Anatomical and Biological Considerations to Determine Resectability in Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Diagnostic Workup for Pancreatic Cancer
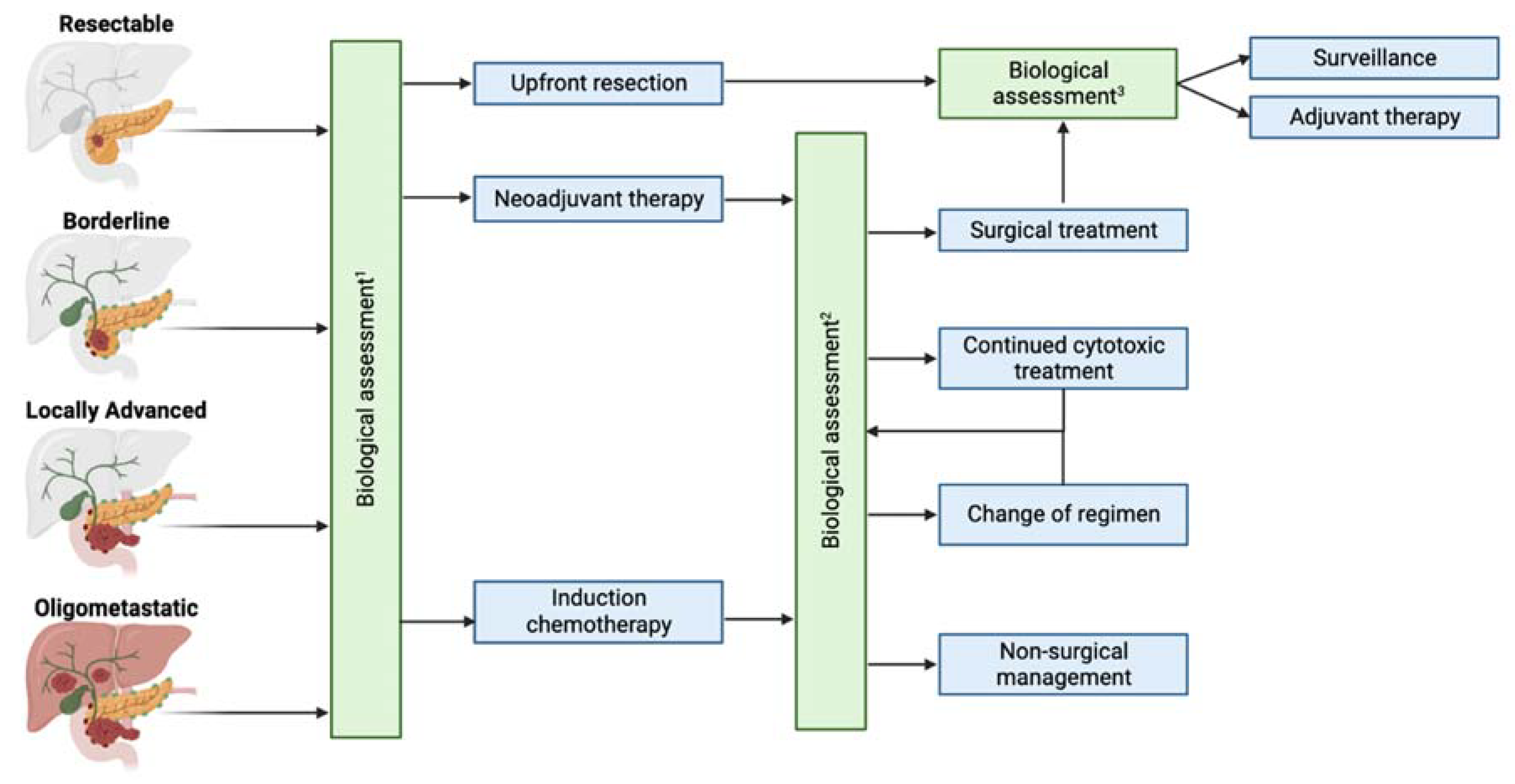
3. Anatomical Assessment of Resectability and Implications for Treatment
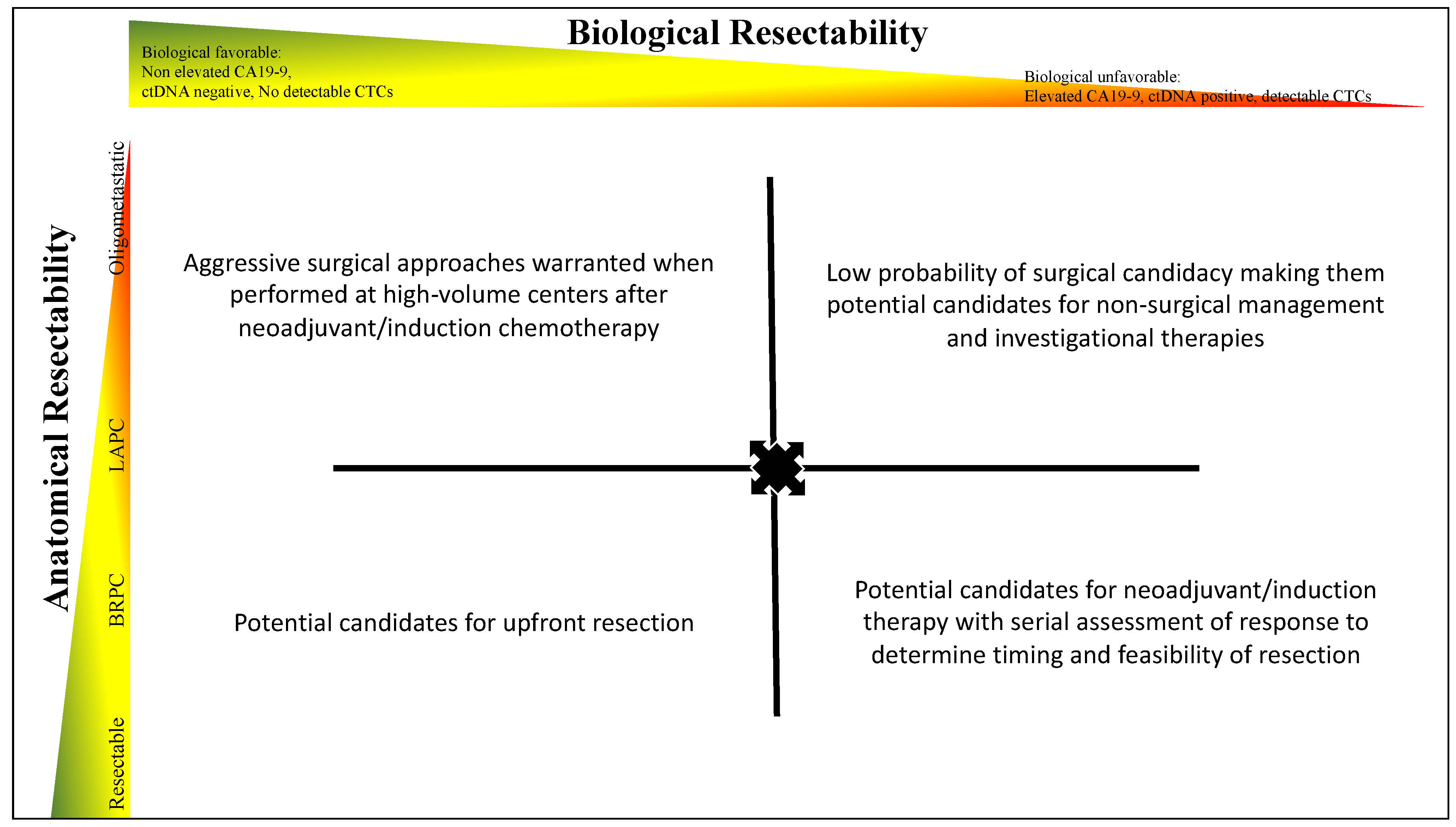
4. Biological Assessment of Resectability
5. Current Limitations of Assessment of Anatomical and Biological Resectability
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Buchler, M.; Charnley, R.M.; et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Rompen, I.F.; Levine, J.; Habib, J.R.; Sereni, E.; Mughal, N.; Hewitt, D.B.; Sacks, G.D.; Welling, T.H.; Simeone, D.M.; Kaplan, B.; et al. Progression of Site-Specific Recurrence of Pancreatic Cancer and Implications for Treatment. Ann. Surg. 2023; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.P.; Psarelli, E.E.; Jackson, R.; Ghaneh, P.; Halloran, C.M.; Palmer, D.H.; Campbell, F.; Valle, J.W.; Faluyi, O.; O’Reilly, D.A.; et al. Patterns of Recurrence after Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg. 2019, 154, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, E.G.; Chiaro, M.D.; Tempero, M.A.; Malafa, M.P.; Benson, A.B.; Cardin, D.B.; Christensen, J.A.; Chung, V.; Czito, B.; Dillhoff, M.; et al. Ampullary Adenocarcinoma, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2023, 21, 753–782. [Google Scholar] [CrossRef]
- Kinny-Köster, B.; van Oosten, F.; Habib, J.R.; Javed, A.A.; Cameron, J.L.; Lafaro, K.J.; Burkhart, R.A.; Burns, W.R.; He, J.; Fishman, E.K.; et al. Mesoportal bypass, interposition graft, and mesocaval shunt: Surgical strategies to overcome superior mesenteric vein involvement in pancreatic cancer. Surgery 2020, 168, 1048–1055. [Google Scholar] [CrossRef]
- Kinny-Köster, B.; Habib, J.R.; van Oosten, F.; Javed, A.A.; Cameron, J.L.; Burkhart, R.A.; Burns, W.R.; He, J.; Wolfgang, C.L. Conduits in Vascular Pancreatic Surgery: Analysis of Clinical Outcomes, Operative Techniques, and Graft Performance. Ann. Surg. 2023, 278, e94–e104. [Google Scholar] [CrossRef]
- Loos, M.; Kester, T.; Klaiber, U.; Mihaljevic, A.L.; Mehrabi, A.; Müller-Stich, B.M.; Diener, M.K.; Schneider, M.A.; Berchtold, C.; Hinz, U.; et al. Arterial Resection in Pancreatic Cancer Surgery: Effective After a Learning Curve. Ann. Surg. 2022, 275, 759–768. [Google Scholar] [CrossRef]
- Hackert, T.; Sachsenmaier, M.; Hinz, U.; Schneider, L.; Michalski, C.W.; Springfeld, C.; Strobel, O.; Jäger, D.; Ulrich, A.; Büchler, M.W. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy with Folfirinox Results in Resectability in 60% of the Patients. Ann. Surg. 2016, 264, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Gemenetzis, G.; Groot, V.P.; Blair, A.B.; Laheru, D.A.; Zheng, L.; Narang, A.K.; Fishman, E.K.; Hruban, R.H.; Yu, J.; Burkhart, R.A.; et al. Survival in Locally Advanced Pancreatic Cancer after Neoadjuvant Therapy and Surgical Resection. Ann. Surg. 2019, 270, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.A.; Wright, M.J.; Siddique, A.; Blair, A.B.; Ding, D.; Burkhart, R.A.; Makary, M.; Cameron, J.L.; Narang, A.; Herman, J.; et al. Outcome of Patients with Borderline Resectable Pancreatic Cancer in the Contemporary Era of Neoadjuvant Chemotherapy. J. Gastrointest. Surg. 2019, 23, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Oba, A.; Del Chiaro, M.; Fujii, T.; Okano, K.; Stoop, T.F.; Wu, Y.H.A.; Maekawa, A.; Yoshida, Y.; Hashimoto, D.; Sugawara, T.; et al. “Conversion surgery” for locally advanced pancreatic cancer: A position paper by the study group at the joint meeting of the International Association of Pancreatology (IAP) & Japan Pancreas Society (JPS) 2022. Pancreatology 2023, 23, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.-W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef]
- Oba, A.; Del Chiaro, M.; Satoi, S.; Kim, S.W.; Takahashi, H.; Yu, J.; Hioki, M.; Tanaka, M.; Kato, Y.; Ariake, K.; et al. New criteria of resectability for pancreatic cancer: A position paper by the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS). J. Hepatobiliary Pancreat. Sci. 2022, 29, 725–731. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, J.M. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J. Gastroenterol. 2014, 20, 7864–7877. [Google Scholar] [CrossRef]
- Tanaka, M.; Mihaljevic, A.L.; Probst, P.; Heckler, M.; Klaiber, U.; Heger, U.; Büchler, M.W.; Hackert, T. Meta-analysis of recurrence pattern after resection for pancreatic cancer. Br. J. Surg. 2019, 106, 1590–1601. [Google Scholar] [CrossRef]
- Søreide, K.; Rangelova, E.; Dopazo, C.; Mieog, S.; Stättner, S. Pancreatic cancer. Eur. J. Surg. Oncol. 2023, 49, 521–525. [Google Scholar] [CrossRef]
- Habib, J.R.; Kinny-Köster, B.; van Oosten, F.; Javed, A.A.; Cameron, J.L.; Lafaro, K.J.; Burkhart, R.A.; Burns, W.R.; He, J.; Thompson, E.D.; et al. Periadventitial dissection of the superior mesenteric artery for locally advanced pancreatic cancer: Surgical planning with the “halo sign” and “string sign”. Surgery 2021, 169, 1026–1031. [Google Scholar] [CrossRef]
- Hackert, T.; Strobel, O.; Michalski, C.W.; Mihaljevic, A.L.; Mehrabi, A.; Müller-Stich, B.; Berchtold, C.; Ulrich, A.; Büchler, M.W. The TRIANGLE operation—Radical surgery after neoadjuvant treatment for advanced pancreatic cancer: A single arm observational study. HPB 2017, 19, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Werner, J.; Weitz, J.; Schmidt, J.; Büchler, M.W. Uncinate process first--a novel approach for pancreatic head resection. Langenbecks Arch. Surg. 2010, 395, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Rahbari, N.; Koch, M.; Büchler, M.W. The “artery first” approach for resection of pancreatic head cancer. J. Am. Coll. Surg. 2010, 210, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.C.; Müller, B.P.; Hackert, T. Contemporary artery-first approaches in pancreatoduodenectomy. Br. J. Surg. 2023, 110, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.; Fishman, E.K. Pancreatic ductal adenocarcinoma staging: A narrative review of radiologic techniques and advances. Int. J. Surg. 2023, 10–1097. [Google Scholar] [CrossRef]
- Javed, A.A.; Young, R.W.C.; Habib, J.R.; Kinny-Köster, B.; Cohen, S.M.; Fishman, E.K.; Wolfgang, C.L. Cinematic Rendering: Novel Tool for Improving Pancreatic Cancer Surgical Planning. Curr. Probl. Diagn. Radiol. 2022, 51, 878–883. [Google Scholar] [CrossRef]
- Asbun, H.J.; Conlon, K.; Fernandez-Cruz, L.; Friess, H.; Shrikhande, S.V.; Adham, M.; Bassi, C.; Bockhorn, M.; Büchler, M.; Charnley, R.M.; et al. When to perform a pancreatoduodenectomy in the absence of positive histology? A consensus statement by the International Study Group of Pancreatic Surgery. Surgery 2014, 155, 887–892. [Google Scholar] [CrossRef]
- van Veldhuisen, E.; van den Oord, C.; Brada, L.J.; Walma, M.S.; Vogel, J.A.; Wilmink, J.W.; del Chiaro, M.; van Lienden, K.P.; Meijerink, M.R.; van Tienhoven, G.; et al. Locally Advanced Pancreatic Cancer: Work-Up, Staging, and Local Intervention Strategies. Cancers 2019, 11, 976. [Google Scholar] [CrossRef]
- Allen, V.B.; Gurusamy, K.S.; Takwoingi, Y.; Kalia, A.; Davidson, B.R. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst. Rev. 2016, 7, Cd009323. [Google Scholar] [CrossRef]
- van Dongen, J.C.; Versteijne, E.; Bonsing, B.A.; Mieog, J.S.D.; de Hingh, I.; Festen, S.; Patijn, G.A.; van Dam, R.; van der Harst, E.; Wijsman, J.H.; et al. The yield of staging laparoscopy for resectable and borderline resectable pancreatic cancer in the PREOPANC randomized controlled trial. Eur. J. Surg. Oncol. 2023, 49, 811–817. [Google Scholar] [CrossRef]
- Michiels, N.; Doppenberg, D.; Groen, J.V.; van Veldhuisen, E.; Bonsing, B.A.; Busch, O.R.; Crobach, A.; van Delden, O.M.; van Dieren, S.; Farina, A.; et al. Intraoperative Ultrasound During Surgical Exploration in Patients with Pancreatic Cancer and Vascular Involvement (ULTRAPANC): A Prospective Multicenter Study. Ann. Surg. Oncol. 2023, 30, 3455–3463. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Callery, M.P.; Chang, K.J.; Fishman, E.K.; Talamonti, M.S.; William Traverso, L.; Linehan, D.C. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann. Surg. Oncol. 2009, 16, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.; Shi, Q.; Ahmad, S.A.; Herman, J.M.; Marsh Rde, W.; Collisson, E.; Schwartz, L.; Frankel, W.; Martin, R.; Conway, W.; et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016, 151, e161137. [Google Scholar] [CrossRef] [PubMed]
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.; Evans, D.B.; Wolff, R.A. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Springfeld, C.; Ferrone, C.R.; Katz, M.H.G.; Philip, P.A.; Hong, T.S.; Hackert, T.; Büchler, M.W.; Neoptolemos, J. Neoadjuvant therapy for pancreatic cancer. Nat. Rev. Clin. Oncol. 2023, 20, 318–337. [Google Scholar] [CrossRef]
- Wu, W.; He, J.; Cameron, J.L.; Makary, M.; Soares, K.; Ahuja, N.; Rezaee, N.; Herman, J.; Zheng, L.; Laheru, D.; et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 2873–2881. [Google Scholar] [CrossRef]
- Versteijne, E.; Vogel, J.A.; Besselink, M.G.; Busch, O.R.C.; Wilmink, J.W.; Daams, J.G.; van Eijck, C.H.J.; Groot Koerkamp, B.; Rasch, C.R.N.; van Tienhoven, G.; et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br. J. Surg. 2018, 105, 946–958. [Google Scholar] [CrossRef]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar] [CrossRef]
- Seufferlein, T.; Uhl, W.; Kornmann, M.; Algül, H.; Friess, H.; König, A.; Ghadimi, M.; Gallmeier, E.; Bartsch, D.K.; Lutz, M.P.; et al. Perioperative or only adjuvant gemcitabine plus nab-paclitaxel for resectable pancreatic cancer (NEONAX)—A randomized phase II trial of the AIO pancreatic cancer group. Ann. Oncol. 2023, 34, 91–100. [Google Scholar] [CrossRef]
- Schwarz, L.; Bachet, J.-B.; Meurisse, A.; Bouché, O.; Assenat, E.; Piessen, G.; Terrebonne, E.; Hammel, P.; Regenet, N.; Turco, C.; et al. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy: A multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). J. Clin. Oncol. 2022, 40 (Suppl. S16), 4134. [Google Scholar] [CrossRef]
- Labori, K.J.; Bratlie, S.O.; Biörserud, C.; Björnsson, B.; Bringeland, E.; Elander, N.; Grønbech, J.E.; Haux, J.; Hemmingsson, O.; Nymo, L.; et al. Short-course neoadjuvant FOLFIRINOX versus upfront surgery for resectable pancreatic head cancer: A multicenter randomized phase-II trial (NORPACT-1). J. Clin. Oncol. 2023, 41 (Suppl. S17), LBA4005. [Google Scholar] [CrossRef]
- Ghaneh, P.; Palmer, D.; Cicconi, S.; Jackson, R.; Halloran, C.M.; Rawcliffe, C.; Sripadam, R.; Mukherjee, S.; Soonawalla, Z.; Wadsley, J.; et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): A four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Siveke, J.T.; Algül, H.; Goekkurt, E.; Siegler, G.; Martens, U.; Waldschmidt, D.; Pelzer, U.; Fuchs, M.; Kullmann, F.; et al. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): A multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 128–138. [Google Scholar] [CrossRef]
- Fietkau, R.; Ghadimi, M.; Grützmann, R.; Wittel, U.A.; Jacobasch, L.; Uhl, W.; Croner, R.S.; Bechstein, W.O.; Neumann, U.P.; Waldschmidt, D.; et al. Randomized phase III trial of induction chemotherapy followed by chemoradiotherapy or chemotherapy alone for nonresectable locally advanced pancreatic cancer: First results of the CONKO-007 trial. J. Clin. Oncol. 2022, 40 (Suppl. S16), 4008. [Google Scholar] [CrossRef]
- Brada, L.J.H.; Daamen, L.A.; Magermans, L.G.; Walma, M.S.; Latifi, D.; van Dam, R.M.; de Hingh, I.H.; Liem, M.S.L.; de Meijer, V.E.; Patijn, G.A.; et al. Survival Benefit Associated with Resection of Locally Advanced Pancreatic Cancer after Upfront FOLFIRINOX Versus FOLFIRINOX Only: Multicenter Propensity Score-matched Analysis. Ann. Surg. 2021, 274, 729–735. [Google Scholar] [CrossRef]
- Ushida, Y.; Inoue, Y.; Oba, A.; Mie, T.; Ito, H.; Ono, Y.; Sato, T.; Ozaka, M.; Sasaki, T.; Saiura, A.; et al. Optimizing Indications for Conversion Surgery Based on Analysis of 454 Consecutive Japanese Cases with Unresectable Pancreatic Cancer Who Received Modified FOLFIRINOX or Gemcitabine Plus Nab-paclitaxel: A Single-Center Retrospective Study. Ann. Surg. Oncol. 2022, 29, 5038–5050. [Google Scholar] [CrossRef]
- Kaslow, S.R.; Sacks, G.D.; Berman, R.S.; Lee, A.Y.; Correa-Gallego, C. Natural History of Stage IV Pancreatic Cancer. Identifying Survival Benchmarks for Curative-intent Resection in Patients with Synchronous Liver-only Metastases. Ann. Surg. 2022, 278, e798–e804. [Google Scholar] [CrossRef]
- Nagai, M.; Wright, M.J.; Ding, D.; Thompson, E.D.; Javed, A.A.; Weiss, M.J.; Hruban, R.H.; Yu, J.; Burkhart, R.A.; He, J.; et al. Oncologic resection of pancreatic cancer with isolated liver metastasis: Favorable outcomes in select patients. J. Hepatobiliary Pancreat Sci. 2023, 30, 1025–1035. [Google Scholar] [CrossRef]
- Hackert, T.; Niesen, W.; Hinz, U.; Tjaden, C.; Strobel, O.; Ulrich, A.; Michalski, C.W.; Büchler, M.W. Radical surgery of oligometastatic pancreatic cancer. Eur. J. Surg. Oncol. 2017, 43, 358–363. [Google Scholar] [CrossRef]
- Gebauer, F.; Damanakis, A.I.; Popp, F.; Quaas, A.; Kütting, F.; Lutz, K.; Held, S.; Deuß, B.; Göser, T.; Waldschmidt, D.; et al. Study protocol of an open-label, single arm phase II trial investigating the efficacy, safety and quality of life of neoadjuvant chemotherapy with liposomal irinotecan combined with Oxaliplatin and 5-fluorouracil/Folinic acid followed by curative surgical resection in patients with hepatic Oligometastatic adenocarcinoma of the pancreas (HOLIPANC). BMC Cancer 2021, 21, 1239. [Google Scholar] [CrossRef]
- Ilmer, M.; Schiergens, T.S.; Renz, B.W.; Schneider, C.; Sargut, M.; Waligora, R.; Weniger, M.; Hartwig, W.; Ceyhan, G.O.; Friess, H.; et al. Oligometastatic pulmonary metastasis in pancreatic cancer patients: Safety and outcome of resection. Surg. Oncol. 2019, 31, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, R.; Michalski, C.W.; Liu, B.; Liao, Q.; Kleeff, J. Surgery for synchronous and metachronous single-organ metastasis of pancreatic cancer: A SEER database analysis and systematic literature review. Sci. Rep. 2020, 10, 4444. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.M.; Kirsch, M.J.; Zhuang, Y.; Meguid, C.L.; Sugawara, T.; Colborn, K.L.; Messersmith, W.; Lieu, C.; Gleisner, A.L.; Del Chiaro, M.; et al. Pulmonary metastasectomy is associated with survival after lung-only recurrence in pancreatic cancer. Surgery 2023, 174, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; Blair, A.B.; Gemenetzis, G.; Ding, D.; Burkhart, R.A.; van Oosten, A.F.; Molenaar, I.Q.; Cameron, J.L.; Weiss, M.J.; Yang, S.C.; et al. Isolated pulmonary recurrence after resection of pancreatic cancer: The effect of patient factors and treatment modalities on survival. HPB 2019, 21, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Rompen, I.F.; Merz, D.C.; Alhalabi, K.T.; Klotz, R.; Kalkum, E.; Pausch, T.M.; Strothmann, H.; Probst, P. Perioperative Drug Treatment in Pancreatic Surgery-A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1750. [Google Scholar] [CrossRef] [PubMed]
- Rompen, I.F.; Nienhuser, H.; Crnovrsanin, N.; Musa, J.; Haag, G.M.; Longerich, T.; Fiedler, T.; Muller-Stich, B.P.; Sisic, L.; Billeter, A.T. Clinical Characteristics and Oncological Outcomes of Surgically Treated Early-Onset Gastric Adenocarcinoma—A Retrospective Cohort Study. J. Cancer 2023, 14, 1470–1478. [Google Scholar] [CrossRef]
- Katz, M.H.; Pisters, P.W.; Evans, D.B.; Sun, C.C.; Lee, J.E.; Fleming, J.B.; Vauthey, J.N.; Abdalla, E.K.; Crane, C.H.; Wolff, R.A.; et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J. Am. Coll. Surg. 2008, 206, 833–846; discussion 846–838. [Google Scholar] [CrossRef]
- Morales-Oyarvide, V.; Rubinson, D.A.; Dunne, R.F.; Kozak, M.M.; Bui, J.L.; Yuan, C.; Qian, Z.R.; Babic, A.; Da Silva, A.; Nowak, J.A.; et al. Lymph node metastases in resected pancreatic ductal adenocarcinoma: Predictors of disease recurrence and survival. Br. J. Cancer 2017, 117, 1874–1882. [Google Scholar] [CrossRef]
- Ivey, G.D.; Shoucair, S.; Delitto, D.J.; Habib, J.R.; Kinny-Köster, B.; Shubert, C.R.; Lafaro, K.J.; Cameron, J.L.; Burns, W.R.; Burkhart, R.A.; et al. Postoperative Chemotherapy is Associated with Improved Survival in Patients with Node-Positive Pancreatic Ductal Adenocarcinoma after Neoadjuvant Therapy. World J. Surg. 2022, 46, 2751–2759. [Google Scholar] [CrossRef]
- van Roessel, S.; van Veldhuisen, E.; Klompmaker, S.; Janssen, Q.P.; Abu Hilal, M.; Alseidi, A.; Balduzzi, A.; Balzano, G.; Bassi, C.; Berrevoet, F.; et al. Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment. JAMA Oncol. 2020, 6, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Kinny-Köster, B.; Habib, J.R.; Wolfgang, C.L.; He, J.; Javed, A.A. Favorable tumor biology in locally advanced pancreatic cancer—Beyond CA19-9. J. Gastrointest. Oncol. 2021, 12, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Yamada, S.; Tashiro, M.; Sonohara, F.; Takami, H.; Hayashi, M.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Nakayama, G.; et al. Biological and conditional factors should be included when defining criteria for resectability for patients with pancreatic cancer. HPB 2019, 21, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Doppenberg, D.; van Dam, J.L.; Han, Y.; Bonsing, B.A.; Busch, O.R.; Festen, S.; van der Harst, E.; de Hingh, I.H.; Homs, M.Y.V.; Kwon, W.; et al. Predictive value of baseline serum carbohydrate antigen 19-9 level on treatment effect of neoadjuvant chemoradiotherapy in patients with resectable and borderline resectable pancreatic cancer in two randomized trials. Br. J. Surg. 2023, 110, 1374–1380. [Google Scholar] [CrossRef]
- Schorn, S.; Demir, I.E.; Samm, N.; Scheufele, F.; Calavrezos, L.; Sargut, M.; Schirren, R.M.; Friess, H.; Ceyhan, G.O. Meta-analysis of the impact of neoadjuvant therapy on patterns of recurrence in pancreatic ductal adenocarcinoma. BJS Open 2018, 2, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hartlapp, I.; Valta-Seufzer, D.; Siveke, J.T.; Algül, H.; Goekkurt, E.; Siegler, G.; Martens, U.M.; Waldschmidt, D.; Pelzer, U.; Fuchs, M.; et al. Prognostic and predictive value of CA 19-9 in locally advanced pancreatic cancer treated with multiagent induction chemotherapy: Results from a prospective, multicenter phase II trial (NEOLAP-AIO-PAK-0113). ESMO Open 2022, 7, 100552. [Google Scholar] [CrossRef]
- Heger, U.; Sun, H.; Hinz, U.; Klaiber, U.; Tanaka, M.; Liu, B.; Sachsenmaier, M.; Springfeld, C.; Michalski, C.W.; Büchler, M.W.; et al. Induction chemotherapy in pancreatic cancer: CA 19-9 may predict resectability and survival. HPB 2020, 22, 224–232. [Google Scholar] [CrossRef]
- Servin-Rojas, M.; Fong, Z.V.; Fernandez-Del Castillo, C.; Ferrone, C.R.; Lee, H.; Lopez-Verdugo, F.; Qiao, G.; Rocha-Castellanos, D.M.; Lillemoe, K.D.; Qadan, M. Tumor Size Reduction and Serum Carbohydrate Antigen 19-9 Kinetics After Neoadjuvant FOLFIRINOX in Patients with Pancreatic Ductal Adenocarcinoma. Surgery 2024, 175, 471–476. [Google Scholar] [CrossRef]
- van Veldhuisen, E.; Vogel, J.A.; Klompmaker, S.; Busch, O.R.; van Laarhoven, H.W.M.; van Lienden, K.P.; Wilmink, J.W.; Marsman, H.A.; Besselink, M.G. Added value of CA19-9 response in predicting resectability of locally advanced pancreatic cancer following induction chemotherapy. HPB 2018, 20, 605–611. [Google Scholar] [CrossRef]
- Alva-Ruiz, R.; Yohanathan, L.; Yonkus, J.A.; Abdelrahman, A.M.; Gregory, L.A.; Halfdanarson, T.R.; Mahipal, A.; McWilliams, R.R.; Ma, W.W.; Hallemeier, C.L.; et al. Neoadjuvant Chemotherapy Switch in Borderline Resectable/Locally Advanced Pancreatic Cancer. Ann. Surg. Oncol. 2022, 29, 1579–1591. [Google Scholar] [CrossRef]
- van Manen, L.; Groen, J.V.; Putter, H.; Vahrmeijer, A.L.; Swijnenburg, R.-J.; Bonsing, B.A.; Mieog, J.S.D. Elevated CEA and CA19-9 serum levels independently predict advanced pancreatic cancer at diagnosis. Biomarkers 2020, 25, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Schlieman, M.G.; Ho, H.S.; Bold, R.J. Utility of Tumor Markers in Determining Resectability of Pancreatic Cancer. Arch. Surg. 2003, 138, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Distler, M.; Pilarsky, E.; Kersting, S.; Grützmann, R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas—A retrospective tumor marker prognostic study. Int. J. Surg. 2013, 11, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Duraker, N.; Hot, S.; Polat, Y.; Höbek, A.; Gençler, N.; Urhan, N. CEA, CA 19-9, and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. J. Surg. Oncol. 2007, 95, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.E.; Mellotte, G.S.; Mylod, E.; O’Brien, R.M.; O’Connell, F.; Buckley, C.E.; Arlow, J.; Nguyen, K.; Mockler, D.; Meade, A.D.; et al. Diagnostic Accuracy of Blood-based Biomarkers for Pancreatic Cancer: A Systematic Review and Meta-analysis. Cancer Res. Commun. 2022, 2, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Omiya, K.; Oba, A.; Inoue, Y.; Kobayashi, K.; Wu, Y.H.A.; Ono, Y.; Sato, T.; Sasaki, T.; Ozaka, M.; Sasahira, N.; et al. Serum DUPAN-2 could be an Alternative Biological Marker for CA19-9 Non-secretors with Pancreatic Cancer. Ann. Surg. 2022, 277, e1278–e1283. [Google Scholar] [CrossRef] [PubMed]
- Kawa, S.; Oguchi, H.; Kobayashi, T.; Tokoo, M.; Furuta, S.; Kanai, M.; Homma, T. Elevated serum levels of Dupan-2 in pancreatic cancer patients negative for Lewis blood group phenotype. Br. J. Cancer 1991, 64, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Liu, C.; Guo, M.; Cheng, H.; Lu, Y.; Jin, K.; Liu, L.; Long, J.; Xu, J.; Lu, R.; et al. Potential Biomarkers in Lewis Negative Patients with Pancreatic Cancer. Ann. Surg. 2017, 265, 800–805. [Google Scholar] [CrossRef]
- Doppenberg, D.; Stoop, T.F.; van Dieren, S.; Katz, M.H.G.; Janssen, Q.P.; Nasar, N.; Prakash, L.R.; Theijse, R.T.; Tzeng, C.D.; Wei, A.C.; et al. Serum CEA as a Prognostic Marker for Overall Survival in Patients with Localized Pancreatic Adenocarcinoma and Non-Elevated CA19-9 Levels Treated with FOLFIRINOX as Initial Treatment: A TAPS Consortium Study. Ann. Surg. Oncol. 2024, 1–14. [Google Scholar] [CrossRef]
- Cheng, H.; Long, F.; Jaiswar, M.; Yang, L.; Wang, C.; Zhou, Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis. Sci. Rep. 2015, 5, 11026. [Google Scholar] [CrossRef]
- Shrotriya, S.; Walsh, D.; Bennani-Baiti, N.; Thomas, S.; Lorton, C. C-Reactive Protein Is an Important Biomarker for Prognosis Tumor Recurrence and Treatment Response in Adult Solid Tumors: A Systematic Review. PLoS ONE 2015, 10, e0143080. [Google Scholar] [CrossRef] [PubMed]
- Recio-Boiles, A.; Nallagangula, A.; Veeravelli, S.; Vondrak, J.; Saboda, K.; Roe, D.; Elquza, E.; McBride, A.; Babiker, H.M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios inversely correlate to clinical and pathologic stage in patients with resectable pancreatic ductal adenocarcinoma. Ann. Pancreat. Cancer 2019, 2, 8. [Google Scholar] [CrossRef]
- Mowbray, N.G.; Griffith, D.; Hammoda, M.; Shingler, G.; Kambal, A.; Al-Sarireh, B. A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB 2018, 20, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Yao, W.; Maitra, A.; Ying, H. Recent insights into the biology of pancreatic cancer. EBioMedicine 2020, 53, 102655. [Google Scholar] [CrossRef] [PubMed]
- Helal, C.; Valery, M.; Ducreux, M.; Hollebecque, A.; Smolenschi, C. FGFR2 fusion in metastatic pancreatic ductal adenocarcinoma: Is there hope? Eur. J. Cancer 2022, 176, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; Court, C.M.; Janssen, Q.P.; Tao, A.J.; D’Angelica, M.I.; Drebin, J.A.; Gonen, M.; O’Reilly, E.M.; Jarnagin, W.R.; Wei, A.C. Alterations in Somatic Driver Genes Are Associated with Response to Neoadjuvant FOLFIRINOX in Patients with Localized Pancreatic Ductal Adenocarcinoma. J. Am. Coll. Surg. 2022, 235, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef]
- 90. Pranzini, E.; Raugei, G.; Taddei, M.L. Metabolic Features of Tumor Dormancy: Possible Therapeutic Strategies. Cancers 2022, 14, 547. [Google Scholar] [CrossRef]
- Dreyer, S.B.; Rae, S.; Bisset, K.; Upstill-Goddard, R.; Gemenetzis, G.; Johns, A.L.; Dickson, E.J.; Mittal, A.; Gill, A.J.; Duthie, F.; et al. The Impact of Molecular Subtyping on Pathological Staging of Pancreatic Cancer. Ann. Surg. 2023, 277, e396–e405. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Okamura, R.; Fanta, P.; Patel, C.; Lanman, R.B.; Raymond, V.M.; Kato, S.; Kurzrock, R. Clinical correlates of blood-derived circulating tumor DNA in pancreatic cancer. J. Hematol. Oncol. 2019, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, H.; Chen, N.; Hao, J.; Jin, H.; Ma, X. Diagnostic value of various liquid biopsy methods for pancreatic cancer: A systematic review and meta-analysis. Medicine 2020, 99, e18581. [Google Scholar] [CrossRef] [PubMed]
- Grunvald, M.W.; Jacobson, R.A.; Kuzel, T.M.; Pappas, S.G.; Masood, A. Current Status of Circulating Tumor DNA Liquid Biopsy in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 7651. [Google Scholar] [CrossRef]
- Dawson, S.J.; Tsui, D.W.; Murtaza, M.; Biggs, H.; Rueda, O.M.; Chin, S.F.; Dunning, M.J.; Gale, D.; Forshew, T.; Mahler-Araujo, B.; et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 2013, 368, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Muinelo, L.; Dalmases, A.; Jones, F.; Edelstein, D.; Iglesias, M.; Orrillo, M.; Abalo, A.; Rodríguez, C.; Brozos, E.; et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann. Oncol. 2017, 28, 1325–1332. [Google Scholar] [CrossRef]
- Schraa, S.J.; van Rooijen, K.L.; Koopman, M.; Vink, G.R.; Fijneman, R.J.A. Cell-Free Circulating (Tumor) DNA before Surgery as a Prognostic Factor in Non-Metastatic Colorectal Cancer: A Systematic Review. Cancers 2022, 14, 2218. [Google Scholar] [CrossRef]
- Lapin, M.; Edland, K.H.; Tjensvoll, K.; Oltedal, S.; Austdal, M.; Garresori, H.; Rozenholc, Y.; Gilje, B.; Nordgård, O. Comprehensive ctDNA Measurements Improve Prediction of Clinical Outcomes and Enable Dynamic Tracking of Disease Progression in Advanced Pancreatic Cancer. Clin. Cancer Res. 2023, 29, 1267–1278. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, S.; Pandey, R.M.; Chauhan, S.S.; Saraya, A. High levels of cell-free circulating nucleic acids in pancreatic cancer are associated with vascular encasement, metastasis and poor survival. Cancer Investig. 2015, 33, 78–85. [Google Scholar] [CrossRef]
- McDuff, S.G.R.; Hardiman, K.M.; Ulintz, P.J.; Parikh, A.R.; Zheng, H.; Kim, D.W.; Lennerz, J.K.; Hazar-Rethinam, M.; Van Seventer, E.E.; Fetter, I.J.; et al. Circulating Tumor DNA Predicts Pathologic and Clinical Outcomes Following Neoadjuvant Chemoradiation and Surgery for Patients With Locally Advanced Rectal Cancer. JCO Precis. Oncol. 2021, 5, 123–132. [Google Scholar] [CrossRef]
- Lee, B.; Lipton, L.; Cohen, J.; Tie, J.; Javed, A.A.; Li, L.; Goldstein, D.; Burge, M.; Cooray, P.; Nagrial, A.; et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann. Oncol. 2019, 30, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and Dissemination Precede Pancreatic Tumor Formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.A. Cancer. The metastasis cascade. Science 2008, 321, 1785–1787. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating tumor cell isolation for cancer diagnosis and prognosis. EBioMedicine 2022, 83, 104237. [Google Scholar] [CrossRef] [PubMed]
- Gemenetzis, G.; Groot, V.P.; Yu, J.; Ding, D.; Teinor, J.A.; Javed, A.A.; Wood, L.D.; Burkhart, R.A.; Cameron, J.L.; Makary, M.A.; et al. Circulating Tumor Cells Dynamics in Pancreatic Adenocarcinoma Correlate with Disease Status: Results of the Prospective CLUSTER Study. Ann. Surg. 2018, 268, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.A.; Ding, D.; Hasanain, A.; van Oosten, F.; Yu, J.; Cameron, J.L.; Burkhart, R.A.; Zheng, L.; He, J.; Wolfgang, C.L. Persistent Circulating Tumor Cells at One Year after Oncologic Resection Predict Late Recurrence in Pancreatic Cancer. Ann. Surg. 2022, 277, 859–865. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef]
- Perri, G.; Prakash, L.; Qiao, W.; Varadhachary, G.R.; Wolff, R.; Fogelman, D.; Overman, M.; Pant, S.; Javle, M.; Koay, E.J.; et al. Response and Survival Associated with First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2020, 155, 832–839. [Google Scholar] [CrossRef]
- Ferrone, C.R.; Marchegiani, G.; Hong, T.S.; Ryan, D.P.; Deshpande, V.; McDonnell, E.I.; Sabbatino, F.; Santos, D.D.; Allen, J.N.; Blaszkowsky, L.S.; et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann. Surg. 2015, 261, 12–17. [Google Scholar] [CrossRef]
- Welsh, J.L.; Bodeker, K.; Fallon, E.; Bhatia, S.K.; Buatti, J.M.; Cullen, J.J. Comparison of response evaluation criteria in solid tumors with volumetric measurements for estimation of tumor burden in pancreatic adenocarcinoma and hepatocellular carcinoma. Am. J. Surg. 2012, 204, 580–585. [Google Scholar] [CrossRef]
- Michelakos, T.; Pergolini, I.; Castillo, C.F.; Honselmann, K.C.; Cai, L.; Deshpande, V.; Wo, J.Y.; Ryan, D.P.; Allen, J.N.; Blaszkowsky, L.S.; et al. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment with FOLFIRINOX. Ann. Surg. 2019, 269, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, J.A.; Merchant, N.B. Intensity of follow-up after pancreatic cancer resection. Ann. Surg. Oncol. 2014, 21, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.L.; Garajová, I.; Caparello, C.; Le Large, T.Y.S.; Frampton, A.E.; Vasile, E.; Funel, N.; Kazemier, G.; Giovannetti, E. Plasma miR-181a-5p Downregulation Predicts Response and Improved Survival After FOLFIRINOX in Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2020, 271, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Barenboim, A.; Lahat, G.; Nachmany, I.; Goykhman, Y.; Shacham-Shmueli, E.; Halpern, N.; Brazowski, E.; Geva, R.; Wolf, I.; et al. Increased Rate of Complete Pathologic Response After Neoadjuvant FOLFIRINOX for BRCA Mutation Carriers with Borderline Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2020, 27, 3963–3970. [Google Scholar] [CrossRef]
| Borderline Resectable | NCCN | AHBPA | Alliance | IAP | MD Anderson |
|---|---|---|---|---|---|
| Superior mesenteric Vein/Portal Vein | Reconstructable involvement (distortion, narrowing, occlusion, thrombosis) | Reconstructable abutment, encasement or occlusion | Solid contact >180° or reconstructable occlusion | SMV/PV: tumor contact 180° or greater or bilateral narrowing/occlusion, not exceeding the inferior border of the duodenum | Short-segment occlusion with suitable vessel for reconstruction |
| Superior mesenteric artery | Solid tumor contact ≤180° | Abutment | Interface between tumor and vessel measuring < 180° | Tumor contact of less than 180° without showing deformity/stenosis | Abutment ≤ 180° |
| Common hepatic artery | Solid tumor contact without extension to the coeliac artery or hepatic artery bifurcation | Abutment or short-segment encasement | Reconstructable, short-segment interface between tumor and vessel of any degree | Tumor contact without showing tumor contact of the PHA and/or CA | Short-segment encasement/abutment |
| Celiac trunk | Solid tumor contact <180° | No abutment or encasement | Interface between tumor and vessel measuring <180° | Tumor contact of less than 180° without showing deformity/stenosis | Abutment ≤ 180° |
| Biological | - | Suspicion for distant metastasis, including CA19-9 > 500 U/mL, or regional lymph node metastasis diagnosed by biopsy or PET-CT | CT findings suspicious of metastatic disease; nodal-positive disease. | ||
| Conditional | - | ECOG performance status of 2 or more | Performance status ≥ 3 or severe preexisting medical comorbidity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rompen, I.F.; Habib, J.R.; Wolfgang, C.L.; Javed, A.A. Anatomical and Biological Considerations to Determine Resectability in Pancreatic Cancer. Cancers 2024, 16, 489. https://doi.org/10.3390/cancers16030489
Rompen IF, Habib JR, Wolfgang CL, Javed AA. Anatomical and Biological Considerations to Determine Resectability in Pancreatic Cancer. Cancers. 2024; 16(3):489. https://doi.org/10.3390/cancers16030489
Chicago/Turabian StyleRompen, Ingmar F., Joseph R. Habib, Christopher L. Wolfgang, and Ammar A. Javed. 2024. "Anatomical and Biological Considerations to Determine Resectability in Pancreatic Cancer" Cancers 16, no. 3: 489. https://doi.org/10.3390/cancers16030489
APA StyleRompen, I. F., Habib, J. R., Wolfgang, C. L., & Javed, A. A. (2024). Anatomical and Biological Considerations to Determine Resectability in Pancreatic Cancer. Cancers, 16(3), 489. https://doi.org/10.3390/cancers16030489






