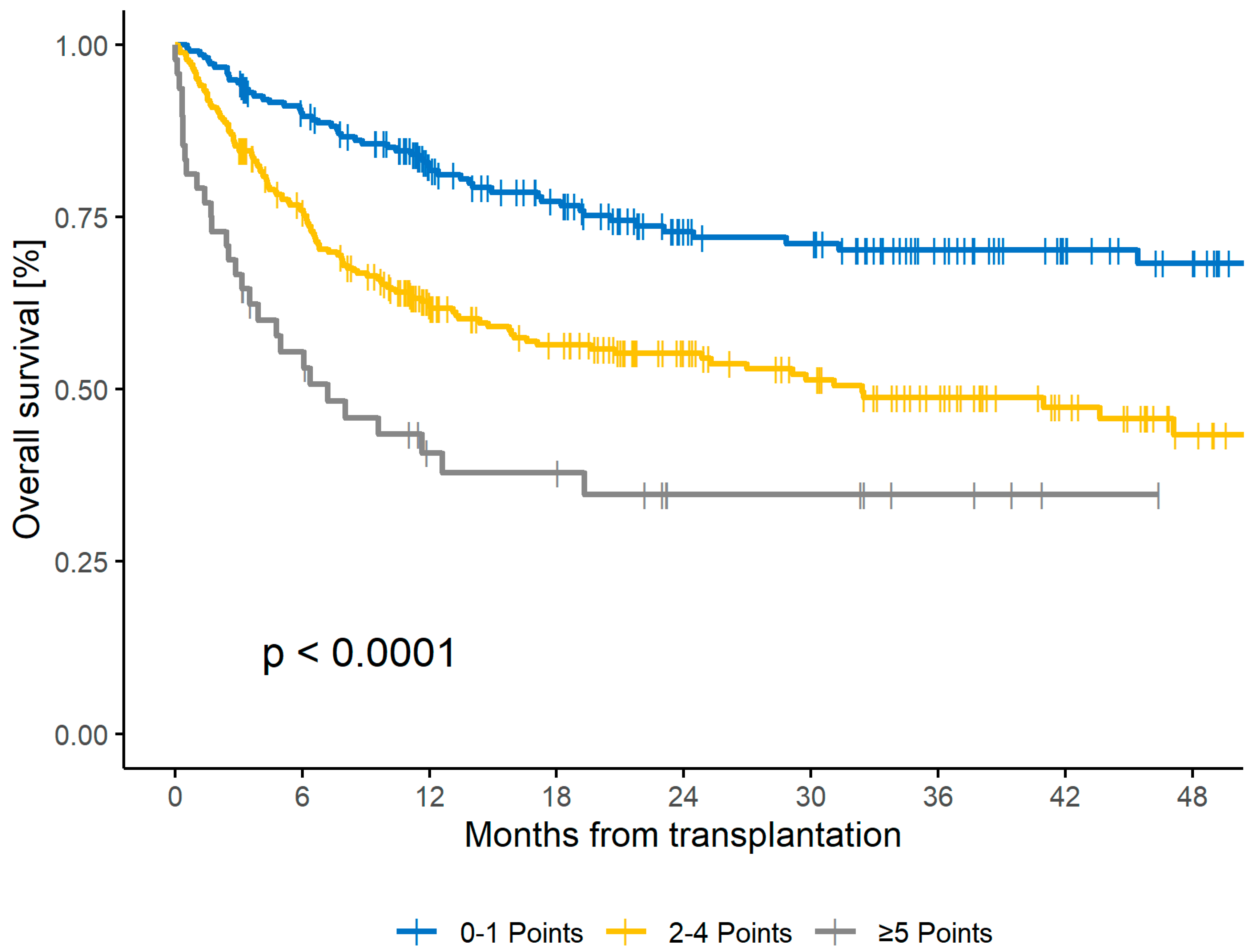
| 0–1 | 39 | reference | 9 | reference | 73 | reference | 27 | |||
| 2–4 | 52 | 2.63 (1.6–4.3) | <0.001 | 22 | 2.24 (1.6–3.1) | <0.001 | 55 | 1.11 (0.8–1.5) | 0.51 | 31 |
| ≥5 | 9 | 6.75 (3.6–12.6) | <0.001 | 46 | 4.19 (2.7–6.6) | <0.001 | 35 | 0.6 (0.3–1.2) | 0.18 | 16 |
| Score | First Validation Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pat. (%) | NRM | OS | Relapse | |||||||
| HR (95% CI) | p (%) | 2-y (%) | HR (95% CI) | p (%) | 2-y (%) | HR (95% CI) | p (%) | 2-y (%) | ||
| Total | <0.001 | <0.001 | 0.571 | |||||||
| 0–1 | 44 | reference | 13 | reference | 73 | reference | 28 | |||
| 2–4 | 49 | 2.03 (1.1–3.8) | 0.03 | 24 | 2.24 (1.5–3.4) | <0.001 | 47 | 1.28 (0.8–2) | 0.29 | 34 |
| ≥5 | 7 | 5.54 (2.2–13.8) | <0.001 | 48 | 5.96 (3.2–11) | <0.001 | 18 | 1.07 (0.5–2.5) | 0.87 | 20 |
| Score | Second Validation Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pat. (%) | NRM | OS | Relapse | |||||||
| HR (95% CI) | p (%) | 2-y (%) | HR (95% CI) | p (%) | 2-y (%) | HR (95% CI) | p (%) | 2-y (%) | ||
| Total | 0.002 | <0.001 | 0.206 | |||||||
| 0–1 | 42 | reference | 7 | reference | 81 | reference | 25 | |||
| 2–4 | 50 | 3.08 (1.3–7.6) | 0.014 | 20 | 3.01 (1.7–5.4) | <0.001 | 55 | 1.61 (0.9–2.9) | 0.11 | 31 |
| ≥5 | 8 | 6.49 (2.1–19.7) | 0.001 | 43 * | 5.87 (2.6–13.1) | <0.001 | 30 * | 1.95 (0.7–5.7) | 0.22 | 31 * |
| n | c-Statistics (SE) | p-Value | |
|---|---|---|---|
| NRM | |||
| CORE, first validation cohort | 267 | 0.63 (0.044) | 0.003 |
| CORE, second validation cohort | 205 | 0.666 (0.05) | 0.001 |
| CORE, all cohorts | 1025 | 0.648 (0.022) | <0.001 |
| CORE and ECOG combined, all cohorts | 937 | 0.675 (0.024) | <0.001 |
| HCT-CI, second validation cohort | 205 | 0.431 (0.057) | 0.223 |
| OS | |||
| CORE, first validation cohort | 267 | 0.659 (0.034) | <0.001 |
| CORE, second validation cohort | 205 | 0.675 (0.039) | <0.001 |
| CORE, all cohorts | 1025 | 0.644 (0.018) | <0.001 |
| CORE and ECOG combined, all cohorts | 937 | 0.669 (0.018) | <0.001 |
| HCT-CI, second validation cohort | 205 | 0.535 (0.042) | 0.411 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weise, G.; Massoud, R.; Krause, R.; Heidenreich, S.; Janson, D.; Klyuchnikov, E.; Wolschke, C.; Zeck, G.; Kröger, N.; Ayuk, F. Development and Validation of a Concise Objectifiable Risk Evaluation Score for Non-Relapse Mortality after Allogeneic Hematopoietic Stem Cell Transplantation. Cancers 2024, 16, 515. https://doi.org/10.3390/cancers16030515
Weise G, Massoud R, Krause R, Heidenreich S, Janson D, Klyuchnikov E, Wolschke C, Zeck G, Kröger N, Ayuk F. Development and Validation of a Concise Objectifiable Risk Evaluation Score for Non-Relapse Mortality after Allogeneic Hematopoietic Stem Cell Transplantation. Cancers. 2024; 16(3):515. https://doi.org/10.3390/cancers16030515
Chicago/Turabian StyleWeise, Gunnar, Radwan Massoud, Rolf Krause, Silke Heidenreich, Dietlinde Janson, Evgeny Klyuchnikov, Christine Wolschke, Gaby Zeck, Nicolaus Kröger, and Francis Ayuk. 2024. "Development and Validation of a Concise Objectifiable Risk Evaluation Score for Non-Relapse Mortality after Allogeneic Hematopoietic Stem Cell Transplantation" Cancers 16, no. 3: 515. https://doi.org/10.3390/cancers16030515
APA StyleWeise, G., Massoud, R., Krause, R., Heidenreich, S., Janson, D., Klyuchnikov, E., Wolschke, C., Zeck, G., Kröger, N., & Ayuk, F. (2024). Development and Validation of a Concise Objectifiable Risk Evaluation Score for Non-Relapse Mortality after Allogeneic Hematopoietic Stem Cell Transplantation. Cancers, 16(3), 515. https://doi.org/10.3390/cancers16030515






