Deep Learning Applications in Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
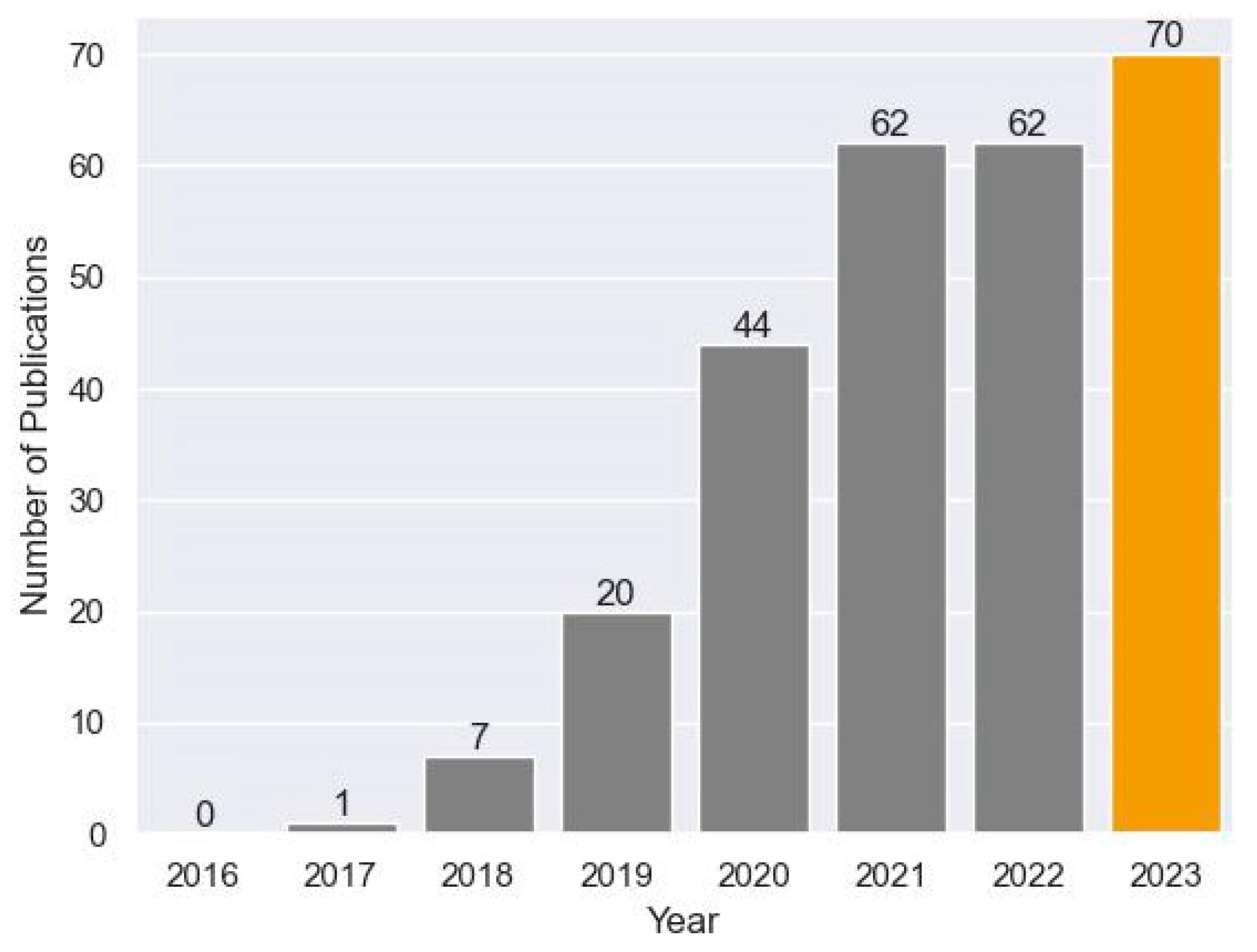
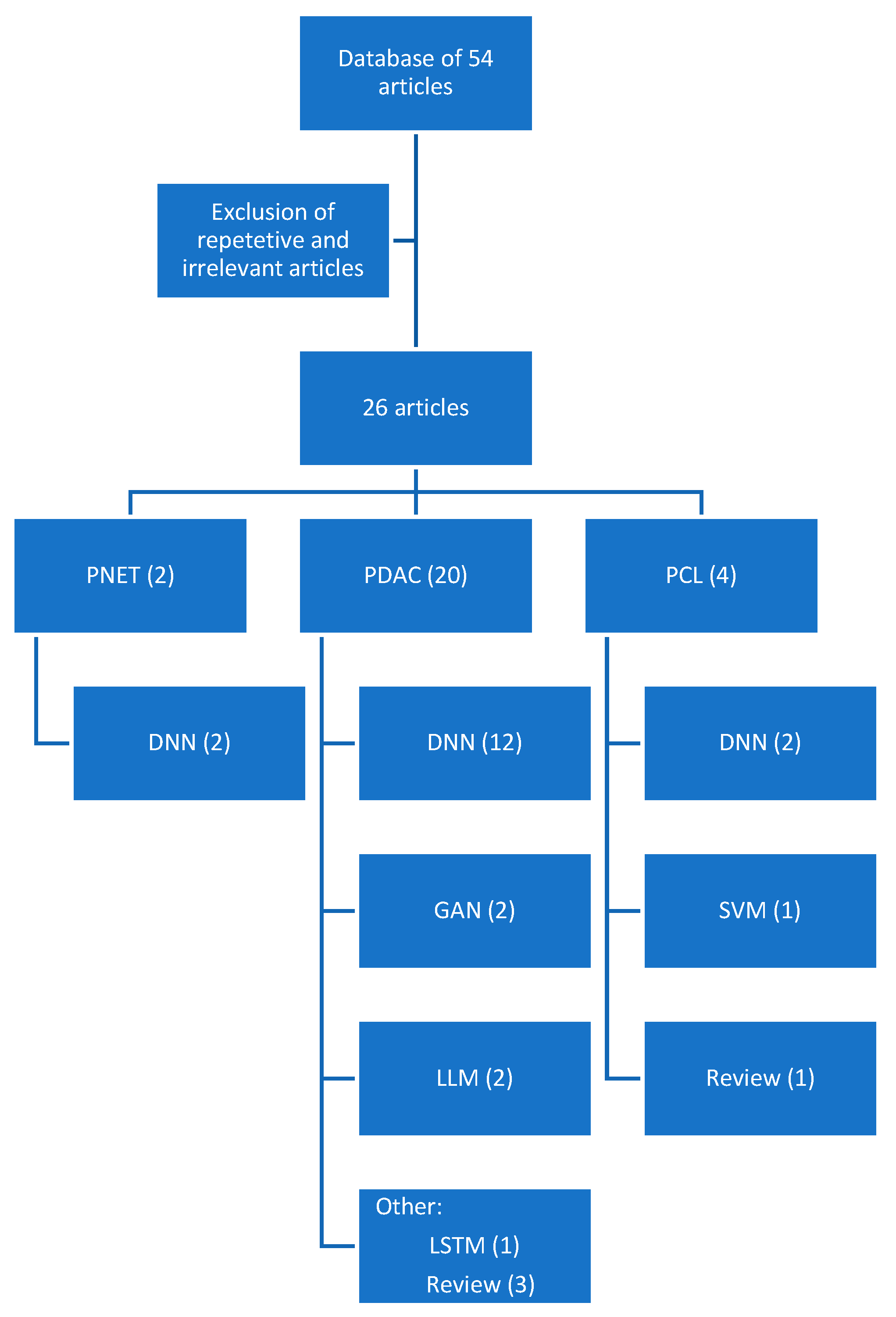
2. Materials and Methods
3. Results
3.1. Pancreatic Ductal Adenocarcinoma
3.1.1. Applications in the Diagnosis of Pancreatic Ductal Adenocarcinoma
3.1.2. Applications in Predicting Postoperative Outcomes
3.1.3. Applications in Treatment Response
3.1.4. Applications of Large Language Models
3.1.5. Applications of Generative Adversarial Networks
3.2. Pancreatic Cystic Lesions
3.3. Pancreatic Neuroendocrine Tumors
3.4. Ethical Considerations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Keane, M.G.; Afghani, E. A Review of the Diagnosis and Management of Premalignant Pancreatic Cystic Lesions. J. Clin. Med. 2021, 10, 1284. [Google Scholar] [CrossRef]
- Marti-Bonmati, L.; Cerdá-Alberich, L.; Pérez-Girbés, A.; Beveridge, R.D.; Orón, E.M.; Rojas, J.P.; Alberich-Bayarri, A. Pancreatic cancer, radiomics and artificial intelligence. Br. J. Radiol. 2022, 95, 20220072. [Google Scholar] [CrossRef]
- Facciorusso, A.; Bajwa, H.; Menon, K.; Buccino, V.; Muscatiello, N. Comparison between 22G aspiration and 22G biopsy needles for EUS-guided sampling of pancreatic lesions: A meta-analysis. Endosc. Ultrasound 2020, 9, 167–174. [Google Scholar] [CrossRef]
- Berbís, M.A.; Godino, F.P.; del Val, J.R.; Mata, L.A.; Luna, A. Clinical impact of artificial intelligence-based solutions on imaging of the pancreas and liver. World J. Gastroenterol. 2023, 29, 1427–1445. [Google Scholar] [CrossRef]
- Singh, S. Cousins of Artificial Intelligence. Towards Data Science. Available online: https://towardsdatascience.com/cousins-of-artificial-intelligence-dda4edc27b55 (accessed on 19 October 2023).
- Fu, N.; Fu, W.; Chen, H.; Chai, W.; Qian, X.; Wang, W.; Jiang, Y.; Shen, B. A deep-learning radiomics-based lymph node metastasis predictive model for pancreatic cancer: A diagnostic study. Int. J. Surg. 2023, 109, 2196–2203. [Google Scholar] [CrossRef]
- Faur, A.C.; Lazar, D.C.; Ghenciu, L.A. Artificial intelligence as a noninvasive tool for pancreatic cancer prediction and diagnosis. World J. Gastroenterol. 2023, 29, 1811–1823. [Google Scholar] [CrossRef]
- Gu, J.; Pan, J.; Hu, J.; Dai, L.; Zhang, K.; Wang, B.; He, M.; Zhao, Q.; Jiang, T. Prospective assessment of pancreatic ductal adenocarcinoma diagnosis from endoscopic ultrasonography images with the assistance of deep learning. Cancer 2023, 129, 2214–2223. [Google Scholar] [CrossRef]
- Kambakamba, P.; Mannil, M.; Herrera, P.E.; Müller, P.C.; Kuemmerli, C.; Linecker, M.; von Spiczak, J.; Hüllner, M.W.; Raptis, D.A.; Petrowsky, H.; et al. The potential of machine learning to predict postoperative pancreatic fistula based on preoperative, non-contrast-enhanced CT: A proof-of-principle study. Surgery 2019, 167, 448–454. [Google Scholar] [CrossRef]
- Chang, J.; Liu, Y.; Saey, S.A.; Chang, K.C.; Shrader, H.R.; Steckly, K.L.; Rajput, M.; Sonka, M.; Chan, C.H.F. Machine-learning based investigation of prognostic indicators for oncological outcome of pancreatic ductal adenocarcinoma. Front. Oncol. 2022, 12, 895515. [Google Scholar] [CrossRef]
- Wei, W.; Jia, G.; Wu, Z.; Wang, T.; Wang, H.; Wei, K.; Cheng, C.; Liu, Z.; Zuo, C. A multidomain fusion model of radiomics and deep learning to discriminate between PDAC and AIP based on 18F-FDG PET/CT images. Jpn. J. Radiol. 2022, 41, 417–427. [Google Scholar] [CrossRef]
- Bian, Y.; Zheng, Z.; Fang, X.; Jiang, H.; Zhu, M.; Yu, J.; Zhao, H.; Zhang, L.; Yao, J.; Lu, L.; et al. Artificial Intelligence to Predict Lymph Node Metastasis at CT in Pancreatic Ductal Adenocarcinoma. Radiology 2023, 306, 160–169. [Google Scholar] [CrossRef]
- Tong, T.; Gu, J.; Xu, D.; Song, L.; Zhao, Q.; Cheng, F.; Yuan, Z.; Tian, S.; Yang, X.; Tian, J.; et al. Deep learning radiomics based on contrast-enhanced ultrasound images for assisted diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis. BMC Med. 2022, 20, 74. [Google Scholar] [CrossRef]
- Gai, T.; Thai, T.; Jones, M.; Jo, J.; Zheng, B. Applying a radiomics-based CAD scheme to classify between malignant and benign pancreatic tumors using CT images. J. X-ray Sci. Technol. 2022, 30, 377–388. [Google Scholar] [CrossRef]
- An, C.; Li, D.; Li, S.; Li, W.; Tong, T.; Liu, L.; Jiang, D.; Jiang, L.; Ruan, G.; Hai, N.; et al. Deep learning radiomics of dual-energy computed tomography for predicting lymph node metastases of pancreatic ductal adenocarcinoma. Eur. J. Nucl. Med. 2021, 49, 1187–1199. [Google Scholar] [CrossRef]
- Zhang, Y.; Lobo-Mueller, E.M.; Karanicolas, P.; Gallinger, S.; Haider, M.A.; Khalvati, F. Improving prognostic performance in resectable pancreatic ductal adenocarcinoma using radiomics and deep learning features fusion in CT images. Sci. Rep. 2021, 11, 1378. [Google Scholar] [CrossRef]
- Ziegelmayer, S.; Kaissis, G.; Harder, F.; Jungmann, F.; Müller, T.; Makowski, M.; Braren, R. Deep Convolutional Neural Network-Assisted Feature Extraction for Diagnostic Discrimination and Feature Visualization in Pancreatic Ductal Adenocarcinoma (PDAC) versus Autoimmune Pancreatitis (AIP). J. Clin. Med. 2020, 9, 4013. [Google Scholar] [CrossRef]
- Do, R.K.G.; Lupton, K.; Andrieu, P.I.C.; Luthra, A.; Taya, M.; Batch, K.; Nguyen, H.; Rahurkar, P.; Gazit, L.; Nicholas, K.; et al. Patterns of Metastatic Disease in Patients with Cancer Derived from Natural Language Processing of Structured CT Radiology Reports over a 10-year Period. Radiology 2021, 301, 115–122. [Google Scholar] [CrossRef]
- Walker, H.L.; Ghani, S.; Kuemmerli, C.; Nebiker, C.A.; Müller, B.P.; Raptis, D.A.; Staubli, S.M. Reliability of Medical Information Provided by ChatGPT: Assessment Against Clinical Guidelines and Patient Information Quality Instrument. J. Med. Internet. Res. 2023, 25, e47479. [Google Scholar] [CrossRef]
- Momin, S.; Lei, Y.; Wang, T.; Zhang, J.; Roper, J.; Bradley, J.D.; Curran, W.J.; Patel, P.; Liu, T.; Yang, X. Learning-based dose prediction for pancreatic stereotactic body radiation therapy using dual pyramid adversarial network. Phys. Med. Biol. 2021, 66, 125019. [Google Scholar] [CrossRef]
- Hooshangnejad, H.; Chen, Q.; Feng, X.; Zhang, R.; Ding, K. deepPERFECT: Novel Deep Learning CT Synthesis Method for Expeditious Pancreatic Cancer Radiotherapy. Cancers 2023, 15, 3061. [Google Scholar] [CrossRef]
- Saillard, C.; Delecourt, F.; Schmauch, B.; Moindrot, O.; Svrcek, M.; Bardier-Dupas, A.; Emile, J.F.; Ayadi, M.; Rebours, V.; de Mestier, L.; et al. Pacpaint: A histology-based deep learning model uncovers the extensive intratumor molecular heterogeneity of pancreatic adenocarcinoma. Nat. Commun. 2023, 14, 3459. [Google Scholar] [CrossRef]
- Al-Fatlawi, A.; Malekian, N.; García, S.; Henschel, A.; Kim, I.; Dahl, A.; Jahnke, B.; Bailey, P.; Bolz, S.N.; Poetsch, A.R.; et al. Deep Learning Improves Pancreatic Cancer Diagnosis Using RNA-Based Variants. Cancers 2021, 13, 2654. [Google Scholar] [CrossRef]
- Huang, C.; Chopra, S.; Bolan, C.W.; Chandarana, H.; Harfouch, N.; Hecht, E.M.; Lo, G.C.; Megibow, A.J. Pancreatic Cystic Lesions. Gastrointest. Endosc. Clin. N. Am. 2023, 33, 533–546. [Google Scholar] [CrossRef]
- Kuwahara, T.; Hara, K.; Mizuno, N.; Okuno, N.; Matsumoto, S.; Obata, M.; Kurita, Y.; Koda, H.; Toriyama, K.; Onishi, S.; et al. Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clin. Transl. Gastroenterol. 2019, 10, e00045-8. [Google Scholar] [CrossRef]
- Chakraborty, J.; Midya, A.; Gazit, L.; Attiyeh, M.; Langdon-Embry, L.; Allen, P.J.; Do, R.K.G.; Simpson, A.L. CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas. Med. Phys. 2018, 45, 5019–5029. [Google Scholar] [CrossRef]
- Liang, W.; Tian, W.; Wang, Y.; Wang, P.; Wang, Y.; Zhang, H.; Ruan, S.; Shao, J.; Zhang, X.; Huang, D.; et al. Classification prediction of pancreatic cystic neoplasms based on radiomics deep learning models. BMC Cancer 2022, 22, 1237. [Google Scholar] [CrossRef]
- Song, C.; Wang, M.; Luo, Y.; Chen, J.; Peng, Z.; Wang, Y.; Zhang, H.; Li, Z.-P.; Shen, J.; Huang, B.; et al. Predicting the recurrence risk of pancreatic neuroendocrine neoplasms after radical resection using deep learning radiomics with preoperative computed tomography images. Ann. Transl. Med. 2021, 9, 833. [Google Scholar] [CrossRef]
- Gao, X.; Wang, X. Deep learning for World Health Organization grades of pancreatic neuroendocrine tumors on contrast-enhanced magnetic resonance images: A preliminary study. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1981–1991. [Google Scholar] [CrossRef]
- Blyuss, O.; Zaikin, A.; Cherepanova, V.; Munblit, D.; Kiseleva, E.M.; Prytomanova, O.M.; Duffy, S.W.; Crnogorac-Jurcevic, T. Development of PancRISK, a urine biomarker-based risk score for stratified screening of pancreatic cancer patients. Br. J. Cancer 2019, 122, 692–696. [Google Scholar] [CrossRef]
- Aung, K.L.; Fischer, S.E.; Denroche, R.E.; Jang, G.-H.; Dodd, A.; Creighton, S.; Southwood, B.; Liang, S.-B.; Chadwick, D.; Zhang, A.; et al. Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin. Cancer Res. 2018, 24, 1344–1354. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Alva-Ruiz, R.; Yohanathan, L.; Yonkus, J.A.; Abdelrahman, A.M.; Gregory, L.A.; Halfdanarson, T.R.; Mahipal, A.; McWilliams, R.R.; Ma, W.W.; Hallemeier, C.L.; et al. Neoadjuvant Chemotherapy Switch in Borderline Resectable/Locally Advanced Pancreatic Cancer. Ann. Surg. Oncol. 2021, 29, 1579–1591. [Google Scholar] [CrossRef]
- Al Abbas, A.I.; Zenati, M.; Reiser, C.J.; Hamad, A.; Jung, J.P.; Zureikat, A.H.; Zeh, H.J.; Hogg, M.E. Serum CA19-9 Response to Neoadjuvant Therapy Predicts Tumor Size Reduction and Survival in Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 2007–2014. [Google Scholar] [CrossRef]
- Watson, M.D.; Baimas-George, M.R.; Murphy, K.J.; Pickens, R.C.; Iannitti, D.A.; Martinie, J.B.; Baker, E.H.; Vrochides, D.; Ocuin, L.M. Pure and Hybrid Deep Learning Models can Predict Pathologic Tumor Response to Neoadjuvant Therapy in Pancreatic Adenocarcinoma: A Pilot Study. Am. Surg. 2020, 87, 1901–1909. [Google Scholar] [CrossRef]
- Buch, P.; Thakkar, A. Reconstructing Medical Images Using Generative Adversarial Networks: A Study; Springer: Singapore, 2022; pp. 81–92. [Google Scholar] [CrossRef]
- Facciorusso, A.; Kovacevic, B.; Yang, D.; Vilas-Boas, F.; Martínez-Moreno, B.; Stigliano, S.; Rizzatti, G.; Sacco, M.; Arevalo-Mora, M.; Villarreal-Sanchez, L.; et al. Predictors of adverse events after endoscopic ultrasound-guided through-the-needle biopsy of pancreatic cysts: A recursive partitioning analysis. Endoscopy 2022, 54, 1158–1168. [Google Scholar] [CrossRef]
- Machado, N.; al Qadhi, H.; al Wahibi, K. Intraductal papillary mucinous neoplasm of pancreas. N. Am. J. Med. Sci. 2015, 7, 160–175. [Google Scholar] [CrossRef]
- El Khoury, R.; Kabir, C.; Maker, V.K.; Banulescu, M.; Wasserman, M.; Maker, A.V. What is the Incidence of Malignancy in Resected Intraductal Papillary Mucinous Neoplasms? An Analysis of Over 100 US Institutions in a Single Year. Ann. Surg. Oncol. 2018, 25, 1746–1751. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Yang, C.-Y.; Wu, J.-M.; Kuo, T.-C.; Tien, Y.-W. Utility of the 2006 Sendai and 2012 Fukuoka guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas: A single-center experience with 138 surgically treated patients. Medicine 2016, 95, e4922. [Google Scholar] [CrossRef]
- Mustard, A. WHO Calls for Safe and Ethical AI for Health; WHO/Blink Media: Brooklyn, NY, USA, 2023. [Google Scholar]
- Naik, N.; Hameed, B.M.Z.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R.; Aggarwal, K.; Ibrahim, S.; Patil, V.; Smriti, K.; et al. Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front. Surg. 2022, 9, 862322. [Google Scholar] [CrossRef]


| Author | Title | Article Type | Pathology | Algorithm | Performance (AUC-ROC) |
|---|---|---|---|---|---|
| Fu et al. [9] | A deep-learning radiomics based lymph node metastasis predictive model for pancreatic cancer: A diagnostic study | Retrospective | PDAC | CNN | 0.85 |
| Faur et al. [10] | Artificial intelligence as a noninvasive tool for pancreatic cancer prediction and diagnosis | Review article | PDAC | n/a | n/a |
| Gu et al. [11] | Prospective assessment of pancreatic ductal adenocarcinoma diagnosis from endoscopic ultrasonography images with the assistance of deep learning | Prospective | PDAC | CNN ResNet50 | 0.9 |
| Kambakamba et al. [12] | The potential of machine learning to predict postoperative pancreatic fistula based on preoperative, non-contrast-enhanced CT: A proof-of-principle study | Retrospective | Postoperative Whipple | Multiple | 0.78 |
| Chang et al. [13] | Machine-learning based investigation of prognostic indicators for oncological outcome of pancreatic ductal adenocarcinoma | Retrospective | PDAC | CNN | 0.79/0.85 (LN/margin status) |
| Wei et al. [14] | A multidomain fusion model of radiomics and deep learning to discriminate between PDAC and AIP based on 18F-FDG PET/CT images | Retrospective | PDAC | CNN VGG11 | 0.96 |
| Bian et al. [15] | Artificial Intelligence to Predict Lymph Node Metastasis at CT in Pancreatic Ductal Adenocarcinoma | Retrospective | PDAC | CNN | 0.91 |
| Tong et al. [16] | Deep learning radiomics based on contrast enhanced ultrasound images for assisted diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis | Retrospective | PDAC | CNN ResNet50 | 0.9 |
| Gai et al. [17] | Applying a radiomics-based CAD scheme to classify between malignant and benign pancreatic tumors using CT images | Retrospective | PDAC | LSTM | 0.7 |
| An et al. [18] | Deep learning radiomics of dual-energy computed tomography for predicting lymph node metastases of pancreatic ductal adenocarcinoma | Retrospective | PDAC | CNN ResNet18 | 0.87 |
| Zhang et al. [19] | Improving prognostic performance in resectable pancreatic ductal adenocarcinoma using radiomics and deep learning features fusion in CT images | Retrospective | PDAC | CNN | 0.84 |
| Zeigelmayer et al. [20] | Deep Convolutional Neural Network-Assisted Feature Extraction for Diagnostic Discrimination and Feature Visualization in Pancreatic Ductal Adenocarcinoma (PDAC) versus Autoimmune Pancreatitis (AIP) | Retrospective | PDAC | CNN VGG19 | 0.9 |
| Do et al. [21] | Patterns of Metastatic Disease in Patients with Cancer Derived from Natural Language Processing of Structured CT Radiology Reports over a 10-year Period | Retrospective | PDAC | NLP | Accuracy 90% |
| Walker et al. [22] | Reliability of Medical Information Provided by ChatGPT: Assessment Against Clinical Guidelines and Patient Information Quality Instrument | Retrospective | PDAC | GPT 4 | 60% Agreement |
| Momin et al. [23] | Learning-based dose prediction for pancreatic stereotactic body radiation therapy using dual pyramid adversarial network | Retrospective | PDAC | GAN | Accuracy 87% |
| Hooshangnejad et al. [24] | deepPERFECT: Novel Deep Learning CT Synthesis Method for Expeditious Pancreatic Cancer Radiotherapy | Prospective | PDAC | 3D UNET GAN | DSC 93% |
| Bonmati et al. [5] | Pancreatic cancer, radiomics and artificial intelligence | Review Article | PDAC | n/a | n/a |
| Saillard et al. [25] | Pacpaint: a histology-based deep learning model uncovers the extensive intratumor molecular heterogeneity of pancreatic adenocarcinoma | Retrospective | PDAC | ANN | 0.8–0.9 |
| Al-Fatlawi et al. [26] | Deep Learning Improves Pancreatic Cancer Diagnosis Using RNA-Based Variants | Retrospective | PDAC | DNN | 0.96 |
| Huang et al. [27] | Pancreatic Cystic Lesions: Next Generation of Radiologic Assessment | Review Article | PCL | n/a | n/a |
| Kuwahara et al. [28] | Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas | Retrospective | PCL | CNN ResNet50 | 0.9 |
| Chakraborty et al. [29] | CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas | Retrospective | PCL | SVM/RF | 0.8 |
| Liang et al. [30] | Classification prediction of pancreatic cystic neoplasms based on radiomics deep learning models | Retrospective | PCL | ANN | 0.9 |
| Song et al. [31] | Predicting the recurrence risk of pancreatic neuroendocrine neoplasms after radical resection using deep learning radiomics with preoperative computed tomography images | Retrospective | PNET | CNN UNET | 0.8 |
| Gao et al. [32] | Deep learning for World Health Organization grades of pancreatic neuroendocrine tumors on contrast-enhanced magnetic resonance images: a preliminary study | Retrospective | PNET | CNN GAN | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, H.; Zanos, T.; Hewitt, D.B. Deep Learning Applications in Pancreatic Cancer. Cancers 2024, 16, 436. https://doi.org/10.3390/cancers16020436
Patel H, Zanos T, Hewitt DB. Deep Learning Applications in Pancreatic Cancer. Cancers. 2024; 16(2):436. https://doi.org/10.3390/cancers16020436
Chicago/Turabian StylePatel, Hardik, Theodoros Zanos, and D. Brock Hewitt. 2024. "Deep Learning Applications in Pancreatic Cancer" Cancers 16, no. 2: 436. https://doi.org/10.3390/cancers16020436
APA StylePatel, H., Zanos, T., & Hewitt, D. B. (2024). Deep Learning Applications in Pancreatic Cancer. Cancers, 16(2), 436. https://doi.org/10.3390/cancers16020436






